Endothelial progenitor cells (EPCs) were coembedded with renal mesenchymal stem cells (MSCs) to further ameliorate renal dysfunction. Their paracrine influence on cytokine/chemokine release and proinflammatory macrophages was examined. Hyaluronic acid-based hydrogel delivery of EPCs alone or codelivered with hypoxic preconditioned MSCs offered significant protection of renal and vascular function during endotoxemia, including reducing the circulating levels of cytokines/chemokines.
Keywords: Endothelial progenitor cells, Hyaluronic acid hydrogel, Acute kidney injury, Macrophage polarization, Mesenchymal stem cells, Lipopolysaccharide
Abstract
We previously reported the delivery of endothelial progenitor cells (EPCs) embedded in hyaluronic acid-based (HA)-hydrogels protects renal function during acute kidney injury (AKI) and promotes angiogenesis. We attempted to further ameliorate renal dysfunction by coembedding EPCs with renal mesenchymal stem cells (MSCs), while examining their paracrine influence on cytokine/chemokine release and proinflammatory macrophages. A live/dead assay determined whether EPC-MSC coculturing improved viability during lipopolysaccharide (LPS) treatment, and HA-hydrogel-embedded delivery of cells to LPS-induced AKI mice was assessed for effects on mean arterial pressure (MAP), renal blood flow (RBF), circulating cytokines/chemokines, serum creatinine, proteinuria, and angiogenesis (femoral ligation). Cytokine/chemokine release from embedded stem cells was examined, including effects on macrophage polarization and release of proinflammatory molecules. EPC-MSC coculturing improved stem cell viability during LPS exposure, an effect augmented by MSC hypoxic preconditioning. The delivery of coembedded EPCs with hypoxic preconditioned MSCs to AKI mice demonstrated additive improvement (compared with EPC delivery alone) in medullary RBF and proteinuria, with comparable effects on serum creatinine, MAP, and angiogenesis. Exposure of proinflammatory M1 macrophages to EPC-MSC conditioned medium changed their polarization to anti-inflammatory M2. Incubation of coembedded EPCs-MSCs with macrophages altered their release of cytokines/chemokines, including enhanced release of anti-inflammatory interleukin (IL)-4 and IL-10. EPC-MSC delivery to endotoxemic mice elevated the levels of circulating M2 macrophages and reduced the circulating cytokines/chemokines. In conclusion, coembedding EPCs-MSCs improved their resistance to stress, impelled macrophage polarization from M1 to M2 while altering their cytokine/chemokines release, reduced circulating cytokines/chemokines, and improved renal and vascular function when MSCs were hypoxically preconditioned.
Significance
This report provides insight into a new therapeutic approach for treatment of sepsis and provides a new and improved strategy using hydrogels for the delivery of stem cells to treat sepsis and, potentially, other injuries and/or diseases. The delivery of two different stem cell lines (endothelial progenitor cells and mesenchymal stem cells; delivered alone and together) embedded in a protective bioengineered scaffolding (hydrogel) offers many therapeutic benefits for the treatment of sepsis. This study shows how hydrogel-delivered stem cells elicit their effects and how hydrogel embedding enhances the therapeutic efficacy of delivered stem cells. Hydrogel-delivered stem cells influence the components of the overactive immune system during sepsis and work to counterbalance the release of many proinflammatory and prodamage substances from immune cells, thereby improving the associated vascular and kidney damage.
Introduction
The therapeutic delivery of stem cells has been demonstrated to enhance the repair and regeneration of the kidney after injury [1–7]. Although various multipotent stem cells have been examined for their repair potential, endothelial progenitor cells (EPCs) have demonstrated remarkable renoprotective potential. EPCs are typically found in the circulation, residing in their niches located in the bone marrow or locally in renal vascular beds. EPCs assist in homeostasis by maintaining normal vascular function, including maintenance and possible replacement of the endothelium [8–10]. However, during kidney and vascular injury, the competence of endogenous EPCs is often compromised, leading to accelerated progression of the injury [7]. Previous studies from our laboratory and others have shown that delivered EPCs counter the vascular impairment that occurs during the course of acute kidney injury (AKI) [1, 6, 7, 11] and improve renal function, attenuate inflammation, decrease tubular and vascular damage, and enhance angiogenesis [1, 4, 6, 7].
Despite the therapeutic advantages stem cell delivery offers for the treatment of AKI, many problems confront current cell therapy application. The delivery of stem cells by intravenous (i.v.) injection, currently the most common method of stem cell delivery, results in less than 1% of delivered cells engrafted in targeted injured tissue [12]. Although this technique of cell therapy enhances the risk of potential embolism, the delivered cells are also subject to programmed cell death (anoikis) and are rapidly cleared from the systemic circulation before they can elicit their therapeutic benefits to target tissues [12]. Insufficient homing is a major limitation of systemically delivered stem cell-based therapies and is caused in part by inadequate expression and activation of cell surface adhesion receptors, including integrins, an effect that also inhibits their anti-inflammatory and proregenerative activities [13]. Additionally, i.v. delivery of stem cells into the circulation subjects these cells to any circulating cytotoxins/endotoxins that might be present and responsible for the initial renal injury. These multiple drawbacks of conventional i.v. delivery of stem cells highlight the inefficiency of this delivery method.
An alternative method of stem cell therapy that we have previously shown to be superior to the conventional i.v. route is the delivery of stem cells embedded in bioengineered scaffolds [1, 6]. Although a variety of bioengineered scaffolds have been used for cell embedding [14–16], we have found that hyaluronic acid-based (HA) hydrogel scaffolds offer significant advantages for the delivery of stem cells. The use of HA-hydrogels for stem cell delivery allows direct delivery and retention of embedded stem cells at precise localities of tissue damage, avoiding the substantial side effects associated with conventional systemic i.v. delivery. Once delivered, the embedded stem cells can be released “on demand” from HA-hydrogels either by endogenous release of hyaluronidase from the injured tissues [17, 18] or by its direct injection into the HA-hydrogel. We have previously demonstrated the therapeutic benefits of EPCs are significantly augmented when these cells are delivered in HA-hydrogels for the treatment of kidney injury [1, 6].
In the present studies, we continued to explore the enhancement of the therapeutic benefits of stem cell delivery using HA-hydrogels by coembedding EPCs with renal mesenchymal stem cells (MSCs). We hypothesized that coembedding EPCs with MSCs might further stimulate the therapeutic effects of both EPCs and MSCs once codelivered into a model of AKI. MSCs might offer significant advantages as a supporting cell to EPCs because of their immunomodulatory properties; their ability to induce neighboring cells to secret cytokines that inhibit inflammation and pathological remodeling [19]; and their ability to improve stem cell niche quality and cell mobilization [19]. In addition, we examined the influence HA-hydrogel embedding has on stem cell release of cytokines/chemokines, and the influence these cells have in modulating the immune response of macrophages, including macrophage polarization and their subsequent release of anti- and/or proinflammatory molecules.
Materials and Methods
Cell Culture
For allogeneic HA-hydrogel implantation and cell culture experiments, we used an established line of mouse embryonic EPCs [20] and MSCs that we isolated from mouse kidneys (as previously described [21]). RAW 264.7 macrophages (American Type Culture Collection, Manassas, VA, http://www.atcc.org) were also used in the experiments. For hypoxic preconditioning of MSCs, the cells were exposed to <5% O2, 5% CO2, 90% N2 for 72 hours in culture before use. The details of cell culturing are described in the supplemental online data.
HA-Hydrogel Formation and Degradation
HA-hydrogels were prepared using the HyStem Hydrogel Kit (BioTime, Inc., Alameda, CA, http://www.esibio.com), as previously described. ProNectin (50 µg/ml; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), EPCs, and MSCs were added before hydrogel solidification with cross-linker. For cell release, the hydrogels were digested with collagenase (300 U/ml) and hyaluronidase (100 U/ml) (Sigma-Aldrich). The HA-hydrogel details are described in the supplemental online data.
Live/Dead Assay
To assess the effects of LPS (1, 10, and 20 μg/ml) on EPCs and MSCs in and out of HA-hydrogels, the LIVE/DEAD Viability/Cytotoxicity Kit (Invitrogen/Life Technologies, Grand Island, NY, http://www.lifetechnologies.com) was used. The details of the LIVE/DEAD assay are described in the supplemental online data.
