The involvement of megestrol acetate (MA), a progesterone analog, in the stimulation of adipose-derived stem cells (ASCs) was investigated and the target receptors underlying stimulation were identified. The adipogenic differentiation potential of MA was inferior to dexamethasone, but MA had mitogenic effects in ASCs, indicating that MA increases the proliferation, migration, and adipogenic differentiation of ASCs via glucocorticoid receptor phosphorylation.
Keywords: Adipose-derived stem cells, Glucocorticoid receptor, Megestrol acetate, Proliferation, Adipogenic differentiation
Abstract
Because adipose-derived stem cells (ASCs) are usually expanded to acquire large numbers of cells for therapeutic applications, it is important to increase the production yield and regenerative potential during expansion. Therefore, a tremendous need exists for alternative ASC stimuli during cultivation to increase the proliferation and adipogenic differentiation of ASCs. The present study primarily investigated the involvement of megestrol acetate (MA), a progesterone analog, in the stimulation of ASCs, and identifies the target receptors underlying stimulation. Mitogenic and adipogenic effects of MA were investigated in vitro, and pharmacological inhibition and small interfering (si) RNA techniques were used to identify the molecular mechanisms involved in the MA-induced stimulation of ASCs. MA significantly increased the proliferation, migration, and adipogenic differentiation of ASCs in a dose-dependent manner. Glucocorticoid receptor (GR) is highly expressed compared with other nuclear receptors in ASCs, and this receptor is phosphorylated after MA treatment. MA also upregulated genes downstream of GR in ASCs, including ANGPTL4, DUSP1, ERRF11, FKBP5, GLUL, and TSC22D3. RU486, a pharmacological inhibitor of GR, and transfection of siGR significantly attenuated MA-induced proliferation, migration, and adipogenic differentiation of ASCs. Although the adipogenic differentiation potential of MA was inferior to that of dexamethasone, MA had mitogenic effects in ASCs. Collectively, these results indicate that MA increases the proliferation, migration, and adipogenic differentiation of ASCs via GR phosphorylation.
Significance
Magestrol acetate (MA) increases the proliferation, migration, and adipogenic differentiation of adipose-derived stem cells (ASCs) via glucocorticoid receptor phosphorylation. Therefore, MA can be applied to increase the production yield during expansion and can be used to facilitate adipogenic differentiation of ASCs.
Introduction
Adipose-derived stem cells (ASCs) are located in the perivascular adipose tissue and can be enriched from culture of the stromal vascular fraction (SVF) [1–4]. ASCs show promise for tissue repair and regeneration because of their differentiation potential and paracrine effects [5–7]. Although an unexpanded SVF has been clinically applied, ASCs are usually expanded to acquire large numbers of cells for therapeutic applications. Pharmacological stimuli such as sphingosylphosphocholine, lysophosphatidic acid, and vitamin C have been used to increase the production yield and regenerative potential of ASCs [8–10]. However, transplanted ASCs encounter harmful environments, which mitigate their building-block function and survival. Treating ASCs with exogenous stimuli before transplantation to enhance their survival and differentiation might overcome this limitation [11, 12]. For example, hypoxia increases proliferation, migration, and adipogenic differentiation of ASCs in vitro, and hypoxia preconditioning enhanced the regenerative potential of ASCs in vivo [13–15]. However, a tremendous need exists for alternative ASC stimuli during cultivation to increase the survival, proliferation, and adipogenic differentiation of ASCs. We propose a progesterone analog as a promising alternative for ASC stimulus.
Because progesterone and estrogen are critical for the growth and proliferation of breast cells during normal development and in the progression of breast cancer [16–18], we hypothesized that these hormones might stimulate the proliferation and differentiation of ASCs. However, estrogen derivatives did not affect ASC proliferation in a preliminary study. Of interest, progesterone slightly increased these functions in a preliminary study, and megestrol acetate (MA), a progesterone derivative, has strong stimulatory effects on ASCs. In previous studies, progesterone increased the proliferation of mammary gland and pancreatic cells [19, 20]. In addition, progesterone stimulated the proliferation of steroid receptor-positive breast cancer and increased the migration of breast cancer cells [21, 22]. Progesterone also enhanced the immunoregulatory activity of mesenchymal stem cells [23]. However, the effect of progesterone derivatives on the proliferation, migration, and adipogenic differentiation of ASCs is unclear.
MA is a synthetic progestogen with progestogenic effects similar to those of progesterone. High-dose MA is often used to boost the appetite and induces weight gain in patients with cancer or HIV/AIDS-associated cachexia [24]. It is also used as a contraceptive with an estrogen at relatively low doses. MA acts predominantly as a potent agonist of the progesterone receptor (PR) [25]. In addition, MA is a high-affinity, weak, partial agonist/antagonist of the androgen receptor (AR), where it binds with similar affinity to the PR [26]. MA is also an agonist of the glucocorticoid receptor (GR), with similar but less affinity than the PR and AR [25, 27]. MA mediates its pharmacological effects via these nuclear receptors; however, its functional role and related receptors in ASCs have not been identified. Therefore, the present study primarily investigated the stimulatory effects of MA in the proliferation, migration, and adipogenic differentiation of ASCs and identified the target receptors mediating the stimulation of these functions.
Materials and Methods
Cell Culture
Human subcutaneous adipose tissue samples were acquired from elective liposuction of two healthy women, and informed consent was received and approved by the institutional review board [2]. The obtained samples were digested with 0.075% collagenase type II (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) under gentle agitation for 45 minutes at 37°C and then centrifuged at 300g for 10 minutes to obtain the stromal cell fraction. The pellet was filtered with a 70-μm nylon mesh filter and resuspended in phosphate-buffered saline (PBS). The cell suspension was layered onto histopaque-1077 (Sigma-Aldrich) and centrifuged at 840g for 10 minutes. The supernatant was discarded, and the cell band that was buoyant over the histopaque was collected. The retrieved cell fraction was cultured overnight at 37°C in 5% CO2 in control medium (Dulbecco’s modified Eagle medium, 10% fetal bovine serum [FBS], and 100 U/ml penicillin). The ASCs were characterized by transdifferentiation and analysis of cell surface markers using flow cytometry, as described previously [2]. The ASCs were grown in minimum essential medium-α (HyClone, Thermo Scientific, Logan, UT, http://www.thermoscientific.com) with 10% FBS (Gibco, Invitrogen, Carlsbad, CA, http://www.invitrogen.com) and 1% penicillin and streptomycin (Gibco, Invitrogen) at 37°C in humidified air plus 5% CO2 and balanced nitrogen.
