Abstract
Skull structure is intimately associated with feeding ability in vertebrates, both in terms of specific performance measures and general ecological characteristics. This study quantitatively assessed variation in the shape of the cranium and mandible in varanoid lizards, and its relationship to structural performance (von Mises strain) and interspecific differences in feeding ecology. Geometric morphometric and linear morphometric analyses were used to evaluate morphological differences, and finite element analysis was used to quantify variation in structural performance (strain during simulated biting, shaking and pulling). This data was then integrated with ecological classes compiled from relevant scientific literature on each species in order to establish structure-function relationships. Finite element modelling results showed that variation in cranial morphology resulted in large differences in the magnitudes and locations of strain in biting, shaking and pulling load cases. Gracile species such as Varanus salvadorii displayed high strain levels during shaking, especially in the areas between the orbits. All models exhibit less strain during pull back loading compared to shake loading, even though a larger force was applied (pull =30N, shake = 20N). Relationships were identified between the morphology, performance, and ecology. Species that did not feed on hard prey clustered in the gracile region of cranial morphospace and exhibited significantly higher levels of strain during biting (P = 0.0106). Species that fed on large prey clustered in the elongate area of mandible morphospace. This relationship differs from those that have been identified in other taxonomic groups such as crocodiles and mammals. This difference may be due to a combination of the open ‘space-frame’ structure of the varanoid lizard skull, and the ‘pull back’ behaviour that some species use for processing large prey.
Introduction
The varanoid lizards comprise three modern genera (Varanus, Heloderma and Lanthanotus) and make up important components of various modern predator guilds [1,2]. For example, the monsoonal tropics of Northern Australia contain up to 11 sympatric varanoid species [2] that function as apex predators, mesopredators, insectivores and scavengers [2,3]. Despite having a conserved body plan, varanoids exhibit considerable variation in body size [4,5], limb dimensions [6] and skull structure [7]. Whilst the influence of varanid (referring to the genus Varanus) body size on locomotion and ecology has been explored, the consequences and contribution of skull variation for biomechanical and ecological performance have received less attention [5,8,9]. Here we quantify variation in cranial morphology between a number of extant varanoid species and examine the biomechanical and ecological consequences of these differences.
Varanoid lizards were previously considered a monophyletic group [10] however some recent phylogenetic analyses group Varanus and Lanthanotus together with Shinisaurus in Paleoanguimorpha, and find Heloderma to be a member of a sister group (the Neoanguimorpha) to these [11]. Within a phylogenetic context, the inclusion of Heloderma represents an outgroup to the other specimens included in the study (which are all part of the Paleoanguimorpha). Recent revisions of squamate relationships indicate that the phylogeny may still not be completely resolved [12,13]. Here we use the term varanoid to refer to Varanus, Lanthanotus and Heloderma as a useful ecological or functional grouping, but note that future consensus of phylogenetic analyses may find it to be polyphyletic.
Varanoid lizards exhibit a range of behaviours during capture and processing of prey. Prey is predominantly killed using a bite. If too large to be swallowed whole, prey must be processed into manageable pieces for consumption. To do this varanoid species can shake prey from side to side to tear off chunks [14], or use a sawing pull-back movement where the teeth are drawn back through the item [15,16]. From a biomechanical perspective, a major role of the skull is to transmit the force generated by jaw and postcranial musculature to the prey whilst resisting the loads induced by these behaviours.
Identifying mechanistic links between form and function is challenging because the complex morphology of biological structures, as well as often complex interplay between morphology and performance, makes analytical approaches difficult [17]. Simulation based techniques, such as finite element analysis (FEA), provide a valuable methodology to investigate the effect of complex differences in cranial structure on performance measures [18]. When combined with modern morphometric analyses, these structural models represent a powerful method for predicting the influence of morphology on mechanical performance [19,20,21,22,23]. Palaeontologists and biologists have gained great insights by utilizing FEA to explore how variations in morphology influence structural performance [21,23,24,25,26,27,28,29,30,31,32,33,34,35]. These studies have greatly increased understanding of how (and to what degree) biological form relates to the structural performance (ability to resist loading) of the skull and the influence this has on the animal’s ecology.
The cranial design of varanoid lizards is highly fenestrated compared to mammalian and crocodilian skulls [36]. In engineering design a highly fenestrated structure is termed a “space frame” in contrast to a “shell” construction. In the context of cranial mechanics, it is not clear whether space frame structures exhibit the same form-function relationships as shell constructions. Logically the strength of space frame constructions will be dictated to a high degree by the thickness, position and orientation of the struts, whereas in shell constructions it will be determined by the thickness of the continuous wall that is used to distribute loads. Presumably space-frame skulls are expected to work in a different manner to shell structured skulls with load being transferred through the struts perpendicular to its long axis. The mechanics of shell-type skulls have been investigated a number of times using finite element analysis [24,26,31,32,33,34,37,38,39,40,41,42,43,44]; space-frame skulls have not been examined to the same degree, with only single specimen studies [45,46,47,48,49].
Here we present a comparative cranial FEA of varanoid species, with the aim of quantitatively assessing the relationship between the shape of varanoid lizard crania and mandibles, their structural performance to biologically relevant loading (biting, shaking and pulling forces) and the dietary ecology of the species. Our study includes high resolution models of 13 skulls, making it one of the largest three dimensional finite element analyses of cranial mechanics performed to date.
Beam theory provides a means of calculating the load carrying characteristics of a simple beam structure [50]. Based on beam theory we predicted that:
Lower strain levels will occur in taller craniums and mandibles during bite loading.
Lower strain levels will occur in wider craniums and mandibles during shake loading.
Lower strain levels will occur in taller and wider craniums and mandibles during pull loading.
When comparing performance and ecology it is predicted that:
Species that feed on hard prey items will exhibit less strain during bite loading than other species.
Species that feed on large prey items will exhibit less strain during shake and pull loading than other species.
Methods
Data acquisition and morphometric analysis
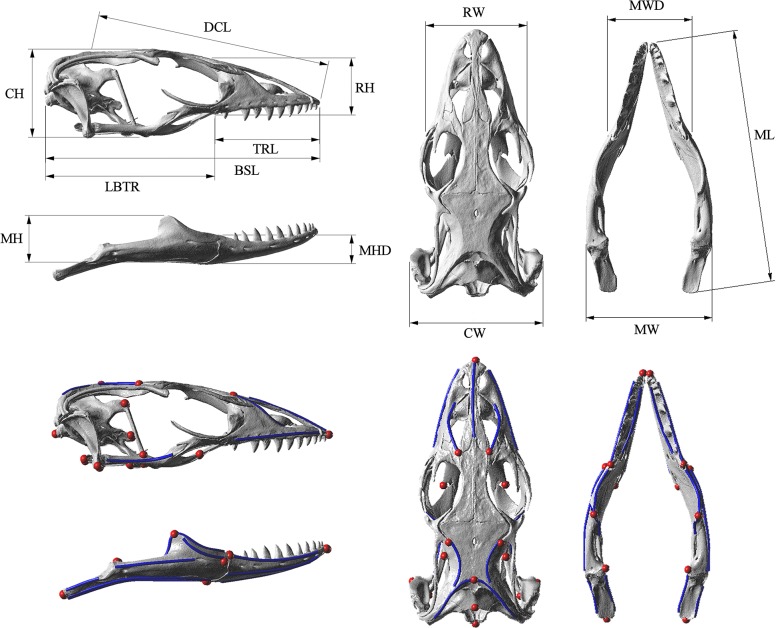
Computer tomography (CT) scans of 13 varanoid skulls, representing 12 species, were obtained from online libraries (www.digimorph.org) or through scanning (at either Newcastle Mater Hospital using a Toshiba Aquilion 64 slice CT or at Sydney University using an Xradia MicroXCT-400 micro CT). The specimens were selected from the approximately 79 possible varanoid species to maximise phylogenetic and morphological variation (Fig 1). These specimens are housed in a number of repositories (Table 1). Two of the specimens (V. gouldii and V. komodoensis) represent sub-adult specimens. Mimics V13 (MATERIALISE, Belgium) was used to convert scan data into three dimensional surface geometry of the cranium and mandible. Morphometrics were undertaken using both 3D and linear methods. Thirteen linear measurements were taken in the 3D CAD software package Rhino [51] (Fig 2). LANDMARK [52] was used to select the location in 3D space of a series of 24 landmarks and 11 curves on the cranium and 14 landmarks and 22 curves on the mandible for geometric morphometric analysis (Fig 2). These locations were exported as.pts files, manually reformatted, and then imported into MOPHOLOGIKA [53] where Procrustes superimposition and principle component analysis were undertaken (S1 File).
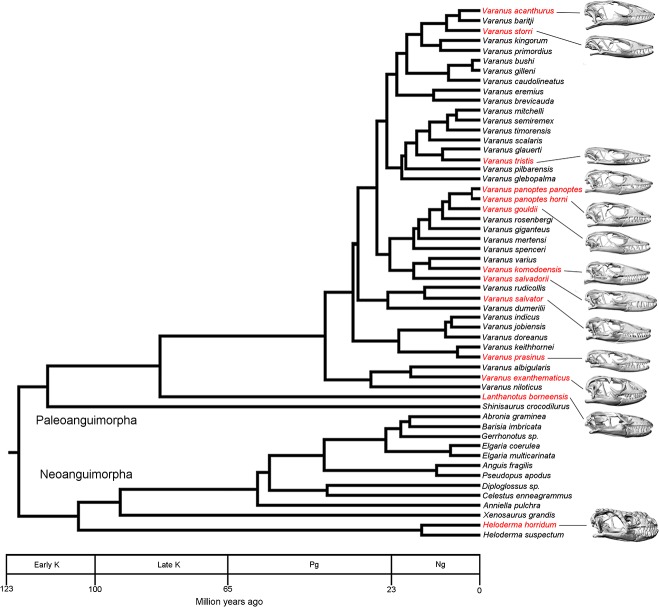
Fig 1. Phylogenetic sampling.
Phylogeny of Paleoanguimorpha and Neoanguimorpha showing the taxa used within this study in red text. Labels on timescale: K, Cretaceous; Pg, Paleogene; Ng, Neogene. Adapted from Vidal [11]. Note that the lateral views of the skulls are not to scale.
Table 1. Specimens used in this study.
| Taxon | Specimen number | Repository of original specimen | Source of digital data | Dorsal cranial length (cm) |
|---|---|---|---|---|
| Heloderma horridum | TNHC 64380 | Texas Memorial Museum | Digimorph online library | 5.7 |
| Lanthanotus borneensis | YPM 6057 | Yale Peabody Museum of Natural History | Digimorph online library | 1.8 |
| Varanus acanthurus | UTA 13015 | The Amphibian and Reptile Diversity Research Centre at the University of Texas at Arlington | Digimorph online library | 2.7 |
| Varanus exanthematicus | FMNH 58288 | Florida Museum of Natural History | Digimorph online library | 4.9 |
| Varanus gouldii | AMR 142826 | The Australian Museum | Digimorph online library | 4.0 |
| Varanus komodoensis | AMR 106933 | The Australian Museum | Sydney University | 11.2 |
| Varanus panoptes panoptes | AMR 75084 | The Australian Museum | Newcastle Mater Hospital | 7.4 |
| Varanus panoptes horni | TMM-M-1295 | Texas Memorial Museum | Newcastle Mater Hospital CT | 5.3 |
| Varanus prasinus | AMR 166380 | The Australian Museum | Sydney University | 3.2 |
| Varanus salvadorii | QMJ 14498 | Queensland Museum | Newcastle Mater Hospital | 11.2 |
| Varanus salvator | FMNH 35144 | Florida Museum of Natural History | Digimorph online library | 11.9 |
| Varanus storri | AMR 143912 | The Australian Museum | Sydney University | 2.2 |
| Varanus tristis | AMR 143919 | The Australian Museum | Sydney University | 3.1 |
Fig 2. Linear measurements and landmarks used in morphometric analysis.