Sepsis Models
The animal study protocol was in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the institutional animal care and use committee. For LPS-induced endotoxemia in male mice (C57/Bl6 age >16 weeks), a single intraperitoneal injection of 10 μg/kg LPS (from Escherichia coli serotype 0111:B8, Sigma-Aldrich) was applied. Details of the animal model are described in the supplemental online data.
In Vivo HA-Hydrogel Implantation
HA-hydrogels with embedded stem cells were implanted subcutaneously in the ears of sedated mice. Subcutaneous implantation of HA-hydrogels with embedded cells was conducted at the same time as the LPS injection. A total of 1 million cells was delivered to each mouse (5 × 105 cells were delivered to each ear). For the coembedding studies, 5 × 105 EPCs were combined with 5 × 105 MSCs in HA-hydrogels, and mice still received a total of 1 million cells. The ear implants were injected with collagenase and hyaluronidase to permit mobilization of the embedded cells 2 hours after LPS injection. Details of the HA-hydrogel implantation are described in the supplemental online data. Blood pressure was measured using a noninvasive blood pressure monitoring system 24 hours after sepsis induction and delivery of stem cells, as described in the supplemental online data.
Renal Blood Flow and Function
At 24 hours after sepsis induction and delivery of the stem cells, renal blood flow was evaluated using laser-Doppler flowmetry. Renal function was evaluated by serum creatinine and proteinuria measurement using commercial kits. Laser-Doppler flowmetry and the serum creatinine and proteinuria assays are described in the supplemental online data.
Engraftment Analysis
Engraftment of CellTracker (Invitrogen/Life Technologies) fluorescently labeled stem cells was examined by microscopy in the kidneys 24 hours after LPS injection and their delivery, as described in detail in the supplemental online data.
Femoral Ligation
Femoral ligation was used to examine the angiogenesis capability of the HA-hydrogel-delivered stem cells. Details of the femoral ligation procedure are described in the supplemental online data.
Flow Cytometry Analysis
Polarization of circulating macrophages in the plasma of LPS-injected mice (treated with HA-hydrogel-embedded stem cells) was evaluated by flow cytometry, as described in detail in the supplemental online data.
Macrophage Polarization
The polarization of macrophages cultured in stem cell-conditioned medium was examined using real time-polymerase chain reaction (RT-PCR), as described in detail in the supplemental online data.
Chemokine/Cytokine Release
The release of cytokines/chemokines was evaluated in the circulation of endotoxemic mice 24 hours after LPS injection (with and without HA-hydrogel-embedded stem cell treatment) and in the cell medium from cultured cells (cultured for 48 hours in or out of HA-hydrogels). The levels of cytokines/chemokines were analyzed using the Luminex 100 system (Luminex Corporation, Austin, TX, http://www.luminexcorp.com). Details of chemokine/cytokine analysis are described in the supplemental online data.
Statistical Analysis
Data are presented as the mean ± SEM. Data presented as a mean of a limited number of replicates (n < 6) were analyzed using nonparametric methods. The Kruskal-Wallis test was used to compare three or more groups of nonparametric data, and the Mann-Whitney U test was subsequently performed to identify significant differences by pairwise comparison of each group. Data taken from larger sampling sizes were examined using a t test for pairwise comparisons and a one-way analysis of variance with Dunnett’s multiple comparison post hoc test to determine the differences among three or more groups. The statistical analysis software used was GraphPad Prism, version 4.00, for Windows (GraphPad Software, Inc., San Diego, CA, http://www.graphpad.com) and NCSS, version 9 (NCSS Statistical Software, Kaysville, UT, http://www.ncss.com). Differences were considered significant at p ≤ .05, unless otherwise denoted. The animal numbers used for each experiment were 5–7 and are indicated in the figure legends in regard to each specific experiment.
Results
In Vitro Studies: Viability of Coembedded EPCs-MSCs
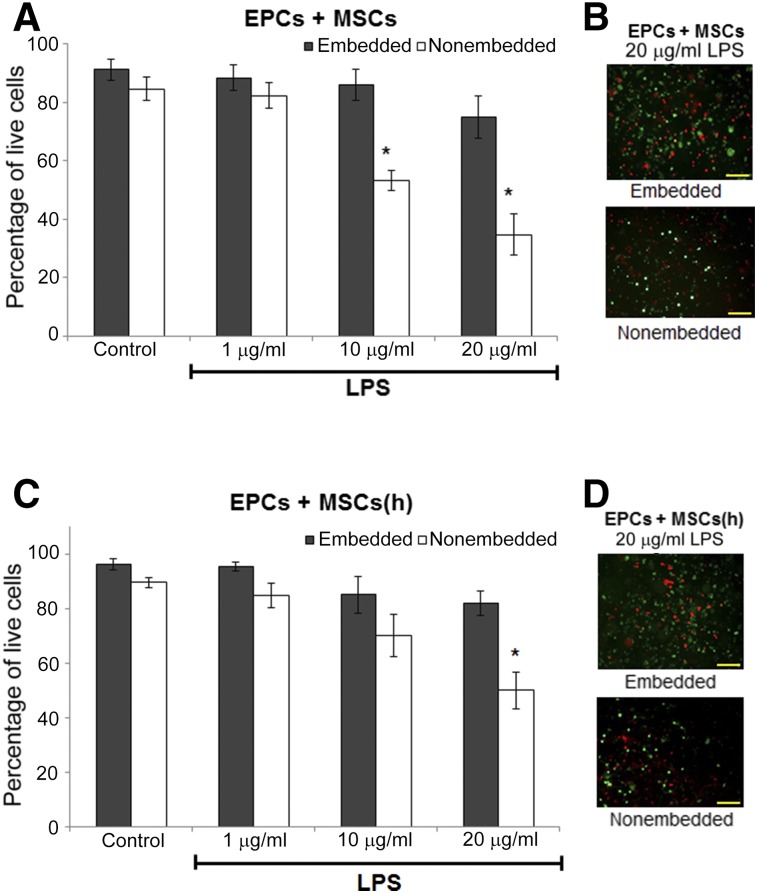
To determine whether the combination of EPCs with MSCs in HA-hydrogels improved cell viability during endotoxemic conditions, the cells were coembedded in HA-hydrogels (4% crosslinking) in vitro and subject to varying concentrations of LPS for 24 hours and subsequently evaluated for viability. At higher concentrations of LPS (10 and 20 μg/ml), nonembedded cells cultured on the surface of dishes sustained a significant loss of viability (53% and 35% EPC and MSC viability, respectively). However, coembedding these cells in HA-hydrogels improved viability to 86% and 75%, respectively (Fig. 1A). Hypoxic preconditioning of MSCs has been shown to improve the anti-inflammatory effects of these cells and enhance their support of nearby cells [22–24]. Coculturing EPCs with hypoxic preconditioned MSCs (72 hours in <5% O2) improved their viability when cultured in 2 dimensions (2D) (70% and 50% viability in response to 10 and 20 μg/ml of LPS, respectively; Fig. 1B), with no further improvement when embedded in hydrogels.
Figure 1.
In vitro LIVE/DEAD assay of EPCs-MSCs cocultures exposed to 24 hours of LPS. EPCs were coembedded in hyaluronic acid-based (HA)-hydrogels with nonhypoxic (A, B) or hypoxic preconditioned (C, D) MSCs and exposed to 24 hours of LPS of varying concentrations. Subsequent cellular vitality was quantified (A, C) using a LIVE/DEAD assay in which live cells stain with calcein (green) and dead cells with ethidium homodimer (red) (B, D). Coembedding EPCs with MSCs in HA-hydrogels (three-dimensional culturing) conferred improved protection against increasing LPS concentrations (compared with two-dimensional coculturing of EPCs with MSCs on the surface of culture dishes instead of embedded in HA-hydrogels). Hypoxic preconditioning of MSCs improved stem cell viability in nonembedded cells. n = 6. ∗, p ≤ .05 vs. embedded. Magnification, ×150. Scale bar = 100 µm. Abbreviations: EPCs, endothelial progenitor cells; (h), hypoxic; LPS, lipopolysaccharide; MSCs, mesenchymal stem cells.
