ABSTRACT
Two licensed serogroup B meningococcal vaccines contain factor H binding protein (FHbp). The antigen specifically binds human FH, which downregulates complement. In wild-type mice whose mouse FH does not bind to FHbp vaccines, the serum anti-FHbp antibody response inhibited binding of human FH to FHbp. The inhibition was important for eliciting broad anti-FHbp serum bactericidal activity. In human FH transgenic mice and some nonhuman primates, FHbp was able to form a complex with FH and FHbp vaccination elicited anti-FHbp antibodies that did not inhibit FH binding. To investigate the human anti-FHbp repertoire, we cloned immunoglobulin heavy- and light-chain-variable-region genes of individual B cells from three adults immunized with FHbp vaccines and generated 10 sequence-distinct native anti-FHbp antibody fragments (Fabs). All 10 Fabs bound to live meningococci; only 1 slightly inhibited binding of human FH, while 4 enhanced FH binding. Affinity-purified anti-FHbp antibody from serum of a fourth immunized adult also enhanced binding of human FH to live meningococcal bacteria. Despite the bound FH, the affinity-purified serum anti-FHbp antibodies elicited human complement-mediated bactericidal activity that was amplified by the alternative pathway. The lack of FH inhibition by the human anti-FHbp Fabs and serum antibodies suggests that binding of human FH to the vaccine antigen skews the anti-FHbp antibody repertoire to epitopes outside the FH-binding site. Mutant FHbp vaccines with decreased FH binding may represent a means to redirect the human antibody repertoire to epitopes within the FH binding site, which can inhibit FH binding and, potentially, increase safety and protective activity.
IMPORTANCE
Two meningococcal vaccines contain factor H binding protein (FHbp). Immunized mice whose mouse factor H (FH) does not bind to FHbp develop serum anti-FHbp antibodies that block binding of human FH to the bacteria. With less bound FH, the bacteria become more susceptible to complement killing. To investigate human responses, we isolated 10 recombinant anti-FHbp antibody fragments (Fabs) from immune cells of three immunized adults. One slightly inhibited binding of FH to the bacteria, and four enhanced FH binding. Purified serum anti-FHbp antibodies from a fourth immunized adult also enhanced FH binding. Although bound FH would be expected to block the alternative pathway, the human anti-FHbp antibodies retained bactericidal activity and the ability to activate the alternative pathway. Mutant FHbp vaccines with decreased binding to human FH may redirect the human antibody repertoire to epitopes within the FH binding site that inhibit FH binding, which are expected to increase protective activity.
INTRODUCTION
Meningococci cause meningitis and sepsis worldwide. The strains can be subdivided into serogroups based on antigenically and chemically distinctive capsular polysaccharides. Conjugate vaccines that target the polysaccharide capsule are available against serogroups A, C, W, and Y (1). Because the group B capsular polysaccharide is an autoantigen (2), two recently licensed serogroup B vaccines target protein antigens (3, 4). Both of these vaccines contain factor H-binding protein (FHbp), which is a surface-exposed lipoprotein that binds human complement factor H (FH) (5). A key property of FHbp is its ability to recruit human FH to the bacterial surface, which downregulates the complement alternative pathway (5). This mechanism is important for the ability of the organism to evade human complement-mediated serum bactericidal activity and invade the host (5, 6).
To date, more than 800 natural amino acid sequence variants of FHbp have been identified and are accessible in the public database at http://pubmlst.org/neisseria/fHbp. Based on amino acid sequence relatedness, FHbp can be classified into two subfamilies, A and B (7), or three variant groups, 1, 2, and 3 (8). In general, serum anti-FHbp bactericidal activity is specific against strains expressing FHbp sequence variants from the homologous antigenic variant group or subfamily as the vaccine antigen.
FHbp immunization of humans (9–11) and mice (3, 7, 8, 12) elicits serum anti-FHbp bactericidal antibodies. However, binding of FH to FHbp is specific for human FH (13) and FH from some nonhuman primates (13, 14). When humans and nonhuman primates are immunized, the vaccine antigen can form a complex with FH, which does not occur in wild-type mice, whose mouse FH does not bind to FHbp. In immunized human FH transgenic mice (15–17) and infant rhesus macaques (18), binding of FH to the vaccine antigen skews the serum anti-FHbp antibody repertoire to epitopes outside the FH binding site. These antibodies have lower complement-mediated bactericidal activity than anti-FHbp antibodies that inhibit human FH binding.
To investigate the anti-FHbp repertoire of immunized humans, we cloned the heavy- and light-chain immunoglobulin variable-region genes (VH and VL genes, respectively) from individual peripheral blood plasmablast cells from three humans immunized with vaccines containing FHbp. The natively paired VH and VL genes were expressed in Escherichia coli, and the resulting recombinant antibody fragments (Fabs) were characterized for antigen binding and the ability to inhibit binding of human FH to live bacteria. We also investigated the ability of affinity-purified serum anti-FHbp antibodies from a fourth immunized adult to inhibit binding of human FH and elicit complement-mediated bactericidal activity.
RESULTS
Binding of recombinant human Fabs to FHbp.
