Mesenchymal stem cells (MSCs) arise predominantly from pericytes released from broken/inflamed blood vessels and appear to function as immunomodulatory and regeneration mediators. MSCs are being tested for their management capabilities to produce therapeutic outcomes for a wide range of clinical conditions. MSC-based therapy has the potential to change how health care is delivered, and health care appears to be entering a new age of cellular medicine.
Summary
It has been assumed that adult tissues cannot regenerate themselves. With the current understanding that every adult tissue has its own intrinsic progenitor or stem cell, it is now clear that almost all tissues have regenerative potential partially related to their innate turnover dynamics. Moreover, it appears that a separate class of local cells originating as perivascular cells appears to provide regulatory oversight for localized tissue regeneration. The management of this regeneration oversight has a profound influence on the use of specific cells for cell therapies as a health care delivery tool set. The multipotent mesenchymal stem cell (MSC), now renamed the medicinal signaling cell, predominantly arises from pericytes released from broken and inflamed blood vessels and appears to function as both an immunomodulatory and a regeneration mediator. MSCs are being tested for their management capabilities to produce therapeutic outcomes in more than 480 clinical trials for a wide range of clinical conditions. Local MSCs function by managing the body’s primary repair and regeneration activities. Supplemental MSCs can be provided from either endogenous or exogenous sources of either allogeneic or autologous origin. This MSC-based therapy has the potential to change how health care is delivered. These medicinal cells are capable of sensing their surroundings. Also, by using its complex signaling circuitry, these cells organize site-specific regenerative responses as if these therapeutic cells were well-programmed modern computers. Given these facts, it appears that we are entering a new age of cellular medicine.
Significance
This report is a perspective from an active scientist and an active entrepreneur and commercial leader. It is neither a comprehensive review nor a narrowly focused treatise. The broad themes and the analogy to the working component of a computer and that of a cell are meant to draw several important scientific principles and health care themes together into the thesis that regenerative medicine is a constant throughout life and its management is the next frontier of health care. Mesenchymal stem cells are used as the central connection in the broad theme, not as multipotent progenitors but rather as an important control element in the natural local regeneration process.
Introduction
The delivery of health care follows three distinct logics: preventive care, maintenance, and crisis intervention (e.g., disease, trauma, tissue failure). How our care-delivery organizations approach these three different logics, and thereby influence the biopharmaceutical world, is driven by historic precedents and hindsight, not necessarily forward thinking. The dominant logic has been “take pills, cure your ills.” For prevention and homeostasis, we take our multivitamin, statins, extra iron, zinc, and whatever else we have been told would be a “useful” additive to our diets. In addition, we have decreased our consumption of butter, eggs, red meat, and so forth and replaced them with “healthy” alternatives, because we have been told that these will forestall our early demise. The reality for these behavioral modifications is that, first, we can financially and socially afford these “extras” and modifications, in contrast to those who live on the edge of starvation and the border of existence. Second, as individuals, we have assumed we are “optimizing” our unique biology by providing an abundance of “minimal essential daily requirements,” supposedly in support of the myriad of chemical pathways that drive and control every cell in our bodies. This is in complete ignorance of our individual genetics and accurate predictors of where we are developmentally and physically on our ever-changing life continuum or life dynamic. For example, whole milk is good for infants, but skim milk thereafter. However, it is unknown whether the fat in milk does good or harm to adolescents or those older than 65 years old. Because persons A and B are quite different genetically, should they follow the dietary and pharmaceutical logic of the “average,” or are they genetically far outside the average?
In the situation of crisis management—a broken femur, an acute myocardial infarct, or a stroke—one question is whether the standard emergency room procedure will work equally for person A and person B. Additional questions are which post-treatment pharmaceutical agents should be taken and what complications will these patients’ unique genetics perpetuate.
These questions are not only an argument for “personalized medicine” but, more importantly, also raise the issue of whether treatments started when a patient is 25 years old must change when that person has reached 35, 55, or 65 years old, because their physiologies will have also macroscopically changed. Thus, how do we personally manage our bodies, both on a daily basis as we continue to develop and age and as we go through various medical fluctuations and crises?
Body Management
Our bodies have a perpetual developmental progression in play as we go from fertilized egg to a senescent adult. The management blueprint and, indeed, the written record is found in and on our genetic material neatly packaged into every cell in our body [1, 2]. Anthropologically, we have tried to manage our body’s functions through the several development phases of our lives. As societal and evolutionary groups, we have done this in several ways:
As nomads, cave dwellers, and hunter-gatherers, humans have systematized our discovery of bioactive molecules (drugs), first verbally through our shamans and medicine men, to provide plants and other preparations to improve our health status [3, 4]. For example, primitive women learned that consuming their placenta directly after child bearing (just as do many animals) has a nutritive and pharmaceutical value. These early humans learned that certain dried grasses and leaves made wonderful medicinal teas and sedatives. Likewise, “locoweed” or cannabis has been used for thousands of years as a recreational drug. Finally, more recently, nitroglycerin has been used for heart pain since the 1880s [5].