Cell Proliferation Assay
ASCs were seeded in a 48-well plate at a density of 5 × 103 cells per well. After 24 hours, the medium was replaced with medium containing 0.2% FBS. The next day, the cells were treated with MA (1, 5, 10, and 50 μM) for 48 hours. The medium was then removed, and cell numbers were counted using the Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies Inc., Rockville, MD, http://www.dojindo.com) [10]. The cells were treated with 10% CCK-8 solution in the medium for 4 hours, and the absorbance was measured at 450 nm using a microplate reader (Tecan, Grödig, Austria, http://www.tecan.com). To inhibit PR and GR, the cells were incubated with 10 μM RU486 (Sigma-Aldrich).
Cell Migration Assay
ASCs were seeded on the upper chamber of Transwell membrane plates (Corning Inc., Corning, NY, http://www.corning.com) at a density of 1.5 × 104 cells per well. After 2 hours, MA (10 μM) or RU486 (10 μM) in α-MEM containing 0.2% FBS was added on the lower side of the Transwell membrane plates for 16–20 hours. The migrated cells remaining in the Transwell membrane were fixed with ice-cold methanol for 20 minutes. Fixed cells were stained using 10% crystal violet (Sigma-Aldrich) for 30 minutes at room temperature. The remaining cells in the upper chamber were removed with cotton swabs, and the cells on the lower side of the membrane were counted using light microscopy [28]. We then counted the number of migrated cells in a 6.5-mm (diameter) Transwell with an 8.0-μm pore polycarbonate membrane.
Adipocyte Differentiation
ASCs (4 × 104) were seeded in 12-well plates in complete medium. When they became confluent, the medium was replaced with adipogenic induction medium (AIM). This medium contained α-MEM, 10% FBS, 1% penicillin and streptomycin, 10 μg/ml insulin, 500 μM isobutyl-1-methylxanthine, and 50 μM indomethacin. In addition, dexamethasone (1 μM) was used and compared with MA as an inducer of adipogenic differentiation. After the medium was changed to AIM, the cells were cultured for 2 weeks with MA. Oil Red O staining was used to assess the lipid accumulation in ASCs [29].
Oil Red O Staining
ASCs were washed with PBS and fixed with 10% formalin for 1 hour. After fixing, the cells were washed with PBS and stained with Oil Red O solution for 2 hours. The stained cells were washed twice with distilled water and examined microscopically. For quantification of Oil Red O, the cells were incubated with isopropanol for 30 minutes, and the color in the supernatant of the decolorized cells was measured by absorbance at 492 nm using a microplate reader (Tecan) [29].
Small Interfering RNA Transfection
Mixtures of GR small interfering RNA (siRNA) (50 nM) were prepared with Lipofectamine 2000 (Invitrogen) before the cell trypsinization step. Next, the cells were seeded on 48-well plates or 60-mm dishes, and the siRNA mixtures were transfected into the cells for 24 hours [10]. Silencing was evaluated using quantitative real-time polymerase chain reaction (qPCR).
RNA Isolation and Quantitative PCR
Total cellular RNA was extracted using an RNA preparation kit (RNeasy; Qiagen, Hilden, Germany, http://www.qiagen.com), and isolated mRNA was reverse transcribed with a cDNA synthesis kit (A2500; Promega, Madison, WI, http://promega.com). cDNA was synthesized from 500 ng of total RNA using 1,000 U of reverse transcriptase and 50 ng/ml oligo(dT) primers. Thermal cycling for 35 cycles consisted of an initial denaturation at 95°C for 5 minutes, 95°C for 30 seconds, 56°C for 20 seconds, and 72°C for 40 seconds, terminated by a final extension at 72°C for 5 minutes. qPCRs were performed using a Step One Plus Real-Time polymerase chain reaction (PCR) system (Applied Biosystems, Invitrogen) using an SYBR Green PCR Master Mix (Takara Bio, Inc., Otsu, Japan, http://www.takara-bio.com). Primers used are listed in supplemental online Table 1. The glyceraldehyde-3-phosphate dehydrogenase mRNA level was used for sample standardization. Analysis of the fold change was calculated by the ΔCt value.
Polymerase Chain Reaction Array
For analysis with the RT2 Profiler PCR array (Human Glucocorticoid Signaling: PAHS-154ZA; SABiosciences, Qiagen), the cells were seeded on 60-mm dishes at a density of 2.5 × 105 cells in 0.2% FBS in α-MEM. Total RNA was harvested using an RNA preparation kit (Qiagen), and cDNA was synthesized using reverse transcriptase. Gene expression was detected using a PCR array kit according to the manufacturer’s instructions (Qiagen) [28].
Statistical Analysis
Data are representative of triplicate independent experiments. The statistical significance of differences among the groups was tested using the Student t test; p < .05 or p < .01 was considered significant.