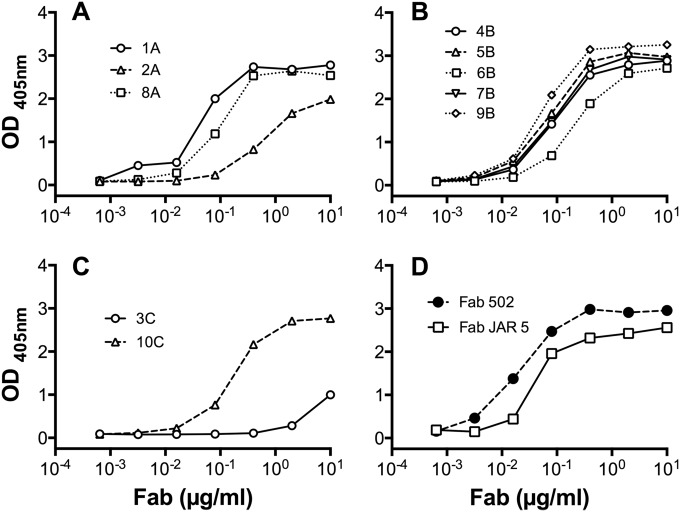
We selected 10 anti-FHbp Fabs for characterization based on their having distinct immunoglobulin gene rearrangements (see Table S1 in the supplemental material). Thus, each Fab expressed a distinct paratope, which was derived from an unrelated B cell. By enzyme-linked immunosorbent assay (ELISA), each of the 10 anti-FHbp Fabs from the three immunized humans showed concentration-dependent binding to recombinant FHbp (ID 1), which is the variant group 1 antigen in the vaccines (Fig. 1A, B, and C for subjects A, B, and C, respectively). Most of the human Fabs had concentration-dependent binding similar to the binding of two control chimeric human-mouse anti-FHbp Fabs, derived from monoclonal antibody (MAb) 502 and MAb JAR 5 (Fig. 1D). The control Fabs were encoded by V region gene segments from murine anti-FHbp MAbs 502 (19) and JAR 5 (20).
FIG 1 .
Binding of anti-FHbp Fabs to recombinant FHbp ID 1 as measured by ELISA. (A) Fabs prepared from subject A. (B) Fabs prepared from subject B. (C) Fabs prepared from subject C. (D) Control human-mouse chimeric anti-FHbp Fabs, 502 and JAR 5, which were derived from murine MAb 502 (19) and JAR 5 (20), respectively.
Cross-reactivity of human anti-FHbp Fabs.
To assess the FHbp cross-reactivity of binding by the human anti-FHbp Fabs, we measured the ability of soluble FHbp amino acid sequence variants from variant groups 1, 2, and 3 to inhibit binding of the Fabs to the nominal FHbp vaccine antigen (ID 1) by ELISA (Table 1). Representative data for four human Fabs and five different FHbp amino acid sequence variants are shown (Fig. 2). Overall, three of the Fabs were inhibited only by FHbp ID 1 (see, for example, Fig. 2A). Five additional Fabs were inhibited by FHbp ID 1 and by one to four additional FHbp sequence variants in variant group 1. For example, Fab 4B was inhibited by FHbp ID 1 and ID 4, and Fab 7B was inhibited by all five FHbp sequence variants tested in variant group 1 (Fig. 2B and C; note that data are shown only for inhibition by FHbp ID 1, 4, and 55 in variant group 1). Two Fabs (9B and 10C), which were from donors B and C, respectively, were broadly cross-reactive; each was inhibited by all of the FHbp sequence variants tested in variant groups 1, 2, and 3 (see, for example, Fig. 2D).
TABLE 1 .
Summary of cross-reactivity of the human anti-FHbp Fabsa
| Fab no.b | Cross-reactivity result for FHbp with indicated IDc |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variant group 1 |
Variant group 2 |
Variant group 3 | ||||||
| 1 | 4 | 13 | 74 | 55 | 22 | 77 | 28 | |
| 1A | +d | − | − | − | − | − | − | − |
| 2A | + | − | − | − | − | − | − | − |
| 3C | + | − | − | − | − | − | − | − |
| 4B | + | + | − | − | − | − | − | − |
| 5B | + | + | − | − | − | − | − | − |
| 6B | + | + | − | + | − | − | − | − |
| 7B | + | + | + | + | + | − | − | − |
| 8A | + | + | + | + | + | − | − | − |
| 9B | + | + | + | + | + | + | + | + |
| 10C | + | + | + | + | + | + | + | + |
Tested by inhibition ELISA using soluble FHbp variants as inhibitors (see Materials and Methods).
Suffixes A, B, and C correspond to the three different human subjects.
FHbp ID number from the public database at http://pubmlst.org/neisseria/fHbp and variant group number as described by Masignani et al. (8).
+, at least 40% inhibition at the highest inhibitor concentration tested (50 µg/ml); −, less than 40% inhibition at the highest inhibitor concentration tested.
FIG 2 .
Cross-reactivity of human anti-FHbp Fabs with divergent FHbp sequence variants. Reactivity was determined by the ability of soluble FHbp sequence variants to inhibit binding of Fabs to immobilized FHbp ID 1 by ELISA. Representative data are shown for four Fabs and five FHbp sequence variants. The key for all four panels is shown in panel D. FHbp ID 1, 4, and 55 are in variant group 1; ID 77 is in variant group 2; ID 28 is in variant group 3. The cross-reactivity data for all 10 Fabs and 8 FHbp sequence variants are summarized in Table 1.
Affinity of Fabs for FHbp.