In Vivo Studies: Therapeutic Effects of HA-Hydrogel-Delivered EPCs-MSCs
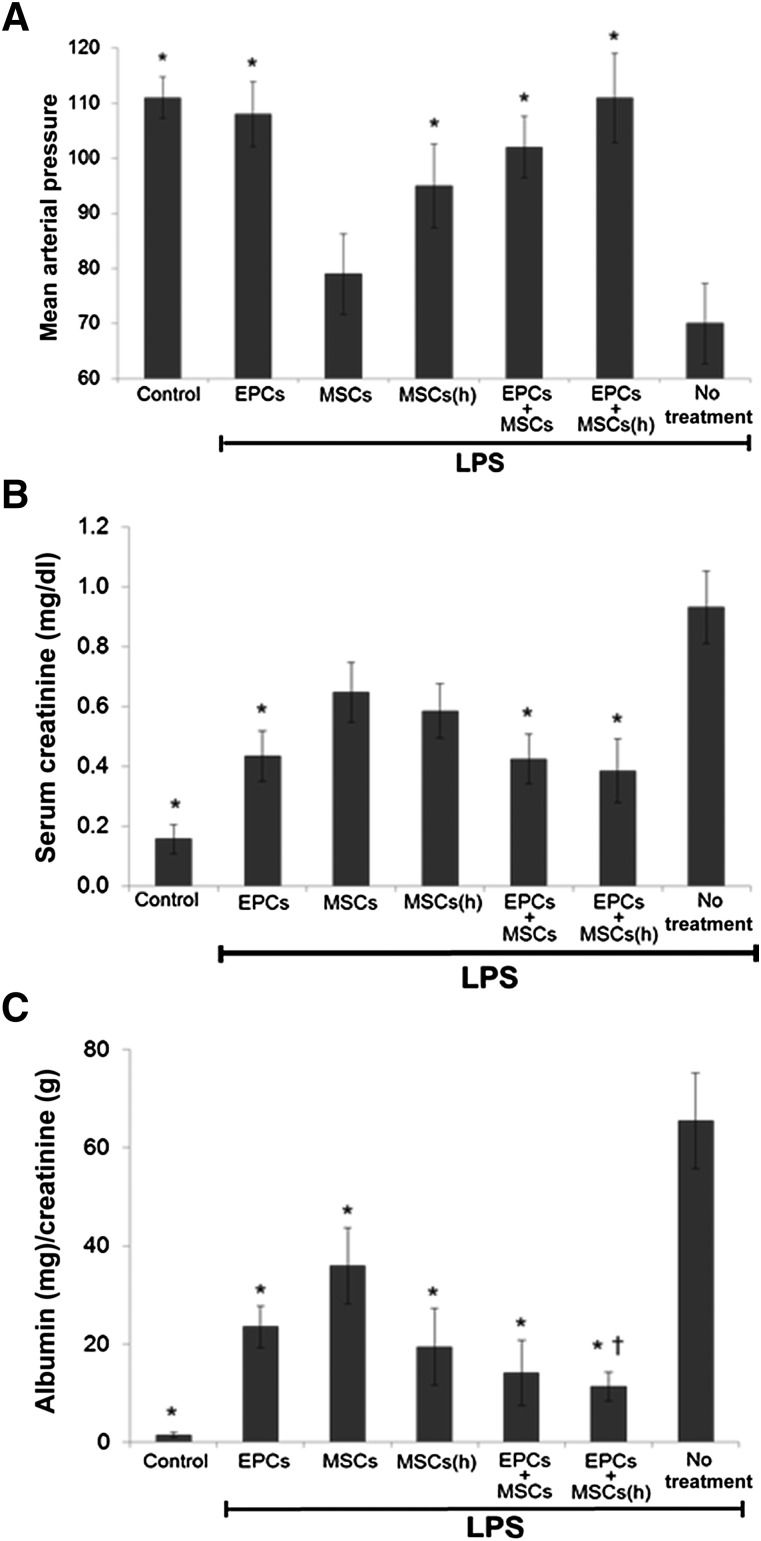
We have previously observed that delivery of EPCs embedded in HA-hydrogels improved their therapeutic effects compared with conventional i.v. delivery for treatment of AKI [1, 6]. Additionally, in our previously published report, we demonstrated the therapeutic benefits of HA-hydrogel-delivered stem cells were mediated by the embedded stem cells themselves and not any direct effect of the HA-hydrogel components that might enter the circulation or tissues of endotoxemic mice. This was indicated by the absence of any therapeutic effects when HA-hydrogels were delivered alone without embedded stem cells to endotoxemic mice [1]. In the present in vivo studies, we sought to determine whether HA-hydrogel coembedding EPCs with MSCs improved their therapeutic efficacy compared with EPC delivery alone. HA-hydrogels with embedded stem cells were transplanted via injection into mice (mouse ears) (Fig. 2A) at the onset of endotoxemia (as induced by 10 μg/kg LPS intraperitoneal injection). At 2 hours after implantation, HA-hydrogels were digested by direct injection of the enzyme mixture (collagenase and hyaluronidase) into the implanted HA-hydrogels, allowing for stem cell mobilization, and monitored microscopically by imaging the site of implantation and the disappearance of fluorescently labeled cells [6]. Administration of LPS typically causes systemic hypotension; however, the delivery of EPCs alone and codelivered with hypoxic preconditioned MSCs preserved systemic blood pressure (mean arterial pressure [MAP]) within 24 hours (Fig. 2B). In contrast, delivery of MSCs alone conferred the least improvement in MAP, despite a significantly enhanced therapeutic effect observed when these cells were preconditioned by hypoxia. We also examined the effect of EPC and MSC codelivery on kidney function during the course of endotoxemic kidney injury. Overall, the delivery of stem cells either alone or in tandem improved renal function during endotoxemia, with some minor differences associated with the application strategy. At 24 hours after LPS injection, HA-hydrogel delivery of EPCs alone lowered the serum creatinine levels more effectively than the delivery of either nonhypoxic or hypoxic MSCs (Fig. 2C). The delivery of coembedded EPCs with MSCs (nonhypoxic and hypoxic) did not further decrease the serum creatinine levels compared with EPCs alone. In contrast, analysis of albuminuria demonstrated that codelivery of embedded EPCs with MSCs resulted in a greater reduction in albuminuria 24 hours after endotoxemia induction compared with EPCs or MSCs delivered alone (Fig. 2D).
Figure 2.
Mean arterial pressure (MAP), serum creatinine, and albuminuria in endotoxemic mice treated with stem cells delivered by hyaluronic acid-based (HA)-hydrogels. Endotoxemia was induced in mice by LPS injection, and MAP, serum creatinine, and albumin were measured 24 hours later. Stem cells embedded in HA-hydrogels were delivered to mice and transplanted into mouse ears during induction of endotoxemia. Stem cells were released from hydrogels by digestive enzyme injection 2 hours after implantation. EPCs delivered alone or codelivered with hypoxic preconditioned MSCs mediated the greatest improvement in MAP (A) and serum creatinine (B) 24 hours after LPS injection. Codelivery attenuated albuminuria (C) the most effectively. n = 5 (5 different mice for each group). ∗, p ≤ .05 vs. no treatment; †, p ≤ .05 vs. MSCs. Abbreviations: EPCs, endothelial progenitor cells; (h), hypoxic; LPS, lipopolysaccharide; MSCs, mesenchymal stem cells.