As modern biochemists, we have worked out almost all the body’s metabolism and energy pathways such that we can tell exactly how many ATPs are generated from that cookie you just ate or the buttered toast you had for breakfast [6]. Likewise, a century ago, physicians ground up dog pancreases to provide a daily therapeutic for patients with diabetes; this was replaced by purifying the active agent from pig pancreas (i.e., pig insulin). Now, the biotech industry has found a way to produce and purify human insulin for use by diabetic patients [7].
We have developed the ability to precisely sequence the human genome [8] and, more importantly, we have developed the technologies summarily referred to as “panomics” to analytically analyze almost every cell function: metabolomics (cellular metabolism), proteomics (which proteins are made), lipidomics, epigenomics, methylomics, microbiomics, and so forth [9]. These technologies allow the quantitative assessment of individual cellular, tissue, and organ functions.
Finally, we have developed complex software and computers with huge memories and lightning-fast computational capabilities to actually model how all the biochemical and signaling pathways interact and coordinately function (on average) [10]. Such computers now control robots that can perform complicated surgical procedures more precisely than can humans.
Thus, we have all the hardware, software, and tools to analyze the physiological status of a living organism, especially humans. Moreover, the dynamic fluctuations that occur with age, disease, changing environment, stress, and so on, can be established once a baseline multimodal database can be established. The management of cellular, tissue, and body functions can thus be accomplished by adjusting key nutrients, metabolic intermediates, environmental parameters, and other key variables to control or modulate our individual bodily functions at any moment of time.
Personalized Management
One part of this encyclopedia of personal information will be data describing how individual cells function in different environments. The functioning of red blood cells is a good example of how detailed structural, metabolic, and functional information has helped us to understand specific diseases and how to better manage such diseases. The detailed structure and function of one main component of red blood cells is, of course, the protein hemoglobin [11]. We now know why and how fetal, adult, and sickle cell hemoglobins function and exactly when, how, and why oxygen binds to and releases from these molecules. Moreover, we know how to increase the circulating numbers of red blood cells and the detailed physiological consequences of increasing or decreasing these parameters [12]. By increasing the number of red blood cells in the circulation, a bicyclist can more easily go up step hills, because more oxygen will be available to those large leg muscles that push the bike’s pedals.
However, we know much less about other cell types, with their diverse molecular constituents. Detailed descriptions of the many variables of interest need to be amassed and provided in a centralized database. It is important to separate fact from current dogma because, as discussed in the present report, such dogma is often not correct and, at best, is misleading. In this context, we focus the remainder of this essay on one cell type, the mesenchymal stem cell (MSC), which will allow us to better understand one specific class of cells that are central to the new management tactics in the human health care arena. Our studies of the MSCs have generated the new logic and insight that involves schemes and tactics to help the body manage its own innate capacities to maintain or regenerate specific tissues and organ systems. In addition, these logics now extend to using exogenously added MSCs to maximize the regenerative capabilities of injured, aged, or diseased tissues. In some cases, these uses will provide curative cell-based therapies [13, 14]. The switch from the “big pharma” logic, one molecule cures all, to understanding how to manage the body’s intrinsic tissue regenerative capacity using the natural capabilities of cells such as MSCs to manage regenerative motifs will change how medicine is practiced in the future [15]. Because MSCs play a central role in the natural repair and regeneration of almost all tissues, we strongly suggest that local MSCs help provide the microenvironment for local tissue regeneration after localized tissue injury and can be thought of as controlling the local “regenerator.”
Regeneration: Lesson to Learn
If a newt’s limb is amputated at any level (at the wrist, elbow, or middle of the humerus), the limb will perfectly regenerate the missing piece, large or small [16]. The wound sets up a clot, and the epithelium grows under the clot and covers the exposed mesenchyme, which subsequently forms a ball of cells called the regeneration blastema. If an adult human has a distal digit, including the nail base and first joint, cut off, it will not normally regenerate. Cases have been published in which young children (younger than 5 years old) have regenerated distal digits that had been cut off [17]. The key to this happening is, first, do not take the child to the emergency room at the local hospital where stitches will be used to close the wound. In rural England, where the distal digit regenerations were reported, no stitches were applied. Instead, a clean bandage was applied, a large clot formed, the epithelia grew under the clot, and a blastema must have formed to supply the cells required for regeneration of the distal structures.
The lessons learned: no stitches, let the clot form, and provide conditions in which the epithelia will grow under the clot and bring about the formation of the mesenchymal blastema, which contains MSCs. In a mouse model, the placement of a decellularized and carefully processed extracellular matrix (ECM), with its full array of associated bioactive molecules, was sufficient to result in the formation of a proper blastema, sufficient to regenerate the severed distal digit [18]. In contrast, no regeneration could be observed in the unsupplemented control [18]. Thus, in an adult animal incapable of regenerating the distal digit, a sufficient number of tissue progenitors can be brought into a blastema structure responsible for digit regeneration. In this context, as discussed below, local MSCs provide the local environment to support regeneration but might not differentiate into various limb phenotypes. Thus, the management of the body’s regenerative capacity can result in the formation of structures not normally possible. Where are these progenitors coming from and what are the signals released by the implanted ECM and the local MSCs that can control these progenitors? Clearly, this is an intricate cell management process.
