Abstract
Introduction:
It is unclear whether health-related quality of life (HRQoL) outcomes are superior in robot-assisted radical prostatectomy (RARP) compared to open prostatectomy (ORP).
Methods:
We retrospectively analyzed records from men who received ORP or RARP at our institution between January 2009 and December 2012. Patients completed a demographics questionnaire and the Patient-Oriented Prostate Utility Scale (PORPUS), a validated disease-specific HRQoL instrument prior to surgery and every 3 months up to 15 months after surgery.
Results:
In total, 974 men met the inclusion criteria (643 ORP and 331 RARP patients). At baseline, RARP patients were significantly younger (p < 0.001), had lower body mass index (BMI) (p < 0.001), lower preoperative prostate-specific antigen (PSA) (p < 0.001), fewer comorbidities (p < 0.004), and higher baseline PORPUS scores (p = 0.024). On follow-up, unadjusted PORPUS scores were significantly higher in the RARP group at each point. On multivariable analysis adjusting for age, ORP versus RARP procedure, Gleason score, BMI, first PSA, comorbidity, ethnicity, and baseline PORPUS scores, PORPUS score was higher for the RARP group at 3 months (p = 0.038) and 9 months (p = 0.037), but not at 6, 12, and 15 months (p = 0.014). No difference met pre-defined thresholds of clinical significant.
Conclusions:
Though unadjusted HRQoL outcomes appeared improved with RARP compared to ORP differences, adjusted differences were seen at only 2 of 5 postoperative time points, and did not meet pre-defined thresholds of clinical significance. Further randomized trials are needed to assess whether one treatment option provides consistently better HRQoL outcomes.
Introduction
Prostate cancer is the most common solid organ tumour in North American men, with an estimated 233 000 diagnoses expected in 2014 in the United States alone.1 Prostate-specific antigen (PSA) testing has facilitated increased early detection of locally confined tumours that are amenable to surgery. In an attempt to decrease the morbidity of open surgery, minimally invasive laparoscopic approaches were developed. Robot-assisted laparoscopic prostatectomy (RARP) has become a widely used and increasingly adopted approach. Multiple case series of RARP by experienced surgeons have suggested short-term benefits over historical controls of open radical prostatectomy (ORP) in terms of better visualization of the surgical field, lower perioperative complication rate, lower stricture rate, fewer transfusions, and shorter hospital stay.2 Indeed, many surgical proponents have unequivocally stated that RARP is the sole standard of care for localized prostate cancer; however, whether health-related quality of life (HRQoL) outcomes are superior to ORP remains unclear.3–5
Major determinants of HRQoL following prostate cancer treatment include long-term side effects of sexual and urinary dysfunction. Comparisons of continence between ORP and RARP are mixed, with demonstration of no significant difference at 3 months following surgery in some cases,2 faster return to continence with RARP in other cases (16 vs. 46 days),6 and no significant difference at the 1-year mark.7 International Prostate Symptom Scores (IPSS) have been demonstrably better at 1 and 3 months postoperatively with RARP.6 Further, sexual function may return more rapidly after RARP compared to ORP.8 However, limitations of previous studies are numerous and include non-randomized, uncontrolled, small, and unbalanced single surgeon case series.
To our knowledge, broader HRQoL outcomes between ORP and RARP have not yet been compared. However, general QoL measures, such as the Medical Outcomes Study SF-36, may not be sensitive to prostate-specific HRQoL outcomes. The Patient-Oriented Prostate Utility Scale (PORPUS) is a validated, sensitive, and specific tool consisting of 10 independent QoL domains that is aggregated as a psychometric score. The PORPUS is highly responsive to small changes not otherwise detected in general (non-prostate cancer-specific) tools.9
We sought to quantify global differences in prostate-specific HRQoL outcomes after RARP and ORP using the PORPUS and to compare our surgical outcomes in the 2 most-quoted treatment-specific domains, potency and voiding function, using the International Index of Erectile Function (IIEF)10 and IPSS, respectively.11 Our secondary objective was to measure baseline differences between the two cohorts to better understand the rationale for surgical selection between treatment groups.
Methods
Patients
After receiving institutional ethics review board approval (University Health Network IRB Study ID number: 13-6495), we retrospectively analyzed the records of men consecutively treated with RP at the University Health Network in Toronto, Canada between January 2009 and December 2012. Five experienced high volume, single academic centre-based surgeons performed the ORPs and 4 of those same 5 surgeons performed the RARPs. We conducted a sensitivity analysis for the 327 RARP operations (after excluding 4 operations with unknown surgeons) to account for physician learning curve for the RARP. We fitted a linear mixed effect model to investigate whether changes of PORPUS-P over time (3, 6, 9, 12, and 15 months) are significantly different between the two groups, one for RARPs that are the first 50 operations by a surgeon and the other for those after the 50th surgery. A surgeon specific effect (random effect) is included in the model to capture the possible correlations within the operations performed by a same surgeon. No statistical significances of difference in the change of PORPUS-P between the two groups are detected (p > 0.3) The first 50 cases for each surgeon were included in our subsequent analysis.
