The authors reviewed recently discovered cell-autonomous and microenvironmental mechanisms that could promote the survival of gastrointestinal stromal tumor (GIST) cells in the presence of tyrosine kinase inhibitor therapy, with a focus on the potential role of adult precursors for interstitial cells of Cajal. Eradication of residual GIST cells and cure of GIST will likely require individualized combinations of several approaches tailored to tumor genotype and phenotype.
Summary
Gastrointestinal stromal tumors (GISTs) represent 20%–40% of human sarcomas. Although approximately half of GISTs are cured by surgery, prognosis of advanced disease used to be poor due to the high resistance of these tumors to conventional chemo- and radiotherapy. The introduction of molecularly targeted therapy (e.g., with imatinib mesylate) following the discovery of the role of oncogenic mutations in the receptor tyrosine kinases KIT and platelet-derived growth factor α (PDGFRA) significantly increased patient survival. However, GIST cells persist in 95%–97% of imatinib-treated patients who eventually progress and die of the disease because of the emergence of clones with drug-resistant mutations. Because these secondary mutations are highly heterogeneous, even second- and third-line drugs that are effective against certain genotypes have only moderately increased progression-free survival. Consequently, alternative strategies such as targeting molecular mechanisms underlying disease persistence should be considered. We reviewed recently discovered cell-autonomous and microenvironmental mechanisms that could promote the survival of GIST cells in the presence of tyrosine kinase inhibitor therapy. We particularly focused on the potential role of adult precursors for interstitial cells of Cajal (ICCs), the normal counterpart of GISTs. ICC precursors share phenotypic characteristics with cells that emerge in a subset of patients treated with imatinib and in young patients with GIST characterized by loss of succinate dehydrogenase complex proteins and lack of KIT or PDGFRA mutations. Eradication of residual GIST cells and cure of GIST will likely require individualized combinations of several approaches tailored to tumor genotype and phenotype.
Significance
Gastrointestinal stromal tumors (GISTs) are one of the most common connective tissue cancers. Most GISTs that cannot be cured by surgery respond to molecularly targeted therapy (e.g., with imatinib); however, tumor cells persist in almost all patients and eventually acquire drug-resistant mutations. Several mechanisms contribute to the survival of GIST cells in the presence of imatinib, including the activation of “escape” mechanisms and the selection of stem-like cells that are not dependent on the expression of the drug targets for survival. Eradication of residual GIST cells and cure of GIST will likely require individualized combinations of several approaches tailored to the genetic makeup and other characteristics of the tumors.
Gastrointestinal Stromal Tumors and Interstitial Cells of Cajal
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract [1,2]. With an estimated annual incidence of 3,300–6,000, GISTs represent a substantial proportion of the ∼15,000 bone and soft tissue sarcomas diagnosed in the U.S. each year [3]. Based on morphological, ultrastructural, and immunophenotypic characteristics including the expression of the receptor tyrosine kinase (RTK) KIT in ∼95% of cases [4], GISTs are thought to originate from stem cells that differentiate toward the lineage of interstitial cells of Cajal (ICCs) [5–8]. ICCs are KIT-expressing (KIT+), mesodermally derived mesenchymal cells that occur throughout the gut tunica muscularis [9]. In concert with the enteric nervous system and smooth muscle cells, ICCs govern gastrointestinal motor functions by generating electrical pacemaker activity, mediating cholinergic excitatory and nitrergic inhibitory neuromuscular neurotransmission, and by setting smooth muscle membrane potential and tone [9–12]. ICCs and most GISTs, including ∼50% of KIT-negative (KIT−) GISTs, also share expression of the calcium-activated chloride channel anoctamin 1 (ANO1; also known as TMEM16A and discovered on GIST-1 [DOG1]) [8, 13–17]. However, the relationship of KIT−ANO1− GIST to the ICC lineage cannot be ascertained based on immunohistochemical criteria. A subset of these tumors have been proposed to arise, possibly due to activated hedgehog signaling [18], from interstitial cells expressing platelet-derived growth factor α (PDGFRA); these cells mediate purinergic inhibitory neuromuscular neurotransmission [9].