Results
Progesterone and MA Increased the Proliferation and Migration of ASCs
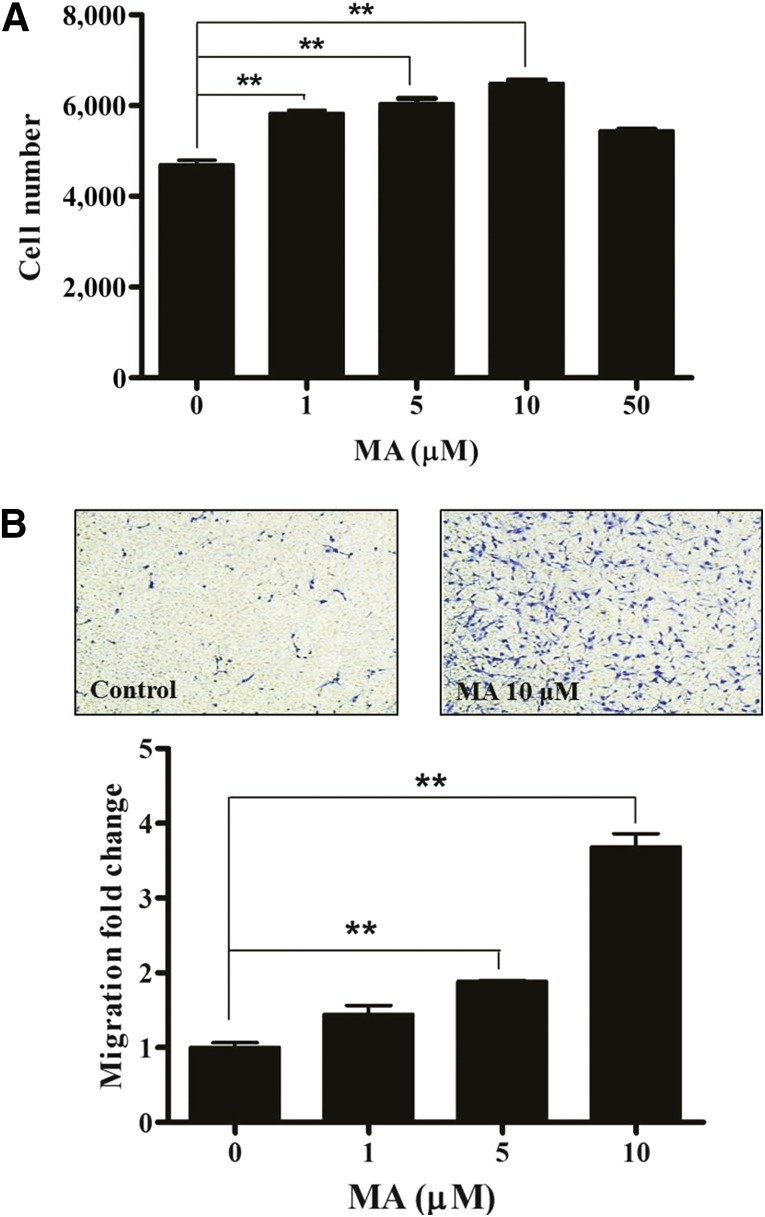
Progesterone slightly increased the proliferation (supplemental online Fig. 1A) and migration (supplemental online Fig. 1B) of ASCs. In contrast, MA, a synthetic derivative, significantly increased the proliferation (Fig. 1A, p < .01, n = 3) and migration (Fig. 1B, p < .01, n = 3) of ASCs in a dose-dependent manner. However, MA showed adverse effects at high concentrations (>50 mM).
Figure 1.
MA increased the proliferation and migration of adipose-derived stem cells (ASCs). MA significantly increased the proliferation (A) and migration (B) of ASCs. However, MA showed adverse effects at a high concentration (>50 mM). ∗∗, p < .01. Abbreviation: MA, megestrol acetate.
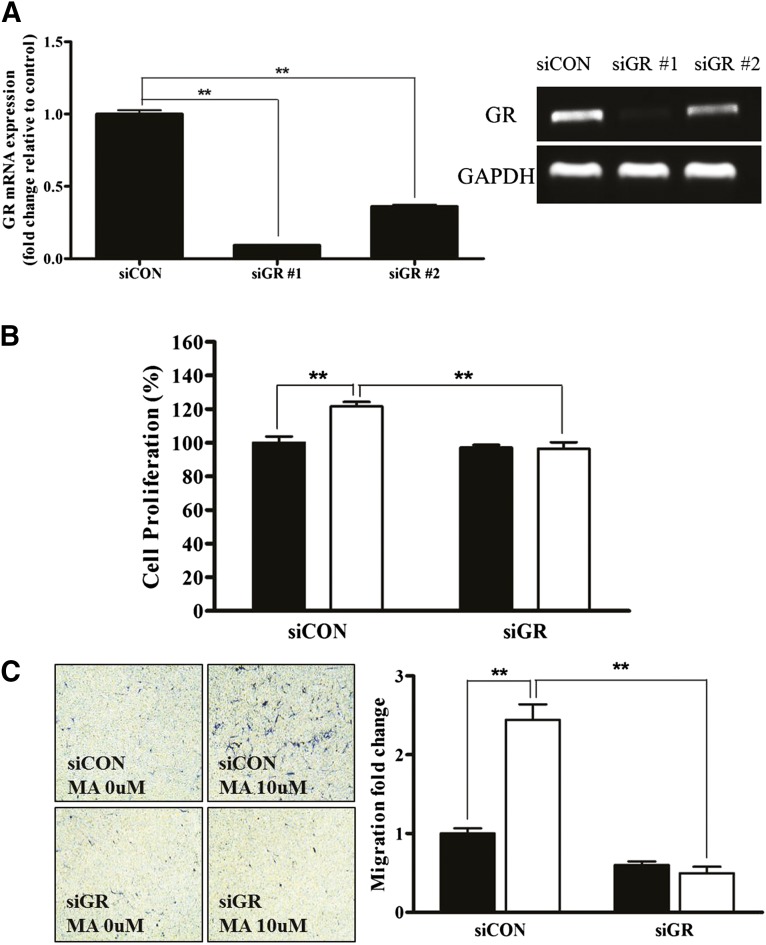
GR Is Highly Expressed and Phosphorylated in ASCs
Because MA acts as an agonist of diverse nuclear receptors (NRs), we measured the mRNA expression of NRs in ASCs with or without MA (10 μM) and progesterone (10 μM) treatment. Unexpectedly, PR was not expressed in ASCs (Fig. 2A). The mineralocorticoid receptor and AR were expressed at low levels, but GR was expressed at high levels (Fig. 2A; supplemental online Table 2). However, MA or progesterone treatment did not alter the expression of these receptors (supplemental online Table 2). In addition, MA (10 μM) and dexamethasone (1 μM, a positive control for GR phosphorylation) significantly increased the phosphorylation of GR in ASCs (Fig. 2B). Phosphorylated GR translocated to the nucleus after MA and dexamethasone treatment (Fig. 2C, green, phosphorylated GR; blue, 4′,6-diamidino-2-phenylindole). Collectively, these results suggest that MA might act as a GR agonist and stimulate ASCs via the GR.
Figure 2.
GR is highly expressed and phosphorylated in adipose-derived stem cells (ASCs). (A): We measured the mRNA expression of nuclear receptors in ASCs with or without MA (10 μM) and progesterone (10 μM) treatment. The progesterone receptor is not expressed in ASCs. The mineralocorticoid receptor and androgen receptor are expressed at low levels. However, the GR is expressed at high levels. (B): On Western blotting, MA significantly increased the phosphorylation of the GR in ASCs. Dex (1 μM) was used as a positive control for GR phosphorylation. (C): Phosphorylated GR translocation to the nucleus after MA and Dex treatment (green, phosphorylated GR; blue, DAPI). Scale bar = 20 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; Dex, dexamethasone; GR, glucocorticoid receptor; p-GR, phosphorylated GR; MA, megestrol acetate.