Men were administered HRQoL, IIEF and IPSS questionnaires at the baseline visit at our centre (preoperatively) and at each follow-up visit, which were manually entered alongside clinical and laboratory variables into our institutional Prostate Centre Database. Baseline demographic data included age, marital status, ethnicity, smoking, and alcohol consumption. Clinical data included body mass index (BMI), biopsy Gleason score, clinical stage, and modified Charlson comorbidity index score.12 Surgical data included type of surgery and nerve-sparing status.
Men were included in our study if they were treated with ORP or RARP. Men were excluded if they received neoadjuvant, adjuvant, or salvage treatments.
To ensure that any missing data was missing at random, we conducted a non-parametric paired test (Wilcoxon signed rank test) and determined that the difference in the percentage of missing between ORP and RARP groups was not significant over the whole time frame (p = 0.094). Therefore, the missing data were not significantly skewed to one or the other surgical approaches and it is safe to assume that the missing is at random.
Outcome measures
We measured HRQoL using the PORPUS, a 10-domain multi-attribute health classification system that measures a patient’s physical and emotional status (Appendix).4 Each domain contains 4 to 5 possible ordinal responses. Domain scores are weighted equally and normalized to provide a score from 0 to 100, where 100 indicates greatest and 0 the lowest HRQoL status (the PORPUS-P [psychometric]).9,13,14 The minimum clinically important difference for the PORPUS-P is 5 points.2 At each time point, patients also filled out the IIEF10 and the IPSS.15 The minimum clinically important difference for IIEF and IPSS were 4 and 6 points, respectively.16 However, the minimum clinically important difference for the IIEF varied according to erectile dysfunction severity (mild: 2; moderate: 5; severe: 7).16
Data analysis
Survey data were grouped based on time of collection after surgery: 3 ± 1.5 months, 6 ± 1.5 months, 9 ± 1.5 months, 12 ± 1.5 months and 15 ± 1.5 months.
Baseline demographic characteristics were described using mean (standard deviation), and median (minimum, maximum) for quantitative variables, and proportions for categorical variables. We compared differences in baseline characteristics between the open and robotic groups using independent two-sample (unpaired) t-tests for the quantitative variables and Pearson’s Chi-squared Test for the categorical data. We then conducted analyses to compare differences in the HRQoL outcomes at baseline and the follow-up times (3, 6, 9, 12 and 15 months), again using two-sample t-tests. Similar comparisons were also applied to the differences of changes of the HRQoL measure from the baseline at the various follow-up times between the open and robotic groups. We constructed univariate and multivariable linear regression models at 3, 6, 9, 12 and 15 months to examine possible predictors of the changes of HRQoL outcomes. We considered the following predictors: age, type of operation (open vs. robotic), nerve-sparing, Gleason score, BMI, first PSA, Charlson score (0 vs. ≥1), ethnicity (Caucasian vs. non-Caucasian), surgeon, and baseline PORPUS. For comparison purposes, we conducted univariate analysis for IIEF and IPSS scores. A p value of 0.05 was used to denote statistical significance. All statistical analyses were done using R version 2.15.1.
Results
Baseline characteristics
A total of 974 men met the inclusion criteria, 643 in the ORP cohort, and 331 in the RARP group. The RARP group was significantly younger, more ethnically diverse, with lower BMI, less comorbidity and lower preoperative PSA compared to the ORP group (all p < 0.05). No significant differences were detected in marital status, smoking, alcohol consumption, or clinical stage between groups. Nerve-sparing surgery was significantly more commonly performed in the RARP group compared to the ORP group (p < 0.001) (Table 1).
Table 1.
Baseline demographics of study patients
| Factors | Open | Robotic | p value | |
|---|---|---|---|---|
| Age, years | N | 643 | 331 | <0.001 |
| Mean (SD) | 61.49 (7.07) | 59.71 (7.03) | ||
| First elevated PSA | N | 432 | 195 | <0.001 |
| Mean (SD) | 7.58 (5.22) | 5.89 (3.28) | ||
| BMI | N | 597 | 299 | <0.001 |
| Mean (SD) | 28.6 (4.0) | 27.3 (3.6) | ||
| Non response rate | 7.2% | 9.7% | ||
| CCS* | 0 | 512 (80) | 289 (87) | 0.004 |
| ≥1 | 131 (20) | 42 (13) | ||
| Biopsy Gleason score N (%)** | Low/intermediate risk: Gleason 4, 5, 6, or 7 | 532 (88) | 310 (99) | <0.001 |
| High risk: Gleason 8, 9, 10 | 76 (12) | 4 (1) | ||
| Ethnicity*** | Caucasian: n(%) | 228 (75) | 98 (63) | 0.008 |
| Non-Caucasian: n(%) | 75 (25) | 58 (37) | ||
| PORPUS-P score | N | 437 | 210 | 0.024 |
| Mean (sd) | 82.59 (11.10) | 84.73(11.26) | ||
| Nerve-sparing status | None: n (%) | 155 (25) | 19 (7) | <0.001 |
| Unilateral: n (%) | 146 (23) | 45 (15) | ||
| Bilateral: n (%) | 326 (52) | 224 (78) |
Data are shown only for statistically significantly different characteristics. PORPUS-P: Patient-Oriented Prostate Utility Scale-Psychometric; PSA: prostate-specific antigen; CCS: Charlson comorbidity score; SD: standard deviation; RARP: robot-assisted radical prostatectomy; ORP: open radical prostatectomy.