Morphometric information used in this study. Top: linear measurements taken from specimens. Bottom: Points and curves used in geometric morphometric analysis. Point landmarks are shown in red and curves are shown in blue. Abbreviations and definitions for linear measurements can be found in S1 File. The specimen shown is Varanus acanthurus: UTA 13015.
Finite element analysis
High resolution versions of the geometries used in the morphometric analysis were converted into solid meshes composed of simple tetrahedral elements (tet4s), here termed bricks, using Harpoon (http://www.sharc.co.uk). Each surface.stl file was imported before being meshed firstly using the “wrapping” function and secondly the “all tet” function. The wrapping function allowed for surface and internal geometry to be standardised between models through adjusting the surface cell size and with it the degree of captured geometry. Only major internal geometry such as the mandibular cavity was included. The solid mesh of each cranium consisted of 1 million bricks (±4%). The mandible was then meshed so that the tetrahedral bricks were approximately equal in size to those of the cranium (±1%). These solid models were imported into Strand 7 V2.4.4 (www.strand7.com) for FEA. Isotropic homogeneous material properties (Young’s Modulus = 22.8 GPa, density = 1050 kg/m3) were used for bone in all models based on published data of a Varanus exanthematicus femur [54]. Homogeneous material properties were chosen over heterogeneous material properties because of: 1) a general lack of data on varanoid bone properties and 2) to better isolate biological shape as a variable within the analysis. A hinge system was implemented to allow the mandible to pivot in relation to the cranium [27] and the gape angle was set to 10 degrees. The jaw hinge was created by extruding beams in the x axis from the lateral side of the bottom of the quadrate. These beams were then subdivided into two equal parts. The end furthest from the skull was released in rotation in the x axis to allow for the jaw to pivot in relation to the cranium. Rigid links were then created to take the place of the half of the beam closest to the skull and to join the freed end to the mandible. A user defined cylindrical co-ordinate system was created around the jaw hinge axis for use in defining the loads (applied forces) and freedoms (used to constrain the model) of each load case.
Muscles were simulated by attaching 120 truss elements between muscle attachment locations based on the diagrams presented in Holliday [55]. The number of truss elements used to represent each muscle was calculated based on the bone surface areas of the various muscles on Varanus panoptes horni (the species of median volume). Muscle elements were defined as either being part of the temporal or pterygoid muscle groups which resulted in 56 temporal truss elements and 64 pterygoid truss elements. Each truss element was assigned a diameter of 1.944423 cm and applied with a tensile force based on the cross sectional area of the two major muscle groups outlined in Walmsley et al.[21] and McHenry [56]. The numbers of beams used to represent each muscle were selected based on the relative surface area of the muscle attachment areas in Varanus panoptes panoptes. Table 2 displays which muscles were classified into each of these groups as well as the number of beams used to represent the muscle. Muscle elements were evenly distributed over the attachment areas. The same force was applied to each truss within its respective muscle group (temporal or pterygoid). Data on the forces calculated for the muscles can be found in S1 Table. In whole, this method of model construction assumes that the muscle attachment sites of the various species are identical whilst including differences in the relative size of the two muscle groups. Load cases were used to simulate the forces generated by relevant feeding behaviours. Each model was scaled to the same volume as V. panoptes horni, following the methods used in Walmsley et al.[21,57]; applied forces were not scaled. The three load cases simulated were:
Bite loading, where pretension was applied through muscle beams. The occipital condyle was restrained in all 6 degrees of freedom and the four largest teeth in the middle of the tooth row were restrained in circumferential translation centred on the jaw hinge axis. The right upper and lower teeth were also restrained in medial-lateral translation. The magnitude of force applied through each muscle beam (constructed as a “truss” element in Strand7) was standardised as to generate the same node reaction forces at the teeth (i.e. the same resultant bite force) of 47.8 N (Predicted bite force for the Varanus panoptes horni model)(Fig 3). The muscle beam forces applied to each model to give that standardised resultant bite force are shown in S1 Table.
Shake loading, where a force of 20N was applied in a lateral direction using a system of beams connecting the four middle teeth. Muscle beams were set with a Young’s Modulus of 15 MPa with no pretension applied to simulate the muscles bracing the skull [21,56]. The occipital condyle was restrained in all 6 degrees of freedom and the centre of the beams connecting the middle teeth was restrained in an arc around the jaw hinge axis (Fig 3).
Pull back loading, where a force of 30N was applied in an anterior direction using a system of beams connecting the four middle teeth. Muscle beams were again set with a Young’s Modulus of 15 with no pretension applied to simulate the muscles bracing the skull. The occipital condyle was restrained in all 6 degrees of freedom and the centre of the beams connecting the middle teeth was restrained in an arc around the jaw hinge axis (Fig 3).
Table 2. Functional classification of jaw muscles into two muscle groups (temporalis and pterygoid).
| Individual muscle | Abbreviation | Muscle group | Number of elements |
|---|---|---|---|
| m. pterygoideus | mPT | Pterygoid | 34 |
| m. levator pterygoideus | mLPt | Pterygoid | 4 |
| m. protractor pterygoideus | mPPt | Pterygoid | 26 |
| m. adductor mandibulae posterior | mAMP | Temporalis | 6 |
| m. adductor mandibulae externus superficialis | mAMES | Temporalis | 16 |
| m. adductor mandibulae externus medialis | mAMEM | Temporalis | 10 |
| m. pseudotemporalis superficialis | mPSTs | Temporalis | 16 |
| m. pseudotemporalis profundus | mPSTp | Temporalis | 2 |
| m. adductor mandibulae externus profundus | mAMEP | Temporalis | 6 |
Fig 3. Load cases.
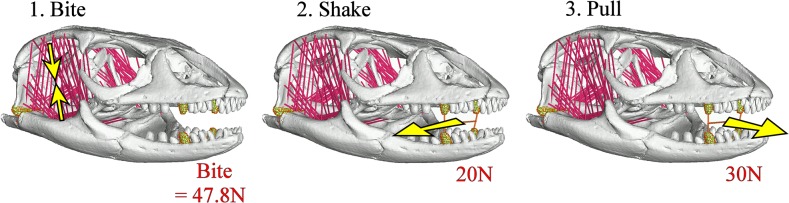
The three load cases undertaken in this study: 1. Biting where the models are constructed to bite with the same resultant force of 47.8 N; 2. Shaking where a force of 20N is applied laterally to the middle teeth and 3. Pulling where a force of 30N is applied anteriorly to the middle teeth. Note that within the Shake and Pull load cases muscles do not apply force but brace the skull. The model also shows muscle beams that were used to apply loads in biting (pink), H beams (orange) that were used to apply load to the teeth during shake and pull load cases and surface beams (green) that distribute load across the restrained teeth, jaw hinge surface and occipital condyle. The specimen shown is Varanus exanthematicus: FMNH 58288.
A series of beams were also used to reinforce areas where artificial (high) strain was expected. These areas include the restrained teeth, the occipital condyle (also a site of restraint), the jaw hinge surfaces and small areas around the site of each muscle beam attachment.
Ecological classifications
Data on the diet of species was compiled from relevant scientific literature [58,59,60,61,62,63,64,65,66,67,68,69,70,71,72]. Because of the nature of much of the published data, we were only able to draw broad categories based on the presence or absence of prey items with certain characteristics (Tables 3 and 4). The two categories were: 1) whether the diet of the species included prey items considered as hard, or harder, than a bird egg (e.g. crabs) and 2) whether the diet of the species included prey items larger than could be swallowed whole (e.g. large mammals such as wallabies).
Table 3. Ecological classifications.
| Species | Hard Prey | Large Prey |
|---|---|---|
| Heloderma horridum | Y | N |
| Lanthanotus bornensis | Y | N |
| Varanus acanthurus | Y | N |
| Varanus exanthematicus | Y | N |
| Varanus gouldii | N | Y |
| Varanus komodoensis | Y | Y |
| Varanus panoptes horni | Y | Y |
| Varanus panoptes panoptes | Y | Y |
| Varanus prasinus | N | Y |
| Varanus salvadorii | Y | Y |
| Varanus salvator | Y | Y |
| Varanus storri | N | N |
| Varanus tristis | N | N |
Table 4. Diet of the study species.
| Food item | H.hor | L.bor | V.exa | V.slt | V.pra | V.sdi | V.kom | V.gou | V.pan | V.stor | V.aca | V.tri |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| worms | 1 | |||||||||||
| other insects | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| beetles | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| orthopterans | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| centipedes | 1 | 1 | 1 | |||||||||
| spiders | 1 | 1 | 1 | 1 | 1 | |||||||
| scorpions | 1 | |||||||||||
| snails | 3 | 3 | ||||||||||
| mussels | 3 | |||||||||||
| crabs | 3 | 3 | ||||||||||
| fish | 1 | 2 | ||||||||||
| small lizards | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| large lizards | 1 | 1 | 1 | |||||||||
| snakes | 1 | |||||||||||
| lizard eggs | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| amphibians | 1 | 1 | 1 | |||||||||
| birds | 1 | 1 | 1 | 1 | 1 | |||||||
| bird eggs | 3 | 3 | 3 | 3 | 3 | 3 | ||||||
| small mammals | 1 | 2 | 1 | 1 | ||||||||
| large mammals | 2 | 2 | ||||||||||
| carrion | 2 | 2 | 2 | 2 |
1 = normal prey item
2 = food items too large to be eaten whole
3 = hard prey items. Data was compiled from relevant scientific literature. Note that dietary record for several species were incomplete.
