Abstract
AIM: To evaluate the protective effect of lysozyme chloride on betel quid chewing (BQC) aggravated gastric oxidative stress and hemorrhagic ulcer in rats with diabetes mellitus (DM).
METHODS: Male Wistar rats were challenged intravenously with streptozotocin (65 mg/kg) to induce DM. Rats were fed with regular pellet food or BQC-containing diets. After 90 d, rats were deprived of food for 24 h. Rat stomachs were irrigated for 3 h with normal saline or simulated gastric juice. Rats were killed and gastric specimens were harvested.
RESULTS: An enhancement of various gastric ulcerogenic parameters, including acid back-diffusion, mucosal lipid peroxide generation, as well as decreased glutathione levels and mucus content, were observed in DM rats. After feeding DM rats with BQC, an exacerbation of these ulcero-genic parameters was achieved. Gastric juice caused a further aggravation of these ulcerogenic parameters. Daily intragastric lysozyme chloride dose-dependently inhibited exacerbation of various ulcerogenic parameters in those BQC-fed DM rats.
CONCLUSION: (1) Gastric juice could aggravate both DM and BQC-fed DM rat hemorrhagic ulcer; (2) BQC exacerbated gastric hemorrhagic ulcer in DM rats via enhancing oxidative stress and reducing defensive factors; (3) lysozyme chloride effectively protected BQC aggravated gastric damage in DM rats.
Keywords: Glutathione, Lipid peroxide, Oxyradical, Prostaglandins, Gastric mucus, Mucosal damage, Acid back-diffusion
INTRODUCTION
Betel quid chewing (BQC) is a habit of human beings in most parts of the world. It has been estimated that more than 10% of the world’s population chew betel quid for its mild psychoactive effects[39]. Ample documents demonstrate that BQC is a carcinogenic agent either in animal[4] or in human beings[7]. BQC can result in cancer of buccal cavity[26], esophagus[31], stomach[8], lung, and liver[4]. Since the stomach is the organ that directly contacts BQC, it is likely that gastric mucosa can also be damaged after its long-term consumption.
On the other hand, diabetes mellitus (DM) is an important fatal disease that is frequently found in the clinic. Gastrointestinal disturbance, such as gastric hemorrhagic ulcer, is one of the main manifestations observed in poor controlled DM patients. Previous document demonstrates the importance of acid back-diffusion in the formation of hemorrhagic ulcer in DM rats[22]. Whether BQC can aggravate DM-induced gastric hemorrhagic ulcer is unknown.
During gastric oxidative stress, the imbalance of aggressive and defensive factors in the stomach plays a pivotal role in gastric hemorrhage and ulcer formation. The aggressive factors include gastric acid back-diffusion and oxyradical generation, while defensive factors involve glutathione (GSH) and mucus biosynthesis[17]. Aggravation of aggressive factors and/or attenuation of defensive substances may result in high risk of gastric hemorrhage and ulcer formation.
When gastric mucosal barriers are inflamed or disrupted by acid, ethanol or aspirin, the intraluminal free acid may diffuse back into gastric mucosa through damaged mucosal barriers and consequently causes exacerbation of hemorrhage and ulceration. This acid back-diffusion is considered as a sensitive marker of gastric mucosal integrity[10,21]. In fact, gastric acid back-diffusion is greatly associated with hemorrhage and ulcer formation in various inflammatory diseases[12,17]. In the disease state, oxidative stress of the stomach may occur and result in an elevation of mucosal lipid peroxide (LPO) that are generated from the reaction of oxyradicals and cellular polyunsaturated fatty acid. Whereas reduced GSH is a sulfhydryl compound, which may act to prevent lipid peroxidation produced by noxious oxyradicals that can damage gastric mucosal cells[3]. Various studies have demonstrated a decrease in GSH level in inflammatory and ulcerated gastric mucosa[14,19]. The mucosal protective effect of GSH on gastric damage induced by nonsteroidal anti-inflammatory drugs[35], ethanol[36] or lipopolysaccharide[17] has been well documented. Nevertheless, the role of mucosal GSH and oxyradicals in BQC-induced hemorrhagic ulcers are unknown. Gastric mucus, which plays an important role in the mucosal protection, can be eliminated by acid or noxious agents[1]. Nevertheless, the influence of BQC on gastric mucus secretion in DM rats also remains obscure.
Lysozyme (muramidase), which widely distributes in various human tissues[27] is a stable polypeptide containing 18 amino acids. High content of lysozyme is found in mammalian milk, chicken eggshell membrane, and matrix[13]. This compound may possess multiple pharmacological actions, including analgesic[5], anti-inflammatory[33], hemostatic, anti-allergy[2], anticancer[25], and antibiotic[24] effects. The aim was to study the influence of chronic consumption of BQC on gastric oxidative stress, mucus production, and mucosal hemorrhagic ulcer in DM rat gastric mucosa. The mucosal protective effects of lysozyme chloride on this ulcer model were also evaluated.