All Charlson comorbidities are not captured in the PCDB. The following are captured: myocardial infarction, congestive heart failure, peripheral vascular disease, liver disease, diabetes mellitus, leukemia, lymphoma, AIDS, cerebrovascular Disease, dementia, pulmonary disease, rheumatologic disease, and peptic ulcer.
Missing biopsy Gleason scores: n = 35 ORP, n = 17 RARP.
Missing ethnicity: n = 340 ORP, n = 175 RARP.
PORPUS-P Scores
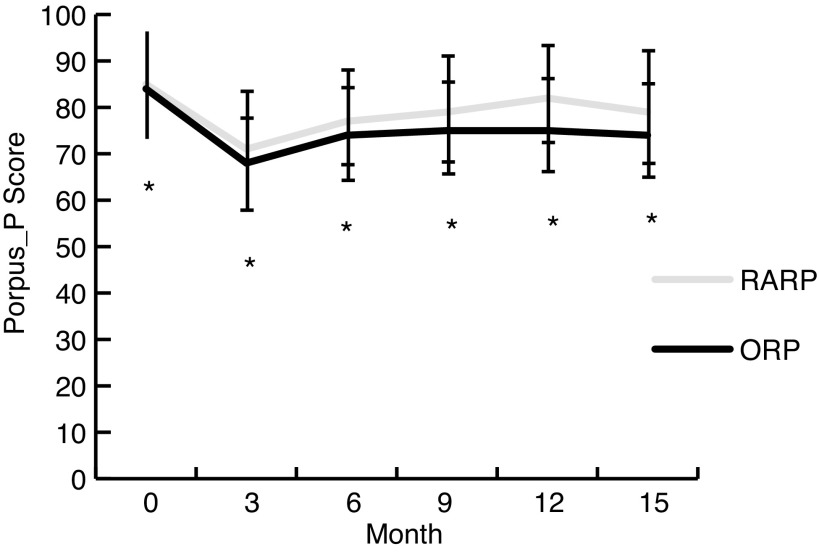
At baseline, unadjusted PORPUS-P scores were significantly higher in the RARP group compared to the ORP group (83 ± 11 ORP, 85 ± 11 RARP, p = 0.024). The RARP group had higher scores at every subsequent time point: 3 months (67 ± 12.5 ORP, 70 ± 13 RARP, p = 0.007), 6 months (74 ± 12 ORP, 77 ± 10, p ≤ 0.001), 9 months (75 ± 12 ORP, 79 ± 11 RARP, p < 0.001), 12 months (76 ±13 ORP, 82 ± 10 RARP, p < 0.001), and 15 months (75 ±13 ORP, 80 ±12 RARP, p = 0.014) (Fig. 1). However, none of the between-group differences were greater than 5 points at any time point, indicating no clinically significant difference between groups.17
Fig. 1.
Psychometric PORPUS-scores (mean and standard deviation) at each time point. Baseline questionnaires plotted at month 0. The response rate for the completed PORPUS-P scores at each time point was: baseline (n = 437 ORP, n = 210 RARP), 3 months (n = 399 ORP, n = 230 RARP), 6 months (n = 342 ORP, n = 208 RARP), 9 months (n = 236 ORP, n = 164 RARP), 12 months (n = 223 ORP, n = 133 RARP), 15 months (n = 101 ORP, n = 63 RARP). Differences between RARP and ORP groups were statistically but not clinically significant at each time point (statistical significance indicated by the asterisk in the figure). PORPUS-P: Patient-Oriented Prostate Utility Scale-Psychometric; RARP: robot-assisted radical prostatectomy; ORP: open radical prostatectomy.
Predictors of baseline HRQOL
On univariate analysis, increased age, higher Gleason score, higher BMI, and higher PSA level were associated with decreased PORPUS-P scores at baseline (preoperatively) (p < 0.05) (Table 2).
Table 2.
Univariate predictors of baseline (preoperative) PORPUS-P
| Variable | Effect | Confidence interval | p value |
|---|---|---|---|
| Age | −0.22 | (−0.34, −0.105) | <0.001 |
| Type of RP* | 2.14 | (0.30, 3.98) | 0.023 |
| Gleason score | −4.35 | (−7.33, −1.37) | 0.004 |
| BMI | −0.025 | (−0.26, 0.21) | 0.836 |
| First positive PSA | −0.24 | (−0.44, −0.04) | 0.017 |
Robot-assisted radical prostatectomy compared to open radical prostatectomy. RP: radical prostatectomy; BMI: body mass index; PSA: prostate-specific antigen; PORPUS-P: Patient-Oriented Prostate Utility Scale-Psychometric.