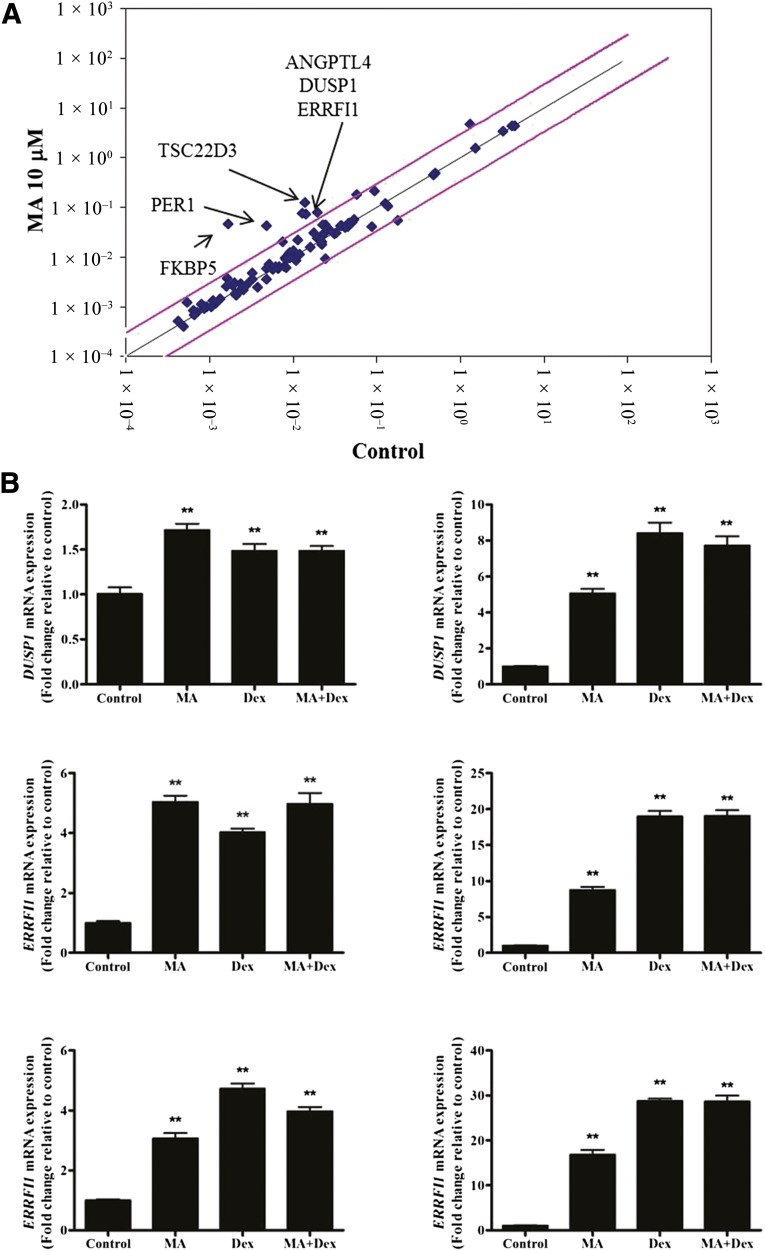
MA Upregulated the Downstream Genes of GR
We examined the effect of MA treatment on GR-related genes using an RT2 Profiler PCR array. Expression of angiopoietin-like 4 (ANGPTL4), dual specificity phosphatase 1 (DUSP1), ERbB receptor feedback inhibitor 1 (ERRF11), FK506 binding protein 5 (FKBP5), glutamate-ammonia ligase (GLUL), and TSC22 domain family, number 3 (TSC22D3) was upregulated in ASCs by MA (10 μM) treatment (>2.5-fold increase, n = 1). In addition, the expression of these genes was significantly upregulated by MA (10 μM), dexamethasone (1 μM), or MA plus dexamethasone in qPCR analysis (Fig. 3B, p < .01, n = 3).
Figure 3.
MA upregulated the downstream genes of GR. (A): Using an RT2 Profiler PCR array, we determined the effects of MA treatment on GR-related gene expression. The expression of ANGPTL4, DUSP1, ERRF11, FKBP5, GLUL, and TSC22D3 was upregulated (>2.5-fold increase) by GR (10 μM) treatment in ASCs. (B): Expression of these genes was confirmed by quantitative real-time polymerase chain reaction and was significantly upregulated after MA, Dex, or MA plus Dex treatment. ∗∗, p < .01. Abbreviations: Dex, dexamethasone; GR, glucocorticoid receptor; MA, megestrol acetate.
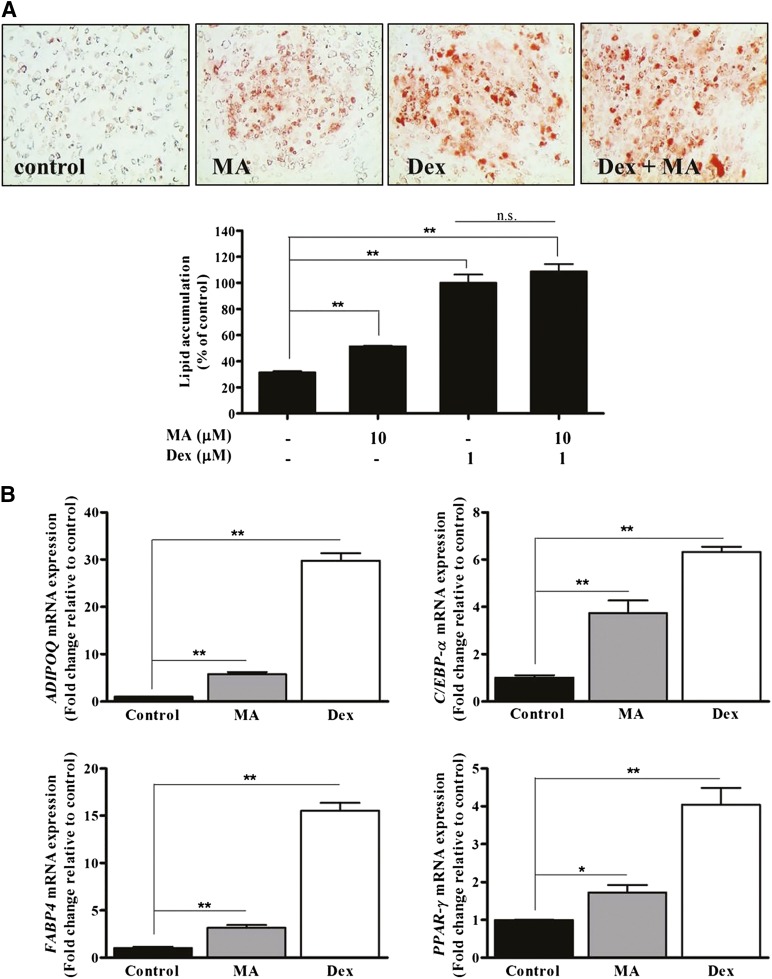
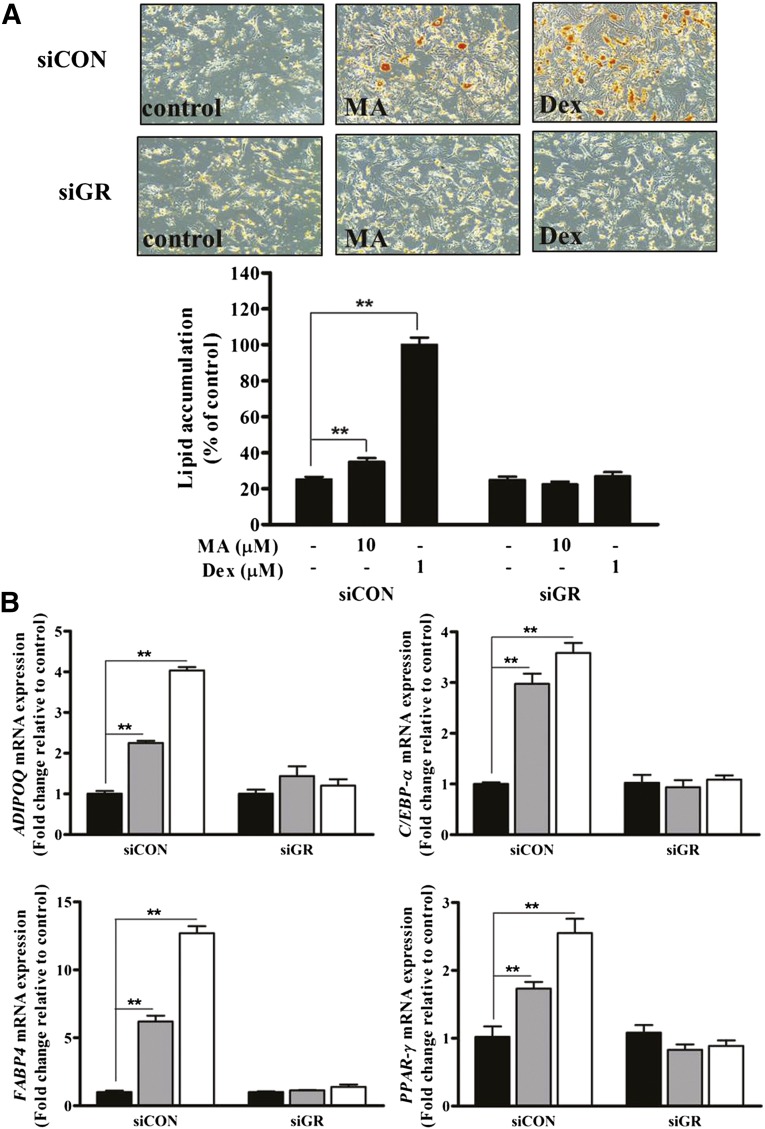
MA Enhanced the Adipogenic Differentiation of ASCs
Because MA might mediate its function via GR, we examined whether MA enhanced the adipogenic differentiation of ASCs. First, we added MA to the commercially available adipogenic differentiation medium (i.e., PDM-2 medium; Lonza, Walkersville, MD, http://www.lonza.com) containing