MATERIALS AND METHODS
Chemicals used
The following chemicals in reagent grade were used: acivicin, alcian blue, lysozyme chloride, 1-butanol, o-phthaldialdehyde, pyridine, rat hemoglobin (Hb), reduced GSH, sodium laurylsulfate, trichloroacetic acid, 1,1,3,3-tetramethoxypropane, and 2-thiobarbiturate were purchased from Sigma (St. Louis, MO, USA). The purity of all drugs was over 98%. All chemical solutions were freshly prepared before use.
Drug administration
Intragastric lysozyme chloride (0-500 mg/kg) was challenged once daily to normal and those rats fed with BQC for 90 d. The control group received normal saline instead of chemicals.
Animals
Male specific pathogen-free Wistar rats, weighing 15020 g, were housed individually in a room with a 12-h dark-light cycle and with central air conditioning (25 °C, 70% humidity). Animal care and experimental protocols were in accordance with the guidelines of the National Sciences Council of Taiwan (NSC 2002). Prior to the experiments, rats were injected intravenously with streptozotocin (65 mg/kg) to induce DM. Non-DM rats received vehicle only. Blood glucose level over 2 500 mg/L was considered as DM. All rats were divided into groups. Control rats received laboratory rodent diet (the Richmond standard, PMI feeds, St. Louis, MO, USA). Test groups were fed with the BQC diet prepared by mixing the laboratory rodent diet with chopped and crushed BQC (30%, w/w), which contained areca nuts (40%, w/w), gambir lime (30%, w/w) and betel stems (30%, w/w). The calories of BQC-diet were compensated near the control pellet diet (4.25 kcal/g) by adding adequate amounts of sugar and eggs. The BQC-diet was then dried in cold air and stored in a refrigerator (4 °C). The rats were given fresh diet every day at 8 a.m. While chewing the test food, rats mimicked human beings in chewing and spitting the fiber contained in the BQC diet. The consumption of the diet, water, and the body weight of the animal were recorded every day before feeding, respectively.
Surgical procedures
After 90 d, rats were deprived of food for 24 h and anesthetized with diethylether. Rat stomachs were surgically exposed for the ligation of pylorus and lower esophagus. To prevent the spontaneous gastric secretion, a bilateral diaphragmatic vagotomy was performed in all rats. A small incision was then made in the forestomach. A polypropylene tube (3.5 mm internal diameter20 mm long) was inserted through the same incision and secured with a ligature. The stomach was subsequently rinsed meticulously with warm normal saline (37 °C). Care was taken to avoid injury to blood vessels. The residues were gently removed.
Measurement of gastric acid back-diffusion
Gastric acid back-diffusion (the H+ loss in the gastric lumen) was quantified by the method previously described[21]. Either normal saline or simulated gastric juice (7 mL) containing 100 mmol/L HCl, 17.4 mmol/L pepsin and 54 mmol/L NaCl was instilled into the cleansed stomach with a syringe. Luminal contents were mixed with the same syringe by three repeated aspirations and injections, and 3 mL of the fluid was taken as an initial sample. The forestomach was tightly closed. The abdominal wound was sutured. After 3 h, rats were killed with an overdose of diethylether. Gastric contents (final sample) were collected. Both initial and final samples were centrifuged at 3 000 r/min for 20 min. The volumes of the initial and final samples were measured. The acidity of samples was assessed by titrating 1.0 mL of gastric contents sample with 0.1 mol/L NaOH to pH 7.0 on an autoburette titrator (Radiometer, Copenhagen, Denmark). The net flux of ions through the gastric mucosa was calculated as follows:
Ionic net flux =Fv×Iv-(7-Iv)×Ic
where Fv and Iv are the volume (mL) of the final sample and initial sample, respectively, and Fc and Ic are the ionic concentrations (in mmol/L) of the final sample and the initial sample, respectively. A negative value for net flux indicates luminal electrolyte loss and a positive value indicates luminal electrolyte gain.
Morphological and histological studies of gastric mucosa
As soon as the final sample was collected, the stomach was filled with 8 mL of 10g/L formaldehyde for 10 min. The mucosa was exposed by opening the stomach along the greater curvature. The length (mm) and the width (mm) of ulcer on the mucosa were measured with a planimeter (1p/4).
The total ulcer area (mm2) of each stomach was recorded. Histological studies of the stomach also were conducted by methods as previously described[21]. Sections were scored as 0-5, in which (0) indicated normal appearance, (1) mild injury in the epithelial cells, (2) mild injury in the upper part of mucosal cells, (3) hemorrhage or edema in the mid or lower part of mucosal cells, (4) degranulation or necrosis of the epithelial cells, and (5) serious cell disruption of lower part of the mucosa. The index score of each section was evaluated on a cumulative basis to give a maximal score of 15.