We measured the binding affinity of the human Fabs to immobilized FHbp ID 1 by surface plasmon resonance (SPR). The 10 human Fabs had a >6,000-fold range in affinity, with equilibrium dissociation constant (KD) values from 0.019 to 130 nM (Table 2). The two human Fabs that showed the lowest binding to FHbp by ELISA, Fabs 2A and 3C (Fig. 1A and C), had the lowest affinities as measured by SPR (KD = 115 and 130 nM, respectively). In comparison, the control chimeric human-mouse Fab JAR 5 had relatively high affinity (KD = 0.05 nM) and Fab 502 had moderate affinity (KD = 4.8 nM).
TABLE 2 .
Summary of kinetic data on binding of human Fabs to FHbp ID 1 as measured by surface plasmon resonancea
| Fab no.b | ka × 10−6 (M−1 s−1) | kd × 103 (s− 1) | KD × 109 (M) | Rmax (RU) | χ2 (RU2) | GenBank accession no. |
|
|---|---|---|---|---|---|---|---|
| Light chain | Heavy chain | ||||||
| 1A | 2.25 | 0.1 | 0.44 | 45 | 0.02 | KP770107 | KP770108 |
| 2A | 0.53 | 61.0 | 115 | 31 | 0.38 | KP770109 | KP770110 |
| 3C | 0.03 | 3.2 | 130 | 11 | 0.28 | KP770111 | KP770112 |
| 4B | 0.72 | 0.6 | 0.86 | 119 | 0.88 | KR017714 | KR017716 |
| 5B | 0.39 | 4.1 | 10.6 | 141 | 2.1 | KP770113 | KP770114 |
| 6B | 0.50 | 0.4 | 0.82 | 98 | 0.20 | KP770115 | KP770116 |
| 7B | 0.18 | 4.1 | 22.9 | 114 | 0.87 | KR017715 | KR017717 |
| 8A | 2.30 | 2.0 | 1.04 | 57 | 0.02 | KP770117 | KP770118 |
| 9B | 1.85 | 0.04 | 0.019 | 58 | 0.55 | KP770119 | KP770120 |
| 10C | 0.93 | 0.5 | 0.68 | 132 | 0.73 | KP770121 | KP770122 |
| JAR 5 | 8.84 | 0.5 | 0.054 | 84 | 0.14 | JF715927 | JF715926 |
| Fab 502 | 1.09 | 5.3 | 4.8 | 105 | 1.1 | EU835941 | EU835942 |
ka, association rate constant; kd, dissociation rate constant; KD, equilibrium dissociation constant; Rmax, maximal binding response units (RU); χ2, chi-square value indicating quality of fit of data to a 1:1 binding model. χ2 was <3% of Rmax.
Binding of the Fabs to live bacteria.
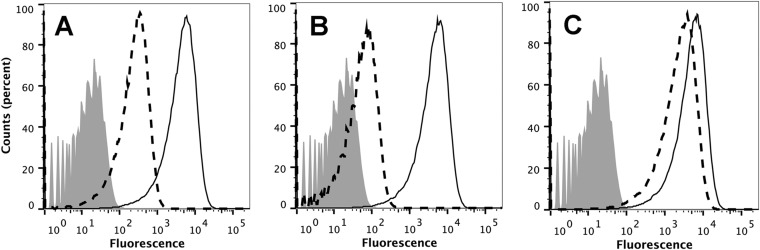
By flow cytometry, all 10 human anti-FHbp Fabs bound to the surface of live bacteria of serogroup B strain H44/76, which expresses FHbp ID 1. The binding of Fab 1A with high affinity for FHbp and of Fab 3C with low affinity is shown in Fig. 3. At 10 µg/ml, the higher-affinity Fab gave greater binding than the lower-affinity Fab (Fig. 3A). At 2 µg/ml, the relative difference in binding between the two Fabs was even greater (Fig. 3B). For comparison, data are shown for binding of 2 µg/ml of the two control chimeric human-mouse Fabs (Fig. 3C).
FIG 3 .
Binding of Fabs to live meningococci as measured by flow cytometry. The test strain was serogroup B strain H44/76 with FHbp ID 1 in variant group 1. (A) Binding of Fabs 1A (solid line) and 3C (dashed line) at 10 µg/ml. (B) Binding of the same Fabs at 2 µg/ml. (C) Binding of control chimeric human-mouse Fabs JAR 5 (solid line) and Fab 502 (dashed line) at 2 µg/ml. Bound Fab was detected with (Fab-specific) biotinylated goat anti-human IgG and streptavidin conjugated to phycoerythrin. Shaded gray histogram, bacteria without FH or Fab.
The anti-FHbp Fabs enhance FH binding to live meningococci.