dexamethasone, but it did not induce adipogenic differentiation of ASCs (data not shown). However, MA significantly increased Oil Red O staining and lipid accumulation in adipogenic induction medium that did not contain dexamethasone (composition is described in Materials and Methods); however, its adipogenic effect was inferior to that of dexamethasone (Fig. 4A). MA and dexamethasone significantly upregulated adipogenic marker genes such as ADIPOQ, C/EBP-α, FABP4, and PPAR-γ (Fig. 4B, p < .05, n = 3) in qPCR.
Figure 4.
MA enhanced the adipogenic differentiation of adipose-derived stem cells (ASCs). (A): MA significantly increased Oil Red O staining and lipid accumulation in adipogenic induction medium that did not contain Dex, but the adipogenic effect was inferior to Dex. (B): MA and Dex significantly upregulated adipogenic marker genes such as ADIPOQ, C/EBP-α, FABP4, and PPAR-γ in differentiation medium. ∗, p < .05; ∗∗, p < .01. Abbreviations: Dex, dexamethasone; MA, megestrol acetate; n.s., no significant difference.
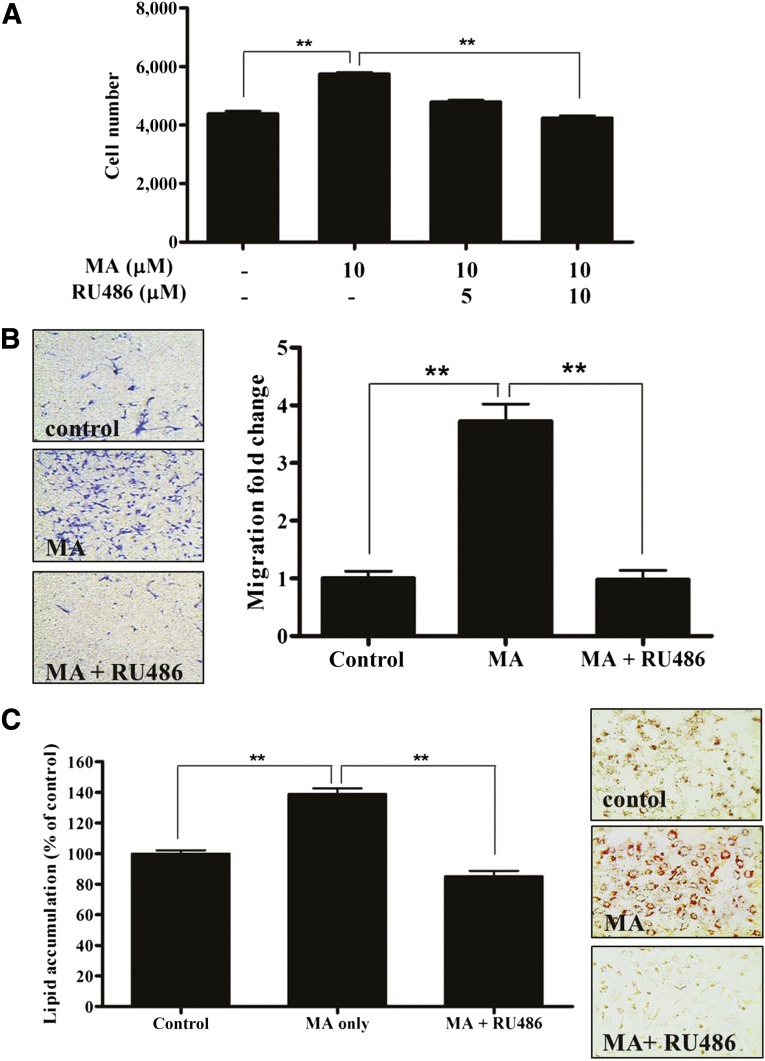
Pharmacological Inhibition by RU486 Attenuated MA-Induced Stimulation
We further examined whether GR mediated the proliferation, migration, and adipogenic differentiation of ASCs. The pharmacological GR inhibitor, RU486 (5 or 10 μM), significantly reduced MA-induced proliferation (Fig. 5A, p < .01, n = 3). In addition, RU486 (10 μM) significantly attenuated MA-induced migration (Fig. 5B, p < .01, n = 3) and adipogenic differentiation (Fig. 5C, p < .01, n = 3). RU486 also inhibited the dexamethasone-induced adipogenic differentiation of ASCs (supplemental online Fig. 3).