Predictors of HRQOL over time
On multivariate analysis, type of RP had a significant effect on PORPUS-P at the 3-month period after adjusting for other predictors (p = 0.038). The mean difference between RARP and ORP was 3.262 points. Type of RP also had a significant effect at the 9-month period after adjusting for other predictors (p = 0.037 mean difference 3.78 points) (Table 3). Type of RP did not have a significant effect at 6, 12, or 15 months. None of these differences met previously defined thresholds of clinical significance.
Table 3.
Univariate analysis of changes to PORPUS-P
| Month | Variable | Effect | p value |
|---|---|---|---|
| 3 | Type of RP | 2.864 | 0.017 |
| 6 | Type of RP | 2.723 | 0.012 |
| 9 | Type of RP | 3.425 | 0.011 |
| Age | −0.202 | 0.032 | |
| 12 | Type of RP | 3.616 | 0.009 |
| BMI | −0.435 | 0.015 | |
| 15 | First Detectable PSA | −0.388 | 0.04 |
Data are reported for significant variables only (p < 0.05). PORPUS-P: Patient-Oriented Prostate Utility Scale-Psychometric; RP: radical prostatectomy; BMI: body mass index; PSA: prostate-specific antigen.
IIEF scores
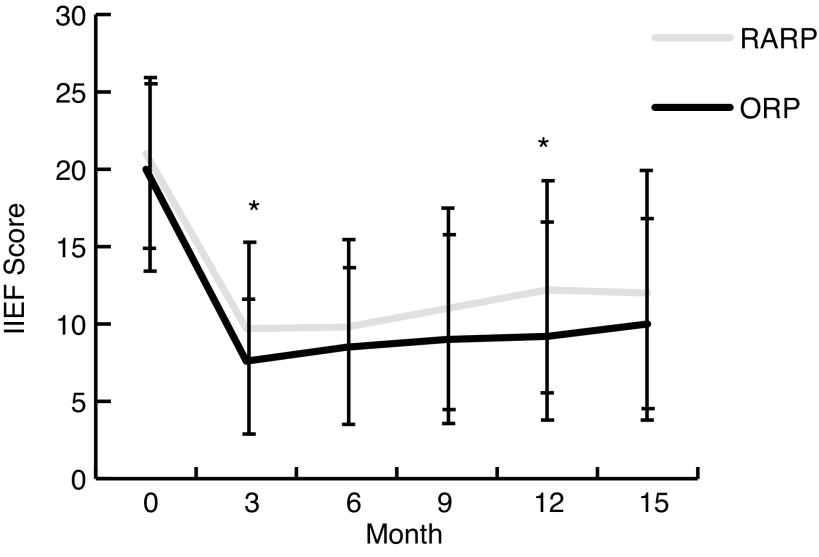
We illustrated the trends in mean (± standard deviation) IIEF scores (the IIEF-5 score is the sum of the ordinal responses to the 5 items: (1) 22–25: No erectile dysfunction; (2) 17–21: Mild erectile dysfunction; (3) 12–16: Mild to moderate erectile dysfunction; (4) 8–11: Moderate erectile dysfunction; and (5) 5–7: Severe erectile dysfunction) over time for the RARP and ORP (Fig. 2). IIEF scores were not significantly different between groups at baseline. They were significantly higher in the RARP group at 3 months (7 ± 4 ORP, 9 ± 6 RARP, p = 0.001) and 12 months (10 ± 6 ORP, 12 ± 7 RARP, p = 0.011), although none of the differences were clinically significant (i.e., all were smaller than the minimum clinically important difference).
Fig. 2.
Mean (± standard deviation) IIEF scores at each time point. Baseline scores plotted at month 0. The response rate for the completed IIEF questionnaires at each time point was: baseline (n = 364 ORP, n = 187 RARP), 3 months (n = 219 ORP, n = 148 RARP), 6 months (n = 213 ORP, n = 148 RARP), 9 months (n = 163 ORP, n = 121 RARP), 12 months (n = 154 ORP, n = 100 RARP), 15 months (n = 66 ORP, n = 46 RARP). Differences between RARP and ORP groups were statistically significant at 3 months (p < 0.001) and 12 months (p = 0.011), but not clinically significant at any time point. Asterisks denote statistical significance. RARP: robot-assisted radical prostatectomy; ORP: open radical prostatectomy; IIEF: International Index of Erectile Function.
IPSS Scores
We illustrated the trends in mean (± standard deviation) IPSS scores (IPSS score categories: (1) 1–7: Mild; (2) 8–19: Moderate; and (3) 20–35: Severe) over time for the RARP and ORP (Fig. 3). IPSS scores were not significantly different between groups at baseline. IPSS scores were statistically significantly lower in RARP patients compared to ORP patients at 3 months (11 ± 7 ORP, 9 ± 6 RARP, p = 0.04), 6 months (8 ± 6 ORP, 6 ± 5 RARP, p < 0.001), 9 months (7 ± 6 ORP, 6 ± 5 RARP, p = 0.007), and 12 months (7± 6 ORP, 5 ± 5 RARP, p = 0.02), but not at 15 months. However, none of the differences were clinically significant.