In the context of not using stitches to close large wounds, one of the outcomes of stitching is the formation of thick scars in adults. As pointed out below, MSCs inhibit scar formation. In this case, it appears that the MSCs inhibit the formative aspects of the production of scar tissue. The implication is that the MSCs can reduce the volume of existing scar tissue by inhibiting the formative aspects of the normal tissue turnover. This would be especially important in MSC treatment of lung, kidney, and liver fibrosis [19–23].
MSCs and Regeneration
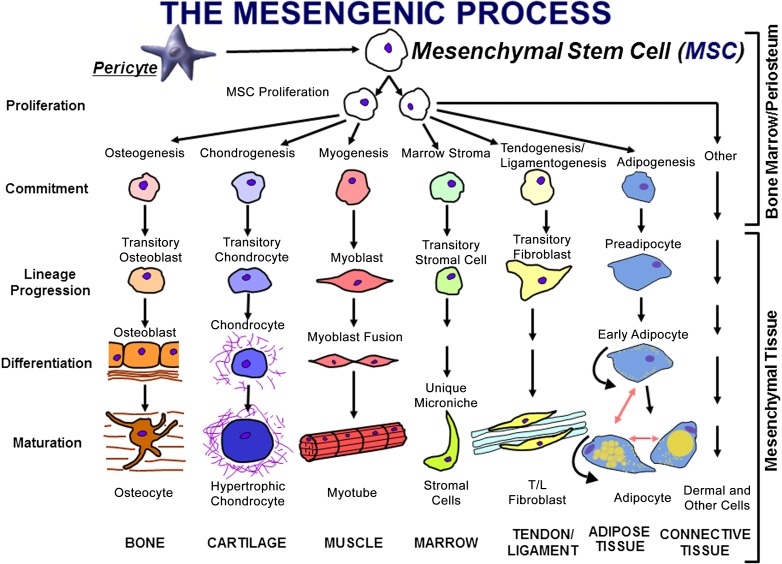
Bone marrow has been used since the days of Aristotle to help repair, regenerate, or reconstruct broken bones. In the late 1980s, Caplan drew up the details shown in the hypothesis diagram pictured in Figure 1 [24]. The thesis represented in this diagram was that special, rare cells in marrow, which Caplan called “mesenchymal stem cells,” could be separately induced to differentiate into bone, cartilage, muscle, bone marrow stroma, tendon ligament, fat, and so on. Separate and unique inductive agents [28] are required for each separate lineage pathway, resulting in phenotypically distinct differentiated mesenchymal tissue. The postulate that marrow contains a multipotent progenitor cell, the mesenchymal stem cell, was against the dogma of the day, which asserted that only one stem cell was present in the marrow and that cell was the hematopoietic stem cell. Furthermore, the dogma of the day asserted that the organs that humans were born with grew larger, but no new cells appeared (i.e., these organs were devoid of stem cells). For example, all the cardiac myocytes that were present at birth were there for life (they grew larger or smaller, but no new descendants of cardiac stem cells were possible). We now know that tissue-specific progenitors (stem cells) exist for every tissue in the body, including neurologic, liver, cardiac, and kidney stem cells, to name of few (clearly at odds with the dogma of the 1980s) [29, 30].
Figure 1.
The mesengenic process was first envisioned in the late 1980s as a pathway for marrow MSCs to differentiate into a number of mesodermal cell types that could contribute to the fabrication of bone, cartilage, muscle, marrow stroma, tendon/ligament, fat, or other connective tissues [13, 19]. It is now clear that MSCs can be isolated from many tissues, because they are derived from perivascular cells, pericytes [25, 26]. Abbreviations: MSC, mesenchymal stem cell; T/L, tendon/ligament. (Adapted from [27].)
The presence of these tissue-specific progenitors or stem cells has several ramifications. First, differentiated cells must have half-lives (i.e., they must expire and must be replaced at specific rates by the descendent of the tissue stem cells). In studies of female hearts transplanted into male recipients (or vice versa), host cells are found in various donor heart structures (cells die and are replaced; thus, specific host progenitors gave rise to these new cells) [30]. Second, the loss of stem cells can affect tissue function or, at the very least, affect the capacity of that tissue to rejuvenate or regenerate itself. Finally, the genetics governing stem cell numbers, potency, and function can have a profound effect on an individual’s health status. As a specific example, a subset of MSCs in the marrow act as osteogenic progenitors and are clearly involved in the maintenance and regeneration potential of the surrounding bone [31]. The marrow MSCs that are leptin receptor positive can be traced directly into bone-forming cells, and their availability is directly responsible for the observed rate of bone healing and turnover [32], known to decrease in older individuals.
During the 1990s, we, and others [25, 26, 33], published many reports describing how MSCs could be induced to form the variety of mesenchymal tissues shown in Figure 1 [34]. The MSC’s multipotency became its most distinguishing characteristic with its in vitro ability to form cartilage, fat, or bone tissue required to allow the designation as an MSC. Because of the dominance of the hematologists and their dogmas, it was presumed that MSCs arose from the bone marrow connective tissue or stroma [35]. Because infused MSCs went “back” to the marrow and because cell culture “lawns” of MSCs provided support for hematopoietic progenitors and their descendents, some investigators referred to MSCs as mesenchymal “stromal” cells [35]. Unexplained through the 1990s was how MSCs could be isolated from nonmarrow tissues such as fat or skin. Then, in 2000, Hariri began to explore the most vasculogenic tissue found under normal conditions and discovered that the placenta was a virtual MSC factory, containing unexpectedly large numbers of MSCs [36].