The majority of GISTs arise on the basis of mutually exclusive, most often heterozygous activating mutations in KIT (75%–80%) [6] or PDGFRA (<10%) [19], which encodes a closely related RTK often coexpressed with KIT [20, 21]. In rare cases, GIST develops in patients carrying germline autosomal dominant KIT or PDGFRA mutations (familial GIST syndrome) [22]. Constitutively activated KIT cooperates with ETV1, a master regulator of the ICC transcription program, to bring about GIST oncogenesis [23]. RTK-mutant GIST may be composed of spindle-shaped and/or epithelioid cells, have no predilection for either sex, mainly occur in patients older than 50 years, and can arise anywhere in the gastrointestinal tract and within the mesentery, omentum, retroperitoneum, and pelvis [4]. Molecular alterations underlying clinical progression of GIST include chromosomal losses or gains and deletions, mutations, or downregulation of cell cycle regulator genes [22, 24]. Intragenic deletion of dystrophin, specifically affecting larger isoforms, often underlies aggressive, metastatic behavior [25]. GISTs that lack KIT or PDGFRA mutations (usually, and most often erroneously, termed “wild-type” GISTs; 10%–15%) may contain driver mutations in BRAF, HRAS, or NRAS or may develop in patients with neurofibromatosis type 1 [26] carrying germline autosomal dominant mutations in NF1 [22, 27]. These tumors are morphologically and clinically indistinguishable from RTK-mutant GISTs including expression and activation of KIT. A small subset of tumors lacking KIT or PDGFRA mutations and the majority (70%–75%) of pediatric GISTs, which represent only 1%–2% of all cases, display unique clinicopathological features including a strong predilection toward female sex, predominant gastric origin, presentation as multifocal nodular growths, epithelioid morphology, and low frequency of cytogenetic aberrations. Importantly, this class of GIST shows increased expression of insulin-like growth factor 1 receptor (IGF1R) [27–31] accompanied by activation of downstream signaling pathways that IGF1R shares with KIT [31]. A key unifying characteristic of these tumors is the loss of mitochondrial succinate dehydrogenase complex iron sulfur subunit B protein (SDHB) [22, 27, 32]. SDHB loss, in turn, causes reduced SDH respiratory complex II enzymatic activity (pseudohypoxia), succinate accumulation, stabilization of hypoxia-inducible factor 1α, and transcriptional activation of hypoxia-inducible genes including vascular endothelial growth factors and IGF2. Furthermore, the reduced α-ketoglutarate:succinate ratios have also been shown to cause DNA hypermethylation, with concomitant reduction of DNA hydroxymethylation likely reflecting substrate-level inhibition of ten-eleven translocation (TET) family methylcytosine dioxygenases [33]. In GIST, SDHB depletion can arise from deleterious mutations leading to biallelic inactivation of SDHA in sporadic cases [27, 34], autosomal dominant SDHB-D mutations in familial cases with associated paragangliomas (Carney-Stratakis syndrome) [35, 36], or epigenetic repression of SDHC transcription from DNA hypermethylation in Carney triad [37, 38]. The latter is a nonfamilial syndrome involving synchronous or metachronous occurrence of GISTs, paragangliomas, and pulmonary chondromas [39]. Even when they occur in adults, SDHB-negative GISTs are characterized by early onset with median age of ∼18–22 years [27]. Although pediatric GISTs often metastasize, they tend to grow more slowly [22].
Disease Persistence and Therapy Resistance in GIST
The standard of care and the only potentially curative therapy for patients with a primary localized GIST is surgery [40]; however, approximately 40% of patients develop tumor recurrence within 5 years and eventually die of the disease [41]. Because GIST is highly resistant to conventional chemo- or radiotherapy, RTK inhibitors are the mainstay of treatment for advanced GIST. Front-line treatment with imatinib, a competitive inhibitor of ATP binding to KIT, PDGFRA, ABL, and BCR-ABL kinases, can achieve disease control in 70%–85% of patients with KIT+ advanced GIST and median progression-free survival of >5 years [22]. Approximately 10% of patients have primary resistance to RTK inhibitors, and that mainly reflects the type of the driving mutation. Resistance developing after an initial benefit is mainly the result of acquired, drug-resistant mutations occurring almost exclusively in the same gene and allele as the primary oncogenic mutation [42]. Unfortunately, resistance mutations show considerable heterogeneity, even within different areas of the same tumor; therefore, even second- and third-line drugs (e.g., sunitinib, regorafenib) that can potently inhibit certain secondary mutations have increased median progression-free survival by only 5–6 months [43].