Statistical analysis
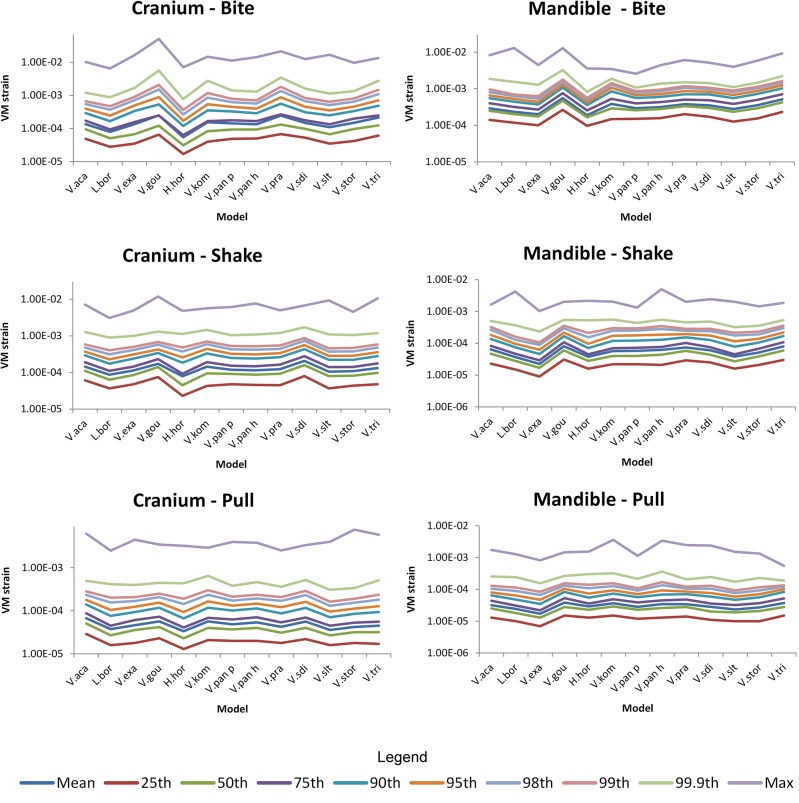
The resulting strains for each brick, in either the cranium or the mandible, during each load case were exported to and analysed in R [73]. R was then used to identify the 95% von Mises (VM) strain values of each case following the methods of Walmsley et al. [21,73]; this 95% von Mises strain constitutes the largest elemental (individual brick) value of strain in the model if the highest 5% of all elemental values are removed. The R code has been uploaded as a supplementary file (S2 File) as well as instructions on its use (S3 File). Examination of resulting strain values using mean, 25%, 50%, 75%, 90%, 95% and 98 values all showed similar patterns in results between models. Patterns between models using 99, 99.9 and maximum values did however vary slightly to the others indicating that localised peaks may be affecting these values (Fig 4). The 95% value presumably represents a way to compare the performance of the models whilst ignoring peak loading artefacts.
Fig 4. Comparison of possible summary values used as performance measures.
Comparison of the pattern between models using various summary values. These were generated from von Mises strain values for each brick within the models. Note that all values, apart from the maximum, 99th and 99.9th values, depict a similar pattern in the results.
Tests for phylogenetic signal in the morphometric results (using the phylogeny presented in Vidal [11]) were undertaken using a permutation test with 10000 randomisation rounds in MorphoJ. Partial least squares analysis (PLS) was undertaken in JMP V11 to test the relationship between morphology and the strain levels resulting from FEA load cases. Only one latent variable was included in each comparison because the addition of extra variables did not greatly improve the percentage of variation explained. Variable importance values and percentage variation values were also calculated in JMP. In the case of linear variables, the analysis was run between each single performance measure (VM strain during biting, shaking and pulling) and all morphological variables of the mandible or cranium, the percentage variation explained is only presented for the best correlated morphological variable. We also tested for intraspecific allometry using the same PLS method, comparing centroid size and PC1 and PC2 of the cranium and mandible. Multivariate analysis of variance (MANOVA), with a repeated measures response specification, was undertaken in JMP V 11 to test the relationship between ecological variables and performance. Univariate tests (ANOVA) were then used to test each individual combination of variable after the initial analysis.
Results
Ecological classification
All species are opportunistic predators, feeding on a range of vertebrate and invertebrate prey including small lizards, small and large mammals, bird and lizard eggs, amphibians, worms, beetles, centipedes, spiders, crabs, fish and birds. However, noticeable variation in diet is also reported in the published literature [58,59,60,61,62,63,64,65,66,67,68,69,70,71,72]. This variation appears to reflect both habitat and capabilities in prey capture (Tables 3 and 4). No species solely feed on invertebrates, however V. acanthurus and V. storri were found to prey on insects along with small prey such as lizards. Other notable specialisation included H. horridum, which preys primarily on eggs and juvenile birds, and V. komodoensis and V. salvadorii, the only species within this analysis to feed on large mammals (Tables 3 and 4).
Morphometrics
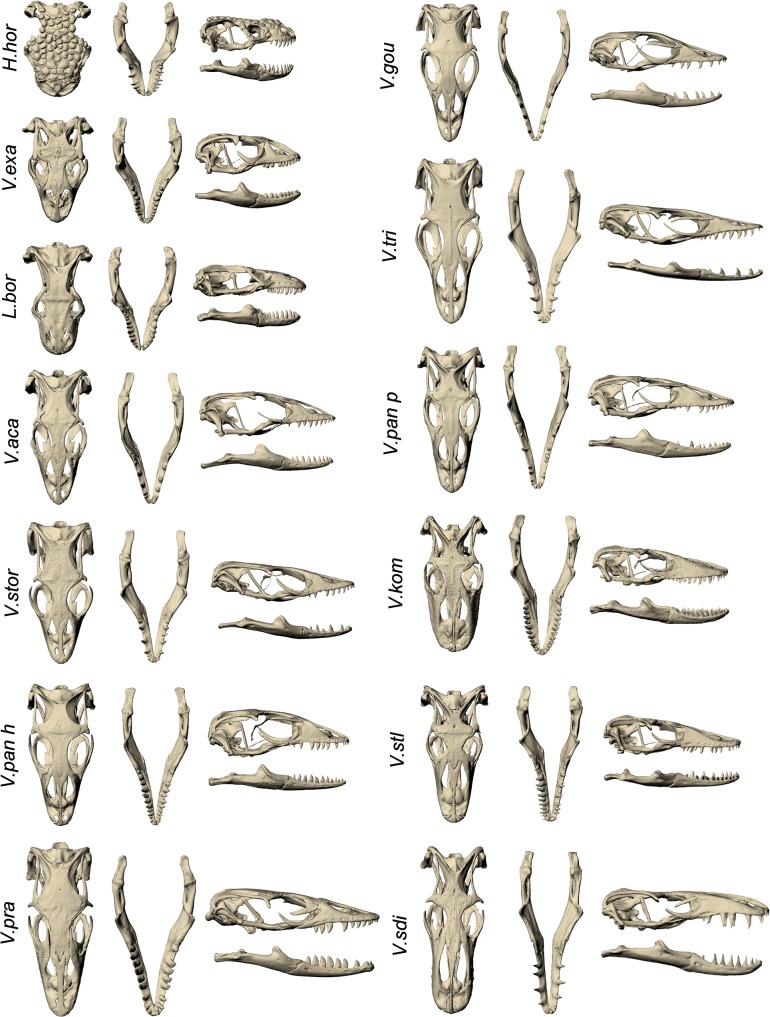
Qualitatively, species of varanoid lizards differ substantially in cranial and mandibular morphology (Fig 5). Linear measurements taken on volume scaled models are shown in Table 5. Geometric morphometric analysis provided a more comprehensive picture of morphological variation. Within the cranium, Principal Component (PC) 1 and PC2 together only explained 58.26% of the differences in shape. PC1 was composed of differences in the height, width, length and curvature of the rostrum as well as the width of the skull roof. PC2 was represented by the height of the back of the skull and the area of the skull roof (Fig 6). Within the mandible, PC1 and PC2 together explained 65.07% of the variation in shape. PC1 was composed of differences in the overall width and height of the mandible as well as the position and height of the coronoid process. PC2 represented changes in the height of the coronoid process and comparative length of the tooth row (Fig 7).
Fig 5. Visual comparison of cranial and mandibular morphology between specimens.
The morphology of each specimen is shown in dorsal and lateral view for both the cranium and mandible. Species are ordered by cranial PC1 values. Abbreviations: H.hor, Heloderma horridum; L.bor, Lanthanotus borneensis; V.aca, Varanus acanthurus; V.exa, Varanus exanthematicus; V.gou, Varanus gouldii; V.kom, Varanus komodoensis; V.pan h, Varanus panoptes horni; V.pan p, Varanus panoptes panoptes; V.pra, Varanus prasinus; V.sdi, Varanus salvadorii; V.slt, Varanus salvator; V.stor, Varanus storri; V.tri, Varanus tristis.
Table 5. Linear measurements (cm) from volume scaled models.
| Species | BSL | DCL | CH | CW | RH | RW | TRL | IOD | ML | MH | MW | MHD | MWD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heloderma horridum | 4.11 | 3.97 | 3.77 | 3.06 | 2.69 | 3.57 | 3.11 | 3.17 | 4.11 | 2.58 | 3.73 | 2.38 | 3.20 |
| Lanthanotus bornensis | 4.30 | 4.02 | 3.83 | 3.03 | 2.47 | 3.49 | 3.27 | 2.77 | 4.22 | 2.55 | 3.78 | 2.37 | 3.41 |
| Varanus acanthurus | 4.53 | 4.37 | 3.83 | 3.40 | 2.90 | 3.54 | 3.54 | 2.18 | 4.50 | 2.72 | 3.80 | 2.28 | 3.41 |
| Varanus exanthematicus | 4.20 | 4.02 | 3.80 | 3.19 | 2.90 | 3.46 | 3.37 | 2.27 | 4.22 | 2.73 | 3.78 | 2.11 | 3.38 |
| Varanus gouldii | 4.55 | 4.40 | 3.81 | 3.32 | 2.93 | 3.51 | 3.72 | 2.14 | 4.51 | 2.72 | 3.91 | 2.24 | 3.51 |
| Varanus komodoensis | 4.39 | 4.26 | 3.65 | 3.17 | 2.79 | 3.55 | 3.62 | 2.37 | 4.48 | 2.57 | 3.71 | 2.33 | 3.23 |
| Varanus panoptes horni | 4.45 | 4.30 | 3.68 | 3.37 | 2.94 | 3.45 | 3.61 | 2.13 | 4.45 | 2.66 | 3.68 | 2.10 | 3.30 |
| Varanus panoptes panoptes | 4.50 | 4.35 | 3.59 | 3.31 | 2.91 | 3.44 | 3.73 | 2.25 | 4.51 | 2.76 | 3.75 | 2.35 | 3.32 |
| Varanus prasinus | 4.52 | 4.35 | 3.53 | 3.17 | 2.81 | 3.41 | 3.73 | 2.37 | 4.45 | 2.46 | 3.74 | 2.08 | 3.40 |
| Varanus salvadorii | 4.55 | 4.39 | 3.54 | 3.15 | 2.75 | 3.30 | 3.78 | 2.10 | 4.52 | 2.46 | 3.59 | 2.22 | 3.01 |
| Varanus salvator | 4.38 | 4.23 | 3.71 | 3.15 | 2.72 | 3.33 | 3.60 | 2.31 | 4.41 | 2.64 | 3.69 | 2.41 | 3.24 |
| Varanus storri | 4.50 | 4.34 | 3.71 | 3.11 | 2.79 | 3.47 | 3.49 | 2.12 | 4.45 | 2.54 | 3.76 | 2.11 | 3.28 |
| Varanus tristis | 4.63 | 4.46 | 3.73 | 3.12 | 2.79 | 3.50 | 3.73 | 2.11 | 4.59 | 2.26 | 3.84 | 1.89 | 3.20 |
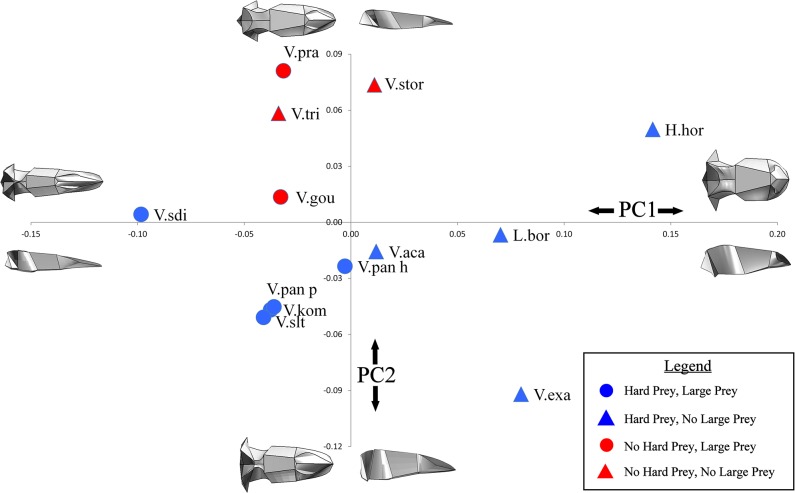
Fig 6. Cranial morphospace.