Likewise, it became clear that MSCs secreted molecules that had profound effects on the cell components of the immune system [37]. This raised such questions as why a multipotent progenitor would have the capacity to shutdown T-cell mobility and the interrogating function, and, in the context of lineage progression down a tissue pathway (Fig. 1), why would the need to modulate immune cells exist.
All MSCs Are Pericytes
These questions can now be addressed, because, we and others, have determined that most MSCs arise from perivascular cells, pericytes, as shown in Figure 1, after the addition of this arrow from pericyte to MSC in 2010 [38–42]. The identification of pericytes as the source of MSCs easily explains how MSCs can be isolated from every vascularized tissue of the human body [38]. We now know that when a blood vessel is injured or inflamed, the pericyte detaches from the basement membrane of that blood vessel or sinusoid to become an MSC. This newly formed MSC responds to the local environment by secreting site-specific bioactive molecules that have two very different functions. From the front of the MSCs (facing away from the damaged tissue), a curtain of molecules is secreted that stops the body’s overaggressive immune system from surveying the injured tissue behind the MSC [43]. This is the body’s first line of defense against the establishment of a chronic autoimmune reaction. Thus, MSCs are naturally and intensely immunomodulatory; in a simplistic sense, MSCs have been called the “guardians of inflammation” [44]. Indeed, this curtain from human MSCs appears to function in rodent models of disease without immunosuppression of the rodent hosts. From the back of the MSCs toward the damaged tissue, a different set of bioactive molecules are secreted that function as “trophic” agents [37], which are grouped into 4 categories that contribute to a regenerative microenvironment. The first group consists of molecules that inhibit ischemia-stimulated apoptosis (a broken blood vessel cannot deliver oxygen to the surrounding tissue, thus stimulating ischemia-caused cell death). The second group consists of molecules that inhibit the formation of scar tissue. The third group consists of molecules, such as vascular endothelial growth factor, that bring in endothelial cells to form new blood vessels. The MSCs can then revert to their pericyte phenotype and attach to these new, fragile vessels to stabilize them. Indeed, it has been shown that MSC engraftment into injured marrow involves the MSCs taking up a perivascular location [45]. The final group includes molecules that function as mitogens for tissue-specific progenitors. In summary, we further hypothesize that these secretory or paracrine activities of MSCs will themselves change as a function of time and the local milieu.
With this information available, Caplan [13] has suggested that the acronym MSCs be retained for this class of cells, but that it should stand for “medicinal signaling cells.” Clearly, the major function of MSCs in vivo is not as multipotent progenitor stem cells, but as cells that help to manage repair and/or regeneration at sites of injury [14, 15, 40, 41]. What confuses this issue is that within the MSC population from bone marrow are cells that readily form osteoblasts but within the MSC population from fat are cells that readily form adipocytes and some MSCs from muscle readily form muscle, and so on. Thus, Figure 1 shows an oversimplified hypothesis diagram that represents this general class of cells but is not related to how and why MSCs (medicinally) function in vivo.
The management of these composite trophic activities is an inherent capacity of MSCs independent of the tissue of origin. Each tissue houses MSCs/pericytes that have different chemistries because of the local environments in which they are housed. The small amount of data now available suggest they all have the same sensory and response elements (immunomodulatory and trophic) even when expanded in culture under very different culture conditions [46–49]. We were struck by this latter fact, because the differing variables, such as plating density, culture medium constituents, multiple passages, low or high oxygen, and so forth, used to culture expand MSCs from various tissues do not destroy this core capacity of immunomodulation and trophic activity, which seems to be hard-wired into these cells. In contrast, the progenitor or stem cell capacity is lost after only a few passages in culture.
The optimization of the use of MSC-based therapies is far from current practice [50]. A huge number of variables must be addressed. Simply stated, the detailed characteristics of both the effector MSCs and the responding host cells must be both quantitatively and qualitatively known. The key elements that require immediate experimental attention are how and when to deliver the proper dose of the optimized MSCs to a known injury or diseased tissue site [51–54]. For instance, if a patient experiences an acute myocardial infarct and MSCs are the therapy of choice, the optimal delivery route must be known. Current clinical trials involve two approaches: either venous delivery or corkscrew catheter directly into the ischemic heart tissue [55, 56]. In the first case, the cells must be protected from their first entrance into the blood stream where they will be coated with deleterious serum proteins [53]. This protection must either enhance or introduce heart-specific targeting molecules to ensure that the MSCs properly dock in the heart. One question is whether the MSCs must also dock in the lymph nodes servicing the heart to perform their natural immunomodulatory function. In the second case, the coating of the MSCs will be quite different to ensure cell survival and docking within the catheter-damaged heart tissue. These are not trivial technical issues.