In patients who respond to imatinib, substantial reduction in tumor size occurs as a result of apoptosis, consistent with the unique dependency of KIT-mutant GIST on oncogenic KIT signaling, termed “oncogene addiction” [44]. However, although long-term disease control lasting more than a decade can be achieved in some patients, RTK inhibitors fail to eradicate GIST cells in 95%–97% of patients who eventually progress and succumb to the disease [22]. The surviving cells are metabolically quiescent and do not proliferate [45, 46]; however, their exit from the cell cycle is reversible, as indicated by in vitro data [47] and the increased rate of progression in patients in whom treatment was discontinued [22], necessitating life-long therapy. The significance of GIST persistence despite continuing RTK inhibitor therapy is that it sets the stage for the selection of clones with secondary drug-resistant mutations, which cannot be effectively controlled by current pharmacological approaches [43]. Therefore, it can be argued that targeting disease persistence should take precedence over the development of additional pharmacological agents against secondary mutations.
Cell-Autonomous Mechanisms of GIST Persistence
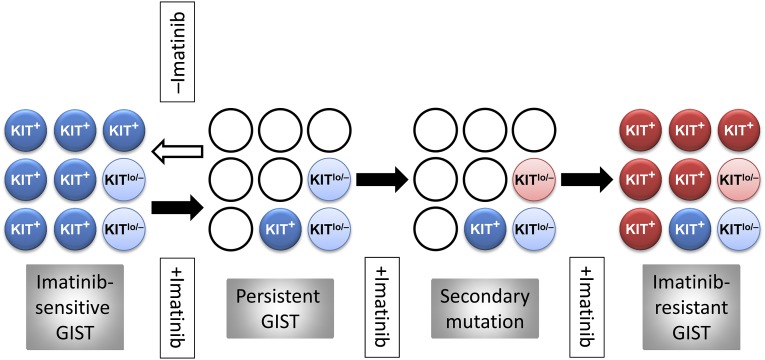
The persistence during RTK inhibitor therapy of GIST cells carrying imatinib-sensitive mutations could result from “escape” mechanisms expressed by the tumor cells. Alternatively, a pre-existing subset of cells not addicted to oncogenic RTK signaling, for example, due to lack of significant expression of the mutant receptor, could selectively survive the treatment. In six studies that investigated KIT expression in patients who underwent imatinib or sunitinib treatment prior to surgery, 15 of 131 samples lacked KIT expression detectable by conventional immunohistochemistry [46, 48–52] (further samples studied in [46] displayed reduced KIT expression [KITlow]), whereas the remainder showed no obvious phenotypic change. Thus, both mechanisms may contribute to GIST persistence. Typically, the cells with low or no expression of KIT (KITlow/−) had epithelioid morphology [46, 50, 52], which also predominates in untreated KIT− GISTs [53]. Previously, we described a rare Kitlow/− cell type with nondescript epithelioid morphology in the tunica muscularis of postnatal mice and demonstrated their ability to self-renew and differentiate into ICCs in cell culture, in tissue explants, and following allogeneic transplantation in vivo [7, 8, 54]. In mice carrying germline oncogenic Kit mutation, the population of these ICC stem cells (ICC-SCs) was dramatically increased, paralleling ICC hyperplasia [8]. Furthermore, clonally derived, spontaneously transformed ICC-SCs gave rise to GIST-like tumors in immunocompromised mice [8]. Besides epithelioid, Kitlow cells, these tumors contained spindle-shaped or ICC-like Kit+ cells, indicating that both phenotypes can arise from the same Kitlow/− precursor. Importantly, despite the competitive growth advantage that the Kit mutation seemed to confer on these Kitlow/− cells over their non-neoplastic counterparts, neither normal nor transformed (including Kit-mutant) ICC-SCs were dependent on Kit signaling for survival, as indicated by their low sensitivity to imatinib. These findings are consistent with a GIST model in which a small number of mutated ICC-SCs gives rise to KIT+ cells, representing the bulk of the tumors (Fig. 1). Although RTK inhibition can control the differentiation, proliferation, and survival of KIT+ GIST cells, it may not eradicate the inherently imatinib-resistant KITlow/− stem cell pool from which the tumor is re-established following the cessation of therapy. Acquisition of an imatinib-resistant mutation by the surviving precursors would again permit their differentiation into KIT-expressing cells and uncontrolled GIST growth [8]. It follows that stimulation of KIT expression in the surviving KITlow/− GIST precursors before the emergence of drug-resistant mutations could potentially restore the sensitivity of these cells to imatinib. Although this model bears remarkable similarities to the model proposed to underlie disease persistence in chronic myeloid leukemia [55, 56], its applicability to human GIST remains to be established.
Figure 1.