Principal component plot of cranial morphospace. The diagrams at the end of each axis represent the theoretical geometry of the cranium in dorsal and lateral view. Each marker depicts the location of that specimen in morphospace as well as whether the animal was classed as feeding on hard prey (blue = yes, red = no) and whether the animal was classed as feeding on comparatively large prey (circle = yes, triangle = no). Abbreviations: H.hor, Heloderma horridum; L.bor, Lanthanotus borneensis; V.aca, Varanus acanthurus; V.exa, Varanus exanthematicus; V.gou, Varanus gouldii; V.kom, Varanus komodoensis; V.pan h, Varanus panoptes horni; V.pan p, Varanus panoptes panoptes; V.pra, Varanus prasinus; V.sdi, Varanus salvadorii; V.slt, Varanus salvator; V.stor, Varanus storri; V.tri, Varanus tristis.
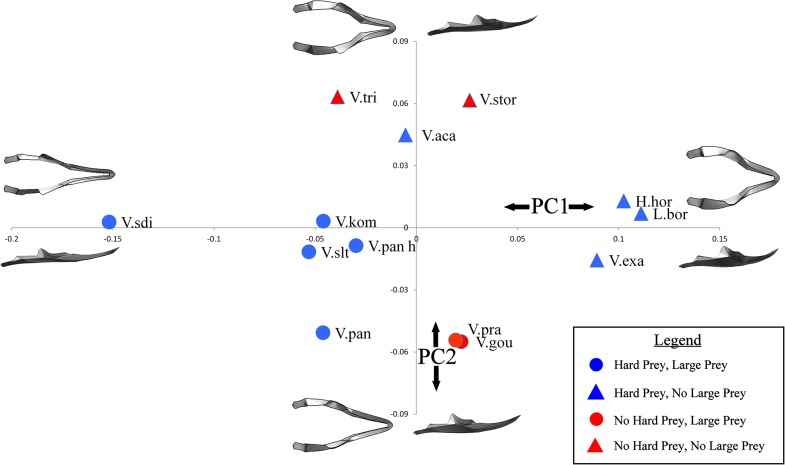
Fig 7. Mandibular morphospace.
Principal component plot of mandibular morphospace. The diagrams at the end of each axis represent the theoretical geometry of the mandible in dorsal and lateral view. Each marker depicts the location of that specimen in morphospace as well as whether the animal was classed as feeding on hard prey (blue = yes, red = no) and whether the animal was classed as feeding on comparatively large prey (circle = yes, triangle = no). Abbreviations: H.hor, Heloderma horridum; L.bor, Lanthanotus borneensis; V.aca, Varanus acanthurus; V.exa, Varanus exanthematicus; V.gou, Varanus gouldii; V.kom, Varanus komodoensis; V.pan h, Varanus panoptes horni; V.pan p, Varanus panoptes panoptes; V.pra, Varanus prasinus; V.sdi, Varanus salvadorii; V.slt, Varanus salvator; V.stor, Varanus storri; V.tri, Varanus tristis.
Both phylogenetic and functional patterns are present within the results; a permutation test using a time calibrated phylogeny produced a P value of 0.0402 in the cranium and a P value of 0.0018 in the mandible indicating that the dataset contains significant phylogenetic influence [74]. Heloderma horridum, Lanthanotus borneensis and Varanus exanthematicus clustered in each of the PC plots, the Australian dwarf monitors (subgenus Odatria; included here are V. acanthurus, V. storri, and V. tristis) also clustered, occupying the top of the mandible morphospace. The test for interspecific allometry using PLS identified one pair of singular warp vectors that were poorly correlated (Total variation explained for Y effects = 13.467), indicating that allometric trends in shape are small. The species that fed on prey larger than can be swallowed whole clustered in the centre left of the cranial morphospace and in the bottom left of the mandible morphospace. This morphospace is associated with thin and shallow mandibles and thin, long crania with comparatively long tooth rows. The species that feed on large prey items occupied a larger area of morphospace in the mandible analysis than in the cranial analysis (Figs 6 and 7).
Finite element analysis
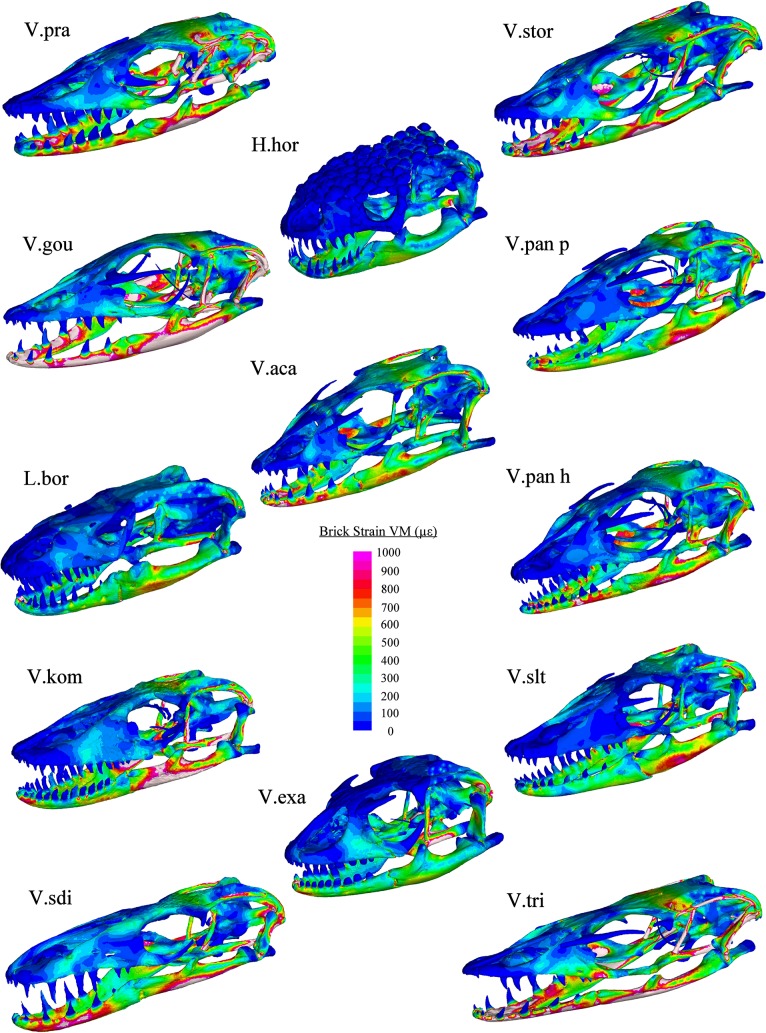
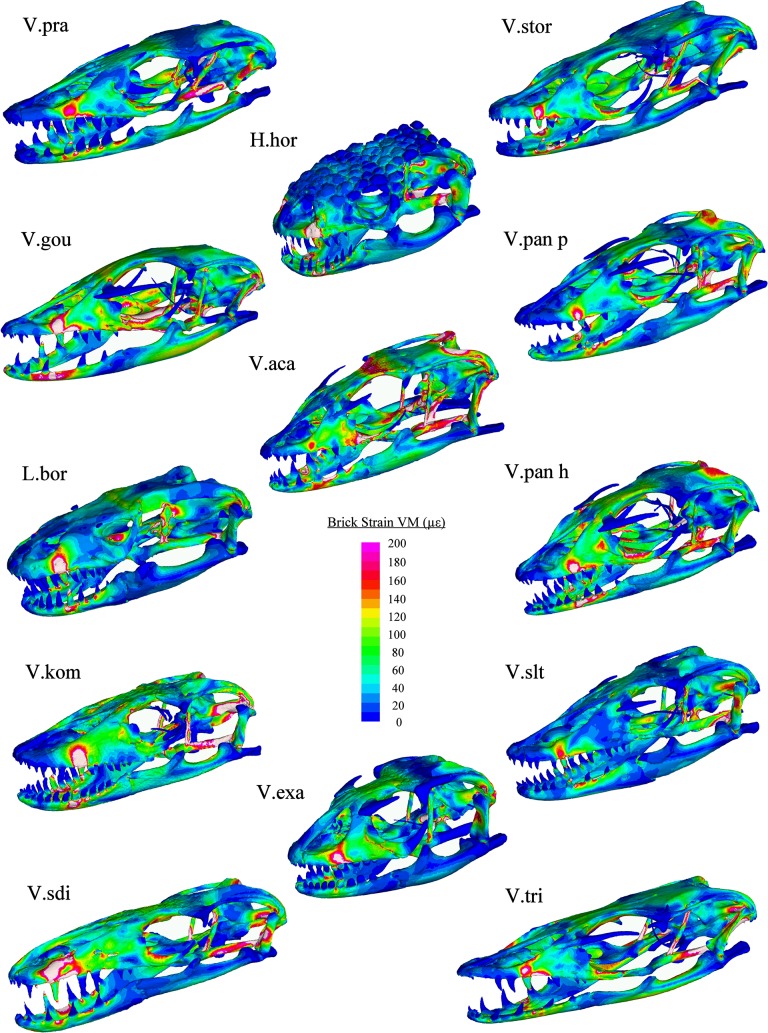
Finite element modelling results showed that variation in cranial morphology resulted in large differences in the magnitudes and locations of strain in each load case (Figs 8–11). During bite loading, strain occurred in high levels within the pterygoid, epipterygoid, sides and front of the rostrum as well as in the mandible anterior to the jaw hinge axis (Fig 8). Low magnitudes of strain were observed in the crania of H. horridum, L. borneensis and V. salvator and the mandibles of H. horridum, L. borneensis, and V. exanthematicus in this load case.
Fig 8. VM Strain plots: Bite loading.
Cool colours (blues) represent areas of low stain, hot colours (reds) represent areas of high strain and white areas represent areas where the upper strain threshold (1000 μԐ) was exceeded. Load was applied through muscle beams at a magnitude that standardised bite force at the teeth to 47.8 N (see S1 Table for muscle forces used for each model). Abbreviations: H.hor, Heloderma horridum; L.bor, Lanthanotus borneensis; V.aca, Varanus acanthurus; V.exa, Varanus exanthematicus; V.gou, Varanus gouldii; V.kom, Varanus komodoensis; V.pan h, Varanus panoptes horni; V.pan p, Varanus panoptes panoptes; V.pra, Varanus prasinus; V.sdi, Varanus salvadorii; V.slt, Varanus salvator; V.stor, Varanus storri; V.tri, Varanus tristis.
Fig 11. Magnitudes of strain.