Determination of hemoglobin (Hb)
Cleansed rat stomachs were irrigated for 3 h with either normal saline or gastric juice. Initial and final samples were collected by methods described above.
The blood attached to the gastric mucosa was carefully scraped and added to the final sample. Subsequently, both initial and final samples were adjusted to pH 1.5 with 0.1 mol/L HCl. The Hb content of the samples was measured spectrophotometrically[22]. The absorption maximum of Hb was measured at 376 nm. Appropriate irrigating solutions, adjusted to pH 1.5, were used as blank. Absorbances of the samples were measured against a standard curve (r2>0.98) constructed with freshly prepared rat Hb (0.05-1.00 mg/mL) treated in the same manner as gastric samples. The luminal Hb content was calculated as:
Hb content =Fv×FHb (7-Iv)×IHb,
where Fv and Iv are the volume (mL) of final sample and initial sample, respectively, and FHb and IHb are the luminal Hb concentrations (mg/mL) in the final and initial samples, respectively. The results obtained from gastric samples were expressed in mg Hb per stomach.
Mucosal GSH assay
The quantitation of gastric mucosal GSH was performed by following the methods that were described previously[19]. As soon as the final sample was collected, the rat stomach was dissected. The corpus mucosa was scraped using two glass slides on ice, weighed and homogenized immediately in 2 mL phosphate buffer (0.1 mol/L NaH2PO4 plus 0.25 mol/L sucrose, pH 7.4). Acivicin (250 mmol/L), an irreversible inhibitor of g-glutamyltransferase, was added to the homogenate to inhibit the catabolism of GSH. Samples were then centrifuged at 4 000 r/min for 15 min at 4 °C.
To determine the recovery of reduced thiol, GSH (200 mmol reduced GSH contained in phosphate buffer solution, pH 7.0) was added to the supernatant. Subsequently, 0.5 mL, 0.25 mol/L trichloroacetic acid was added to 1.0 mL of the supernatant. The mixture was kept for 30 min at 4 °C. After centrifugation for 15 min at 3 000 r/min, the supernatant was used to determine GSH using 2,2-dinitro-5,5-dithio-dibenzoic acid. The optical density was measured at 412 nm on a spectrophotometer (model U-3210, Hitachi, Tokyo, Japan). All samples were measured in duplicate. Recovery of added internal standard was greater than 90% in all experiments. Absorbances of the samples were measured against a standard curve constructed with freshly prepared GSH solutions (0.05-0.5 mmol/L) that were treated in the same manner as the tissue samples. The results obtained from tissue samples were expressed as µmol GSH/g wet tissue.
Determination of lipid peroxides (LPO)
The concentration of gastric mucosal LPO was determined by estimating malondialdehyde using the thiobarbituric acid test[30]. Briefly, the stomachs of rats were promptly excised and rinsed with cold normal saline. To minimize the possibility of interference of Hb with free radicals, any blood adhering to the mucosa was carefully removed. The corpus mucosa was scraped, weighed and homogenized in 10 mL 100 g/L KCl. The homogenate (0.5 mL) was added with a solution containing 0.2 mL 80 g/L sodium laurylsulfate, 1.5 mL 200 g/L acetic acid,1.5 mL 8 g/L 2-thiobarbiturate and 0.3 mL distilled water. The mixture was incubated at 98 °C for 1 h. Upon cooling, 5 mL of 1-butanol/pyridine (15:1) was added. The mixture was vortexed for 1 min and centrifuged for 10 min at 4000 r/min. The supernatant was measured at 532 nm. The standard curve was obtained by using 1,1,3,3-tetramethoxypropane. The recovery was over 90%. All samples were measured in duplicate. The results were expressed as nmol malondialdehyde/g wet tissue.
Measurement of gastric mucus
Gastric mucus was assessed by the method as described by Corne et al[9]. Briefly, the rat stomach was excised and washed with tap water. The sample was immersed in 10 mL of a solution containing alcian blue (1.0 mg/L), sucrose (0.16 mol/L) and sodium acetate (0.05 mol/L) for 2 h. To remove the unbound dye, the sample was washed for 15 min and then for 45 min in a 0.25 mol/L sucrose solution. The mucus bound dye was eluted by immersing gastric mucosa in 10 mL of 0.5 mol/L MgCl2solution for 2 h. The obtained solution was mixed with 10 mL diethylether. The absorbance of the color aqua solution was measured on a spectrophotometer (U3210, Hitachi) at 605 nm. The amount of alcian blue was extracted from known graded concentrations (5-50 mg/L) of alcian blue solution. All samples were measured in duplicate. The results were expressed as µgalcian blue/g wet tissue.
Statistical analysis
The data were expressed as mean±SE. Significant differences in single measurement traits were analyzed statistically by using the Tukey truly significant difference test for pairwise comparison after ANOVA or by using the Student’s t-test[28]. Statistical significance was set at P < 0.05.