Fig. 3.
Mean (± standard deviation) IPSS scores at each time point. Baseline scores plotted at month 0. The response rate for the completed IPSS questionnaires at each time point was: baseline (n = 431 ORP, n = 213 RARP), 3 months (n = 382 ORP, n = 215 RARP), 6 months (n = 326 ORP, n = 201 RARP), 9 months (n = 228 ORP, n = 161 RARP), 12 months (n = 222 ORP, n = 135 RARP), 15 months (n = 101 ORP, n = 60 RARP). Differences between RARP and ORP groups were statistically significant at 3 months (p = 0.04) 6 months (p < 0.001), 9 months (p = 0.007) and 12 months (p = 0.02), but not clinically significant at any time point. Asterisks denote statistical significance. RARP: robot-assisted radical prostatectomy; ORP: open radical prostatectomy; IIEF: International Index of Erectile Function; IPSS: International Prostate Symptom Scores.
Discussion
What drives technical innovation in surgery? The objective of any surgical intervention is to eliminate disease with minimal operative morbidity and without persistent detrimental effects in HRQoL. RP was historically associated with significant HRQoL losses (impotence, incontinence, bowel injury) and had been largely abandoned to radiation therapy until the anatomical prostatectomy with the possibility of potency sparing nerve-sparing technology was introduced and popularized by Walsh.18
However, in spite of the popularization of the anatomic nerve-sparing operation and its increasing use by many, many studies suggest that the quoted almost perfect continence and potency rates were either a result of routine selection bias of younger, thinner, more potent men for the operation. At most academic and community medical centres where similar procedures were performed by experienced high volume cancer surgeons, these outcomes were routinely inferior to the results claimed by the few “ masters” of this operation, or perhaps these masters of the procedure really had developed superior technique. The advent of the Intuitive Surgery l robot suggested a leveling of differences because of its many unique attributes. The daVinci robot’s unique dual camera system gave the surgeon the ability to work in a familiar visual environment – direct 3-dimensional vision with real depth of field. Furthermore, the robotic system placed the surgeon, away from the patient, seated in a comfortable, ergonometrically designed console area where his fingers and feet were attached in a master slave manner where each movement of the surgeon fingers would cause scaled, tremor-reduced emulated movements to the miniature tools that were placed through traditional laparoscopic ports. The miniaturized tool’s articulated wrist offered six degrees of freedom of movement. These and other unique features allowing for a visualized technically perfect urethral anastomosis. Further, the device’s variable magnification, multiple working arms, and positive pressure environment suggested a blood-free operative field leading to the possibility of markedly improved results by every surgeon in performing a difficult, often bloody dissection of the neurovascular bundles. Indeed, the initial published results for RARP suggested exactly that, and the plethora of superb case series by the pioneers in robotic surgery suggested it was true.19
To attempt to understand whether these initially reported results with RARP were due to selection bias or a truly superior surgical technique, we examined whether HRQoL outcomes differed between RARP and ORP among experienced uro-oncologic surgeons. We found that disease-specific HRQoL, as measured by the PORPUS, was statistically better after RARP compared to ORP at all 5 time points examined between 3 months and 15 months after surgery. However, these differences persisted at only 3 and 9 months after adjusting for baseline differences. Moreover, none of the differences in HRQoL between RARP and ORP groups were clinically important based on the patient’s ability to perceive a meaningful difference at any time point after surgery (i.e. the minimum clinically important difference). Similar trends were noted using both the IIEF and IPSS to measure erectile function and urinary function, respectively. Again, for both latter measures, none of the differences were clinically important based on the minimum clinically important difference for each measure.
Baseline demographic differences may have contributed to the difference in QoL outcomes in ORP versus RARP. At our centre, younger males with lower BMI, Gleason scores, and total PSA, and men with less comorbidity tended to choose or be selected for RARP. Given the relatively small differences in adjusted HRQoL over time between groups, unmeasured baseline differences between the two groups may still be a factor in the differences postoperatively.
Numerous studies have measured quality of life after ORP and RARP with mixed results.3–5,20,21 Selection bias has been a factor in previous QoL studies comparing these procedures, where surgeons would limit their RARP patients to younger groups, lower-grade tumours, or those lacking comorbidities.4 This phenomenon might be then considered appropriate clinical practice if RARP had been demonstrated in a convincing fashion to unequivocally better preserve potency than ORP because of its better visualization of the nerve bundles. Further, older patients with waning potency, a higher BMI and a higher volume of disease would appropriately have a higher likelihood of having their nerve bundles sacrificed for oncological efficacy in ORP.