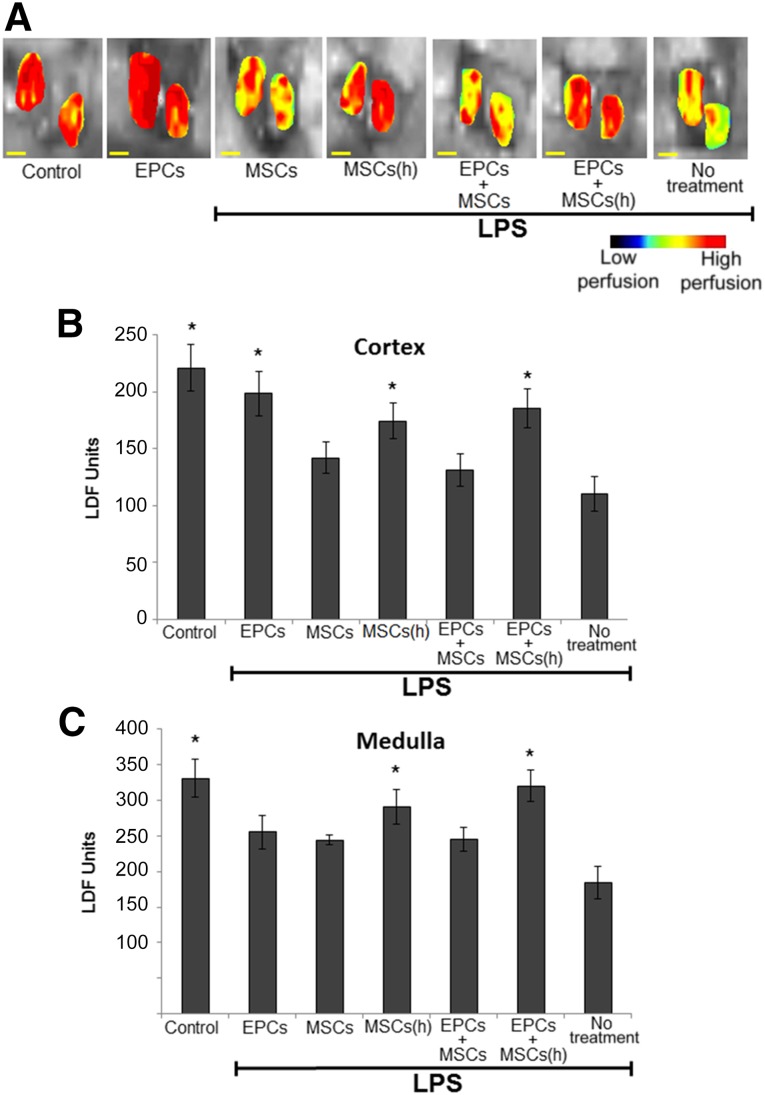
In addition to systemic hypotension, endotoxemia also reduces renal cortical and medullary blood flow, further impairing renal function. Delivery of EPCs enhanced cortical blood flow (measured using laser-Doppler flowmetry) (Fig. 3A, 3B). Codelivery with MSCs did not further enhance the ability of EPCs to increase cortical blood flow; however, when MSCs were primed by hypoxic preconditioning, EPC and MSC codelivery improved medullary blood flow (Fig. 3C).
Figure 3.
Renal blood flow measured using LDF in endotoxemic mice after stem cell treatment. Stem cells embedded in hyaluronic acid-based (HA)-hydrogels were delivered to mice (via ear transplant) during induction of endotoxemia (LPS injection). Stem cells were released from hydrogels by digestive enzyme injection 2 hours after implantation. Renal blood flow was imaged 24 hours after LPS injection (A). EPC delivery improved cortical blood flow (B), and hypoxic preconditioning of MSCs enhanced their ability to improve medullary blood flow (C). n = 5 (5 different mice for each group). ∗, p ≤ .05 vs. no treatment. Scale bar = 3 mm. Abbreviations: EPCs, endothelial progenitor cells; (h), hypoxic; LDF, laser-Doppler flowmetry; LPS, lipopolysaccharide; MSCs, mesenchymal stem cells.
We have previously shown that delivery of EPCs by HA-hydrogel enhances their engraftment in the injured kidney [1] compared with conventional i.v. delivery. We therefore questioned whether codelivery of embedded EPCs with MSCs further improved their engraftment and is a factor in their therapeutic efficacy. However, we did not observe any enhancement in renal engraftment of the delivered cells during coembedding (supplemental online Fig. 1).
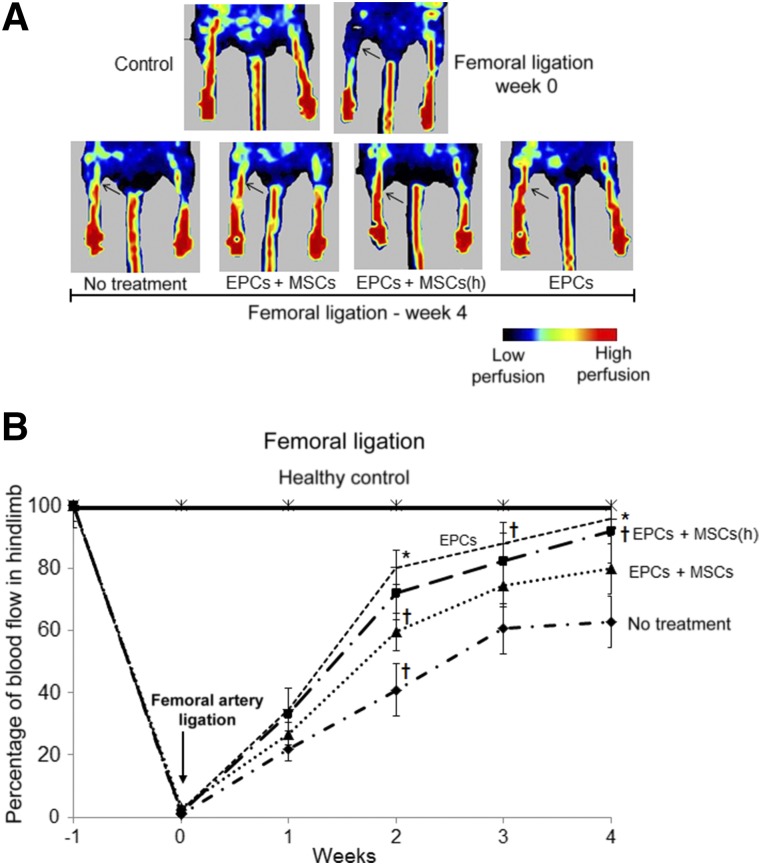
The therapeutic qualities of EPCs include their ability to mediate angiogenesis in the face of tissue and vascular damage [1, 6, 7]. We sought to determine whether codelivery of embedded EPCs with MSCs could enhance the ability of EPCs to mediate angiogenesis and vascular repair. We used the femoral ligation technique and evaluated blood flow in the ligated hindlimb of mice treated with codelivery of HA-hydrogel-embedded EPCs and MSCs. As previously, we implanted HA-hydrogels with embedded stem cells during the onset of femoral ligation, followed by scaffold-degrading enzyme administration 2 hours after implantation. In these experiments, although EPC delivery demonstrated significantly superior neovascularization effects, no statistically significant difference was found between the effects of EPC delivery alone and when these cells were codelivered with MSCs (nonhypoxic or hypoxic) (Fig. 4A, 4B). In contrast, delivery of MSCs alone (nonhypoxic or hypoxic) did not induce significant neoangiogenesis and was comparable to no treatment (data not shown).
Figure 4.
Blood perfusion in the hindlimb of mice after femoral arterial ligation. Blood perfusion, indicative of neoangiogenesis, in hindlimbs was imaged (A) and quantified (B) using laser-Doppler flowmetry over the course of 4 weeks after femoral ligation and delivery of stem cells embedded in hyaluronic acid-based (HA)-hydrogels. HA-hydrogels were transplanted into mouse ears during femoral ligation and were subsequently released by digestive enzyme injection 2 hours after implantation. Hindlimb perfusion was enhanced the greatest during HA-hydrogel delivery of EPCs alone. MSC (normoxic and hypoxic preconditioned) delivery alone (data not shown) resulted in perfusion values comparable to that of no treatment. n = 5 (5 different mice for each group). ∗, p ≤ .05 vs. no treatment; †, p ≤ .10 vs. no treatment. Abbreviations: EPCs, endothelial progenitor cells; (h), hypoxic; LPS, lipopolysaccharide; MSCs, mesenchymal stem cells.