RESULTS
Changes in gastric parameters in normal, DM, and BQC-fed DM rat stomachs irrigated with either normal saline or gastric juice
Table 1 indicated that in normal diet rat stomachs irrigated with normal saline no gastric acid back-diffusion but only little gastric acid secretion was achieved. Mucosal GSH and LPO generation were at normal levels. No mucosal ulceration and hemorrhage was observed in these rats (photo not shown). In saline-irrigated stomachs of DM rats, an enhancement of LPO generation as well as an attenuation of GSH and mucus secretion was obtained. After receiving the BQC-diet, marked necrotic ulcers accompanied by a further decrease in mucosal GSH and mucus were achieved in DM rats. The generation of mucosal LPO also was further aggravated in those rats, despite that no acid back-diffusion occurred.
Table 1.
Gastric juice-aggravated various gastric biochemical parameters in stomachs of normal, DM and BQC fed DM rats
| Acid ack-diffusion μmol /stomach | Glutathione μmol/g tissue | Lipid peroxide nmol MDA/g tissue | Mucus μg/g tissue | Hemoglobin mg/stomach | Ulcer area mm2 /stomach | |
| Normal saline | ||||||
| Control | 8.6±1.2 | 3.8±0.4 | 45.6±4.2v | 230.2±18.4 | 0.2±0.2 | 1.4±0.6 |
| DM | 5.0±0.8a | 2.4±0.3a | 95.4±5.2a | 186.1±10.2a | 2.2±0.5a | 64.6±8.5a |
| BQC+DM | 3.2±0.2a | 1.6±0.2a | 32±11.4a | 155.0±14.8a | 4.6±0.6 a | 54.8±18.4 a |
| Gastric juice | ||||||
| Control | -164.2±5.4 | 3.4±0.2 | 54.0±7.8 | 220.0±14.5 | 0.6±0.2b | 2.2±1.2 |
| DM | -235.4±10.6ac | 2.8±0.1ac | 120.0±10.2ac | 140.0±12.6ac | 5.3±0.7ac | 264.8±27.1ac |
| BQC+DM | -384.2±18.6ac | 0.8±0.1ac | 184.0±16.3ac | 75.0±6.8ac | 9.0±1.2ac | 78.6±45.6ac |
Data are mean±SE (n = 8).
P < 0.05 vs corresponding control group.
P < 0.05 vs corresponding group in saline-treatment. MDA = malondialdehyde, BQC = betel quid chewing, DM = diabetes mellitus.
When gastric juice was present in the stomachs of normal diet rats, the mucosa looked normal. Except when a normal level of acid back-diffusion occurred, various gastric parameters were not significantly different from those in saline-irrigated stomachs of normal rats. When DM rat stomachs were irrigated with gastric juice, a significant (P < 0.05) increased acid back-diffusion and LPO generation accompanied with an attenuation of mucosal GSH and mucus secretion was observed and an exacerbated hemorrhagic ulcer also was produced. Further aggravation of gastric acid back-diffusion and LPO generation as well as decreased GSH and mucus production was found in those BQC-fed DM rats. More severe gastric hemorrhage and ulcer also was observed in these rats.
Histological examination of gastric mucosa of DM and BQC-fed DM rats
Figure 1 shows the microscopic examination of normal, DM, and BQC-fed DM rat stomachs irrigated with gastric juice. Marked upper gastric mucosal damage was observed in DM rats. More severe mucosal damage in BQC-fed DM rat stomachs than those in DM rats was obtained. The comparisons of degrees of histological tissue damage in normal, DM and BQC-fed DM rat stomachs irrigated with saline or gastric juice are illustrated in Figure 2. When the stomachs of normal diet rats were irrigated with either saline or gastric juice, no appreciable damage of gastric mucosal cells was observed. In DM rats, normal saline-irrigation produced more mucosal cell damage than those were irrigated with normal saline or gastric juice in normal diet rats. A pronounced aggravation of mucosal cell damage was observed in gastric juice-irrigated stomach of DM rats. Further exacerbation of gastric damage was achieved in DM rats fed with BQC. In most cases, gastric edema also was observed in DM and BQC-fed DM rats.
Figure 1.
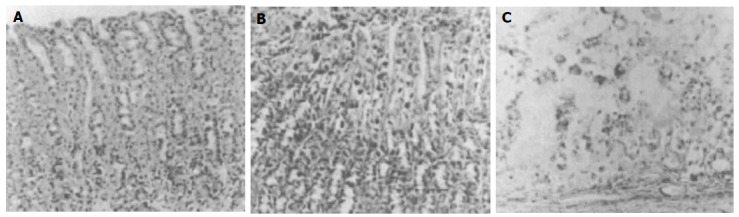
Histological studies of gastric mucosa exposed for 3 h gastric juice in normal, DM, and BQC-fed DM rats. Note that in normal rat stomachs irrigated with gastric juice (A), gastric mucosal cells look intact. However, gastric juice- irrigated DM rat mucosa (B), a disruption of gastric epithelial layer is observed. When stomachs of BQC-fed DM rats are irrigated with gastric juice (C), a complete disruption of the upper mucosal cells and lamina propria is obtained. The injured cells are characterized by karyorrhexis and dense homogenous acidophilic cytoplasm. In most cases, gastric edema also is observed (×150).