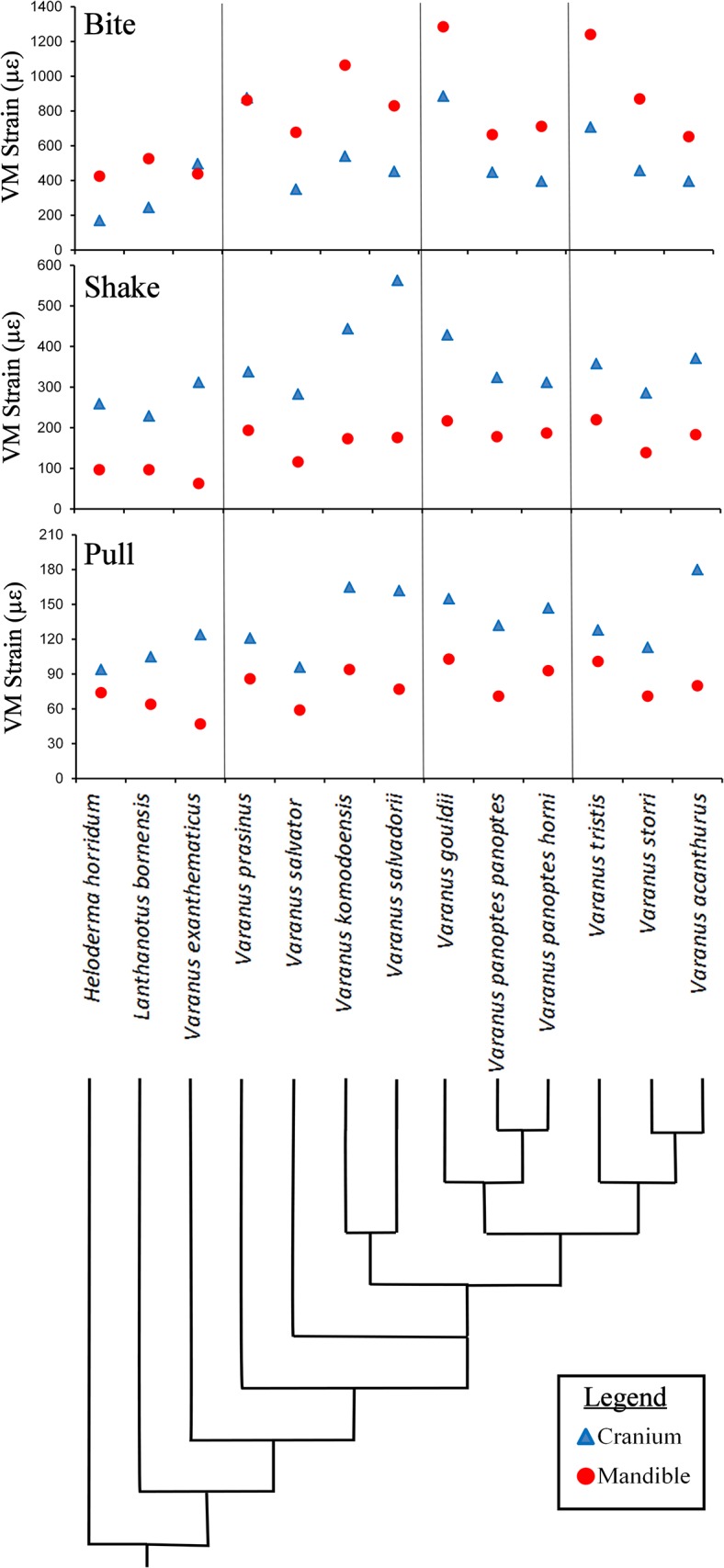
Comparative performance (95% von Mises strain levels) of the cranium (blue) and mandible (red) models in bite, shake and pull loading. The species are in phylogenetic order with a simplified phylogenetic tree based on Vidal [11] showing the relative position of each species.
There was considerably less strain associated with the mandible in shake loading compared to bite loading (Fig 11). Patterns of strain are similar in each model during shaking with high levels in the anterior of the mandible and across parts of the cranium, specifically in-between the orbits, the posterior part of the pterygoid bone, the epiterygoid, and the opisthotic and in localised areas close to the teeth at which forces were applied (Fig 9). The magnitude of strain within these areas varied considerably between species (Figs 8, 9 and 10). Gracile species such as V. salvadorii displayed high strain levels during shaking, especially in the areas between the orbits. V. salvadorii displayed strain values substantially higher than the other models. The V. salvadorii, V. komodoensis and V. gouldii models exhibited high VM strain values within the cranium. Considerably less variation in strain was observed in mandibles as opposed to the crania during this load case (Fig 11).
Fig 9. VM Strain plots: Shake loading.
Cool colours (blues) represent areas of low stain, hot colours (reds) represent areas of high strain and white areas represent areas where the upper strain threshold (500 μԐ) was exceeded. An arbitrary load of 20N was applied laterally through H beams connecting the four middle teeth. Abbreviations: H.hor, Heloderma horridum; L.bor, Lanthanotus borneensis; V.aca, Varanus acanthurus; V.exa, Varanus exanthematicus; V.gou, Varanus gouldii; V.kom, Varanus komodoensis; V.pan h, Varanus panoptes horni; V.pan p, Varanus panoptes panoptes; V.pra, Varanus prasinus; V.sdi, Varanus salvadorii; V.slt, Varanus salvator; V.stor, Varanus storri; V.tri, Varanus tristis.
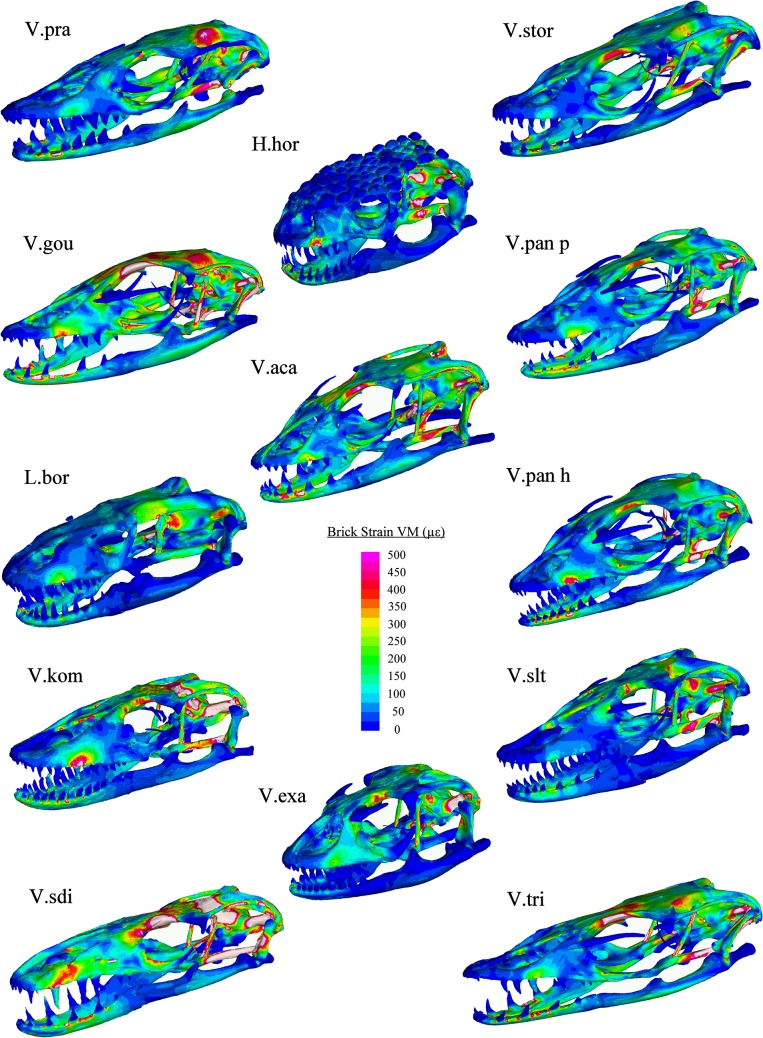
Fig 10. VM Strain plots: Pull loading.
Cool colours (blues) represent areas of low stain, hot colours (reds) represent areas of high strain and white areas represent areas where the upper strain threshold (200 μԐ) was exceeded. An arbitrary load of 30N was applied anteriorly through H beams connecting the four middle teeth. Abbreviations: H.hor, Heloderma horridum; L.bor, Lanthanotus borneensis; V.aca, Varanus acanthurus; V.exa, Varanus exanthematicus; V.gou, Varanus gouldii; V.kom, Varanus komodoensis; V.pan h, Varanus panoptes horni; V.pan p, Varanus panoptes panoptes; V.pra, Varanus prasinus; V.sdi, Varanus salvadorii; V.slt, Varanus salvator; V.stor, Varanus storri; V.tri, Varanus tristis.
The areas of high strain during pull back loading include the pterygoid, epipterygoid and opisthotic bones as well as the sides of the rostrum and areas surrounding the teeth where forces were applied (Fig 10). All models exhibit less strain during pull back loading compared to shake loading, even though a larger force was applied (pull = 30N, shake = 20N). During pull back load cases strain values varied between each model. Within the cranium the highest observed strain values were in V. acanthurus followed by V. komodoensis, V. salvadorii and V. gouldii. Within the mandible strain magnitudes were high in the V.gouldii and V.tristis models (Fig 10). Within intrinsic (bite) loading strain was higher in the mandible than the cranium. In contrast extrinsic (shake and pull) load cases resulted in higher levels of strain in the cranium than the mandible. Overall the results show that each species varies considerably in terms of its ability to resist applied load and that, in some instances, the various structures are carrying load in different parts of the skull.
The relationship between morphology and performance
Comparisons between linear measurements of shape and performance showed that, within the cranium, tooth row length (TRL) was the best predictive variable for strain during bite and shake loading whilst dorsal cranial length (DCL) was the best predictive variable for pull loading. Mandible length (ML) was found to be the best predictor for strain levels during bite, shake and pull loading in the mandible (Table 6). In all cases larger values corresponded with higher levels of strain.
Table 6. Best linear morphometric predictor variables for each load case.
| Cranium | Mandible | ||||||
|---|---|---|---|---|---|---|---|
| Load Case | Best Predictor | n.o of factors | % explained | Load Case | Best Predictor | n.o of factors | % explained |
| Bite | TRL | 1 | 90.24 | Bite | ML | 1 | 69.96 |
| Shake | TRL | 1 | 92.11 | Shake | ML | 1 | 81.99 |
| Pull | DCL | 1 | 83.28 | Pull | ML | 1 | 65.68 |
Partial least squares regression identified that the cranial shape was closely related to the response of models to bite and shake loading (Table 7). The relationships were however less strong in the mandible (Percentage variation explained for cumulative Y = 40.58 and 26.22 for the cranium and mandible respectively). In both the cranium and the mandible shake loading had a stronger relationship to shape than biting, and both of these were stronger than pulling (Table 7). Variable Importance Values of 1.4130 and 1.4142 for the PC1 in the cranium and mandible respectively, and 0.0582 and 0.0068 for PC2 in the cranium and mandible respectively indicate that PC1 values were far more important in these relationships.