Hypothetical stem cell model of GIST persistence and acquired therapy resistance. Light blue circles: KIT-independent precursor cells carrying imatinib-sensitive KIT mutation but expressing very little or no KIT protein (KITlow/−). Dark blue circles: KIT+ cells arising from the KITlow/− cells carrying imatinib-sensitive KIT mutation. Open circles: dead cells. Pink circles: KITlow/−, KIT-independent precursors with acquired secondary imatinib-resistant mutation. Red circles: KIT+ cells differentiated from the KITlow/− precursors with secondary imatinib-resistant mutation. Filled arrow: imatinib treatment. Open arrow: cessation of imatinib treatment. Abbreviations: GIST, gastrointestinal stromal tumor; KITlo/−, cells with low or no expression of KIT; KIT+, cells expressing KIT.
In GIST cells dependent on imatinib-sensitive mutations, disease persistence may reflect incomplete apoptosis response to RTK inhibition [24]; however, relative to other solid tumors, loss-of-function mutations in the multifunctional proapoptotic protein p53 (TP53) are rare in GIST [24]. Instead, GIST cells may escape apoptosis by upregulating macroautophagy, which can facilitate cell survival during stress [47]. In quiescent GIST cells, apoptosis sensitivity could be restored by RNA interference-mediated (RNAi-mediated) knockdown of key autophagy regulators and by the antimalarial lysosomotropic agents chloroquine and quinacrine. Withdrawal from the cell cycle and consequent resistance to the proapoptotic action of RTK inhibition also involves the nuclear accumulation of the cyclin-dependent kinase inhibitor p27Kip1 due to APCCDH1 complex-mediated proteasomal degradation of the p27Kip1-regulator SKP2 [57]. Another recent study identified a role for the DREAM complex (DP, p130/RBL2, E2F4, and MuvB) in GIST cell quiescence induced by imatinib treatment [58]. Importantly, the authors found that quiescence could be abrogated and apoptosis sensitivity restored by the inhibition of dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A), which plays an essential role in DREAM complex formation. Together, these results suggest that GIST could be sensitized to RTK inhibition-induced apoptosis by pharmacological inhibition of several cell-autonomous mechanisms that underlie cell cycle exit or stimulation of differentiation of KITlow/− precursors.
The effects of these interventions may be limited by changes other than TP53 inactivation. We recently reported that in both imatinib-sensitive and imatinib-resistant GIST cells, and in tumorigenic ICC-SCs, reduced apoptosis sensitivity could also reflect loss of family with sequence similarity 96, member A (FAM96A) [59]. FAM96A is a member of the cytosolic iron-sulfur protein assembly machinery and a regulator of cellular iron homeostasis [60], but we found its proapoptotic action to be dependent on its evolutionarily conserved ability to bind apoptotic peptidase activating factor 1 (APAF1). Although FAM96A was robustly expressed in human and mouse ICCs, murine ICC-SCs, and PDGFRA-expressing interstitial cells, FAM96A protein or mRNA was dramatically reduced or lost in 106 of 108 GIST samples due to genomic deletion or other causes. Re-expression of FAM96A in GIST cells and transformed ICC-SCs increased apoptosis sensitivity and diminished tumorigenicity; however, it is unclear whether pharmacological sensitization to mitochondrial apoptosis (e.g., by TP53-modulating agents [24]) could overcome the effects of FAM96A loss.
Microenvironmental Mechanisms of GIST Persistence
GIST cells not dependent on constitutively active RTK signaling must draw on alternative, ligand-activated signaling pathways for survival. Pharmacological targeting of these mechanisms may provide additional means to eliminate cells contributing to disease persistence and to treat SDHB-deficient, RTK wild-type GISTs, which respond very poorly to imatinib and only slightly better to sunitinib [27, 61]. IGF1R is variably expressed and activated in several molecular subtypes of GIST [31]. Most robust expression has been observed in pediatric and pediatric-like adult GISTs [27–31, 61] reflecting either genomic amplification [31] or constitutive high expression [29, 30]. The latter may signify the less differentiated nature of these tumors and their possible relationship to ICC-SCs [27, 61], which, unlike mature ICCs, strongly express both Igf1r and insulin receptor (Insr) in mice [7]. Furthermore, a recent study reported that at least two other subgroups of adult RTK wild-type GISTs and even some KIT-mutant tumors may also overexpress members of insulin/IGF-activated pathways [61]. Both RNAi-mediated IGF1R knockdown and selective pharmacological inhibition of IGF1R tyrosine kinase activity led to cytotoxicity in KIT-mutant cell lines [31], and IGF1R-targeted treatment of GISTs lacking KIT, PDGFRA, or BRAF mutations is currently under investigation in a phase II clinical trial (NCT01560260).