Figure 2.
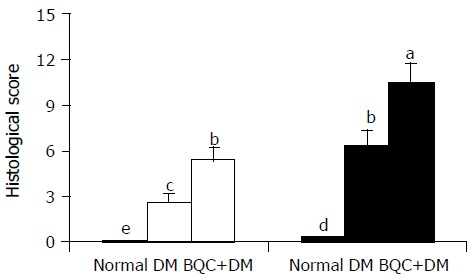
Histological scores of gastric mucosa in normal, DM, and BQC-fed DM rats. Rats were fed with normal diet or BQC diet for 90 d. Diabetes was induced by intravenous streptozotocin. Rat stomachs were irrigated for 3 h with normal saline (□) or gastric juice (■). Values are mean SE, n = 6-8. Bars labeled with different letters are significantly different at P < 0.05 based on Tukey statistic method.
Effects of lysozyme chloride on gastric parameters in gastric juice-irrigated stomachs of BQC-fed DM rats
As demonstrated in Table 2, low mucosal GSH levels and mucus secretion accompanied with high degree of acid back-diffusion and LPO generation were observed in 90-d BQC-fed DM rat stomachs irrigated with gastric juice. Severe gastric hemorrhage and mucosal ulceration also were achieved. Daily intragastric lysozyme chloride dose-dependently suppressed gastric hemorrhage and ulcer. This drug also inhibited the decreased mucosal GSH and mucus liberation as well as increased acid back-diffusion and mucosal LPO generations in BQC-fed DM rats in a dose-related manner. No visible side effects or toxicity of lysozyme chloride were observed in all experiments.
Table 2.
Effects of lysozyme chloride on various biochemical parameters in gastric juice-irrigated stomachs of BQC-fed DM rats
| mg/kg | Acid back-diffusion μmol /stomach | Glutathione μmol/g tissue | Lipid peroxide nmol MD/g tissue | Mucus μg/g tissue | Hemoglobin mg /stomach | Ulcer area mm2 /stomach | |
| Control | |||||||
| Saline | -132.0±3.4 | 3.6±0.3 | 62.6±7.0 | 206.4±14.6 | 0.4±0.2 | 3.2±0.5 | |
| Lysozyme chloride | 500 | -112.4±5.6a | 4.6±0.5a | 40.4±4.2a | 245.4±16.5a | 0.0±0.0a | 0.0±0.0a |
| Indomethacin | 5 | ||||||
| + lysozyme chloride | 500 | -140.0±7.2 | 2.8±0.3a | 74.5±6.8a | 164.6±14.2 | 1.0±0.5 | 5.6±08 |
| BQC+DM | |||||||
| Saline | -365.2±20.4 | 0.8±0.2 | 160.4±11.6 | 83.2±10.2 | 8.0±0.8 | 396.8±34.2 | |
| Lysozyme chloride | 50 | -292.0±11.2a | 1.7±0.3a | 132.0±12.5a | 126.6±7.4a | 4.0±0.5a | 306.0±25.2a |
| 100 | 220.2±13.0 | 2.4±0.3a | 108.4±10.6 | 182.8±9.2 | 2.2±0.3 | 168.6±12.4 | |
| 500 | -186.8±10.2a | 3.4±0.3a | 74.8±6.2 | 216.3±16.4 | 1.7±0.3a | 44.5±6.4 | |
| Indomethacin | 5 | ||||||
| + lysozyme chloride | 500 | -308.6±18.8a | 1.5±0.2a | 154.0±12.6a | 140.8±11.5a | 4.8±0.8a | 320.4±30.4a |
Data are mean±SE (n = 8).
P < 0.05 vs corresponding saline-group. MDA = malondialdehyde. MDA = malondialdehyde, DM = diabetes mellitus, BQC = betel quid chewing.