EPC-MSC Effect on Macrophage Polarization
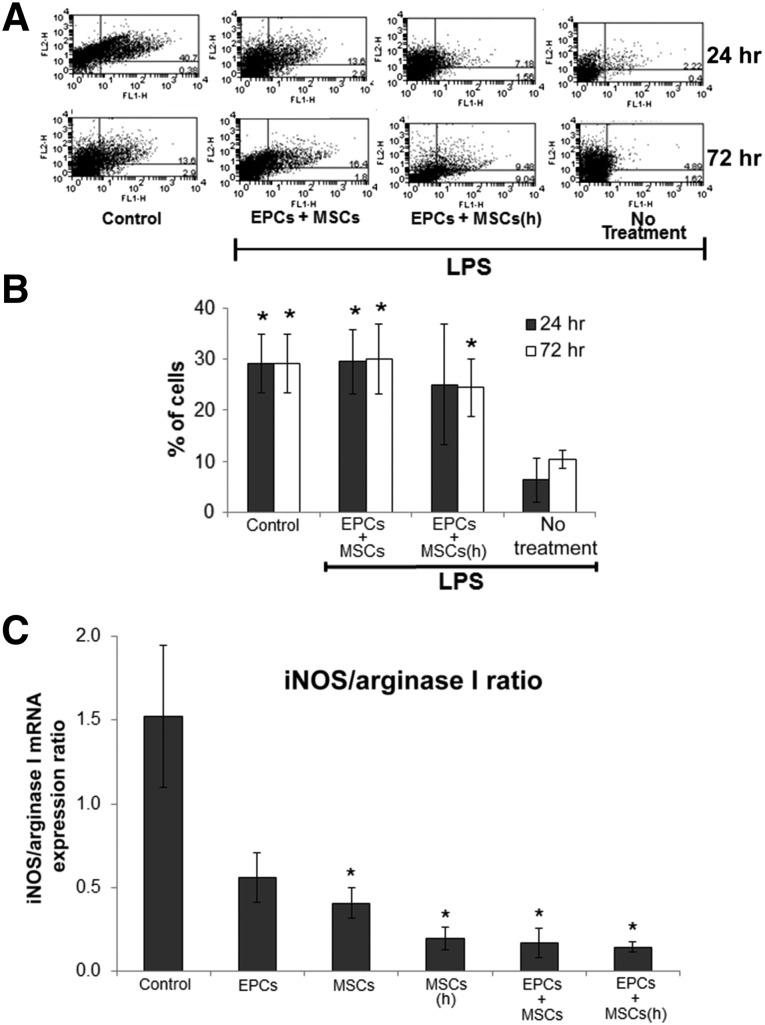
We questioned whether the paracrine effects of EPCs and/or MSCs could alter the polarization of macrophages causing a transition of these cells from the classic M1 proinflammatory phenotype to the alternative anti-inflammatory M2 phenotype, an effect that could participate in the therapeutic efficacy of these stem cells. In animals, the circulating levels of M2 macrophages were significantly reduced 24 and 72 hours after LPS injection (Fig. 5A). However, when EPCs and MSCs were codelivered by HA-hydrogel during LPS injection, the level of M2 macrophages remained preserved in the circulation (Fig. 5A), as determined by flow cytometry analysis using the macrophage marker F4/80 and the M2 marker CD206.
Figure 5.
The polarization of macrophages is influenced by stem cells during endotoxemia. During LPS-induced endotoxemia in mice (A, B), levels of circulating M2 macrophages remained elevated when EPCs and MSCs were codelivered by hyaluronic acid-based (HA)-hydrogels, determined by flow cytometry analysis 24 and 72 hours after LPS injection and stem cell delivery. MSCs were preconditioned by 72 hours of hypoxia (<5% O2). In the flow cytometry analysis dot plots (A), the x-axis represents F4/80 staining (total macrophages) and the y-axis CD206 staining (mannose receptor [M2 polarization]). The percentage of double positive cells in the upper right quadrant of the dot plots (indicative of M2 macrophages) is presented in the graph (B). n = 5–7 (5–7 different mice were used for each treatment group, except for the EPCs + MSCs(h) group, which had an n value of 4). ∗, p ≤ .05 vs. no treatment. In in vitro experiments (C), macrophages were pushed to M2 anti-inflammatory polarization after 48 hours of treatment with stem cell-conditioned culture medium. Stem cell-conditioned medium was obtained from cultures of HA-hydrogel-embedded EPCs and MSCs (normoxic and hypoxic preconditioned) and subsequently used for treatment of macrophages. Macrophages treated for 48 hours with the conditioned medium demonstrated a reduced ratio of iNOS to arginase I mRNA expression (representative of reduced proinflammatory M1 marker iNOS and/or enhanced anti-inflammatory M2 marker arginase I), indicative of a phenotypical change of macrophages from M1 to M2 polarization during their culturing in stem cell-conditioned medium. Before culturing with stem cell-conditioned medium, macrophages were pushed to M1 polarization by 24 hours of treatment with interferon-γ (50 ng/ml). n = 4–5 (4–5 different samples were used for each treatment group, except for EPCs alone, which had an n value of 3). ∗, p ≤ .05 vs. control; †, p ≤ .10 vs. control. Abbreviations: EPCs, endothelial progenitor cells; (h), hypoxic; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; MSCs, mesenchymal stem cells.
In the follow-up in vitro experiments, we collected serum-free conditioned medium from EPCs and MSCs (normoxic and hypoxic preconditioned) and subsequently treated macrophages with the stem cell-conditioned medium for 48 hours. Before treatment with the conditioned medium, the macrophages were impelled to M1 polarization by 24 hours of treatment with 50 ng/ml interferon-γ. After treatment with stem cell-conditioned medium, we determined macrophage polarization by measuring the mRNA expression ratio of 2 classic markers of M1 and M2, inducible nitric oxide synthase (iNOS) and arginase I, respectively. The ratio of iNOS/arginase I expression illustrates the effect the conditioned medium treatment had on macrophage polarization. A high iNOS/arginase I ratio indicates macrophages assumed M1 polarization, while a low ratio indicates an alternative anti-inflammatory M2 phenotype. Although treatment with conditioned medium from EPCs and normoxic MSCs tended to decrease the iNOS/arginase I ratio, the most robust reductions occurred when macrophages were treated with hypoxic MSCs or EPCs with MSCs cocultured conditioned medium (Fig. 5B). The results demonstrated stem cell-conditioned medium influenced macrophage conversion from M1 to M2, with MSCs alone and EPCs with MSCs cocultured medium resulting in the most significant conversion of macrophages to M2.
In Vitro: Coembedded EPC-MSC Secretory Profile
To further evaluate the effects of EPCs and MSCs on the innate immune system, we examined the changes in cytokine/chemokine release from macrophages that were cultured with and without EPCs and MSCs coembedded in HA-hydrogels. We first examined the cytokine/chemokine release of macrophages cultured alone after stress induced by 100 µM hydrogen peroxide (H2O2). We used H2O2 to induce stress for multiple reasons. For technical reasons, we did not want to stress cells with LPS in vitro because of the requirement for serum in the culture medium to activate LPS (LPS requires LPS-binding protein present in serum for its activation). Animal serum routinely contains inconsistent levels of endogenous cytokine/chemokine levels. We wanted to avoid adding serum to our stressed stem cell cultures to avoid this aspect and the presence of unwanted contaminating cytokines/chemokines at varying levels, in particular, because the goal of our in vitro studies was to examine the release of cytokines/chemokines from the stem cells into the culture medium. Additionally, we normally culture EPCs in bovine serum, and MSCs are cultured in horse serum. Thus, in experiments that examined the release of cytokines/chemokines from combined EPCs with MSC cocultures, to activate LPS, we would have to add serum from either bovine or horse and at a variable concentration, which would have changed the conditions in which these stem cells are normally cultured. Furthermore, the use of H2O2 is exceptionally relevant to LPS examination, because the very early effects of LPS exposure to cells and animals includes rapidly enhanced upregulation of reactive oxygen species, particularly H2O2, which has been shown to mediate many of the devastating effects associated with LPS and endotoxemia [25–31].