Figure 5.
Pharmacological inhibition by RU486 attenuated MA-induced stimulation. We examined whether glucocorticoid receptor (GR) mediated the proliferation, migration, and adipogenic differentiation of adipose-derived stem cells (ASCs) using the pharmacological GR inhibitor, RU486. As expected, RU486 significantly reduced MA-induced proliferation (A), migration (B), and adipogenic differentiation (C) of ASCs. ∗∗, p < .01. Abbreviation: MA, megestrol acetate.
GR Silencing Attenuated MA-Induced Stimulation
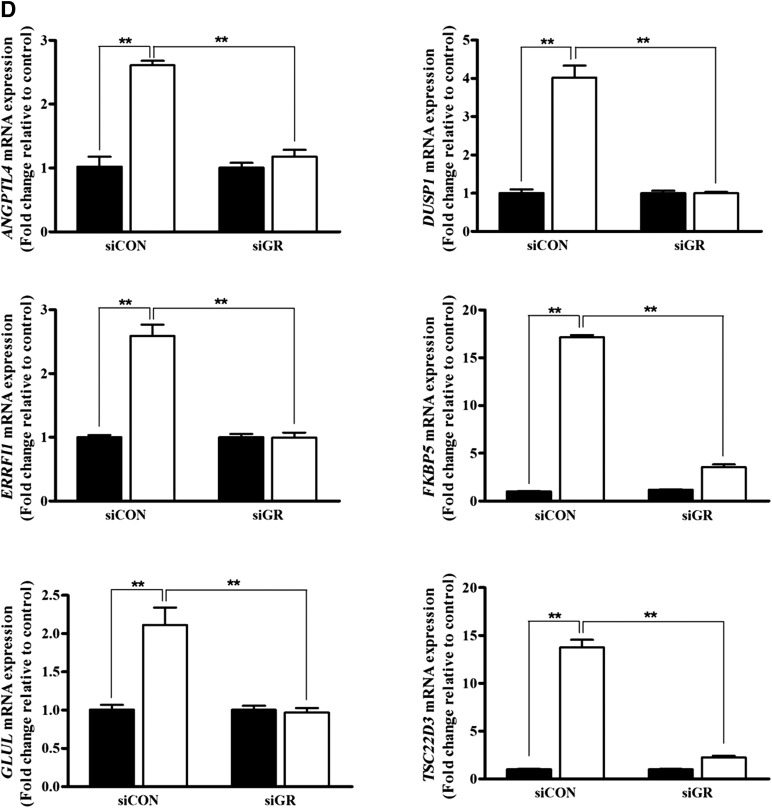
To measure the direct involvement of GR in MA-induced ASC stimulation, siRNA was used to downregulate GR expression. Transfection of siGR significantly downregulated the mRNA expression of GR (Fig. 6A, p < .01, n = 3), and siGR#1 was used throughout our study. siGR significantly attenuated the MA-induced proliferation (Fig. 6B, p < .01, n = 3) and migration (Fig. 6C, p < .01, n = 3) of ASCs. In addition, siGR significantly downregulated the MA-induced mRNA expression of ANGPTL4, DUSP1, ERRF11, FKBP5, GLUL, and TSC22D3 in ASCs (Fig. 6D, p < .01, n = 3).
Figure 6.
GR silencing attenuated the MA-induced proliferation and migration. We used small interfering RNA to downregulate GR expression to measure the direct stimulatory effect of MA on GR. (A): Transfection of siGR significantly downregulated the mRNA expression of GR. siGR significantly attenuated MA-induced proliferation (B) and migration (C) of adipose-derived stem cells (black bars, control; white bars, MA treatment). (D): In addition, siGR significantly downregulated MA-induced mRNA expression of ANGPTL4, DUSP1, ERRF11, FKBP5, GLUL, and TSC22D3 in quantitative real-time polymerase chain reaction (black bars, control; white bars, MA treatment). ∗∗, p < .01. Abbreviations: GR, glucocorticoid receptor; MA, megestrol acetate; siCON, small interfering control; siGR, small interfering GR.
In addition, siGR significantly attenuated the MA- and dexamethasone-induced Oil Red O staining and lipid accumulation that occurs in adipogenic induction medium (Fig. 7A, p < .01, n = 3). In addition, qPCR showed that siGR significantly downregulated MA- and dexamethasone-induced adipogenic marker genes, such as ADIPOQ, C/EBP-α, FABP4, and PPAR-γ (Fig. 7B, p < .01, n = 3). These results collectively indicate that MA increases the proliferation, migration, and adipogenic differentiation of ASCs via GR.
Figure 7.
GR silencing attenuated MA-induced adipogenic differentiation. (A): siGR significantly attenuated MA- and Dex-induced Oil Red O staining and lipid accumulation in adipogenic induction medium. (B): In addition, siGR significantly downregulated MA- and Dex-induced adipogenic marker genes such as ADIPOQ, C/EBP-β, FABP4, and PPAR-γ in quantitative real-time polymerase chain reaction (black bars, control; gray bars, MA treatment; white bars, Dex treatment). ∗∗, p < .01. Abbreviations: GR, glucocorticoid receptor; MA, megestrol acetate; siCON, small interfering control; siGR, small interfering GR.