Table 7. Statistical comparison (PLS) between morphology (PC1 and PC2) and Performance (Bite, Shake and Pull load cases).
| Cranium | n.o of factors | % explained | Mandible | n.o of factors | % explained |
|---|---|---|---|---|---|
| Bite | 1 | 41.02 | Bite | 1 | 27.87 |
| Shake | 1 | 52.19 | Shake | 1 | 38.46 |
| Pull | 1 | 28.53 | Pull | 1 | 12.34 |
The relationship between performance and ecology
No relationships were observed between the presence of large prey in the diet of species and the structural performance of either the cranium or the mandible in any type of loading (Table 8). Relationships were observed between the presence of hard prey items within the diet of the species and the structural performance of cranial and mandible models; species that fed on hard prey items performed with less strain in bite loading than other species.
Table 8. Univariate Statistical comparison (ANOVA) between ecological class and performance in each load case.
| Cranium | Mandible | ||||
|---|---|---|---|---|---|
| Hard | Prob>F | Hard | Prob>F | ||
| Bite | 0.0106 | Bite | 0.0064 | ||
| Shake | 0.7469 | Shake | 0.1207 | ||
| Pull | 0.9016 | Pull | 0.1083 | ||
| Large | Prob>F | Large | Prob>F | ||
| Bite | 0.1788 | Bite | 0.1636 | ||
| Shake | 0.0887 | Shake | 0.0886 | ||
| Pull | 0.2995 | Pull | 0.2660 |
Discussion
We aimed to investigate the relationships between the cranial morphology, biomechanical performance (VM strain under biting, pulling and shaking loads) and feeding ecology of varanoid lizards. There were strong relationships between shape and performance; crania and mandibles with larger widths and heights (high PC1 values) performed better at biting and shaking. Further, there was some evidence for relationships between performance and diet; species that performed well in bite loading were also found to have hard prey within their diet. However, the relationships between morphology and performance were not as evident as that reported in previous studies [21,75], and did not match predictions from mechanical first principles, or those identified by FEA in other taxa [21].
Ecomorphology
Species that feed on large prey clustered together in the bottom right of both the cranial and mandible morphospace (gracile with long rostrum). Species that do not feed on hard prey items clustered in the centre top of cranial morphospace exhibiting low cranial height. There was a clear relationship between prey size and mandible shape, with macrophagous species having lower mandible PC1 and PC2 values. Phylogenetic patterns were also present, for example dwarf monitor (subgenus Odatria) mandibles clustered together in the morphospace.
The most extreme form of feeding upon hard prey is durophagy, Varanus exanthematicus and L. borneensis are both durophagous, feeding on hard prey items (molluscs and crabs respectively) [59,76]. Heloderma horridum, although not distinctively durophagous, does also feed on harder prey items such as eggs. These three species have the highest PC1 scores for both crania and mandibles (Figs 6 & 7), indicating that, as expected, the shortest and most robust skulls are those suited for durophagy. Note, however, that phylogeny is also important here; durophagy may be a plesiomorphic trait of the varanoid lizards as Varanus exanthematicus and L. borneensis are basal species.
Loading type
In simulations of biting, the models consistently showed higher strain levels in the mandible than the cranium. Biomechanical performance during biting may thus be limited primarily by mandibular morphology, as has been suggested in previous analyses [77]. This may also be a result of differing evolutionary forces influencing the two structures; as the cranium houses the brain and capsules, its morphology is a result of multivariate selection, whereas the mandible is principally involved in feeding [78]. The high strain levels observed in the brain case and in between the orbits during shake loading indicate that these areas may be important in this behaviour (Fig 9). This raises the possibility of a trade-off between eye size (sensory function) and strength during shaking (structural function), consistent with a previous study on theropod dinosaurs [79]. The two sub-adult specimens included within the analysis (V. komodoensis and V. gouldii) exhibited higher magnitudes of strain than most other specimens. Eyes are proportionately larger in juveniles, leaving less space for bone in the interorbital region, and that region exhibits high level of strain during biting and shaking. Thus fine scale structural characteristics such as allometric differences between juveniles and adults may be of particular importance to trade-offs in cranial structural performance and neurocranial anatomy.
We examined correlations between morphology and performance using two measures of morphology: linear (euclidean distances), and multivariate (geometric morphometrics).
Linear morphometrics vs performance
Walmsley et al. (2013) found that relationships between linear morphometrics and performance match well with predictions from mechanical first principles in a comparative analysis of crocodile mandibles. However, relationships between linear measures of shape and performance results from FEA in our study were inconsistent with our predictions: the length of the tooth row and mandible correlated better with skull strength (Table 6) than the measures we hypothesised a priori (i.e. rostral/mandibular height for biting, rostral/mandibular width for shaking, and the combination of cranial/mandibular height and width for pull back loads).
Multivariate morphometrics and performance
In the principle components analysis of skull shape, PC1 values correlated negatively with skull length and positively with skull width, so that species with high PC1 values had shorter, wider skulls with taller snouts (Figs 3 and 4). A priori, these would be expected to be stronger under biting and especially shaking loads, and the results agree; two blocked partial least squares regression showed that crania and mandibles with high PC1 values performed better than others, particularly in bite and shake loading (Table 7). Species with lower PC2 values have taller skulls post-orbitally, which is also expected to increase skull strength, but this had a smaller influence on strain levels as indicated by comparable lower variable importance values in the 2-block partial least squares analysis. The fact that there were higher explanation values for biting and shaking load cases may mean that performance in these behaviours is more important than performance in pulling behaviours in driving patterns of morphological variation in the varanoid skull.
Overall
The apparent mismatch between the linear measurements that were predicted to influence skull performance, and those that actually do, may relate to the space frame structure of the varanoid lizard cranial system [36]. Compared with the shell constructions, it seems that the performance of varanoid skulls is difficult to predict from simple measurements chosen with reference to basic beam theory. However, more complex multivariate measures of skull shape do show correlations with performance that make sense in terms of fundamental mechanical principles. If this is the case, then the landmarks used in the multivariate measures must somewhere incorporate aspects of shape that do influence performance. The higher correlation between morphology and performance in the cranium than in the mandible may indicate that the cranium is under a higher selective pressure for strength; intuitively this is difficult to understand as cranial morphology is expected to represent compromise between various functional demands (feeding, protecting the brain, olfaction etc.) whilst the mandible primarily functions in feeding [78].
V. komodoensis exhibited high levels of strain to bite, shake and pull loading. This result is may be a consequence of our model being constructed from a sub-adult specimen (a size that cannot exploit large prey) that has no ecological need for a robust skull. Alternatively it is also possible that the use of toxic effects (whether produced by bacteria or venom glands) to incapacitate prey may free the Komodo dragon from the need to have a highly robust skull [45,80,81]. Clearly further research that measures cranial performance across ontogeny may detail allometric changes in performance consistent with dietary transitions from small prey in hatchlings to large ungulates (e.g. water buffalo) in adults [82].
Do macropredaceous varanoids have longer, weaker skulls?
Species that fed on large prey items compared to their size possess unique morphological characteristics such as elongate crania and mandibles. Predatory mammals and crocodiles that feed on large prey exhibit the opposite trend with shorter, more robust cranial structures [24,75]. This may be due to the influence of other performance considerations such as the need for a long mandible to better tear flesh during pull back prey processing. At least some of the varanid species that feed on large prey use pull back behaviours to process their food. In V. komodoensis the tooth row acts as a saw blade which cuts food as the teeth are drawn back [15]. Logically, an elongate mandible can carry more teeth and thus increases the efficiency of prey processing. Many of these species also feed on carrion which may mean that the skull does not need to handle the same level of extrinsic forces that can occur in high levels during prey capture [59]. Alternatively the elongate skulls could prove useful in allowing the predator to better access hard to reach parts of the carcass.
Methodological limitations and agenda for future research
The complexity of varanoid morphology, behaviour and ecology represented a significant challenge for this study. The relationships identified between morphology and performance were not as strong as those identified in other taxonomic groups [21,24,75]. Either there is only a weak link between morphology, performance and ecology within the group (with behaviour acting as a wild card), or there are limitations on the approach that we used here.
Sample size
Many previous FEA studies have characterised the biomechanics of a single specimen or qualitatively compared between a small number of species [25,26,27,37,45,48]. The complex morphological differences that exist between the varanoid specimens mean that a higher number of individuals are more suitable to tease apart the relationship between form and function. Additionally, a limited sample size prevents us from characterising the effects of intraspecific variation such as allometry or sexual dimorphism. Ontogenetic changes in skull shape have been shown to be large within some varanid species [83]. Sample size thus represents a substantial hurdle for the area of ecological biomechanics given that finite element models take considerable time to produce.
Structural complexity
The space-frame nature of the varanoid cranial system represented another significant challenge for this study. The strength of a structure is dependent on many characteristics of the shape such as the thickness of bones or their orientation. Gross morphology was found to have a large influence (PC 1 correlated with some measures of performance). However, subtler morphological characteristics, such as thicknesses of particular struts, that were not measured, are likely to also be important in dictating strength. It is logical that finer morphological characteristics also play a larger role in dictating strength in space frame structures compared to shell structures. This is because space-frame structures rely on fine scale and localised morphological features more than shell structures that distribute load across a wide surface. Sutures were not included in the analysis; these features have been found to have considerable influence on FEA results in previous studies [40,49,84,85,86,87]. Due to a lack of information on the nature and extent of cranial kinesis (movement between the bones of the cranium) in varanoids it was excluded in FEA models and it is likely that this has also influenced the results to some degree. The low percentage of total variance explained by the PC analyses could also be due to the occurrence of cranial kinesis which would alter the relative position of landmarks between specimens.
Muscle anatomy
Because detailed data on muscle activation and anatomy was not available for the majority of these species we were forced to assume that all species possess identical muscle attachment sites and that each muscle is used at the same time in biting. This may not be a realistic assumption; it would be useful for future studies to address the function of specific muscles and the role of muscle activation patterns.
Loading conditions
Recent sensitivity studies have identified that biological assumptions may have more influence on FEA results then those about material properties or scaling [57]. As no kinematic data was collected for this study it is possible that the forces applied were not exactly biologically equivalent. Future studies should aim to collect data on the exact loading conditions encountered in each species through observation of either wild or captive animals.
Ecological data
The performance and shape data used in this study are fine grained whereas the ecological data are quite course, with only qualitative categories being drawn. It is possible (even likely) that clear relationships between skull shape, biomechanics and ecology do exists but that fine scale ecological data are required to demonstrate them statistically. However this hypothesis cannot be tested without improving the available ecological data, and so quantitative studies of varanid ecology are a priority for investigating form-function relationships in this taxon.
Performance metrics
Within this study we have only examined strength (limited to three load cases) as a performance measure. As a complex integrated system other factors such as the ability of the skull to protect vital organs, the ability of the cranial system to produce bite forces and for certain sensory organs to function, are likely to also influence the shape of the skull [88].
Conclusions
This study identifies that varanoid lizards do exhibit considerable variation in cranial and mandibular morphology that has specific influences on structural performance during biologically relevant loading. This could be an important characteristic that allows for these closely related taxa to exploit diverse ecological habits. The relationships between the morphology of varanoid lizard skulls, their structural performance and their ecology are not as would be predicted from our previous knowledge of cranial form and function. The space frame structure of the varanoid lizard skull as well as the unique behaviours that are used by these species to capture and process prey items may be the cause of this discrepancy. It is hoped that the insights gained from this study will help to guide future research on space-frame skull structures and more broadly in the area of ecological biomechanics. The results may also provide useful context for future paleobiological studies on taxa such as theropod dinosaurs that may use varanoids as extant morphological analogues.