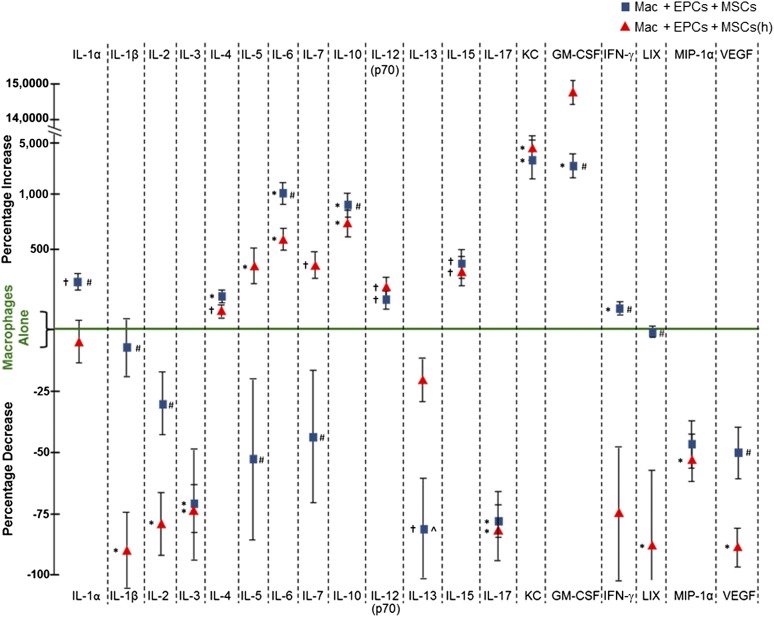
After establishing a cytokine/chemokine release baseline for stressed macrophages cultured alone, we next cultured macrophages with EPCs and MSCs that were coembedded in HA-hydrogels and exposed the 3 cell types to H2O2 stress. Coculturing macrophages with HA-hydrogel-embedded EPCs and MSCs altered the release of the 19 cytokines/chemokines examined, including observable differences when MSCs were hypoxic preconditioned (Fig. 6). The release of many cytokines/chemokines was reduced when macrophages were cocultured with stem cells. However, in contrast, some cytokines/chemokines were enhanced, including anti-inflammatory interleukin (IL)-10 and cytokines associated with macrophage polarization conversion from M1 to M2, such as IL-4, IL-6, and keratinocyte-derived chemokine (KC) (Fig. 6) (reviewed by Gordon and Martinez [32]).
Figure 6.
Comparison of in vitro cytokine/chemokine release from macrophages cultured alone or in coculture with hyaluronic acid-based (HA)-hydrogel-coembedded EPCs and MSCs (normoxic and hypoxic preconditioned) after stress (100 µM hydrogen peroxide [H2O2]). Cytokine/chemokine release was evaluated in the cell culture medium after 48 hours of coculturing. Cells were treated for 4 hours with 100 µM H2O2 before the 48-hour collection period. During coculturing with embedded stem cells, macrophages were not coembedded in HA-hydrogels. After stress, cytokine/chemokine release into the cultured medium was modified when macrophages were cocultured with HA-hydrogel-embedded EPCs and MSCs (normoxic and hypoxic preconditioned), compared with when macrophages were cultured alone. Changes in cytokine/chemokine release are represented in the graph as the percentage of increase or decrease relative to the values obtained from macrophages cultured alone. ∗, p ≤ .05 vs. Mac alone; †, p ≤ .10 vs. Mac alone; #, p ≤ .05 vs. Mac + EPCs + MSCs(h); ^, p ≤ .10 vs. Mac + EPCs + MSCs(h). n = 5–6. Abbreviations: EPCs, endothelial progenitor cells; GM-CSF, granulocyte macrophage colony-stimulating factor; (h), hypoxic; IFN-γ, interferon-γ; IL, interleukin; LIX, lipopolysaccharide-induced CXC; Mac, macrophages; MIP-1α, macrophage-inflammatory protein 1α; MSCs, mesenchymal stem cells; VEGF, vascular endothelial growth factor.
We also measured the release of cytokines/chemokines into the cell culture from EPCs and MSCs (normoxic and hypoxic preconditioned) cultured alone and in combination under control conditions and after H2O2 stress. Stress altered the release of various cytokines/chemokines from EPCs and MSCs cultured in 2D on the surface of the culture dishes, as schematically illustrated in supplemental online Figure 2. We then embedded EPCs and MSCs (alone and combined) in HA-hydrogel and repeated the experiments to determine the effect that HA-hydrogel embedding has on the cytokine/chemokine release from stressed stem cells. Embedding significantly altered the release of numerous cytokine/chemokine from stem cells after stress (supplemental online Fig. 2).
In Vivo: EPC-MSC Effect on Circulating Cytokines/Chemokines During Endotoxemia
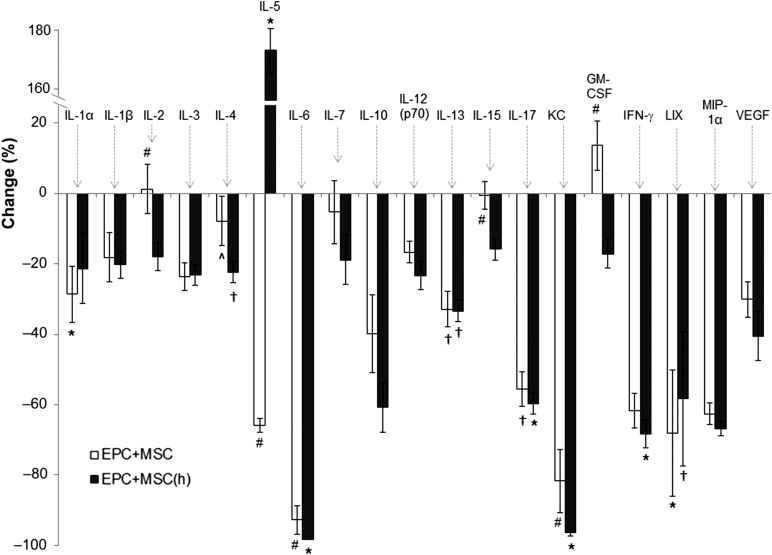
In additional in vivo experiments, we delivered HA-hydrogel-coembedded EPCs with MSCs to LPS-induced endotoxemic mice and examined the levels of circulating cytokines/chemokines after 24 hours. Numerous cytokines/chemokines examined were reduced in the circulation of endotoxemic mice by stem cell treatment (Fig. 7), including many proinflammatory cytokines/chemokines, an effect that was potentiated when MSCs were hypoxically preconditioned before their delivery. Remarkably, when we examined the effects of EPC and/or MSC delivery alone in HA-hydrogels, we observed the most robust attenuation in circulating cytokines/chemokines to occur when EPCs were delivered alone in HA-hydrogels to endotoxemic animals (supplemental online Fig. 3). EPC delivery alone was characterized by a significant reduction in 15 of the 19 cytokines/chemokines examined. Furthermore, the effects of HA-hydrogel-delivered stem cells in altering the circulating levels of cytokines/chemokines were confirmed to be the result of the embedded stem cells and not the HA-hydrogel itself, which was illustrated by the lack of HA-hydrogel delivery alone mediating any reduction in the cytokines/chemokines examined (supplemental online Fig. 3).
Figure 7.
Cytokine/chemokine release into the circulation of endotoxemic mice was measured 24 hours after injection of LPS and treatment with hyaluronic acid-based (HA)-hydrogel-coembedded EPCs and MSCs (normoxic and hypoxic preconditioned). Delivery of HA-hydrogel-coembedded EPCs and MSCs (normoxic and hypoxic preconditioned) reduced the vast majority of cytokines/chemokines that were elevated during LPS-induced endotoxemia. In the graph, changes in circulating cytokines/chemokines during endotoxemia and with EPC and MSC treatment are represented as the percentage of increases or decreases relative to endotoxemia without stem cell treatment. ∗, p ≤ .05 vs. no treatment; †, p ≤ .10 vs. no treatment; #, p ≤ .05 vs. EPCs + MSCs(h) treatment; ^, p ≤ .10 vs. EPCs + MSCs(h) treatment. n = 5 (5 different mice for each group). Abbreviations: EPCs, endothelial progenitor cells; GM-CSF, granulocyte macrophage colony-stimulating factor; (h), hypoxic; IFN-γ, interferon-γ; IL, interleukin; LIX, lipopolysaccharide-induced CXC; LPS, lipopolysaccharide; MIP-1α, macrophage-inflammatory protein 1α; MSCs, mesenchymal stem cells; VEGF, vascular endothelial growth factor.
Discussion
We previously reported that embedding EPCs in protective HA-hydrogel scaffolding increases their viability against LPS and Adriamycin in vitro [1, 6]. Therefore, we hypothesized that coembedding EPCs with kidney-derived MSCs might enhance the therapeutic effects of both these stem cells. Although in vitro treatment with 10 μg/ml of LPS for 24 hours previously resulted in 70% EPC viability [1], EPC and MSC coembedding increased viability to 85% at the same LPS concentration. When EPCs and MSCs were cocultured together without the protection of HA-hydrogel embedding, the contrast in viability was even more pronounced during exposure to LPS (10% viability for EPCs cultured alone vs. 40% viability when EPCs were cocultured with MSCs). Additionally, it appears that the niche-supportive capabilities of MSCs can be augmented by priming these cells. When MSCs were subject to hypoxic preconditioning (<5% O2) for 72 hours before coembedding/culturing with EPCs, the viability of LPS-treated stem cells was further enhanced.