Besides directly activating signaling pathways important for cell survival and proliferation, INSR/IGF1R may also promote the survival of GIST cells via indirect actions. We previously found that Insr/Igf1r-dependent survival [62] and differentiation of murine ICCs [7] was mediated by stem cell factor (KIT ligand; mouse: Kitl; human: KITLG) expressed by cells of the ICC microenvironment. We also demonstrated that in murine gastric muscles and KIT-mutant GIST cells, IGF1R activation stimulated Kitl/KITLG expression by inducing chromatin-level changes favoring transcriptional activation at the Kitl/KITLG promoter [63]. Furthermore, KITLG immunoneutralization inhibited the proliferation of GIST cells carrying a wild-type KIT allele including the imatinib-resistant GIST-T1-5R cell line. Together, these results indicate a role for an IGF1-induced autocrine/paracrine loop in the stimulation of GIST growth mediated by KITLG-induced wild-type KIT activation, a mechanism that remains active in tumors treated with imatinib due to the preferential targeting of mutant receptors by this drug [21]. A similar role has been proposed for ligand-dependent activation of wild-type PDGFRA found coexpressed with mutant KIT or PDGFRA [21].
In GIST882 cells made KIT− by long-term exposure to escalating concentrations of imatinib, phenotypic transition from spindle-shaped to epithelioid and concomitant overexpression of the RTKs AXL and MET together with their cognate ligands (growth arrest-specific 6 and hepatocyte growth factor, respectively) have been reported [64, 65]. AXL and/or MET were also upregulated and activated in three patients with KIT−, imatinib-resistant GISTs [65]. In three RTK wild-type GISTs, including one pediatric case, Agaram et al. described wild-type epidermal growth factor receptor (EGFR) expression and activation [28]. EGFR expression and activation appear to be common in both imatinib-treated and untreated GISTs, along with the expression of EGFR ligands amphiregulin, heparin-binding epidermal growth factor, betacellulin, and epiregulin and matrix metalloproteinases that can liberate them from the cell membrane [65, 66]. Together, these results indicate that autocrine/paracrine activation of alternative RTK pathways may be common in GISTs including tumors not dependent on KIT/PDGFRA signaling.
Conclusion
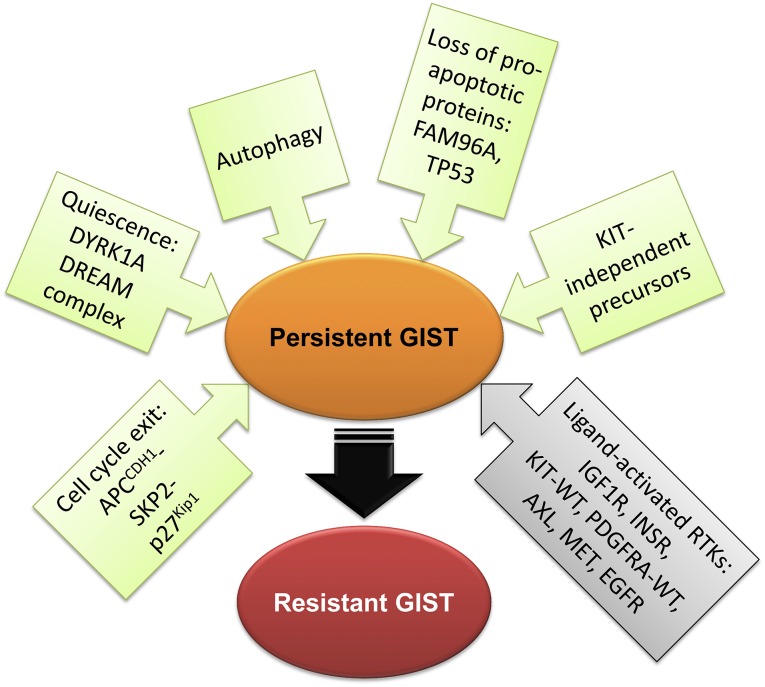
Disease persistence in GIST involves multiple mechanisms including activation of signaling pathways triggering the cells’ exit from the cell cycle, autophagy, loss of proapoptotic proteins, downregulation of KIT/PDGFRA expression, or selection of GIST stem cells that do not depend on KIT/PDGFRA signaling for survival due to expression of alternative wild-type receptor tyrosine kinases (Fig. 2). In view of the diversity of the gene expression profiles of GISTs exposed to long-term imatinib treatment, eradication of residual tumor cells and cure of GIST will likely require individualized combinations of several approaches tailored to tumor genotype and phenotype.
Figure 2.
Mechanisms of disease persistence in GIST. Green and gray boxes signify cell-autonomous and microenvironmental mechanisms, respectively. The depicted mechanisms are not mutually exclusive. Abbreviations: GIST, gastrointestinal stromal tumor; WT, wild type.