In the context of therapeutic cells, the postpartum placenta is recognized as an abundant source of a variety of stem and progenitor cells [36, 57]. A deeper understanding of how these cell constituents originate, propagate, populate, and, ultimately, migrate from the placenta to the developing fetus and mimic and support the broad range of activities seen from stem cells has emerged from the work at the Cellular Therapeutics Division of the Celgene Corporation, which has brought several unique placental-derived stem cell products to the clinic. This trafficking of placental cells is comparable to the trafficking observed in rodent parabiosis experiments [58]. The trafficking phenomenon also illustrates a general principle that certain stem/progenitor/therapeutic cells are mobilized and dock at tissue sites of injury. This trafficking of fetal and adult cells requires that the cells have unique targeting and docking functions [51, 52, 59]. This intrinsic functionality is observed in the therapeutic properties of adult MSCs, regardless of the tissue of origin.
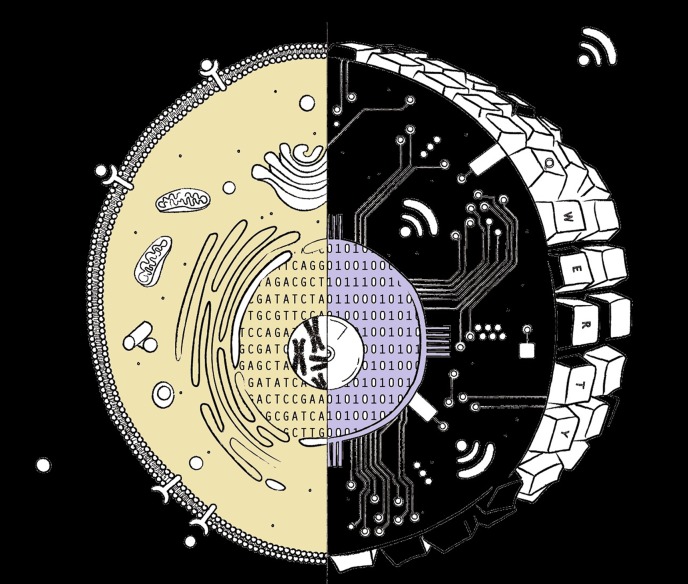
We see MSCs and stem/pericytes cells as sensory [60] (highly receptive to local biologic information), synthetic (capable of producing biomolecules in response to the received information), and secretory (capable of fabricating and delivering those biomolecules) units characterized by their innate therapeutic potential. These units or cells have acquired these unique capabilities through evolution and provide the organism with unique, highly adaptive, survival characteristics. In the analogy to a computer proposed by Hariri [61], these stem and progenitor cells have a design in which the nucleus houses the operational software, resident in the DNA of the chromosomes, at the core of a complex processor and complex information transferring apparatus within the cell cytoplasm (Fig. 2). Hariri proposed that an important way in which these stem and progenitor cells differ from differentiated, specialized cells is in the quality of the software. He further proposed that stem and progenitor cells have, as a fundamental characteristic, access to the entire transcribable genome, or as an analogy, all the code needed to run the complete computer program. As stem cells differentiate, a series of gene silencing events work to transform the cell from a general, versatile, but inefficient, phenotype to a specialized, less versatile, but highly efficient, form [1]. This means that a stem cell has all the “software” to produce the entire transcribable proteome but that specialized cells, such as a neuron, have refined their DNA, or “computer code” to just retain certain synthetic abilities necessary to perform those specialized functions. We see in this design, the membrane of the cell serving as the site for input/output: input similar to how a computer keyboard functions, and output similar to how peripheral devices provide connectivity. Thus, the therapeutic output of cell-based therapies relies both on the docking or sensing and on the cellular output in the form of secreted factors [62], which exert their effects in our genomically controlled endocrine or paracrine regeneration/repair capacity. By the time humans reach the age of 40–50 years, this regenerative and repair potential is greatly diminished and accounts for “late-onset” diseases or tissue dysfunction. The medical challenge is to learn how to optimize the remaining innate regenerative potential and to understand how to supplement these unique functionalities. Such supplementation should not be viewed as the “fountain-of youth,” but rather as a means of enhancing a “good health” status while influencing human longevity, with the objective of not only living longer but also and more importantly forestalling catastrophic health status collapse.
Figure 2.
A eukaryotic cell is a continuum of components for sensing the external environment and translating these signals through signaling pathways with both positive and negative gates. The entire signaling and transmission system is controlled by the native programs included in the DNA. The chromosome/chromatin and their capacity to respond are controlled by the history of the cell and its current anatomic location and microchemistry. For example, the Wnt-caused signaling cascade can have many components in progressive sequence that could culminate in an activation of transcription assembly on several different genes on a number of different chromosomes. The precision of the signaling and the cell’s response are comparable to typing commands into the computer, which initiates a translational cascade involving multiple components and, eventually, relying on the code elements housed and modified within multiple elements of the hard drive. Touch the keys, receive a response—expose the cell surface to a signaling molecule, receive a response.