Supporting Information
(DOCX)
(TXT)
(RTF)
Natural forces represent the force per truss element predicted for each of the muscle beams based on muscle cross sectional area at natural size. Adjusted forces represent the force per truss element adjusted so that the models produced the same node reaction force at the bite location in volume scaled models. Total muscle forces represent the total force applied to each model to generate the same node reaction forces at bite location.
(XLSX)
Acknowledgments
We thank Cecilie Beatson and Ross Sadlier of The Australian Museum, Heather Janetzki and Patrick Couper of Queensland Museum and Matthew Colbert and Jesse Maisano of The University of Texas Digital Morphology Library (Digimorph) for access to specimens. Digimorph specimens were scanned at The University of Texas High-Resolution X-ray CT Facility (UTCT), using NSF funding from the Deep Scaly Project awarded to M. Kearney, T. Reeder, O. Rieppel, J. Sites, and J. Wiens (NSF EF-0334961). We also thank Associate Professor Alan Jones (The Australian Centre for Microscopy and Microanalysis, Sydney University) and Eleanor Cunningham (The Newcastle Mater Hospital) for access to scanning facilities as well as Will Parr for advice on morphometrics.
Data Availability
All data is included within the paper and/or Supporting Information files.
Funding Statement
This study was funded by a summer research scholarship from UNSW awarded to MRM. The funding body had no role in study design, data collection, analysis, decision to publish or preparation of the manuscript.
References
- 1. Fitch AJ, Goodman AE, Donnellan SC. A molecular phylogeny of the Australian monitor lizards (Squamata: Varanidae) infered from mitochondrial DNA sequences. Australian Journal of Zoology. 2006;54:253–69. [Google Scholar]
- 2. Pianka ER, King DR, King RA. Varanoid lizards of the world: Indiana University Press; 2004. [Google Scholar]
- 3. Pianka ER. Comparative ecology of Varanus in the Great Victoria Desert. Australian Journal of Ecology. 1994;19:395–408. [Google Scholar]
- 4.Pianka ER. Evolution of body size: varanid lizards as a model system. American Naturalist. 1995:398–414.
- 5. Clemente CJ, Withers PC, Thompson G, Lloyd D. Evolution of limb bone loading and body size in varanid lizards. The Journal of Experimental Biology. 2011;214(18):3013–20. 10.1242/jeb.059345 [DOI] [PubMed] [Google Scholar]
- 6. Thompson GG, Withers PC. Comparative Morphology of Western Australian Varanid Lizards (Squamata: Varanidae). Journal of Morphology. 1997;233:127–52. [DOI] [PubMed] [Google Scholar]
- 7. Stayton TS. Morphological evolution of the lizard skull: A geometric morphometrics survey. Journal of Morphology. 2005;263:47–59. [DOI] [PubMed] [Google Scholar]
- 8. Clemente CJ, Thompson GG, Withers PC. Evolutionary relationships of sprint speed in Australian varanid lizards. Journal of Zoology. 2009;278(4):270–80. 10.1111/j.1469-7998.2009.00559.x [DOI] [Google Scholar]
- 9. Openshaw G, Keogh J. Head shape evolution in monitor lizards (Varanus): interactions between extreme size disparity, phylogeny and ecology. Journal of Evolutionary Biology. 2014;27(2):363–73. 10.1111/jeb.12299 [DOI] [PubMed] [Google Scholar]
- 10. Ast JC. Mitochondrial DNA evidence and evolution in Varanoidea (Squamata). Cladistics. 2001;17(3):211–26. [DOI] [PubMed] [Google Scholar]
- 11. Vidal N, Marin J, Sassi J, Battistuzzi FU, Donnellan S, Fitch AJ, et al. Molecular evidence for an Asian origin of monitor lizards followed by Tertiary dispersals to Africa and Australasia. Biology Letters. 2012;8(5):853–5. 10.1098/rsbl.2012.0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones ME, Anderson CL, Hipsley CA, Müller J, Evans SE, Schoch RR. Integration of molecules and new fossils supports a Triassic origin for Lepidosauria (lizards, snakes, and tuatara). BMC evolutionary biology. 2013;13(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reeder TW, Townsend TM, Mulcahy DG, Noonan BP, Wood PL Jr., Sites JW Jr., et al. Integrated Analyses Resolve Conflicts over Squamate Reptile Phylogeny and Reveal Unexpected Placements for Fossil Taxa. PLOS one. 2015;10(3):e0118199 10.1371/journal.pone.0118199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Loop MS. The Effect of Relative Prey Size on the Ingestion Behavior of the Bengal Monitor, Varanus bengalensis (Sauria: Varanidae). Herpetologica. 1974;30(2):123–7. 10.2307/3892027 [DOI] [Google Scholar]
- 15. D'Amore DC, Blumenschine RJ. Komodo monitor (Varanus komodoensis) feeding behavior and dental function reflected through tooth marks on bone surfaces, and the application to ziphodont paleobiology. Paleobiology. 2009;35(4):525–52. [Google Scholar]
- 16. D'Amore DC, Moreno K, McHenry CR, Wroe S. The effects of biting and pulling on the forces generated during feeding in the Komodo Dragon (Varanus komodoensis). PLOS one. 2011;6(10):e26226 10.1371/journal.pone.0026226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wainwright PC. Functional morphology as a tool in ecological research. Ecological morphology. 1994:42–59.
- 18. Rayfield EJ. Finite element analysis and understanding the biomechanics and evolution of living and fossil organisms. Annual Review of Earth and Planetary Sciences. 2007;35:541–76. [Google Scholar]
- 19. Parr W, Wroe S, Chamoli U, Richards H, McCurry M, Clausen P, et al. Toward integration of geometric morphometrics and computational biomechanics: New methods for 3D virtual reconstruction and quantitative analysis of Finite Element Models. Journal of Theoretical Biology. 2012;301:1–14. 10.1016/j.jtbi.2012.01.030 [DOI] [PubMed] [Google Scholar]
- 20. O’Higgins P, Cobb SN, Fitton LC, Gröning F, Phillips R, Liu J, et al. Combining geometric morphometrics and functional simulation: an emerging toolkit for virtual functional analyses. Journal of Anatomy. 2011;218(1):3–15. 10.1111/j.1469-7580.2010.01301.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walmsley CW, Smits PD, Quayle MR, McCurry MR, Richards HS, Oldfield CC, et al. Why the Long Face? The Mechanics of Mandibular Symphysis Proportions in Crocodiles. PLOS ONE. 2013;8(1):e53873 10.1371/journal.pone.0053873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pierce SE, Angielczyk KD, Rayfield EJ. Patterns of morphospace occupation and mechanical performance in extant crocodilian skulls: a combined geometric morphometric and finite element modeling approach. Journal of morphology. 2008;269(7):840–64. 10.1002/jmor.10627 [DOI] [PubMed] [Google Scholar]
- 23. Piras P, Maiorino L, Teresi L, Meloro C, Lucci F, Kotsakis T, et al. Bite of the Cats: Relationships between Functional Integration and Mechanical Performance as Revealed by Mandible Geometry. Systematic biology. 2013;62(6):878–900. 10.1093/sysbio/syt053 [DOI] [PubMed] [Google Scholar]
- 24. McHenry CR, Clausen PD, Daniel WJ, Meers MB, Pendharkar A. Biomechanics of the rostrum in crocodilians: A comparative analysis using finite-element modeling. The Anatomical Record Part A. 2006;288:827–49. [DOI] [PubMed] [Google Scholar]
- 25. Wroe S, Clausen P, McHenry C, Moreno K, Cunningham E. Computer simulation of feeding behaviour in the thylacine and dingo: a novel test for convergence and niche overlap. Proceedings of the Royal Society Series B. 2007;274:2819–28. 10.1098/rspb.2007.0906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wroe S, Ferrara TL, McHenry C, Curnoe D, Chamoli U. The craniomandibular mechanics of being human. Proceedings of the Royal Society of London B. 2010;277:3579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McHenry CR, Wroe S, Clausen PD, Moreno K, Cunningham E. Supermodeled sabercat, predatory behavior in Smilodon fatalis revealed by high-resolution 3D computer simulation. Proceedings of the National Academy of Sciences. 2007;104(41):16010–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tseng ZJ, Stynder D. Mosaic functionality in a transitional ecomorphology: skull biomechanics in stem Hyaeninae compared to modern South African carnivorans. Biological Journal of the Linnean Society. 2011;102(3):540–59. [Google Scholar]
- 29. Tseng ZJ. Testing Adaptive Hypotheses of Convergence with Functional Landscapes: A Case Study of Bone-Cracking Hypercarnivores. PLOS one. 2013;8(5):e65305 10.1371/journal.pone.0065305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lautenschlager S, Witmer LM, Altangerel P, Rayfield EJ. Edentulism, beaks, and biomechanical innovations in the evolution of theropod dinosaurs. Proceedings of the National Academy of Sciences. 2013. 110; 51: 20657–20662. 10.1073/pnas.1310711110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soons J, Herrel A, Genbrugge A, Aerts P, Podos J, Adriaens D, et al. Mechanical stress, fracture risk and beak evolution in Darwin's ground finches (Geospiza). Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365(1543):1093–8. 10.1098/rstb.2009.0280 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ross CF, Berthaume MA, Dechow PC, Iriarte-Diaz J, Porro LB, Richmond BG, et al. In vivo bone strain and finite-element modeling of the craniofacial haft in catarrhine primates. Journal of Anatomy. 2011;218(1):112–41. 10.1111/j.1469-7580.2010.01322.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Slater GJ, Van Valkenburgh B. Allometry and performance: the evolution of skull form and function in felids. Journal of Evolutionary Biology. 2009;22(11):2278–87. 10.1111/j.1420-9101.2009.01845.x [DOI] [PubMed] [Google Scholar]
- 34. Dumont ER, Piccirillo J, Grosse IR. Finite-element analysis of biting behavior and bone stress in the facial skeletons of bats. The Anatomical Record Part A. 2005;283A:319–30. [DOI] [PubMed] [Google Scholar]
- 35. Snively E, Cox A. Structural mechanics of pachycephalosaur crania permitted head-butting behavior. Palaeontologia Electronica. 2008;11(1):3A. [Google Scholar]
- 36. Moreno K, Wroe S, Clausen P, McHenry C, D’Amore DC, Rayfield EJ, et al. Cranial performance in the Komodo dragon (Varanus komodoensis) as revealed by high-resolution 3-D finite element analysis. Journal of Anatomy. 2008;212(6):736–46. 10.1111/j.1469-7580.2008.00899.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wroe S. Cranial mechanics compared in extinct marsupial and extant african lions using a finite-element approach. Journal of Zoology. 2008;274:332–9. [Google Scholar]
- 38. Bourke J, Wroe S, Moreno K, McHenry C, Clausen P. Effects of gape and tooth position on bite force and skull stress in the dingo (Canis lupus dingo) using a 3-dimensional finite element approach. PLOS One. 2008;3(5):e2200 10.1371/journal.pone.0002200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oldfield CC, McHenry CR, Clausen PD, Chamoli U, Parr WCH, Stynder DD, et al. Finite element analysis of ursid cranial mechanics and the prediction of feeding behaviour in the extinct giant Agriotherium africanum . Journal of Zoology. 2012;286(2):163–170. 10.1111/j.1469-7998.2011.00862.x [DOI] [Google Scholar]
- 40. Bright JA. The Importance of Craniofacial Sutures in Biomechanical Finite Element Models of the Domestic Pig. PLOS one. 2012;7(2):e31769 10.1371/journal.pone.0031769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chalk J, Richmond BG, Ross CF, Strait DS, Wright BW, Spencer MA, et al. A finite element analysis of masticatory stress hypotheses. American Journal of Physical Anthropology. 2011;145(1):1–10. 10.1002/ajpa.21416 [DOI] [PubMed] [Google Scholar]
- 42. Wang Q, Wright BW, Smith A, Chalk J, Byron CD. Mechanical Impact of Incisor Loading on the Primate Midfacial Skeleton and its Relevance to Human Evolution. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2010;293(4):607–17. 10.1002/ar.21123 [DOI] [PubMed] [Google Scholar]
- 43. Curtis N, Witzel U, Fitton L, O'Higgins P, Fagan M. The Mechanical Significance of the Temporal Fasciae in Macaca fascicularis: An Investigation Using Finite Element Analysis. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2011;294(7):1178–90. 10.1002/ar.21415 [DOI] [PubMed] [Google Scholar]
- 44. Dumont ER, Davis JL, Grosse IR, Burrows AM. Finite element analysis of performance in the skulls of marmosets and tamarins. Journal of Anatomy. 2011;218(1):151–62. 10.1111/j.1469-7580.2010.01247.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moreno K, Wroe S, Clausen P, McHenry C, D'Amore DC, Rayfield E, et al. Cranial performance in the Komodo dragon (Varanus komodoensis) as revealed by high-resolution 3-D finite element analysis. Journal of Anatomy. 2008;212(6):736–46. 10.1111/j.1469-7580.2008.00899.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Curtis N, Jones MEH, Shi J, O'Higgins P, Evans SE, Fagan MJ. Functional Relationship between Skull Form and Feeding Mechanics in Sphenodon, and Implications for Diapsid Skull Development. PLOS one. 2011;6(12):e29804 10.1371/journal.pone.0029804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rayfield EJ, Norman DB, Horner CC, Horner JR, Smith PM, Thomason JJ, et al. Cranial design and function in a large theropod dinosaur. Nature. 2001;409:1033–7. [DOI] [PubMed] [Google Scholar]
- 48. Rayfield E. Cranial mechanics and feeding in Tyrannosaurus rex . Proceedings of the Royal Society of London B. 2004;271:1451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rayfield EJ. Using finite-element analysis to investigate suture morphology: A case study using large carnivorous dinosaurs. The Anatomical Record Part A. 2005;283:349–65. [DOI] [PubMed] [Google Scholar]
- 50. Richards R Jr. Principles of solid mechanics: CRC Press; 2010. [Google Scholar]
- 51.McNeel. Rhino3d.