Acknowledgments
Research support was provided by NIH Grants R01DK058185, P30DK084567, and P30CA015083; the Life Raft Group; and the Mayo Clinic Center for Individualized Medicine.
Author Contributions
T.O.: conception and design, financial support, manuscript writing; M.Z.: manuscript writing, final approval of manuscript; Y.H.: assembly of data, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1. OMIM #606764: Gastrointestinal stromal tumor. Available at http://www.omim.org/entry/606764. Accessed April 22, 2015.
- 2.Ma GL, Murphy JD, Martinez ME, et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: Results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298–302. doi: 10.1158/1055-9965.EPI-14-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demetri GD. Sarcomas of soft tissue and bone, and other neoplasms of connective tissues. In: Goldman L, Schafer AI, editors. Goldman’s Cecil Medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2012. pp. 1327–1329. [Google Scholar]
- 4.Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731–1741. doi: 10.1016/S0140-6736(07)60780-6. [DOI] [PubMed] [Google Scholar]
- 5.Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): Gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 6.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 7.Lorincz A, Redelman D, Horváth VJ, et al. Progenitors of interstitial cells of cajal in the postnatal murine stomach. Gastroenterology. 2008;134:1083–1093. doi: 10.1053/j.gastro.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bardsley MR, Horváth VJ, Asuzu DT, et al. Kitlow stem cells cause resistance to Kit/platelet-derived growth factor alpha inhibitors in murine gastrointestinal stromal tumors. Gastroenterology. 2010;139:942–952. doi: 10.1053/j.gastro.2010.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders KM, Ward SM, Koh SD. Interstitial cells: Regulators of smooth muscle function. Physiol Rev. 2014;94:859–907. doi: 10.1152/physrev.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20(suppl 1):54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 11.Klein S, Seidler B, Kettenberger A, et al. Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nat Commun. 2013;4:1630. doi: 10.1038/ncomms2626. [DOI] [PubMed] [Google Scholar]
- 12.Huizinga JD, Chen JH, Zhu YF, et al. The origin of segmentation motor activity in the intestine. Nat Commun. 2014;5:3326. doi: 10.1038/ncomms4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Ordög T, Chen J, et al. Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine. Physiol Genomics. 2007;31:492–509. doi: 10.1152/physiolgenomics.00113.2007. [DOI] [PubMed] [Google Scholar]
- 14.Espinosa I, Lee CH, Kim MK, et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008;32:210–218. doi: 10.1097/PAS.0b013e3181238cec. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen M, Wang ZF, Lasota J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: A study of 1840 cases. Am J Surg Pathol. 2009;33:1401–1408. doi: 10.1097/PAS.0b013e3181a90e1a. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–G1381. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu MH, Kim TW, Ro S, et al. A Ca(2+)-activated Cl(-) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009;587:4905–4918. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelczar P, Zibat A, van Dop WA, et al. Inactivation of Patched1 in mice leads to development of gastrointestinal stromal-like tumors that express Pdgfrα but not kit. Gastroenterology. 2013;144:134.e6–144.e6. doi: 10.1053/j.gastro.2012.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 20.Zhu MJ, Ou WB, Fletcher CD, et al. KIT oncoprotein interactions in gastrointestinal stromal tumors: Therapeutic relevance. Oncogene. 2007;26:6386–6395. doi: 10.1038/sj.onc.1210464. [DOI] [PubMed] [Google Scholar]
- 21.Negri T, Bozzi F, Conca E, et al. Oncogenic and ligand-dependent activation of KIT/PDGFRA in surgical samples of imatinib-treated gastrointestinal stromal tumours (GISTs) J Pathol. 2009;217:103–112. doi: 10.1002/path.2450. [DOI] [PubMed] [Google Scholar]
- 22.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: Origin and molecular oncology. Nat Rev Cancer. 2011;11:865–878. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 23.Chi P, Chen Y, Zhang L, et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature. 2010;467:849–853. doi: 10.1038/nature09409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henze J, Mühlenberg T, Simon S, et al. p53 modulation as a therapeutic strategy in gastrointestinal stromal tumors. PLoS One. 2012;7:e37776. doi: 10.1371/journal.pone.0037776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Marino-Enriquez A, Bennett RR, et al. Dystrophin is a tumor suppressor in human cancers with myogenic programs. Nat Genet. 2014;46:601–606. doi: 10.1038/ng.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. OMIM #162200: Neurofibromatosis, type I; NF1. Available at http://www.omim.org/entry/162200. Accessed April 22, 2015.