Management of Resident MSCs
Children have a huge capacity to regenerate injured or diseased tissues. The thesis we have developed would contend that this results from the very high relative vascular densities and, thus, large relative numbers of pericytes and, hence, large numbers of local MSCs to help coordinate tissue regeneration and recovery. As we grow older, the relative vascular density in various tissues decreases. This is most easily quantified by accessing the vascular density in a skin biopsy. The presence and depth of the rete ridges in the papillary dermis is an excellent marker of age and, indeed, a marker for diabetes, for which the vascular density is about one half that of age-matched specimens. The question, thus, becomes how to increase the local concentrations of pericytes/MSCs in adult fields of tissue injury or disease or, in other words, how to manage the body’s local regenerative potential.
One such pharmaceutical approach involves the discovery that the molecule stromal cell-derived factor 1 (SDF-1) is a powerful and natural chemotactic agent for MSCs, and its local delivery could serve to cause the migration of MSCs to increase local levels at sites of injury or disease. In experimental acute myocardial infarcts (AMI), it is known that the injured heart tissue secretes SDF-1 during the first few days after the injury (by 1 week, almost none can be detected). The presence of SDF-1 at the sites of injury causes venous-introduced exogenous MSCs to accumulate and dock in the injured heart tissue.
Given these observations, Penn [59], and others, have started clinical trials in which the local management of SDF-1 levels is being used therapeutically in cases of AMI and skin wound treatment. Penn has produced a plasmid that is directly introduced into the injured tissue. The plasmid enters local cells and codes for the production of SDF-1, which is secreted to stimulate the therapeutic cascade, presumably involving the attraction and accumulation of local MSCs. The plasmid can be assumed to be active for a short time before it is inactivated by natural cellular machinery.
Although the SDF-1 plasmid is a “drug” approach, this drug is designed to manage the body’s local MSCs and, thus, circumvent the use of exogenous MSCs. Again, this is a complex and relatively uncharted sequela of events. It can be anticipated that in the human population, a subset of patients will be nonresponders to either or both SDF-1 or MSC-based therapies. As suggested, an encyclopedia of data on the genomics and panomics will allow us to develop tailored treatment by adjusting the doses and potency characteristics of the responding MSCs. Likewise, by adding exogenous MSCs or managing the local endogenous MSC numbers or their positions, we will be able to exert a profound effect on plastic surgeries and the size of all surgical incisions [63, 64].
Longevity
Personalized medicine, cell-based therapies, and quantitative panomics will give rise to, and stimulate adoption of, new logics for health care. Such logics will allow us to grow old more gracefully. This does not mean that these new logics will allow us to live forever, but it might allow us to delay or arrest the degenerative processes that lead to the decay in cognitive performance, impairment of mobility, and accumulation of subtle, but clinically significant, functional abnormalities in every organ system, synonymous with the frail, aged condition. These new logics will lead to treatment protocols to decrease osteoporosis, eliminate rheumatoid arthritis, and decrease the destructive effects of osteoarthritis, diabetes, macular degeneration, multiple sclerosis, and so forth. Cell-based therapies will change how medicine is practiced. For example, a person in rural Ohio experiences an AMI. The helicopter picks the patient up and brings him to an urban medical center for treatment. In 5–10 years, that same patient might drive to a local clinic or urgent care center and be given an infusion of MSCs intravenously as the total or first-line treatment [65]. In such cases, it might be that patients “bank” frozen bags of their own MSCs for use in crisis management. Stem cell banking is certainly not new; an individual’s cord blood has been banked for almost 3 decades. In this context, one could imagine several types of adult MSC banks. However, from a business approach, the allogeneic repositories of companies such as Mesoblast or Athersys might provide the cell therapies of choice just in terms of the cost of goods for mass-produced therapeutic cells versus autologous cell banks. These stem cell treatments will be cost, resource, and time efficient for patients and the society as whole.
Unexpected and unpredicted by most of the cell therapy community is the finding that MSCs constitute a powerful family of antibiotic proteins when they contact bacteria [66, 67]. These proteins have been cloned and studied in detail in the oral cavity and are known summarily as “defensins,” which function to control the identity and number of bacteria that enter the gastrointestinal tract. The MSCs have been shown to quantitatively upregulate the synthesis of LL37 as controlled by the number of bacterial particles in contact with the MSCs [66]. Clinical trials are now using MSCs to treat sepsis, and soon from our clinical colleagues, an MSC trial of patients with cystic fibrosis and lung infections will be started. Finally, the infrequent infections in patients with organ transplants treated with MSCs can now be explained [68].
Conclusion
Managing the body’s natural repair and regeneration capacities is the new frontier for modern medicine and the basis for the science of cell therapies. Body management is now, and the use and study of MSCs is but one avenue being pursued. Long live the body.
Acknowledgments
Support was provided by the L. David and E. Virginia Baldwin Fund (to A.I.C.) and Celgene Cellular Therapeutics, Inc. (Warren, NJ).
Author Contributions
A.I.C. and R.H.: manuscript writing, final approval of the manuscript.
Disclosure of Potential Conflicts of Interest
A.I.C. has uncompensated employment, has compensated royalty from Osiris to Case Western Reserve University, is an uncompensated consultant for Celgene Cellular Therapeutics, and has uncompensated stock options in Cell Bank Therapeutics. R.H. has compensated employment with Cellgene Cellular Therapeutics and compensated stock options in Cellgene Cellular Therapeutics.