Discussion
The present study investigated the effects of MA on the proliferation, migration, and adipogenic differentiation of ASCs and identified the target receptors involved in these functions. MA significantly increased the proliferation, migration, and adipogenic differentiation of ASCs in a dose-dependent manner. GR expression is increased compared with other NRs in ASCs and is phosphorylated after MA treatment. MA upregulates the downstream genes associated with GR, such as ANGPTL4, DUSP1, ERRF11, FKBP5, GLUL, and TSC22D3 in ASCs. Pharmacological inhibition and siRNA techniques were used to identify the molecular mechanisms involved in MA-induced proliferation, migration, and adipogenic differentiation of ASCs. As expected, the pharmacological inhibitor RU486 and transfection of siGR significantly attenuated MA-induced proliferation, migration, and adipogenic differentiation.
MA induces adipogenic differentiation via GR. Therefore, we compared the adipogenic differentiation potential of MA and dexamethasone (Fig. 4). Compared with MA treatment (10 μM), dexamethasone (1 μM) showed more efficient adipogenic differentiation based on Oil Red O staining and lipid accumulation. In addition, dexamethasone resulted in higher expression of adipogenic differentiation markers than MA (Fig. 4B). Therefore, MA treatment did not enhance adipogenic differentiation in PDM-2 medium, which contains dexamethasone (data not shown). However, MA significantly induced adipogenic differentiation in adipogenic differentiation medium without dexamethasone (Fig. 4A, 4B). It is of interest that dexamethasone (<20 μM) treatment did not alter the proliferation of ASCs, but MA did (supplemental online Fig. 2). Although we could not identify the different regulatory mechanisms between MA and dexamethasone and they share the same receptor in ASCs, MA appears to affect ASC proliferation with slight adipogenic differentiation potential and dexamethasone to strongly influence adipogenic differentiation.
In a pharmacological inhibition study, RU486 significantly attenuated MA-induced proliferation, migration, and adipogenic differentiation of ASCs. RU486 also inhibited the dexamethasone-induced adipogenic differentiation of ASCs (supplemental online Fig. 3). RU486 is reportedly a PR antagonist used in small doses as an emergency contraceptive [30, 31]. It is also a powerful GR antagonist and has occasionally been used in refractory Cushing syndrome [30]. Therefore, RU486 can act as a dual antagonist of PR and GR. Because ASCs do not express PR but have high GR, RU486 might have acted as a GR antagonist in the present study. Therefore, functional inhibition of MA by RU486 suggests GR involvement in ASC stimulation.
Although GR is well-known for its involvement in adipogenic differentiation, it also plays a role in osteogenic and chondrogenic differentiation. For example, osteogenic differentiation of mesenchymal stem cells (MSCs) was significantly decreased by glucocorticoids [32]. In contrast, glucocorticoids promote chondrogenic differentiation of adult human MSCs by enhancing expression of cartilage extracellular matrix genes [33]. Although we did not investigate these functions of MA in the present study, MA might have additional effects on the osteogenic and chondrogenic differentiation of ASCs.
A tremendous need exists for alternative ASC stimuli during cultivation to increase the proliferation or adipogenic differentiation of ASCs. We have previously demonstrated that hypoxia significantly increases the proliferation of ASCs via NADPH oxidase 4-generated reactive oxygen species (ROS) [34, 35]. Hypoxia also accelerated the adipogenic differentiation of ASCs via mitochondrial ROS generation [29]. In addition, vitamin C significantly increased ASC proliferation through the mitogen-activated protein kinase pathway and enhanced the hair-growth promoting effect of ASCs [10]. Furthermore, platelet-derived growth factor (PDGF)-B and -D increased the proliferation of ASCs via ROS generation, and preconditioning by PDGF-D significantly enhanced the hair-growth promoting effect of ASCs [28, 36]. Although we did not examine the preconditioning effect of MA on ASCs in vivo, MA can be used as a promising ASC preconditioning agent because MA has mitogenic and differentiating effects in ASCs.
Conclusion
We investigated the stimulation of ASCs by MA and identified the target receptors involved in the stimulation of ASCs. MA significantly increased the proliferation, migration, and adipogenic differentiation of ASCs via GR phosphorylation. Therefore, MA can be applied to increase the production yield and regenerative potential during expansion. Although the adipogenic differentiation potential of MA is inferior to that of dexamethasone, MA can be used to facilitate adipogenic differentiation of ASCs.
Supplementary Material
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grants 2013R1A1A2061722 and 2014054836) and Yonsei University (Grant 2014-12-0040).
Author Contributions
J.-H.S.: conception and design, financial support, data analysis and interpretation, manuscript writing; H.-S.A.: collection and/or assembly of data, data analysis, manuscript writing; J.-H.J.: provision of study material; S.S.: data interpretation; S.Y.S.: conception and design, financial support, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Kim WS, Han J, Hwang SJ, et al. An update on niche composition, signaling and functional regulation of the adipose-derived stem cells. Expert Opin Biol Ther. 2014;14:1091–1102. doi: 10.1517/14712598.2014.907785. [DOI] [PubMed] [Google Scholar]
- 2.Yi T, Kim WK, Choi JS, et al. Isolation of adipose-derived stem cells by using a subfractionation culturing method. Expert Opin Biol Ther. 2014;14:1551–1560. doi: 10.1517/14712598.2014.943661. [DOI] [PubMed] [Google Scholar]
- 3.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 5.Kim WS, Park BS, Sung JH, et al. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Song SY, Chung HM, Sung JH. The pivotal role of VEGF in adipose-derived-stem-cell-mediated regeneration. Expert Opin Biol Ther. 2010;10:1529–1537. doi: 10.1517/14712598.2010.522987. [DOI] [PubMed] [Google Scholar]
- 7.Yang JA, Chung HM, Won CH, et al. Potential application of adipose-derived stem cells and their secretory factors to skin: Discussion from both clinical and industrial viewpoints. Expert Opin Biol Ther. 2010;10:495–503. doi: 10.1517/14712591003610598. [DOI] [PubMed] [Google Scholar]