- 27.Belinsky MG, Rink L, von Mehren M. Succinate dehydrogenase deficiency in pediatric and adult gastrointestinal stromal tumors. Front Oncol. 2013;3:117. doi: 10.3389/fonc.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agaram NP, Laquaglia MP, Ustun B, et al. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res. 2008;14:3204–3215. doi: 10.1158/1078-0432.CCR-07-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janeway KA, Zhu MJ, Barretina J, et al. Strong expression of IGF1R in pediatric gastrointestinal stromal tumors without IGF1R genomic amplification. Int J Cancer. 2010;127:2718–2722. doi: 10.1002/ijc.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pantaleo MA, Astolfi A, Di Battista M, et al. Insulin-like growth factor 1 receptor expression in wild-type GISTs: A potential novel therapeutic target. Int J Cancer. 2009;125:2991–2994. doi: 10.1002/ijc.24595. [DOI] [PubMed] [Google Scholar]
- 31.Tarn C, Rink L, Merkel E, et al. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci USA. 2008;105:8387–8392. doi: 10.1073/pnas.0803383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janeway KA, Kim SY, Lodish M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci USA. 2011;108:314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Killian JK, Kim SY, Miettinen M, et al. Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov. 2013;3:648–657. doi: 10.1158/2159-8290.CD-13-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pantaleo MA, Astolfi A, Indio V, et al. SDHA loss-of-function mutations in KIT-PDGFRA wild-type gastrointestinal stromal tumors identified by massively parallel sequencing. J Natl Cancer Inst. 2011;103:983–987. doi: 10.1093/jnci/djr130. [DOI] [PubMed] [Google Scholar]
- 35. OMIM #606864: Paraganglioma and gastric stromal sarcoma. Available at http://www.omim.org/entry/606864. Accessed April 22, 2015.
- 36.Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: A new syndrome distinct from the Carney triad. Am J Med Genet. 2002;108:132–139. doi: 10.1002/ajmg.10235. [DOI] [PubMed] [Google Scholar]
- 37. OMIM 604287: Carney triad. Available at http://www.omim.org/entry/604287. Accessed April 22, 2015.
- 38.Haller F, Moskalev EA, Faucz FR, et al. Aberrant DNA hypermethylation of SDHC: A novel mechanism of tumor development in Carney triad. Endocr Relat Cancer. 2014;21:567–577. doi: 10.1530/ERC-14-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carney JA, Sheps SG, Go VL, et al. The triad of gastric leiomyosarcoma, functioning extra-adrenal paraganglioma and pulmonary chondroma. N Engl J Med. 1977;296:1517–1518. doi: 10.1056/NEJM197706302962609. [DOI] [PubMed] [Google Scholar]
- 40.Demetri GD, Morgan J, Raut CP. Adjuvant and neoadjuvant imatinib for gastrointestinal stromal tumors. Available at http://www.uptodate.com/contents/adjuvant-and-neoadjuvant-imatinib-for-gastrointestinal-stromal-tumors. Accessed December 10, 2014.
- 41.Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265–274. doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]
- 42.Heinrich MC, Corless CL, Blanke CD, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24:4764–4774. doi: 10.1200/JCO.2006.06.2265. [DOI] [PubMed] [Google Scholar]
- 43.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Tseng M, Perdreau SA, et al. Histone H2AX is a mediator of gastrointestinal stromal tumor cell apoptosis following treatment with imatinib mesylate. Cancer Res. 2007;67:2685–2692. doi: 10.1158/0008-5472.CAN-06-3497. [DOI] [PubMed] [Google Scholar]
- 45.Van den Abbeele AD, Gatsonis C, de Vries DJ, et al. ACRIN 6665/RTOG 0132 phase II trial of neoadjuvant imatinib mesylate for operable malignant gastrointestinal stromal tumor: Monitoring with 18F-FDG PET and correlation with genotype and GLUT4 expression. J Nucl Med. 2012;53:567–574. doi: 10.2967/jnumed.111.094425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agaram NP, Besmer P, Wong GC, et al. Pathologic and molecular heterogeneity in imatinib-stable or imatinib-responsive gastrointestinal stromal tumors. Clin Cancer Res. 2007;13:170–181. doi: 10.1158/1078-0432.CCR-06-1508. [DOI] [PubMed] [Google Scholar]
- 47.Gupta A, Roy S, Lazar AJ, et al. Autophagy inhibition and antimalarials promote cell death in gastrointestinal stromal tumor (GIST) Proc Natl Acad Sci USA. 2010;107:14333–14338. doi: 10.1073/pnas.1000248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauer S, Hartmann JT, de Wit M, et al. Resection of residual disease in patients with metastatic gastrointestinal stromal tumors responding to treatment with imatinib. Int J Cancer. 2005;117:316–325. doi: 10.1002/ijc.21164. [DOI] [PubMed] [Google Scholar]