DISCUSSION
The pathological mechanisms underlying BQC-fed DM ulcer may be complex. Our previous paper demonstrates that chronic consumption of BQC can result in severe gastric hemorrhagic ulcer in rats[20]. In the present study, a remarkably increased acid back-diffusion was observed in those DM rat stomachs irrigated with gastric juice. Further aggravation of these ulcerogenic parameters was obtained in those DM rats fed with BQC. Obviously, chronic consumption of BQC diet could enhance various ulcerogenic parameters and result in greater gastric inflammation and breaking effects of gastric mucosal barriers in DM rats. Mechanisms of barrier-disruptive effect of BQC in DM rats are totally unknown. However, since BQC contained large amounts of gambir lime (which contains about 7-30% catechol, 20-50% catechu tannic acid and small amounts of quercetin waxes and oils; Merck Index, 1983) and calcium, the damage of mucosal barrier probably results from caustic injury from this component of BQC and DM-induced gastric cell degeneration. In clinical studies, it is reported that calcium polysulfite ingestion produces hemorrhagic necrosis of gastric mucosa[16]. BQC also contained arecoline, the main ingredient of areca fruit. Arecoline is a cholinergic agent that can cause gastric contraction and induce gastric secretion. Our previous paper demonstrates that arecoline causes seizure and results in gastric mucosal damage[17]. Therefore, after chronic consumption of BQC, gastric mucosal barriers might be severely disrupted and thus allowed copious free acid to diffuse back into the gastric mucosa during ulcer development.
Aggravation in LPO generation also was observed in gastric mucosa of BQC-fed DM rats. Apparently, the disruption of the mucosal barrier accompanied by gastric oxidative stress occurred in DM rats receiving BQC. In practice, oxyradicals can directly cause cell injury[3]. This cell damaging effect may associate with the occurrence of many diseases, including tissue inflammation and sepsis[17], hemorrhagic shock[11], and DM[29]. The initiation of gastric mucosal oxidative stress and inflammation might result from direct BQC-induced damage and degeneration of gastric mucosal cells induced by DM.
The lower mucosal GSH levels found in stomachs of DM or BQC-fed DM rats might be due to an increase in their consumption for scavenging oxyradicals and a decrease in cellular biosynthesis in damaged mucosal cells. In fact, depletion of neuronal GSH can cause an increase of neuronal nitric oxide synthase activity and cell death[6]. Moreover, GSH is used as a substrate or as a co-factor by several cytosolic and membrane-bound enzymes. A previous study demonstrated that exogenous GSH can produce a significant increase in mucosal GSH levels and a reduction in concentrations of mucosal LPO that associate with severe gastric hemorrhagic ulcers in acid-irrigated stomachs of septic rats[17]. Taken together, the imbalance between mucosal GSH biosynthesis and oxyradicals generation (gastric oxidative stress) was important in modulating BQC-induced ulcer in DM rats. A decreased gastric mucus also was found in DM or BQC-fed DM rats. Since BQC is an ulcerogenic substance, it might also damage gastric mucus cells and lead to attenuation of mucus biosynthesis. In fact, ulcerogens, such as aspirin and other non-steroidal anti-inflammatory drugs, have been reported to attenuate gastric mucus secretion[32]. Increase in mucus secretion can retard acid back-diffusion and is beneficial to the healing of mucosal damage[37].
In the present study, lysozyme chloride dose-dependently inhibited various aggressive parameters and elevated defensive factors in BQC-fed DM rats. It also ameliorated BQC-induced hemorrhagic ulcer in DM rats. This compound remarkably enhanced biosynthesis and secretion of gastric mucus, which is a potent gastroprotective substance. Lysozyme chloride also can enhance antral ulcer healing via stimulating mucus biosynthesis[18]. Indomethacin, a specific antagonist of prostaglandins, effectively reversed lysozyme chloride-induced cytoprotection of gastric mucosa. This implied that gastric mucosal protective effect of lysozyme chloride was partly mediated by stimulating biosynthesis and release of prostaglandins. Additionally, this drug has been reported to have analgesic and anti-inflammatory effects on pulpectomy and root canal filling[15]. It has also been shown to contribute to the advantageous effects in the treatment of patients and experimental animals with cancer[34,38]. The attenuation of LPO generation produced by lysozyme chloride in BQC-fed DM rats explained that this compound might possess potent antioxidant effect, thus reduced consumption of GSH. Moreover, lysozyme chloride may stimulate GSH biosynthesis in mucosal cells. Considering the above-mentioned, this drug can increase the total levels of BQC-fed DM rat mucosal GSH and contribute to the ulcer healing.