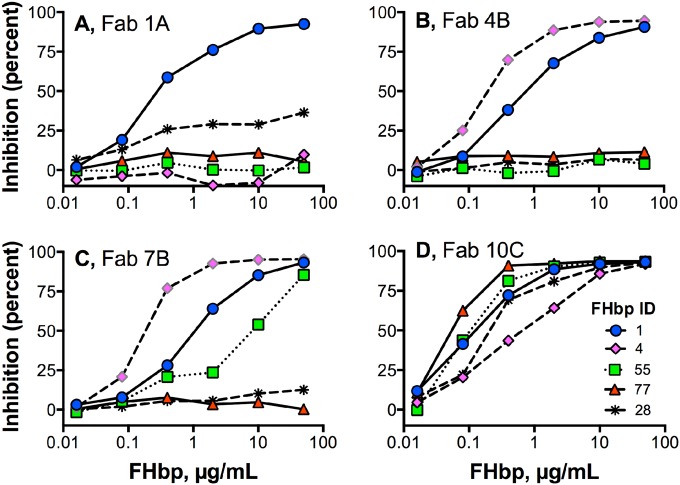
In previous studies, the ability of mouse anti-FHbp MAbs or serum anti-FHbp antibodies to inhibit FH binding to FHbp was important for eliciting broad human complement-mediated anti-FHbp bactericidal activity (12, 21). With less human FH bound to the bacterial surface, there was less complement downregulation, which resulted in greater C3b deposition and greater bactericidal activity (12, 21, 22). We therefore used flow cytometry to measure the ability of the human Fabs to inhibit binding of human FH to the surface of live bacteria from serogroup B strain H44/76. Of the 10 Fabs, only 1 (Fab 5B) showed slight (~50%) inhibition of human FH binding (Fig. 4A). Four Fabs showed 3- to 6-fold enhancement of human FH binding; representative data for Fab 2A are shown (Fig. 4B). The remaining five Fabs showed less-than-2-fold enhancement of human FH binding (representative data for Fab 10C are shown; Fig. 4C). As controls, the chimeric human-mouse JAR 5 Fab showed strong inhibition of human FH binding (Fig. 4D) and the 502 Fab enhanced human FH binding (Fig. 4E).
FIG 4 .
Effect of anti-FHbp Fabs on binding of FH to live meningococci. The test strain was serogroup B strain H44/76. Solid line, FH alone (2 µg/ml); dashed line, FH plus Fab (10 µg/ml); shaded gray histogram, bacteria without FH or Fab. (A) Slight inhibition of FH binding by human anti-FHbp Fab 5B (see also Fig. 5). (B) Enhancement of FH binding by Fab 2A. (C) Slight enhancement of FH binding by Fab 10C (see also Fig. 5). (D) Inhibition of FH binding by control chimeric human-mouse chimeric Fab JAR 5. (E) Enhancement of FH binding by control chimeric human-mouse Fab 502.
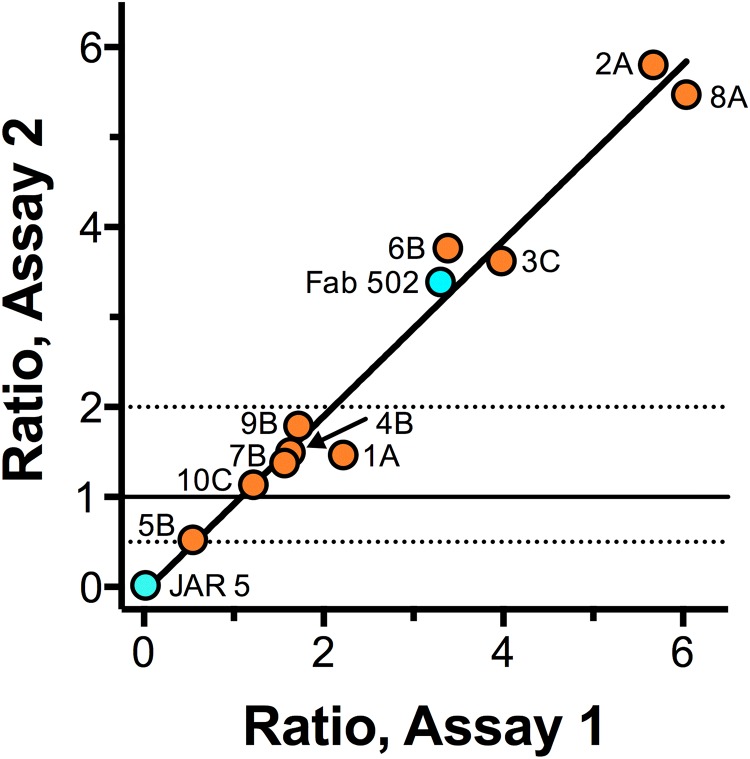
The results of testing inhibition or enhancement of FH binding to the bacteria as measured by flow cytometry were reproducible; the respective results from two independent assays are shown (Fig. 5). The results are expressed as the ratio of the median fluorescence intensity measured when the bacteria were incubated with the Fab and human FH to that measured when the bacteria were incubated with human FH alone. In this assay, the control chimeric human-mouse JAR 5 Fab gave nearly 100% inhibition (ratio of 0.02) and the control human-mouse 502 Fab gave enhancement (ratio of 3.4). The goodness of fit of a linear equation for the data for all 10 human Fabs in the two assays showed an r2 value of 0.97 (P < 0.001).
FIG 5 .
Effect of Fab on FH binding to the bacteria measured in two independent flow cytometric assays. Data are shown as the ratios of the median fluorescence for bound FH in the presence of Fab to the median fluorescence for bound FH without Fab. Ratios of ≥2 represent significant enhancement; ratios of ≤0.5 represent significant inhibition. Control chimeric human-mouse Fabs 502 and JAR 5 (aqua) showed enhancement and inhibition, respectively. Of 10 human Fabs (orange) tested, 1 Fab, 5B, showed slight inhibition, 5 showed no significant inhibition or enhancement, and 4 showed 3- to 6-fold enhancement of FH binding. There was at least one Fab that enhanced FH binding from each of the three immunized human subjects tested.
Serum anti-FHbp antibodies elicited by vaccination also enhance binding of FH.