References
- 1.Caplan AI, Ordahl CP. Irreversible gene repression model for control of development. Science. 1978;201:120–130. doi: 10.1126/science.351805. [DOI] [PubMed] [Google Scholar]
- 2.Caplan AI, Fiszman MY, Eppenberger HM. Molecular and cell isoforms during development. Science. 1983;221:921–927. doi: 10.1126/science.6348946. [DOI] [PubMed] [Google Scholar]
- 3.Vermeer DE, Ferrell RE., Jr Nigerian geophagical clay: A traditional antidiarrheal pharmaceutical. Science. 1985;227:634–636. doi: 10.1126/science.3969552. [DOI] [PubMed] [Google Scholar]
- 4.Bahary N, Zon LI. Development: endothelium—chicken soup for the endoderm. Science. 2001;294:530–531. doi: 10.1126/science.1066282. [DOI] [PubMed] [Google Scholar]
- 5.Fye WB. William Murrell. Clin Cardiol. 1995;18:426–427. doi: 10.1002/clc.4960180714. [DOI] [PubMed] [Google Scholar]
- 6.Hommes FA, Drost YM, Geraets WXM, et al. The energy requirement for growth: An application of Atkinson’s metabolic price system. Pediatr Res. 1975;9:51–55. [Google Scholar]
- 7.Banting FG, Best CH, Collip JB, et al. Pancreatic extracts in the treatment of diabetes mellitus. Can Med Assoc J. 1922;12:141–146. [PMC free article] [PubMed] [Google Scholar]
- 8.Venter JC, Adams MD, Myers EW, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 9.Wikipedia. Panomics Available at http://en.wikipedia.org/wiki/Panomics. Accessed June 2013.
- 10.Müller T, Schrötter A, Loosse C, et al. Sense and nonsense of pathway analysis software in proteomics. J Proteome Res. 2011;10:5398–5408. doi: 10.1021/pr200654k. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi CT, Schechter AN. The intracellular polymerization of sickle hemoglobin and its relevance to sickle cell disease. Blood. 1981;58:1057–1068. [PubMed] [Google Scholar]
- 12.Karkouti K, McCluskey SA, Evans L, et al. Erythropoietin is an effective clinical modality for reducing RBC transfusion in joint surgery. Can J Anaesth. 2005;52:362–368. doi: 10.1007/BF03016277. [DOI] [PubMed] [Google Scholar]
- 13.Caplan AI. What’s in a name? Tissue Eng Part A. 2010;16:2415–2417. doi: 10.1089/ten.TEA.2010.0216. [DOI] [PubMed] [Google Scholar]
- 14.Caplan AI, Correa D. The MSC: An injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caplan AI. MSCs: The new medicine. In: Vertes A, editor. Stem Cells in Regenerative Medicine: Science, Regulation, and Business Strategies. New York: John Wiley & Sons, Ltd; 2013. [Google Scholar]
- 16.Mescher AL, Tassava RA. Denervation effects on DNA replication and mitosis during the initiation of limb regeneration in adult newts. Dev Biol. 1975;44:187–197. doi: 10.1016/0012-1606(75)90386-3. [DOI] [PubMed] [Google Scholar]
- 17.Muller TL, Ngo-Muller V, Reginelli A, et al. Regeneration in higher vertebrates: Limb buds and digit tips. Semin Cell Dev Biol. 1999;10:405–413. doi: 10.1006/scdb.1999.0327. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal V, Tottey S, Johnson SA, et al. Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng Part A. 2011;17:2435–2443. doi: 10.1089/ten.tea.2011.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao DC, Lei JX, Chen R, et al. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005;11:3431–3440. doi: 10.3748/wjg.v11.i22.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 21.Moseley TA, Zhu M, Hedrick MH. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg. 2006;118(suppl):121S–128S. doi: 10.1097/01.prs.0000234609.74811.2e. [DOI] [PubMed] [Google Scholar]
- 22.Sung HM, Suh IS, Lee HB, et al. Case reports of adipose-derived stem cell therapy for nasal skin necrosis after filler injection. Arch Plast Surg. 2012;39:51–54. doi: 10.5999/aps.2012.39.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metcalfe AD, Ferguson MWJ. Tissue engineering of replacement skin: The crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface. 2007;4:413–437. doi: 10.1098/rsif.2006.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caplan AI. Cell delivery and tissue regeneration. J Control Release. 1989;11:157–165. [Google Scholar]
- 25.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 26.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 27.Caplan AI, Correa D. PDGF in bone formation and regeneration: New insights into a novel mechanism involving MSCs. J Orthop Res. doi: 10.1002/jor.21462. 2011;29:1795–1803. [DOI] [PubMed] [Google Scholar]
- 28.Gronthos S, Zannettino ACW, Hay SJ, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 29.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 30.Laflamme MA, Myerson D, Saffitz JE, et al. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ Res. 2002;90:634–640. doi: 10.1161/01.res.0000014822.62629.eb. [DOI] [PubMed] [Google Scholar]
- 31.Maes C, Kobayashi T, Selig MK, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu X, Wu R, Jiang Z, et al. Leptin signaling is required for augmented therapeutic properties of mesenchymal stem cells conferred by hypoxia preconditioning. Stem Cells. 2014;32:2702–2713. doi: 10.1002/stem.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 35.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 36.Hariri RJ. inventor. Method of collecting placental stem cells. US Patent Publication number CA2430989A1. June 13, 2002. PCT number PCT/US2001/046506. December 5, 2001.