In our experience, patients are often told that their quality of life will return to normal shortly after surgery. Our data indicate that this is not the case for most men. HRQoL did not return to normal (preoperative) levels throughout the 15-month follow-up period. Patient regret after surgery is documented in those who underwent RARP, possibly due to inflated expectations. In one study, patients were more likely to experience regret after RARP compared to ORP.22
Our results need to be interpreted in the light of several limitations. The follow-up questionnaire response rate decreased over time. Patients who felt well may have neglected to attend later appointments to avoid wasting their time. This may introduce selection bias among respondents at later time points, and artificially lower overall HRQoL scores. However, the converse may also be true in that patients who were discouraged by their HRQoL outcomes or suffered from questionnaire fatigue may not have responded.
Surgical robotic platforms appear to be here to stay. They truly are the alluring, engineering and surgical wonders that are the soul of disruptive change. These devices continue to improve with each new generation, offering the combination of the master surgeon’s judgment and experience with the machines tireless, tremorless reliability, and may offer significant advantages over traditional surgery in the right hands and for the appropriate indications. However, despite these advantages, we have found that HRQoL outcomes do not appear to be significantly different after RARP compared to ORP over the first 15 months postoperatively. Further studies are needed to define in which patient subgroup is robotics likely to significantly advance patient care (improved oncologic outcomes, improved HRQoL) at an acceptable cost. We believe only at that point should new technology be widely introduced.
Conclusion
Although HRQoL was statistically superior after RARP at selected time periods within the first 15 months after surgery, differences were not clinically significant.
Table 4.
Multivariate model for significant, but not clinically significant data predicting change in PORPUS-P scores
| Time period | Variable | Effect | Confidence interval | p value |
|---|---|---|---|---|
| Baseline to 3 months (n = 280) | Age | −0.015 | (−0.202,0.173) | 0.879 |
| Type of RP | 3.262 | (0.176,6.348) | 0.038 | |
| Gleason Score | 0.753 | (−4.574,6.08) | 0.781 | |
| Charlson Count | 1.565 | (−1.41,4.541) | 0.301 | |
| Ethnicity | −4.047 | (−6.998,−1.096) | 0.007 | |
| Nerve sparing 1* | 1.078 | (−3.395,5.551) | 0.635 | |
| Nerve sparing 2* | 1.992 | (−2.059,6.042) | 0.334 | |
| Nerve sparing 3* | 2.567 | (−3.991,9.125) | 0.442 | |
| Surgeon 2 | −0.396 | (−3.866,3.074) | 0.822 | |
| Surgeon 3 | −6.666 | (−15.892,2.56) | 0.156 | |
| Surgeon 4 | −8.096 | (−23.78,7.588) | 0.31 | |
| Surgeon 5 | −0.991 | (−4.53,2.548) | 0.582 | |
| Surgeon 6 | −0.458 | (−22.305,21.389) | 0.967 | |
| PORPUS-P | −0.376 | (−0.503,−0.25) | 0 | |
|
| ||||
| Baseline to 6 months (n = 234) | Age | 0.055 | (−0.12,0.231) | 0.535 |
| Type of RP | 2.031 | (−0.84,4.902) | 0.165 | |
| Gleason score | −1.015 | (−6.229,4.199) | 0.702 | |
| Charlson count | 1.665 | (−1.201,4.532) | 0.253 | |
| Ethnicity | −1.439 | (−4.203,1.326) | 0.306 | |
| Nerve sparing 1* | 4.768 | (0.52,9.017) | 0.028 | |
| Nerve sparing 2* | 5.067 | (1.031,9.102) | 0.014 | |
| Nerve sparing 3* | 3.766 | (−2.134,9.667) | 0.21 | |
| Surgeon 2 | −3.501 | (−6.716,−0.287) | 0.033 | |
| Surgeon 3 | −12.564 | (−22.215,−2.914) | 0.011 | |
| Surgeon 4 | −14.589 | (−28.121,−1.057) | 0.035 | |
| Surgeon 5 | −1.759 | (−4.973,1.456) | 0.282 | |
| PORPUS-P | −0.455 | (−0.571,−0.338) | 0 | |
|
| ||||
| Baseline to 9 months (n = 165) | Age | −0.131 | (−0.362,0.099) | 0.262 |
| Type of RP | 3.78 | (0.225,7.335) | 0.037 | |
| Gleason score | 7.061 | (0.147,13.975) | 0.045 | |
| Charlson count | 2.024 | (−1.678,5.727) | 0.282 | |
| Ethnicity | −1.483 | (−5.18,2.214) | 0.429 | |
| Nerve sparing 1* | 1.009 | (−4.695,6.713) | 0.727 | |
| Nerve sparing 2* | 4.374 | (−0.655,9.403) | 0.088 | |