- 8.Jeon ES, Song HY, Kim MR, et al. Sphingosylphosphorylcholine induces proliferation of human adipose tissue-derived mesenchymal stem cells via activation of JNK. J Lipid Res. 2006;47:653–664. doi: 10.1194/jlr.M500508-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Jeon ES, Moon HJ, Lee MJ, et al. Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblast-like cells. Stem Cells. 2008;26:789–797. doi: 10.1634/stemcells.2007-0742. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Kim WK, Sung YK, et al. The molecular mechanism underlying the proliferating and preconditioning effect of vitamin C on adipose-derived stem cells. Stem Cells Dev. 2014;23:1364–1376. doi: 10.1089/scd.2013.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rota C, Imberti B, Pozzobon M, et al. Human amniotic fluid stem cell preconditioning improves their regenerative potential. Stem Cells Dev. 2012;21:1911–1923. doi: 10.1089/scd.2011.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis KR, Wei L. Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010;1:e22. doi: 10.1038/cddis.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee EY, Xia Y, Kim WS, et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: Increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540–547. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 14.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 15.Thangarajah H, Vial IN, Chang E, et al. IFATS collection: Adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells. 2009;27:266–274. doi: 10.1634/stemcells.2008-0276. [DOI] [PubMed] [Google Scholar]
- 16.Strong AL, Ohlstein JF, Jiang Q, et al. Novel daidzein analogs enhance osteogenic activity of bone marrow-derived mesenchymal stem cells and adipose-derived stromal/stem cells through estrogen receptor dependent and independent mechanisms. Stem Cell Res Ther. 2014;5:105. doi: 10.1186/scrt493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calis M, Demirtas TT, Atilla P, et al. Estrogen as a novel agent for induction of adipose-derived mesenchymal stem cells for osteogenic differentiation: In vivo bone tissue-engineering study. Plast Reconstr Surg. 2014;133:499e–510e. doi: 10.1097/PRS.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 18.Ihunnah CA, Wada T, Philips BJ, et al. Estrogen sulfotransferase/SULT1E1 promotes human adipogenesis. Mol Cell Biol. 2014;34:1682–1694. doi: 10.1128/MCB.01147-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beleut M, Rajaram RD, Caikovski M, et al. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci USA. 2010;107:2989–2994. doi: 10.1073/pnas.0915148107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieuwenhuizen AG, Schuiling GA, Liem SM, et al. Progesterone stimulates pancreatic cell proliferation in vivo. Eur J Endocrinol. 1999;140:256–263. doi: 10.1530/eje.0.1400256. [DOI] [PubMed] [Google Scholar]
- 21.Kariagina A, Xie J, Langohr IM, et al. Progesterone stimulates proliferation and promotes cytoplasmic localization of the cell cycle inhibitor p27 in steroid receptor positive breast cancers. Horm Cancer. 2013;4:381–390. doi: 10.1007/s12672-013-0159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz J, Aranda E, Henriquez S, et al. Progesterone promotes focal adhesion formation and migration in breast cancer cells through induction of protease-activated receptor-1. J Endocrinol. 2012;214:165–175. doi: 10.1530/JOE-11-0310. [DOI] [PubMed] [Google Scholar]
- 23.Zhao X, Liu L, Liu D, et al. Progesterone enhances immunoregulatory activity of human mesenchymal stem cells via PGE2 and IL-6. Am J Reprod Immunol. 2012;68:290–300. doi: 10.1111/j.1600-0897.2012.01163.x. [DOI] [PubMed] [Google Scholar]
- 24.Rance LB, Tseng AL, Bayoumi A. Megestrol for appetite stimulation and as a progestin in hormone replacement therapy. Arch Intern Med. 1996;156:2498. [PubMed] [Google Scholar]
- 25.Teulings FA, van Gilse HA, Henkelman MS, et al. Estrogen, androgen, glucocorticoid, and progesterone receptors in progestin-induced regression of human breast cancer. Cancer Res. 1980;40:2557–2561. [PubMed] [Google Scholar]
- 26.Luthy IA, Begin DJ, Labrie F. Androgenic activity of synthetic progestins and spironolactone in androgen-sensitive mouse mammary carcinoma (Shionogi) cells in culture. J Steroid Biochem. 1988;31:845–852. doi: 10.1016/0022-4731(88)90295-6. [DOI] [PubMed] [Google Scholar]
- 27.Poyet P, Labrie F. Comparison of the antiandrogenic/androgenic activities of flutamide, cyproterone acetate and megestrol acetate. Mol Cell Endocrinol. 1985;42:283–288. doi: 10.1016/0303-7207(85)90059-0. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Park SG, Kim WK, et al. Functional regulation of adipose-derived stem cells by PDGF-D. Stem Cells. 2015;33:542–556. doi: 10.1002/stem.1865. [DOI] [PubMed] [Google Scholar]
- 29.Kim JH, Kim SH, Song SY, et al. Hypoxia induces adipocyte differentiation of adipose-derived stem cells by triggering reactive oxygen species generation. Cell Biol Int. 2014;38:32–40. doi: 10.1002/cbin.10170. [DOI] [PubMed] [Google Scholar]
- 30.Tang OS, Ho PC. Clinical applications of mifepristone. Gynecol Endocrinol. 2006;22:655–659. doi: 10.1080/09513590601005946. [DOI] [PubMed] [Google Scholar]
- 31.Piaggio G, von Hertzen H, Heng Z, et al. Meta-analyses of randomized trials comparing different doses of mifepristone in emergency contraception. Contraception. 2003;68:447–452. doi: 10.1016/s0010-7824(03)00142-2. [DOI] [PubMed] [Google Scholar]
- 32.Wei N, Yu Y, Schmidt T, et al. Effects of glucocorticoid receptor antagonist, RU486, on the proliferative and differentiation capabilities of bone marrow mesenchymal stromal cells in ovariectomized rats. J Orthop Res. 2013;31:760–767. doi: 10.1002/jor.22298. [DOI] [PubMed] [Google Scholar]
- 33.Derfoul A, Perkins GL, Hall DJ, et al. Glucocorticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes. Stem Cells. 2006;24:1487–1495. doi: 10.1634/stemcells.2005-0415. [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, Park SH, Park SG, et al. The pivotal role of reactive oxygen species generation in the hypoxia-induced stimulation of adipose-derived stem cells. Stem Cells Dev. 2011;20:1753–1761. doi: 10.1089/scd.2010.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JH, Song SY, Park SG, et al. Primary involvement of NADPH oxidase 4 in hypoxia-induced generation of reactive oxygen species in adipose-derived stem cells. Stem Cells Dev. 2012;21:2212–2221. doi: 10.1089/scd.2011.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JH, Park SG, Song SY, et al. Reactive oxygen species-responsive miR-210 regulates proliferation and migration of adipose-derived stem cells via PTPN2. Cell Death Dis. 2013;4:e588. doi: 10.1038/cddis.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.