- 37.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 38.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 40.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 41.Bianco P, Riminucci M, Gronthos S, et al. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 42.Charbord P, Livne E, Gross G, et al. Human bone marrow mesenchymal stem cells: A systematic reappraisal via the genostem experience. Stem Cell Rev. 2011;7:32–42. doi: 10.1007/s12015-010-9125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caplan AI. MSCs as therapeutics. In: Hematti P, Keating A, editors. Stem Cell Biology and Regenerative Medicine: Mesenchymal Stromal Cells: Biology and Clinical Applications. New York: Human Press; 2012. pp. 79–90. [Google Scholar]
- 44.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin P, Correa D, Kean TJ, et al. Serial transplantation and long-term engraftment of intra-arterially delivered clonally derived mesenchymal stem cells to injured bone marrow. Mol Ther. 2014;22:160–168. doi: 10.1038/mt.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shehadah A, Chen J, Pal A, et al. Human placenta-derived adherent cell treatment of experimental stroke promotes functional recovery after stroke in young adult and older rats. PLoS One. 2014;9:e86621. doi: 10.1371/journal.pone.0086621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Penn MS, Ellis S, Gandhi S, et al. Adventitial delivery of an allogeneic bone marrow-derived adherent stem cell in acute myocardial infarction: Phase I clinical study. Circ Res. 2012;110:304–311. doi: 10.1161/CIRCRESAHA.111.253427. [DOI] [PubMed] [Google Scholar]
- 48.See F, Seki T, Psaltis PJ, et al. Therapeutic effects of human STRO-3-selected mesenchymal precursor cells and their soluble factors in experimental myocardial ischemia. J Cell Mol Med. 2011;15:2117–2129. doi: 10.1111/j.1582-4934.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gir P, Oni G, Brown SA, et al. Human adipose stem cells: Current clinical applications. Plast Reconstr Surg. 2012;129:1277–1290. doi: 10.1097/PRS.0b013e31824ecae6. [DOI] [PubMed] [Google Scholar]
- 50.Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sackstein R, Merzaban JS, Cain DW, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 52.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Moll G, Rasmusson-Duprez I, von Bahr L, et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells. 2012;30:1565–1574. doi: 10.1002/stem.1111. [DOI] [PubMed] [Google Scholar]
- 54.Dennis JE, Cohen N, Goldberg VM, et al. Targeted delivery of progenitor cells for cartilage repair. J Orthop Res. 2004;22:735–741. doi: 10.1016/j.orthres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Traverse JH, Henry TD, Ellis SG, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: The LateTIME randomized trial. JAMA. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hariri RJ. inventor. Post-partum mammalian placenta, its use and placental stem cells therefrom. US Patent Publication number CA2438153A1. August 22, 2002. PCT/US2002/004282. February 13, 2002.
- 58.Schindeler A, Liu R, Little DG. The contribution of different cell lineages to bone repair: Exploring a role for muscle stem cells. Differentiation. 2009;77:12–18. doi: 10.1016/j.diff.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Penn MS. Importance of the SDF-1:CXCR4 axis in myocardial repair. Circ Res. 2009;104:1133–1135. doi: 10.1161/CIRCRESAHA.109.198929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 61.Hariri RJ. Imagining tomorrow’s health & medicine. The Walker Library of Human Imagination. TEDMED Leadership Conversation. Available at: http://www.youtube.com/watch?v=M4s1zV0TjlM. Accessed July 2014.
- 62.Zimmerlin L, Park TS, Zambidis ET, et al. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie. 2013;95:2235–2245. doi: 10.1016/j.biochi.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu YL, Liu WH, Sun J, et al. Mesenchymal stem cell-mediated suppression of hypertrophic scarring is p53 dependent in a rabbit ear model. Stem Cell Res Ther. 2014;5:136. doi: 10.1186/scrt526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jackson WM, Nesti LJ, Tuan RS. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res Ther. 2012;3:20. doi: 10.1186/scrt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen HJ, Chen CH, Chang MY, et al. Human placenta-derived adherent cells improve cardiac performance in mice with chronic heart failure. Stem Cells Translational Medicine. 2015;4:269–275. doi: 10.5966/sctm.2014-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bonfield TL, Lennon D, Ghosh SK, et al. Cell based therapy aides in infection and inflammation resolution in the murine model of cystic fibrosis lung disease. Stem Cell Discovery. 2013;3:139–153. [Google Scholar]
- 68.Tan J, Wu W, Xu X, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: A randomized controlled trial. JAMA. 2012;307:1169–1177. doi: 10.1001/jama.2012.316. [DOI] [PubMed] [Google Scholar]