| Nerve sparing 3* | 5.298 | (−3.527,14.123) | 0.237 | |
| Surgeon 2 | −0.385 | (−4.141,3.372) | 0.84 | |
| Surgeon 3 | −2.036 | (−16.564,12.491) | 0.782 | |
| Surgeon 4 | 3.874 | (−10.713,18.461) | 0.601 | |
| Surgeon 5 | −2.737 | (−6.898,1.423) | 0.196 | |
| Surgeon 6 | 2.367 | (−18.622,23.356) | 0.824 | |
| PORPUS-P | −0.466 | (−0.605,−0.327) | 0 | |
|
| ||||
| Baseline to 12 months (n = 139) | Age | −0.106 | (−0.365,0.154) | 0.422 |
| Type of RP | 1.307 | (−2.995,5.609) | 0.549 | |
| Gleason score 1 | 0.08 | (−6.588,6.749) | 0.981 | |
| Charlson count 1 | 0.989 | (−2.956,4.933) | 0.621 | |
| Ethnicity | −0.918 | (−5.112,3.276) | 0.666 | |
| Sparing recorded 1* | 0.102 | (−5.732,5.936) | 0.972 | |
| Sparing recoded 2* | 3.807 | (−1.77,9.385) | 0.179 | |
| Sparing recoded 3* | 1.551 | (−14.124,17.226) | 0.845 | |
| Surgeon 2 | −0.247 | (−4.72,4.226) | 0.913 | |
| Surgeon 3 | −16.003 | (−31.314,−0.693) | 0.041 | |
| Surgeon 5 | −4.893 | (−9.744,−0.042) | 0.048 | |
| PORPUS-P | −0.251 | (−0.414,−0.087) | 0.003 | |
|
| ||||
| Baseline to 15 months (n = 68) | Age | −0.067 | (−0.457,0.323) | 0.732 |
| Type of RP | 4.706 | (−1.478,10.889) | 0.133 | |
| Gleason score | 6.033 | (−5.955,18.021) | 0.318 | |
| Charlson count | −1.062 | (−8.758,6.635) | 0.783 | |
| Ethnicity | −5.399 | (−11.546,0.748) | 0.084 | |
| Nerve sparing 1* | −3.078 | (−13.239,7.083) | 0.546 | |
| Nerve sparing 2* | −5.133 | (−15.033,4.768) | 0.303 | |
| Nerve sparing 3* | −19.707 | (−44.364,4.95) | 0.115 | |
| Surgeon 2 | 2.296 | (−5.116,9.708) | 0.537 | |
| Surgeon 3 | 4.39 | (−10.772,19.552) | 0.564 | |
| Surgeon 5 | −0.574 | (−7.741,6.592) | 0.873 | |
| PORPUS-P | −0.342 | (−0.611,−0.072) | 0.014 | |
“Unilateral”: Nerve sparing 1; “Bilateral”: Nerve sparing 2; “Unknown”: Nerve sparing 3. PORPUS-P: Patient-Oriented Prostate Utility Scale-Psychometric.
Acknowledgments
The paper and our efforts were funded through the generosity of an unrestricted grant of The Weinbaum Family Prostate Cancer Prevention Fund of The Prostate Centre at Princess Margaret Cancer Centre and made possible through the invaluable fund raising and administrative efforts of The Princess Margaret Cancer Centre Foundation. Robin Kalnin of Meridian Software Inc. provided database support.
Appendix. The Prostate-Oriented Record of Psychometric and Utility-Based Outcomes Scale
I. Pain and disturbing body sensations
| |
II. Energy
| |
III. Support from family and friends
| |
IV. Communication with doctor (primary caregiver for prostate cancer, may be specialist or family doctor)
| |
V. Emotional well-being
| |
VI. Urinary frequency (need to pass urine frequently during the day or night) and urgency (difficulty delaying urination after the urge is felt to urinate and ability to “hold it”)
| |
VII. Leaking urine/poor bladder control
| |
VIII. Sexual function (problems with achieving/maintaining an erection)
| |
IX. Sexual interest/drive
| |
X. Bowel problems: diarrhea, rectal discomfort (pain, burning or irritation), or constipation
| |
Taken from Ku et al. Can Urol Assoc J 2009;3(6):445–52.
Footnotes
Competing interests: Ms. Rush, Dr. Alibhai, Dr. L. Xu, Dr. W. Xu, Ms. Louis, Dr. Matthew, Mr. Nesbitt, Dr. Hamilton and Dr. Trachtenberg declare no competing financial or personal interests. Dr. Zlotta is a member of the advisory boards for Amgen, Ferring, and Astellas. He has also received grants from Sanofi, Red Leaf Medical and is currently participating in a clinical trial with Sanofi Aventis. Dr. Kulkarni has received a grant from Astellas and he is currently participating in a clinical trial with Spectrum Pharmaceuticals. Dr. Finelli has participated in clinical trials in the past 2 years for Amgen, Astellas, Janssen and Ferring. He is also Chair of the CUA Guidelines Committee. Dr. Fleshner is a member of the advisory boards for Amgen, Janssen, Astellas and Eli Lily. He has received honoraria from Amgen, Janssen, Astellas, and Eli Lily. He is and has participated in clinical trials for Amgen, Janssen, Medivation, OICR, and Prostate Cancer Canada. Dr. Jewett has received grants from, and is participating in clinical trials with Novartis, GSK, and Pfizer.