Despite the enhanced protection against endotoxins, the delivery of EPCs embedded alone in HA-hydrogel was more therapeutically effective against LPS-induced AKI compared with when these cells were codelivered with MSCs. This was particularly observed when EPCs embedded and delivered alone significantly reduced 15 of the 19 cytokines/chemokines examined in the circulation of endotoxemic mice (supplemental online Fig. 3). However, when MSCs were preconditioned by 72 hours of hypoxia (<5% O2), their therapeutic efficacy was drastically improved. For instance, hypoxic preconditioning of MSCs before their coembedding and codelivery with EPCs led to enhanced maintenance of blood pressure, renal blood flow, and renal function, with more prominent attenuation of circulating cytokines/chemokines during endotoxemia, and also improved angiogenesis after femoral ligation. This finding strongly suggests MSCs should first be primed for their full renoprotective potential to be activated.
Despite the improvement in renal and vascular function, renal homing and engraftment of codelivered HA-hydrogel-embedded stem cells by HA-hydrogels remained very low (less than 1% of total cells delivered, comparable to that with i.v. delivery). This finding suggests that most of the therapeutic effects offered by the delivered stem cells are most likely mediated although paracrine signaling rather than their engraftment and transdifferentiation. In support of this, we observed reduced levels of cytokines/chemokines in the circulation of endotoxemic animals after the delivery of stem cells. Furthermore, we also observed the macrophage secretome is influenced by EPCs and MSCs. When HA-hydrogel-embedded EPCs and MSCs were cocultured with macrophages, IL-4, IL-6, IL-10, and KC release was enhanced. Increased release of IL-4, IL-6, and KC strongly suggests EPCs and MSCs interact with macrophages, effectively altering the latter’s polarization to an anti-inflammatory wound healing phenotype (reviewed by Shi et al. [33]). This was confirmed by the results of our RT-PCR analysis, which indicated in vitro coincubation with stem cells stimulated macrophage polarization from proinflammatory M1 to anti-inflammatory M2. Similarly, in our in vivo experiments, we observed an increase in circulating M2 macrophages during endotoxemia when EPCs and MSCs were codelivered by HA-hydrogels. These findings are in concert with data from other laboratories, who have reported that stromal cells (including bone marrow-derived MSCs) promote M2 macrophage polarization in both in vitro and in vivo settings [34–40]. The stem cell-induced “push” of macrophage polarization would be very beneficial for injury repair, because M2 macrophages not only secrete anti-inflammatory molecules [41–43] but also produce growth factors and control extracellular matrix degradation, thereby effectively promoting tissue repair and regeneration [44, 45]. Thus, it appears that at least a part of the therapeutic benefits of EPCs and MSCs are promoted through their paracrine interaction with components of the innate immune system, in particular, through modulation of monocyte/macrophage phenotype.
As indicated in the present study, HA-hydrogel embedding altered the secretome of both EPCs and MSCs. It appears the HA-hydrogel embedding-induced changes in EPC and MSC secretomes are a result of the activation of cell surface integrins and intracellular cytoskeleton properties owing to the enhanced rigidity (elastic module) offered by HA-hydrogel embedding [46]. In addition, the inclusion of ProNectin into the composition of our HA-hydrogels provides an RGD sequence (an arginine-glycine-aspartic acid-conserved motif) for integrins on the surface of stem cells to bind to [47, 48]. When stem cell integrins bind to the RGD sequence, cytoskeleton properties are altered and the cell’s release of anti-inflammatory and proregenerative factors are stimulated [13]. Activation of the EPC surface integrin α4β1 plays a critical role in stimulating the release of anti-inflammatory and proregenerative factors from EPCs, thereby enhancing the cell’s ability to mediate tissue repair after injury [13]. Therefore, activation of critical integrins and cytoskeleton properties induced in HA-hydrogel-immobilized stem cells, along with the improved viability of embedded stem cells during exposure to endotoxins, seems to be responsible for the superior therapeutic benefits associated with stem cell delivery by HA-hydrogels compared with conventional i.v. delivery.
Importantly, the therapeutic effects of stem cells are not quenched while they remain embedded in the HA-hydrogel. While embedded in the protective scaffold, the therapeutic paracrine factors secreted from stem cells are able to escape from the HA-hydrogel into the circulation. This was evident in our in vitro experiments in which HA-hydrogel-embedded stem cells significantly released a variety of cytokines/chemokines into the culture medium. This further implies the therapeutic benefits of stem cells for treatment of AKI are mediated through their paracrine signaling and not specific renal homing and engraftment.
Another advantage of HA-hydrogels is that, in contrast to i.v. delivery, HA-hydrogel stem cell delivery is not associated with a high risk of embolism. In our previous experiences, i.v. delivery of MSCs to mice was associated with an approximate 25% mortality rate because of embolism. In contrast, when we delivered EPCs and/or MSCs embedded in HA-hydrogels, we did not observe any deaths from embolism. However, it is critical that HA-hydrogels should not be delivered directly into large vessels, because this would increase the risk of embolism. HA-hydrogels should be delivered subcutaneously or superficially to tissues that preferably have small vessel or capillary networks, such that the paracrine factors released by embedded stem cells can be distributed locally and systemically via the circulation.
Although MSCs reside in a variety of tissues and organs [49, 50], we used kidney-derived MSCs in the present experiments. Although MSC populations of different origins have similar morphology and surface marker profiles, they also have dissimilarities in their differentiation to various cell lineages [49]. Despite such discrepancies, MSCs isolated from the bone marrow, cord blood, amniotic fluid, and kidneys have all been much more effective than adipose-derived MSCs in alleviating kidney injury when delivered to various models of AKI (reviewed by Morigi and Benigni [51]) [52]. Although the transcriptome and immunophenotype analysis by Pelekanos et al. [50] indicated similarities between bone marrow- and kidney-derived MSCs, the patterns of gene and protein expression strongly suggest tissue-derived MSCs retain unique properties specific to their tissue of origin rendering them more therapeutic for the specific tissues from which they originate. This suggests kidney-derived MSCs would be more effective in repairing kidney damage compared with MSCs obtained from other tissues. However, additional studies are required to determine functional and regenerative similarities and differences among MSCs of different origins.
Conclusion
HA-hydrogel delivery of EPCs alone or codelivered with hypoxically preconditioned MSCs offered significant protection of renal and vascular function during endotoxemia, including reducing circulating levels of cytokines/chemokines. The improvement of stem cell therapeutic efficacy when these cells are delivered by HA-hydrogels appears to be a result of the alteration in the paracrine profile of the embedded cells, which alters key responses of the innate immune system that occur both on a systemic and on a local level at specific sites of injury. One of the key effects is the ability of embedded stem cells to alter the macrophage secretome and to induce macrophage polarization to an M2 anti-inflammatory phenotype.
Supplementary Material
Acknowledgments
The present experiments were supported by NIH Grants DK54602, DK052783, and DK45462 (to M.S.G.), American Heart Association Grant 12SDG9080006, and American Society of Nephrology Grant 010973-101 (to B.B.R.), and the Westchester Artificial Kidney Foundation.
Author Contributions
J.A.Z.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; E.P.N., M.M.R., M.J.B., M.A.R., C.M.D., R.V., S.S.C., and R.L.: collection and/or assembly of data; M.S.G.: conception and design, financial support, administrative support, data analysis and interpretation, manuscript writing, final approval of manuscript; B.B.R.: conception and design, financial support, administrative support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
S.S.C. has compensated honoraria as a speaker. The other authors indicated no potential conflicts of interest.