- 49.Liegl B, Kepten I, Le C, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol. 2008;216:64–74. doi: 10.1002/path. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pauwels P, Debiec-Rychter M, Stul M, et al. Changing phenotype of gastrointestinal stromal tumours under imatinib mesylate treatment: A potential diagnostic pitfall. Histopathology. 2005;47:41–47. doi: 10.1111/j.1365-2559.2005.02179.x. [DOI] [PubMed] [Google Scholar]
- 51.Dudeja V, Armstrong LH, Gupta P, et al. Emergence of imatinib resistance associated with downregulation of c-kit expression in recurrent gastrointestinal stromal tumor (GIST): Optimal timing of resection. J Gastrointest Surg. 2010;14:557–561. doi: 10.1007/s11605-009-1121-2. [DOI] [PubMed] [Google Scholar]
- 52.Vassos N, Agaimy A, Schlabrakowski A, et al. An unusual and potentially misleading phenotypic change in a primary gastrointestinal stromal tumour (GIST) under imatinib mesylate therapy. Virchows Arch. 2011;458:363–369. doi: 10.1007/s00428-010-1034-1. [DOI] [PubMed] [Google Scholar]
- 53.Medeiros F, Corless CL, Duensing A, et al. KIT-negative gastrointestinal stromal tumors: Proof of concept and therapeutic implications. Am J Surg Pathol. 2004;28:889–894. doi: 10.1097/00000478-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Dave M, Hayashi Y, Gajdos GB, et al. Stem cells for murine interstitial cells of Cajal suppress cellular immunity and colitis via prostaglandin E2 secretion. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.01.036. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corbin AS, Agarwal A, Loriaux M, et al. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121:396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Perdreau SA, Chatterjee P, et al. Imatinib mesylate induces quiescence in gastrointestinal stromal tumor cells through the CDH1-SKP2-p27Kip1 signaling axis. Cancer Res. 2008;68:9015–9023. doi: 10.1158/0008-5472.CAN-08-1935. [DOI] [PubMed] [Google Scholar]
- 58.Boichuk S, Parry JA, Makielski KR, et al. The DREAM complex mediates GIST cell quiescence and is a novel therapeutic target to enhance imatinib-induced apoptosis. Cancer Res. 2013;73:5120–5129. doi: 10.1158/0008-5472.CAN-13-0579. [DOI] [PubMed] [Google Scholar]
- 59.Schwamb B, Pick R, Fernández SB, et al. FAM96A is a novel pro-apoptotic tumor suppressor in gastrointestinal stromal tumors. Int J Cancer. 2015 doi: 10.1002/ijc.29498. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stehling O, Mascarenhas J, Vashisht AA, et al. Human CIA2A-FAM96A and CIA2B-FAM96B integrate iron homeostasis and maturation of different subsets of cytosolic-nuclear iron-sulfur proteins. Cell Metab. 2013;18:187–198. doi: 10.1016/j.cmet.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beadling C, Patterson J, Justusson E, et al. Gene expression of the IGF pathway family distinguishes subsets of gastrointestinal stromal tumors wild type for KIT and PDGFRA. Cancer Med. 2013;2:21–31. doi: 10.1002/cam4.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Horváth VJ, Vittal H, Lörincz A, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–770. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 63.Hayashi Y, Asuzu DT, Gibbons SJ, et al. Membrane-to-nucleus signaling links insulin-like growth factor-1- and stem cell factor-activated pathways. PLoS One. 2013;8:e76822. doi: 10.1371/journal.pone.0076822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mahadevan D, Cooke L, Riley C, et al. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene. 2007;26:3909–3919. doi: 10.1038/sj.onc.1210173. [DOI] [PubMed] [Google Scholar]
- 65.Mahadevan D, Theiss N, Morales C, et al. Novel receptor tyrosine kinase targeted combination therapies for imatinib-resistant gastrointestinal stromal tumors (GIST) Oncotarget. 2015;6:1954–1966. doi: 10.18632/oncotarget.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakagawa M, Nabeshima K, Asano S, et al. Up-regulated expression of ADAM17 in gastrointestinal stromal tumors: Coexpression with EGFR and EGFR ligands. Cancer Sci. 2009;100:654–662. doi: 10.1111/j.1349-7006.2009.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]