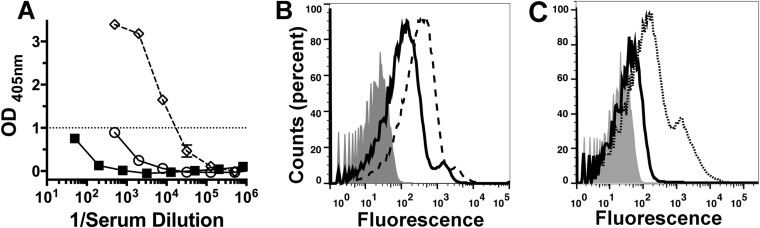
We did not have serum samples from the three immunized adults whose B cells were used to isolate the anti-FHbp Fabs. To investigate whether serum antibodies from a vaccinated human enhance binding of human FH to FHbp, we investigated serum from an adult immunized with the licensed Novartis serogroup B vaccine (4CMenB) 6 years after receiving two doses as part of a clinical trial. Two weeks before the booster dose, the serum IgG anti-FHbp titer was 1:800, which increased to 1:25,000 3 weeks after the booster (Fig. 6A). Compared with the prebooster serum, there was an increase in binding of human FH to live bacteria of wild-type serogroup B strain H44/76 with the serum obtained after vaccination (Fig. 6B). To confirm whether the enhanced FH binding was the result of the presence of serum anti-FHbp antibodies, as opposed to antibodies elicited by other antigens in the 4CMenB vaccine, we purified anti-FHbp antibodies from the post-dose 3 serum using an FHbp affinity column. According to ELISA results, the serum anti-outer membrane vesicle (OMV) and anti-neisserial heparin binding antigen (NHba) titers of the adsorbed serum were not affected by adsorption with FHbp, and the affinity-purified anti-FHbp antibody contained no detectable antibodies to OMV or NHba (data not shown). With a 1:600 dilution of the adsorbed serum as a source of antibodies to 4CMenB antigens other than FHbp and endogenous human FH, there was low-level binding of FH to the bacteria (solid line, Fig. 6C). Following the addition of a 1:600 dilution (~0.1 µg/ml) of the affinity-purified anti-FHbp antibodies to the 1:600 dilution of the adsorbed serum, there was enhanced binding of FH to the meningococcal surface (dotted line).
FIG 6 .
Serum antibodies from an adult human boosted with a serogroup B vaccine containing FHbp enhance FH binding. A healthy adult was immunized with a third dose of a serogroup B vaccine (4CMenB; Novartis Vaccines) which contained FHbp ID 1 in variant group 1. The vaccine was given 6 years after doses 1 and 2. (A) IgG binding to FHbp (ELISA) in serum samples obtained 2 weeks before (open circles) and 3 weeks after (open diamonds) dose 3. Negative-control serum (filled squares) was from a nonvaccinated adult. (B) Binding of serum FH to serogroup B strain H44/76. All sera were heated to inactivate endogenous complement (FH is stable under the conditions used [49]). Gray histogram, bacteria without serum; solid line, 1:600 dilution of serum obtained 2 weeks before dose 3; dashed line, 1:600 dilution of serum obtained 3 weeks after dose 3. (C) Binding of human FH to strain H44/76 incubated with a 1:600 dilution of post-dose 3 serum that had been depleted of anti-FHbp antibodies (solid line) or with a 1:600 dilution of the adsorbed serum mixed with 0.1 µg/ml of affinity-purified anti-FHbp antibodies eluted from the FHbp-Sepharose used for antibody depletion (dotted line). The gray histogram represents background binding to bacteria in the absence of FH or serum (secondary antibody alone).
Anti-FHbp bactericidal activity.
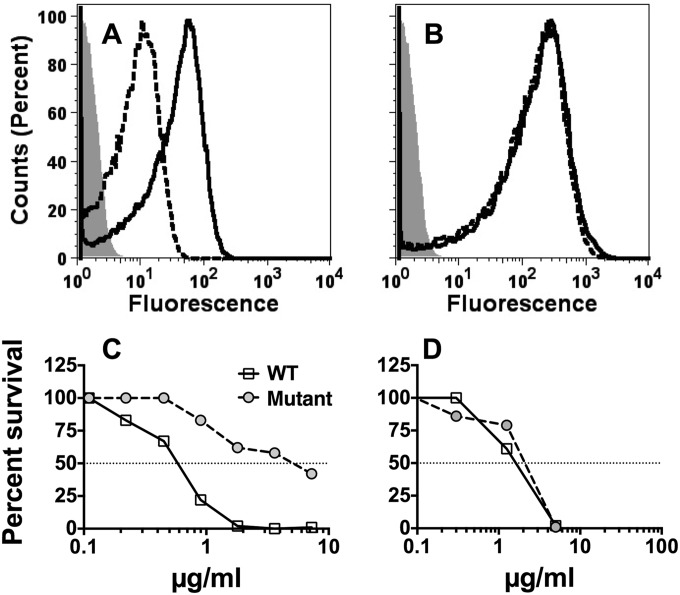
We tested bactericidal activity of the affinity-purified anti-FHbp antibodies against a wild-type serogroup B strain, H44/76, with naturally high FHbp expression and a previously described mutant of H44/76 with lower FHbp expression (23) (Fig. 7). The mutant had about 10-fold-lower binding with a mixture of two anti-FHbp MAbs than the wild-type strain (Fig. 7A) but had binding similar to that seen with a control anti-PorA P1.7 MAb (Fig. 7B). Despite the enhanced FH binding, the affinity-purified anti-FHbp antibodies were bactericidal with human complement, particularly against the wild-type strain with higher FHbp expression (Fig. 7C). The wild-type and mutant strains were equally susceptible to the complement-mediated bactericidal activity of a control anti-PorA MAb (Fig. 7D).