References
- 1.Ghaly T, Rabadi MM, Weber M, et al. Hydrogel-embedded endothelial progenitor cells evade LPS and mitigate endotoxemia. Am J Physiol Renal Physiol. 2011;301:F802–F812. doi: 10.1152/ajprenal.00124.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kale S, Karihaloo A, Clark PR, et al. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112:42–49. doi: 10.1172/JCI17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morigi M, Introna M, Imberti B, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 4.Park HC, Yasuda K, Ratliff B, et al. Postobstructive regeneration of kidney is derailed when surge in renal stem cells during course of unilateral ureteral obstruction is halted. Am J Physiol Renal Physiol. 2010;298:F357–F364. doi: 10.1152/ajprenal.00542.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patschan D, Plotkin M, Goligorsky MS. Therapeutic use of stem and endothelial progenitor cells in acute renal injury: Ça ira. Curr Opin Pharmacol. 2006;6:176–183. doi: 10.1016/j.coph.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Ratliff BB, Ghaly T, Brudnicki P, et al. Endothelial progenitors encapsulated in bioartificial niches are insulated from systemic cytotoxicity and are angiogenesis competent. Am J Physiol Renal Physiol. 2010;299:F178–F186. doi: 10.1152/ajprenal.00102.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yasuda K, Park HC, Ratliff B, et al. Adriamycin nephropathy: A failure of endothelial progenitor cell-induced repair. Am J Pathol. 2010;176:1685–1695. doi: 10.2353/ajpath.2010.091071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 9.Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minamino T, Miyauchi H, Yoshida T, et al. Endothelial cell senescence in human atherosclerosis: Role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 11.Goligorsky MS, Yasuda K, Ratliff B. Dysfunctional endothelial progenitor cells in chronic kidney disease. J Am Soc Nephrol. 2010;21:911–919. doi: 10.1681/ASN.2009111119. [DOI] [PubMed] [Google Scholar]
- 12.Young PP, Vaughan DE, Hatzopoulos AK. Biologic properties of endothelial progenitor cells and their potential for cell therapy. Prog Cardiovasc Dis. 2007;49:421–429. doi: 10.1016/j.pcad.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caiado F, Dias S. Endothelial progenitor cells and integrins: Adhesive needs. Fibrogenesis Tissue Repair. 2012;5:4. doi: 10.1186/1755-1536-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23:H41–H56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levenberg S, Burdick JA, Kraehenbuehl T, et al. Neurotrophin-induced differentiation of human embryonic stem cells on three-dimensional polymeric scaffolds. Tissue Eng. 2005;11:506–512. doi: 10.1089/ten.2005.11.506. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Skardal A, Prestwich GD. Engineered extracellular matrices with cleavable crosslinkers for cell expansion and easy cell recovery. Biomaterials. 2008;29:4521–4531. doi: 10.1016/j.biomaterials.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey AS, Jiang S, Afentoulis M, et al. Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood. 2004;103:13–19. doi: 10.1182/blood-2003-05-1684. [DOI] [PubMed] [Google Scholar]
- 18.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 19.Ranganath SH, Levy O, Inamdar MS, et al. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatzopoulos AK, Folkman J, Vasile E, et al. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development. 1998;125:1457–1468. doi: 10.1242/dev.125.8.1457. [DOI] [PubMed] [Google Scholar]
- 21.Plotkin MD, Goligorsky MS. Mesenchymal cells from adult kidney support angiogenesis and differentiate into multiple interstitial cell types including erythropoietin-producing fibroblasts. Am J Physiol Renal Physiol. 2006;291:F902–F912. doi: 10.1152/ajprenal.00396.2005. [DOI] [PubMed] [Google Scholar]
- 22.Chacko SM, Ahmed S, Selvendiran K, et al. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol. 2010;299:C1562–C1570. doi: 10.1152/ajpcell.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu X, Yu SP, Fraser JL, et al. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 24.Ohnishi S, Yasuda T, Kitamura S, et al. Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem Cells. 2007;25:1166–1177. doi: 10.1634/stemcells.2006-0347. [DOI] [PubMed] [Google Scholar]
- 25.de Broucker V, Hassoun SM, Hulo S, et al. Non-invasive collection of exhaled breath condensate in rats: Evaluation of pH, H2O2 and NOx in lipopolysaccharide-induced acute lung injury. Vet J. 2012;194:222–228. doi: 10.1016/j.tvjl.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Gorąca A, Huk-Kolega H, Kleniewska P, et al. Effects of lipoic acid on spleen oxidative stress after LPS administration. Pharmacol Rep. 2013;65:179–186. doi: 10.1016/s1734-1140(13)70976-9. [DOI] [PubMed] [Google Scholar]
- 27.Kavoosi G, Teixeira da Silva JA, Saharkhiz MJ. Inhibitory effects of Zataria multiflora essential oil and its main components on nitric oxide and hydrogen peroxide production in lipopolysaccharide-stimulated macrophages. J Pharm Pharmacol. 2012;64:1491–1500. doi: 10.1111/j.2042-7158.2012.01510.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim HJ, Kim SR, Park JK, et al. PI3Kγ activation is required for LPS-induced reactive oxygen species generation in respiratory epithelial cells. Inflamm Res. 2012;61:1265–1272. doi: 10.1007/s00011-012-0526-7. [DOI] [PubMed] [Google Scholar]
- 29.Pai K, Sodhi A. Effect of cisplatin, rIFN-Y, LPS and MDP on release of H2O2, O2- and lysozyme from human monocytes in vitro. Indian J Exp Biol. 1991;29:910–915. [PubMed] [Google Scholar]
- 30.Sodhi A, Geetha B. Effect of cisplatin, lipopolysaccharide, muramyl dipeptide and recombinant interferon-gamma on murine macrophages in vitro. II. Production of H2O2, O2- and interleukin-1. Nat Immun Cell Growth Regul. 1989;8:108–116. [PubMed] [Google Scholar]
- 31.Sodhi A, Gupta P. Increased release of hydrogen peroxide (H2O2) and superoxide anion (O2-) by murine macrophages in vitro after cisplatin treatment. Int J Immunopharmacol. 1986;8:709–714. doi: 10.1016/0192-0561(86)90006-8. [DOI] [PubMed] [Google Scholar]
- 32.Gordon S, Martinez FO. Alternative activation of macrophages: Mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Shi Y, Su J, Roberts AI, et al. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33:136–143. doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maggini J, Mirkin G, Bognanni I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantovani A, Biswas SK, Galdiero MR, et al. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 36.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dayan V, Yannarelli G, Billia F, et al. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol. 2011;106:1299–1310. doi: 10.1007/s00395-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 41.English K, Ryan JM, Tobin L, et al. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149–160. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maccario R, Podestà M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525. [PubMed] [Google Scholar]
- 43.Melief SM, Schrama E, Brugman MH, et al. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31:1980–1991. doi: 10.1002/stem.1432. [DOI] [PubMed] [Google Scholar]
- 44.Chinetti-Gbaguidi G, Staels B. Macrophage polarization in metabolic disorders: Functions and regulation. Curr Opin Lipidol. 2011;22:365–372. doi: 10.1097/MOL.0b013e32834a77b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee S, Huen S, Nishio H, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seib FP, Prewitz M, Werner C, et al. Matrix elasticity regulates the secretory profile of human bone marrow-derived multipotent mesenchymal stromal cells (MSCs) Biochem Biophys Res Commun. 2009;389:663–667. doi: 10.1016/j.bbrc.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 47.Franke K, Pompe T, Bornhäuser M, et al. Engineered matrix coatings to modulate the adhesion of CD133+ human hematopoietic progenitor cells. Biomaterials. 2007;28:836–843. doi: 10.1016/j.biomaterials.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 48.Li YJ, Chung EH, Rodriguez RT, et al. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J Biomed Mater Res A. 2006;79:1–5. doi: 10.1002/jbm.a.30732. [DOI] [PubMed] [Google Scholar]
- 49.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 50.Pelekanos RA, Li J, Gongora M, et al. Comprehensive transcriptome and immunophenotype analysis of renal and cardiac MSC-like populations supports strong congruence with bone marrow MSC despite maintenance of distinct identities. Stem Cell Res (Amst) 2012;8:58–73. doi: 10.1016/j.scr.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Morigi M, Benigni A. Mesenchymal stem cells and kidney repair. Nephrol Dial Transplant. 2013;28:788–793. doi: 10.1093/ndt/gfs556. [DOI] [PubMed] [Google Scholar]
- 52.Chen J, Park HC, Addabbo F, et al. Kidney-derived mesenchymal stem cells contribute to vasculogenesis, angiogenesis and endothelial repair. Kidney Int. 2008;74:879–889. doi: 10.1038/ki.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.