- 52. Wiley D. Landmark Editor 3.0. Institute for Data Analysis and Visualization Davis: University of California; 2006. [Google Scholar]
- 53. O’Higgins P, Jones N. Morphologika 2, v. 2.4 Hull York Medical School, York: 2006. [Google Scholar]
- 54. Erickson GM, Catanese J, Keaveny TM. Evolution of the biomechanical material properties of the femur. The Anatomical Record. 2002;268(2):115–24. 10.1002/ar.10145 [DOI] [PubMed] [Google Scholar]
- 55. Holliday CM. New insights Into dinosaur jaw muscle anatomy. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2009;292(9):1246–65. 10.1002/ar.20982 [DOI] [PubMed] [Google Scholar]
- 56. McHenry C. Devourer of Gods: the palaecology of the Cretaceous pliosaur Kronosaurus queenslandicus Newcastle, NSW, Australia: University of Newcastle; 2009. http://hdl.handle.net/1959.13/935911 [Google Scholar]
- 57.Walmsley CW, McCurry MR, Clausen PD, McHenry CR. Beware the black box: investigating the sensitivity of FEA simulations to modelling factors in comparative biomechanics. Peer J. 2013. 10.7717/peerj.204 [DOI] [PMC free article] [PubMed]
- 58. King D. The diet and foraging strategy of Varanus acanthurus . Biawak. 2008;2(1):11–7. [Google Scholar]
- 59. Losos JB, Greene HW. Ecological and evolutionary implications of diet in monitor lizards. Biological Journal of the Linnean Society. 1988;35:379–407. [Google Scholar]
- 60. Shine R. Food Habits, Habitats and Reproductive Biology of Four Sympatric Species of Varanid Lizards in Tropical Australia. Herpetologica. 1986;42(3):346–60. 10.2307/3892313 [DOI] [Google Scholar]
- 61. Beck D. Heloderma horridum In: Pianka ER, King DR, King RA, editors. Varanoid lizards of the world. Bloomington: Indiana University Press; 2004. p. 521–7. [Google Scholar]
- 62. Dryden G. Varanus acanthurus In: Pianka ER, King DR, King RA, editors. Varanoid lizards of the world. Bloomington: Indiana University Press; 2004. p. 298–307. [Google Scholar]
- 63. Thompson G. Varanus gouldii In: Pianka ER, King DR, King RA, editors. Varanoid Lizards of the World. Bloomington: Indiana University Press; 2004. p. 380–400. [Google Scholar]
- 64. Horn H-G. Varanus salvadorii In: Pianka ER, King DR, King RA, editors. Varanoid lizards of the world. Bloomington: Indiana University Press; 2004. p. 231–3. [Google Scholar]
- 65. Bennett D. Observations of Bosc's monitor lizard (Varanus exanthematicus) in the wild. Bull Chicago Herpetol Soc. 2002;35:177–80. [Google Scholar]
- 66. Bennett D. Varanus exanthematicus In: Pianka ER, King DR, King RA, editors. Varanoid lizards of the world. Bloomington: Indiana University Press; 2004. p. 95–103. [Google Scholar]
- 67. Christian K. Varanus panoptes In: Pianka ER, editor. Varanoid lizards of the world. Bloomington: Indiana University Press; 2004. p. 423–9. [Google Scholar]
- 68. Eidenmuller B. Varanus storri In: Pianka ER, King DR, King RA, editors. Varanoid lizards of the world. Bloomington: Indiana University Press; 2004. p. 472–6. [Google Scholar]
- 69. Gaulke M, Horn H-G. Varanus salvator In: Pianka ER, King DR, King RA, editors. Varanoid lizards of the world. Bloomington: Indiana University Press; 2004. p. 244–57. [Google Scholar]
- 70. Greene HW. Varanus prasinus In: Pianka ER, King DR, King RA, editors. Varanoid lizards of the world. Bloomington: Indiana University Press; 2004. p. 225–39. [Google Scholar]
- 71. Shannon R, Mendyk RW. Aquatic foraging behaviour and freshwater Mussel (Velesunio sp.) predation by Varanus panoptes panoptes in Central-Western Queensland. International Varanid Intrest Group. 2009;3(3):85–7. [Google Scholar]
- 72. Ciofi C. Varanus komodoensis In: Pianka ER, King DR, King RA, editors. Varanoid lizards of the world. Bloomington: Indiana University Press; 2004. p. 197–204. [Google Scholar]
- 73. R Core Team. R: A language and Environment for Statistical Computing Vienna: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 74. Klingenberg CP, Gidaszewski NA. Testing and quantifying phylogenetic signals and homoplasy in morphometric data. Systematic biology. 2010;59(3):245–61. 10.1093/sysbio/syp106 [DOI] [PubMed] [Google Scholar]
- 75. Wroe S, McHenry C, Thomason J. Bite club: comparative bite force in big biting mammals and the prediction of predatory behaviour in fossil taxa. Proceedings of the Royal Society of London, Series B. 2005;272:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pianka E. Lanthanotus borneensis In: Pianka ER, King DR, King RA, editors. Varanoid Lizards of the World. Bloomington: Indiana University Press; 2004. p. 535–8. [Google Scholar]
- 77. Wroe S, Ferrara TL, McHenry CR, Curnoe D, Chamoli U. The craniomandibular mechanics of being human. Proceedings of the Royal Society B: Biological Sciences. 2010;277(1700):3579–86. 10.1098/rspb.2010.0509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Figueirido B, Soibelzon LH. Inferring palaeoecology in extinct tremarctine bears (Carnivora, Ursidae) using geometric morphometrics. Lethaia. 2010;43(2):209–22. [Google Scholar]
- 79. Henderson DM. The eyes have it: the sizes, shapes, and orientations of theropod orbits as indicators of skull strength and bite force. Journal of Vertebrate Paleontology. 2003;22(4):766–78. [Google Scholar]
- 80. Fry BG, Wroe S, Teeuwisse W, van Osch MJP, Moreno K, Ingle J, et al. A central role for venom in predation by Varanus komodoensis (Komodo Dragon) and the extinct giant Varanus (Megalania) priscus . Proceedings of the National Academy of Sciences. 2009;106(22):8969–74. 10.1073/pnas.0810883106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bull J, Jessop TS, Whiteley M. Deathly drool: evolutionary and ecological basis of septic bacteria in Komodo dragon mouths. PLOS one. 2010;5(6):e11097 10.1371/journal.pone.0011097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Imansyah M, Jessop T, Ciofi C, Akbar Z. Ontogenetic differences in the spatial ecology of immature Komodo dragons. Journal of Zoology. 2008;274(2):107–15. [Google Scholar]
- 83.Rieppel O, Labhardt L. Mandibular mechanics in Varanus niloticus (Reptilia: Lacertilia). Herpetologica. 1979:158–63.
- 84. Moazen M, Curtis N, O'Higgins P, Jones ME, Evans SE, Fagan MJ. Assessment of the role of sutures in a lizard skull: a computer modelling study. Proceedings of the Royal Society B: Biological Sciences. 2009;276(1654):39–46. 10.1098/rspb.2008.0863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang Q, Smith AL, Strait DS, Wright BW, Richmond BG, Grosse IR, et al. The Global Impact of Sutures Assessed in a Finite Element Model of a Macaque Cranium. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2010;293(9):1477–91. 10.1002/ar.21203 [DOI] [PubMed] [Google Scholar]
- 86. Jasinoski SC, Rayfield EJ, Chinsamy A. Functional implications of dicynodont cranial suture morphology. Journal of Morphology. 2010;271(6):705–28. 10.1002/jmor.10828 [DOI] [PubMed] [Google Scholar]
- 87. Reed DA, Porro LB, Iriarte‐Diaz J, Lemberg JB, Holliday CM, Anapol F, et al. The impact of bone and suture material properties on mandibular function in Alligator mississippiensis: testing theoretical phenotypes with finite element analysis. Journal of Anatomy. 2011;218(1):59–74. 10.1111/j.1469-7580.2010.01319.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Herrel A, Schaerlaeken V, Meyers JJ, Metzger KA, Ross CF. The evolution of cranial design and performance in squamates: Consequences of skull-bone reduction on feeding behavior. 2007. p. 107–17. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(TXT)
(RTF)
Natural forces represent the force per truss element predicted for each of the muscle beams based on muscle cross sectional area at natural size. Adjusted forces represent the force per truss element adjusted so that the models produced the same node reaction force at the bite location in volume scaled models. Total muscle forces represent the total force applied to each model to generate the same node reaction forces at bite location.
(XLSX)
Data Availability Statement
All data is included within the paper and/or Supporting Information files.