FIG 7 .
The effect of strain expression of FHbp on complement-mediated bactericidal activity of affinity-purified human anti-FHbp antibodies. Data are from wild-type strain H44/76 with naturally high FHbp expression (solid line) and from a mutant of strain H44/76 with lower FH expression (dashed line). (A and B) Flow cytometric detection of bound antibody. (A) Murine anti-FHbp MAbs (JAR 5 and JAR 4; 50 µg/ml). (B) Control anti-PorA P1.7 MAb (5 µg/ml). (C and D) Bactericidal activity with human complement. (C) Affinity-purified human anti-FHbp antibody. (D) Control murine anti-PorA P1.7 MAb. Data were replicated in two or three independent experiments.
With individual chimeric-human anti-FHbp MAbs, blocking of binding of human FH to the bacterial surface was critical for eliciting complement-mediated bactericidal activity, which required alternative pathway amplification (22). The high bactericidal activity of the affinity-purified human anti-FHbp antibodies against the H44/76 wild-type strain, therefore, was surprising in light of the enhanced FH binding induced by these antibodies, which would be expected to block the alternative pathway. To assess the basis for the bactericidal activity, we investigated the relative roles of the different complement pathways.
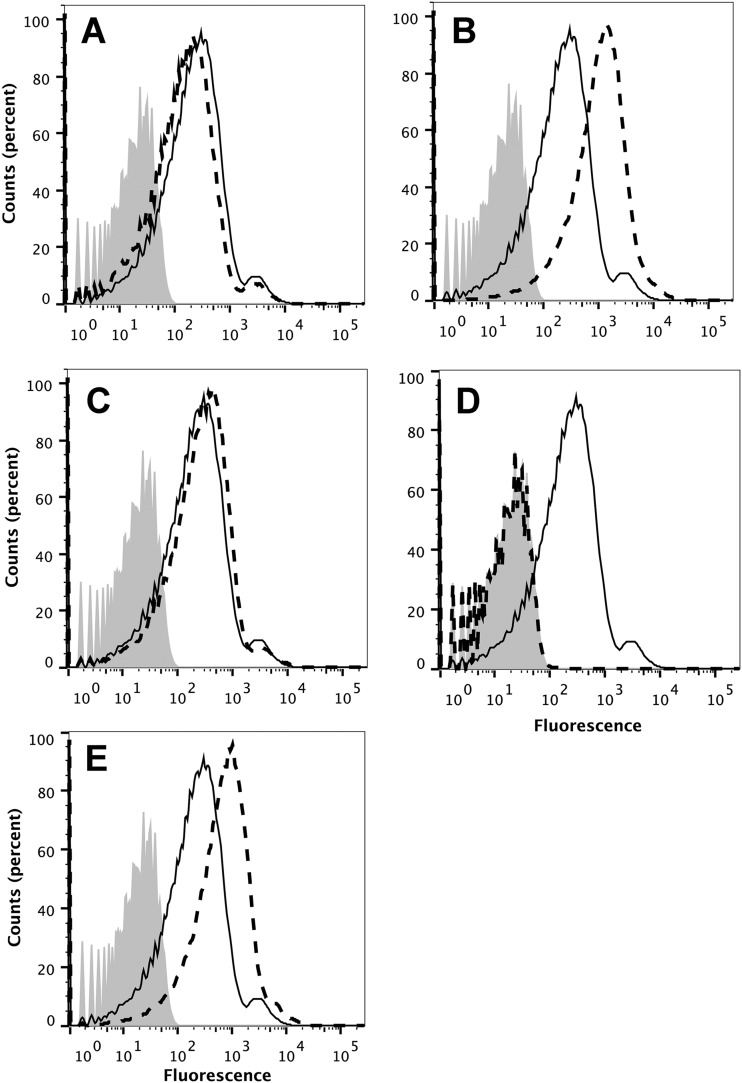
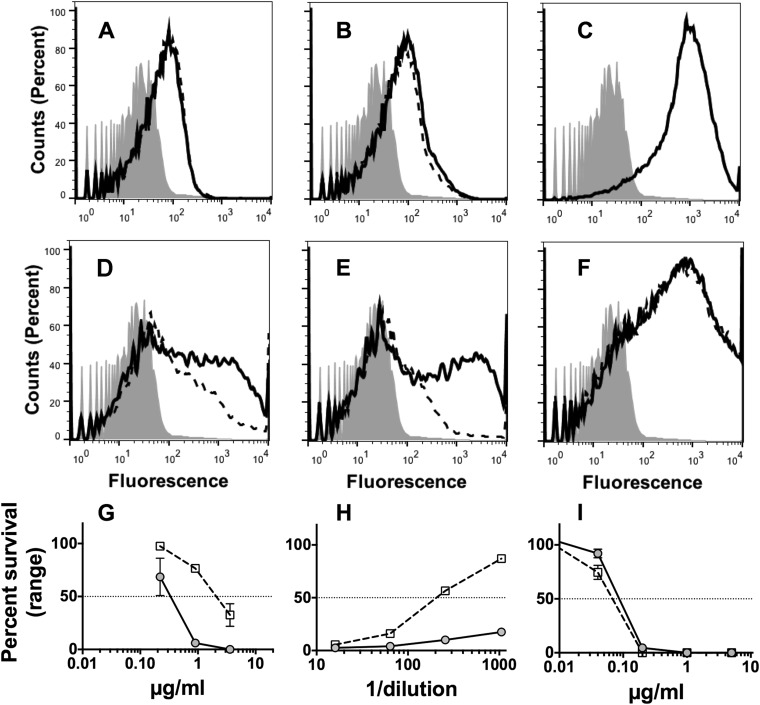
Deposition of human C4b on the surface of live Neisseria meningitidis cells can be used as a marker of lectin and/or classical complement pathway activation (22). The affinity-purified human anti-FHbp antibodies activated C4b deposition on strain H44/76 (Fig. 8A), as did a control mouse polyclonal anti-FHbp antiserum (Fig. 8B). A control chimeric human IgG1 mouse anti-PorA P1.16 MAb (24) gave about 10-fold more C4b deposition than the human or mouse polyclonal anti-FHbp antibodies (Fig. 8C). This result was expected since PorA is more abundant on the bacterial surface than FHbp (25) and since PorA can exist in a trimeric state (26) compared with monomeric FHbp. The resulting anti-PorA classical pathway activity was therefore higher than that elicited by the polyclonal mouse or human anti-FHbp antibodies. As expected, there also was no effect of the addition of an anti-factor B (Bb) MAb on C4b deposition elicited by the anti-PorA MAb (Fig. 8, dashed lines), since the anti-Bb MAb inhibits only the alternative pathway (22).