This paper has been peer-reviewed.
References
- 1.American Cancer Society . Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 2.Ahlering TE, Woo D, Eichel L, et al. Robot-assisted versus open radical prostatectomy: A comparison of one surgeon’s outcomes. Urology. 2004;63:819–22. doi: 10.1016/j.urology.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 3.Gettman MT, Blute ML. Critical comparison of laparoscopic, robotic, and open radical prostatectomy: Techniques, outcomes, and cost. Curr Urol Rep. 2006;7:193–9. doi: 10.1007/s11934-006-0021-1. [DOI] [PubMed] [Google Scholar]
- 4.Berryhill R, Jr, Jhaveri J, Yadav R, et al. Robotic prostatectomy: A review of outcomes compared with laparoscopic and open approaches. Urology. 2008;72:15–23. doi: 10.1016/j.urology.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein J, Eckersberger E, Sadri H, et al. Open versus laparoscopic versus robot-assisted laparoscopic prostatectomy: The European and US experience. Rev Urology. 2012;12:35–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Geraerts I, Van Poppel H, Devoogdt N, et al. Prospective evaluation of urinary incontinence, voiding symptoms and quality of life after open and robot-assisted radical prostatectomy. BJU Int. 2013;112:936–43. doi: 10.1111/bju.12258. [DOI] [PubMed] [Google Scholar]
- 7.Krambeck AE, DiMarco DS, Rangel LJ, et al. Radical prostatectomy for prostatic adenocarcinoma: a matched comparison of open retropubic and robot-assisted techniques. BJU Int. 2009;103:448–53. doi: 10.1111/j.1464-410X.2008.08012.x. [DOI] [PubMed] [Google Scholar]
- 8.Tewari A, Srivasatava A, Menon M. A prospective comparison of radical retropubic and robot-assisted prostatectomy: Experience in one institution. BJU Int. 2003;92:205–10. doi: 10.1046/j.1464-410X.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- 9.Krahn M, Bremner KE, Tomlinson G, et al. Responsiveness of disease-specific and generic utility instruments in prostate cancer patients. Qual Life Res. 2007;16:509–22. doi: 10.1007/s11136-006-9132-x. [DOI] [PubMed] [Google Scholar]
- 10.Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–26. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 11.Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–57. doi: 10.1016/s0022-5347(17)36966-5. http://www.ncbi.nlm.nih.gov/pubmed/1279218. [DOI] [PubMed] [Google Scholar]
- 12.Charlson M, Pompei P, Ales K, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Krahn M, Ritvo P, Irvine J, et al. Construction of the patient-oriented prostate utility scale (PORPUS): A multiattribute health state classification system for prostate cancer. J Clin Epidemiol. 2000;53:920–30. doi: 10.1016/S0895-4356(00)00211-0. [DOI] [PubMed] [Google Scholar]
- 14.Ritvo P, Irvine J, Naglie G, et al. Reliability and validity of the PORPUS, a combined psychometric and utility-based quality-of-life instrument for prostate cancer. J Clin Epidemiol. 2005;58:466–74. doi: 10.1016/j.jclinepi.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 15.O’Connor RC, Bales GT, Avila D, et al. Variability of the International Prostate Symptom Score in men with lower urinary tract symptoms. Scand J Urol Nephrol. 2003;37:35–7. doi: 10.1080/00365590310008668. [DOI] [PubMed] [Google Scholar]
- 16.Rosen RC, Allen KR, Ni X, et al. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function Scale. Eur Urol. 2011;60:e37–48. doi: 10.1016/j.eururo.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 17.Ringash J, O’Sullivan B, Bezjak A, et al. Interpreting clinically significant changes in patient-reported outcomes. Cancer. 2007;110:196–202. doi: 10.1002/cncr.22799. [DOI] [PubMed] [Google Scholar]
- 18.Walsh PC, Mostwin JL. Radical prostatectomy and cystoprostatectomy with preservation of potency: Results using a new nerve-sparing technique. Br J Urol. 1984;56:694–7. doi: 10.1111/j.1464-410X.1984.tb06149.x. [DOI] [PubMed] [Google Scholar]
- 19.Menon M, Tewari A, Peabody J, VIP Team Vattikuti Institute prostatectomy: Technique. J Urol. 2003;169:2289–92. doi: 10.1097/01.ju.0000067464.53313.dd. [DOI] [PubMed] [Google Scholar]
- 20.Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: A systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037–63. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 21.Barry MJ, Gallagher PM, Skinner JS, et al. Adverse effects of robotic-assisted laparoscopic versus open retropubic radical prostatectomy among a nationwide random sample of medicare-age men. J Clin Oncol. 2012;30:513–8. doi: 10.1200/JCO.2011.36.8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroeck FR, Krupski TL, Sun L, et al. Satisfaction and regret after open retropubic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008;54:785–93. doi: 10.1016/j.eururo.2008.06.063. [DOI] [PubMed] [Google Scholar]