In conclusion, long-term consumption of BQC produces aggravation of DM-induced hemorrhage and ulcer in rats via enhancing gastric oxidative stress that can be ameliorated by lysozyme chloride.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
Supported by a grant (NSC 91-2320-B006-103) from National Sciences Council of Taiwan, China
References
- 1.Garner A, Flemström G, Allen A, Heylings JR, McQueen S. Gastric mucosal protective mechanisms: roles of epithelial bicarbonate and mucus secretions. Scand J Gastroenterol Suppl. 1984;101:79–86. [PubMed] [Google Scholar]
- 2.Asakura K, Kojima T, Shirasaki H, Kataura A. Evaluation of the effects of antigen specific immunotherapy on chronic sinusitis in children with allergy. Auris Nasus Larynx. 1990;17:33–38. doi: 10.1016/s0385-8146(12)80018-6. [DOI] [PubMed] [Google Scholar]
- 3.Bhatnagar A. Biochemical mechanism of irreversible cell injury caused by free radical-initiated reactions. Mol Cell Biochem. 1994;137:9–16. doi: 10.1007/BF00926034. [DOI] [PubMed] [Google Scholar]
- 4.Bhide SV, Gothoskar SV, Shivapurkar NM. Arecoline tumorigenicity in Swiss strain mice on normal and vitamin B deficient diet. J Cancer Res Clin Oncol. 1984;107:169–171. doi: 10.1007/BF01032602. [DOI] [PubMed] [Google Scholar]
- 5.Biziulevichius GA, Arestov IG. In vivo studies on lysosubtilin. I. Efficacy for prophylaxis and treatment of gastrointestinal disorders in newborn calves. Vet Res. 1997;28:19–35. [PubMed] [Google Scholar]
- 6.Bolaños JP, Heales SJ, Peuchen S, Barker JE, Land JM, Clark JB. Nitric oxide-mediated mitochondrial damage: a potential neuroprotective role for glutathione. Free Radic Biol Med. 1996;21:995–1001. doi: 10.1016/s0891-5849(96)00240-7. [DOI] [PubMed] [Google Scholar]
- 7.Burton-Bradley BG. Is "betel chewing" carcinogenic? Lancet. 1979;2:903. doi: 10.1016/s0140-6736(79)92716-8. [DOI] [PubMed] [Google Scholar]
- 8.Chang KM. Betel nut chewing and mouth cancer in Taiwan. 2. Observation of the oral mucosa in the betel nut chewer. Taiwan YiXueHui ZaZhi. 1966;65:79–85. [PubMed] [Google Scholar]
- 9.Corne SJ, Morrissey SM, Woods RJ. Proceedings: A method for the quantitative estimation of gastric barrier mucus. J Physiol. 1974;242:116P–117P. [PubMed] [Google Scholar]
- 10.Davenport HW. Destruction of the gastric mucosal barrier by detergents and urea. Gastroenterology. 1968;54:175–181. [PubMed] [Google Scholar]
- 11.Itoh M, Guth PH. Role of oxygen-derived free radicals in hemorrhagic shock-induced gastric lesions in the rat. Gastroenterology. 1985;88:1162–1167. doi: 10.1016/s0016-5085(85)80075-5. [DOI] [PubMed] [Google Scholar]
- 12.Gislason H, Sørbye H, Abdi-Dezfuli F, Waldum HL, Svanes K. Role of prostaglandins and histamine in hyperemic response to superficial and deep gastric mucosal injury and H+ back-diffusion in cats. Dig Dis Sci. 1995;40:1669–1678. doi: 10.1007/BF02212687. [DOI] [PubMed] [Google Scholar]
- 13.Hincke MT, Gautron J, Panheleux M, Garcia-Ruiz J, McKee MD, Nys Y. Identification and localization of lysozyme as a component of eggshell membranes and eggshell matrix. Matrix Biol. 2000;19:443–453. doi: 10.1016/s0945-053x(00)00095-0. [DOI] [PubMed] [Google Scholar]
- 14.Hirokawa K, Kawasaki H. Changes in glutathione in gastric mucosa of gastric ulcer patients. Res Commun Mol Pathol Pharmacol. 1995;88:163–176. [PubMed] [Google Scholar]
- 15.Horiuchi H, Abe S, Maeda K, Watanabe M. Anti-inflammatory, analgesic effect of lysozyme chloride following direct pulpectomy and root canal filling. Shikai Tenbo. 1981;58:1007–1012. [PubMed] [Google Scholar]
- 16.Horowitz BZ, Marquardt K, Swenson E. Calcium polysulfide overdose: a report of two cases. J Toxicol Clin Toxicol. 1997;35:299–303. doi: 10.3109/15563659709001215. [DOI] [PubMed] [Google Scholar]
- 17.Hung CR. Importance of histamine, glutathione and oxyradicals in modulating gastric haemorrhagic ulcer in septic rats. Clin Exp Pharmacol Physiol. 2000;27:306–312. doi: 10.1046/j.1440-1681.2000.03241.x. [DOI] [PubMed] [Google Scholar]
- 18.Hung CR, Wang PS. Role of acid back-diffusion, glutathione, oxyradical, and histamine in antral hemorrhagic ulcer in rats: the protective effect of lysozyme chloride and antioxidants. J Lab Clin Med. 2002;140:142–151. doi: 10.1067/mlc.2002.126412. [DOI] [PubMed] [Google Scholar]