FIG 8 .
Affinity-purified human anti-FHbp antibodies require alternative pathway amplification for maximum complement-mediated bactericidal activity against the wild-type strain of H44/76. (A to C) Effect of the addition of a murine anti-Bb MAb on C4b deposition measured by flow cytometry. Solid line, no anti-Bb MAb; dashed line, addition of anti-Bb MAb (100 µg/ml); filled histograms, bacteria only without antibody or complement. (A) Affinity-purified human anti-FHbp antibody (1:50 dilution, or 1.16 µg/ml). (B) Mouse polyclonal anti-FHbp serum (1:50 dilution). (C) Chimeric human IgG1-mouse anti-PorA P1.16 MAb (1 µg/ml). (D to F) Effect of the addition of a murine anti-Bb MAb on C3b deposition. Symbols and conditions are the same as those described for panels A, B, and C. Note, in panels C and F, solid and dashed lines are superimposed. (D) Affinity-purified human anti-FHbp antibody. (E) Mouse polyclonal anti-FHbp serum. (F) Chimeric human mouse anti-PorA P1.16 MAb. (G to I) Effect of a murine anti-Bb MAb on bactericidal activity. (G) Affinity-purified human anti-FHbp antibody. (H) Mouse polyclonal anti-FHbp serum. (I) Chimeric human mouse anti-PorA P1.16 MAb. Filled circles with solid line, bactericidal activity with no anti-Bb MAb; open squares with dashed line, bactericidal activity in the presence of anti-Bb MAb (100 µg/ml). Representative data from two to three replicate experiments are shown.
We also measured deposition of C3b on H44/76 as a marker of activation of any of the three complement pathways individually or in combination. The anti-Bb MAb downregulated deposition of C3b elicited by the control mouse polyclonal anti-FHbp antiserum, which is known to inhibit binding of human FH to the bacteria (Fig. 8E) (12). This result was expected (22) since, with less bound human FH, classical pathway activation by the anti-FHbp antibodies can recruit alternative pathway amplification of C3b deposition, which would be blocked by the addition of the anti-Bb reagent. However, the anti-Bb MAb also decreased C3b deposition elicited by the affinity-purified human anti-FHbp antibodies (Fig. 8D), which did not inhibit FH binding. This result was unexpected since the bound human FH should have downregulated the alternative pathway and the effect of the addition of anti-Bb MAb on C3b deposition should have been small. These data indicate that the human anti-FHbp antibodies can recruit the alternative pathway despite the presence of bound human FH. As a negative control, we tested the effect of the anti-Bb MAb on C3b deposition elicited by the chimeric human IgG1 mouse anti-PorA P1.16 MAb (Fig. 8F). C3b deposition was not affected by inhibiting the alternative pathway since the anti-PorA MAb does not block FH binding, and with bound FH, the alternative pathway is downregulated and anti-PorA C3b deposition results only from the classical pathway.
We then measured the effect of blocking the alternative pathway on anti-FHbp bactericidal activity. As observed for the C3b deposition, the addition of the anti-Bb MAb markedly decreased the bactericidal activity elicited by the mouse anti-FHbp antiserum or human anti-FHbp antibodies (Fig. 8H and G, respectively) but had no effect on the bactericidal activity of the control chimeric human IgG1 mouse anti-PorA MAb (Fig. 8I). Thus, the bactericidal activity of the affinity-purified human anti-FHbp antibodies, but not the anti-PorA MAb, used the alternative pathway despite the presence of bound human FH.