- 19.Hung CR, Wang PS. Gastric oxidative stress and hemorrhagic ulcer in Salmonella typhimurium-infected rats. Eur J Pharmacol. 2004;491:61–68. doi: 10.1016/j.ejphar.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 20.Hung CR, Cheng JT. Betel quid chewing damaged gastric mucosa: protective effects of cimetidine and sodium bicarbonate. Chin J Physiol. 1994;37:213–218. [PubMed] [Google Scholar]
- 21.Hung CR, Neu SL. Acid-induced gastric damage in rats is aggravated by starvation and prevented by several nutrients. J Nutr. 1997;127:630–636. doi: 10.1093/jn/127.4.630. [DOI] [PubMed] [Google Scholar]
- 22.Hung CR, Huang EY. Role of acid back-diffusion in the formation of mucosal ulceration and its treatment with drugs in diabetic rats. J Pharm Pharmacol. 1995;47:493–498. doi: 10.1111/j.2042-7158.1995.tb05837.x. [DOI] [PubMed] [Google Scholar]
- 23.Hung CR, Cheng JT, Shih CS. Gastric mucosal damage induced by arecoline seizure in rats. Life Sci. 2000;66:2337–2349. doi: 10.1016/s0024-3205(00)00564-6. [DOI] [PubMed] [Google Scholar]
- 24.Iacono VJ, Zove SM, Grossbard BL, Pollock JJ, Fine DH, Greene LS. Lysozyme-mediated aggregation and lysis of the periodontal microorganism Capnocytophaga gingivalis 2010. Infect Immun. 1985;47:457–464. doi: 10.1128/iai.47.2.457-464.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inouye K. The effects of lysozyme chloride on the immune response of patients with head and neck cancer. Gan No Rinsho. 1987;33:627–632. [PubMed] [Google Scholar]
- 26.Lee KW, Chin CT. The effects of betel-nut chewing on the buccal mucosa: a histological study. Br J Cancer. 1970;24:433–441. doi: 10.1038/bjc.1970.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mason DY, Taylor CR. The distribution of muramidase (lysozyme) in human tissues. J Clin Pathol. 1975;28:124–132. doi: 10.1136/jcp.28.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery DC. In: Experiments to compare several treatments: the analysis of variance. Hinkelmann K, Kempthorne O, editors. New York: Design and Analysis of Experiments, Wiley; NY 1984. pp. 43–80. [Google Scholar]
- 29.Oberley LW. Free radicals and diabetes. Free Radic Biol Med. 1988;5:113–124. doi: 10.1016/0891-5849(88)90036-6. [DOI] [PubMed] [Google Scholar]
- 30.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 31.Ohshima H, Friesen M, Bartsch H. Identification in rats of N-nitrosonipecotic acid as a major urinary metabolite of the areca-nut alkaloid-derived nitrosamines, N-nitrosoguvacoline and N-nitrosoguvacine. Cancer Lett. 1989;44:211–216. doi: 10.1016/0304-3835(89)90063-3. [DOI] [PubMed] [Google Scholar]
- 32.Rainsford KD. The effects of aspirin and other non-steroid anti-inflammatory/analgesic drugs on gastro-intestinal mucus glycoprotein biosynthesis in vivo: relationship to ulcerogenic actions. Biochem Pharmacol. 1978;27:877–885. doi: 10.1016/0006-2952(78)90412-4. [DOI] [PubMed] [Google Scholar]
- 33.Sato M, Oe H, Nakano M, Kawasaki H, Hirayama C. A random controlled study of the prophylactic effect of lysozyme chloride on post-transfusion hepatitis. Hepatogastroenterology. 1981;28:135–138. [PubMed] [Google Scholar]
- 34.Sava G, Ceschia V, Pacor S, Zabucchi G. Observations on the antimetastatic action of lysozyme in mice bearing Lewis lung carcinoma. Anticancer Res. 1993;11:1109–1113. [PubMed] [Google Scholar]
- 35.Strubelt O, Hoppenkamps R. Relations between gastric glutathione and the ulcerogenic action of non-steroidal anti-inflammatory drugs. Arch Int Pharmacodyn Ther. 1983;262:268–278. [PubMed] [Google Scholar]
- 36.Mizui T, Sato H, Hirose F, Doteuchi M. Effect of antiperoxidative drugs on gastric damage induced by ethanol in rats. Life Sci. 1987;41:755–763. doi: 10.1016/0024-3205(87)90456-5. [DOI] [PubMed] [Google Scholar]
- 37.Williams SE, Turnberg LA. Retardation of acid diffusion by pig gastric mucosa: a potential role in mucosal protection. Gastroenterology. 1980;79:299–304. [PubMed] [Google Scholar]
- 38.Yamaoka K, Yoshioka T. Effects of lysozyme chloride on immune responses of patients with uterine cervical cancer. Gan To Kagaku Ryoho. 1983;10:1803–1809. [PubMed] [Google Scholar]
- 39.Yoganathan P. Betel chewing creeps into the New World. N Z Dent J. 2002;98:40–45. [PubMed] [Google Scholar]