DISCUSSION
The purpose of our study was to determine the fine antigenic specificity of human anti-FHbp antibodies and the ability of the antibodies to inhibit or enhance FH binding, the importance of which is discussed below. To investigate the human anti-FHbp antibody repertoire, we characterized 10 recombinant human anti-FHbp Fabs derived from individual B cells from three immunized adults. Each of the Fabs had distinct paratopes since they were encoded by different rearrangements of V region immunoglobulin genes. The VH and VL immunoglobulin genes also were mutated as defined by divergence from germ line sequences (see Table S1 in the supplemental material). For each subject, the affinities of the respective Fabs ranged from ~200-fold to 1,200-fold (Table 2). In a polyclonal serum antibody response, the higher-affinity antibodies would be expected to contribute more to the functional antibody response than the lower-affinity antibodies.
Two of the human Fabs, 4B and 5B, which were isolated from the same subject, had the same H-chain and L-chain germ line gene rearrangements except for different diversity (D) regions (see Table S1 in the supplemental material). However, the respective mutated H-chain V regions (VDJ) of the two Fabs differed by 19 amino acids (80% identity), and the mutated L-chain V regions (VJ) differed by 12 amino acid differences (91% identity). Although the two Fabs also had the same pattern of reactivity with different FHbp sequence variants (Table 1), the mutated sequences resulted in Fab 5B having 10-fold-higher affinity for FHbp ID 1 than Fab 4B (Table 2); also, Fab 5B slightly inhibited binding of FH to FHbp whereas Fab 4B showed slight enhancement (Fig. 5). Thus, although the two Fabs had similar respective germ line gene rearrangements, amino acid sequence differences between the mutated antibodies affected the structure of the paratope and antibody function (27–29).
As described in the introduction, binding of FH to FHbp is specific for human (13) and for FH from some nonhuman primates (13, 14). In previous studies in immunized wild-type mice whose mouse FH does not bind to FHbp vaccines, the serum anti-FHbp antibodies inhibited binding of human FH to FHbp (12, 30). The inhibition of binding of human FH was critical for eliciting broad complement-mediated bactericidal activity. The reason is that FHbp is relatively sparsely exposed on the bacterial surface of many meningococcal strains (6, 20); thus, the ability of IgG anti-FHbp antibodies to engage C1q and activate the classical complement pathway may be insufficient for formation of a functional membrane attack complex (5, 21, 31) without alternative pathway amplification (22). In contrast, serum anti-FHbp antibodies of immunized human FH transgenic mice or rhesus macaques did not block binding of human FH (17, 18). For reasons that remain poorly understood, these anti-FHbp antibodies enhanced FH binding. (Relevant studies are summarized in Table S2 in the supplemental material.)
In the present study, we also observed a lack of inhibition by the human anti-FHbp Fabs isolated from three adults immunized with vaccines containing FHbp. The only exception was 1 Fab (of 10) that showed weak (~50%) inhibition. Further, four of the human anti-FHbp Fabs, and serum anti-FHbp antibodies from a fourth immunized adult, markedly enhanced human FH binding. In our previous studies, individual chimeric human IgG1 mouse anti-FHbp MAbs elicited human complement-mediated bactericidal activity only when the MAb inhibited binding of human FH (22). However, mixtures of anti-FHbp MAbs that individually did not elicit bactericidal activity could have bactericidal activity together (6, 30, 32). In general, the MAb combinations with the greatest bactericidal activity included at least one that blocked FH binding. In future studies, we plan to convert the human Fabs to intact human IgG1 antibodies to investigate their ability to elicit human anti-FHbp bactericidal activity individually and in combination, but this issue was beyond the scope of the present study.
To investigate human anti-FHbp bactericidal activity, we purified anti-FHbp antibodies from serum of a vaccinated adult. As observed with the anti-FHbp Fabs, the anti-FHbp antibodies did not inhibit FH binding but did enhance FH binding to N. meningitidis. One hypothesis is that binding of antibodies to epitopes on FHbp results in conformational changes that render FHbp more accessible for FH binding (17). For example, in a previous study, an anti-FHbp MAb (MAb 502) enhanced FH binding to FHbp (33) and, based on results of nuclear magnetic resonance (NMR) studies, binding of this MAb altered the chemical shifts of certain FHbp residues such as Ala206 and Val207 (34), which are located in the FH binding interface (35, 36).
On the basis of the enhanced FH binding, we expected the anti-FHbp bactericidal activity to depend largely on the classical complement pathway since the alternative pathway would be downregulated by the bound FH. However, this prediction proved incorrect in that deposition of C3b and bactericidal activity elicited by the human anti-FHbp antibody were largely dependent on activation of the classical pathway with alternative pathway amplification, as shown by inhibition of C3b deposition and bactericidal activity by the anti-Bb MAb. Since the anti-Bb MAb blocked formation of the C3 convertase C3bBb, the decreased C3b deposition and bactericidal activity suggested either that the bound FH was not fully functional in acceleration of C3bBb decay or that blocking the formation of the C3 convertase had a more potent downregulatory effect than on accelerating decay of the convertase. It also is possible that IgG antibodies bound to the bacterial surface served as sites for deposition of C3b, thus overcoming the downregulatory activities of FH. For example, IgG bound to bacteria can serve as a target for C3b (37) and C3b bound to IgG is resistant to FH inactivation (38). Evidence also suggests that IgG-C3b complexes produce more active C3 convertases than free C3b (39). Understanding the actual basis for the lack of complete downregulation of the alternative pathway despite the bound human FH, however, will require additional study.
The two licensed serogroup B vaccines are efficacious in humans because they can elicit serum bactericidal activity. However, the lack of FH inhibition by the anti-FHbp Fabs in the three subjects given the 4CMenB vaccine and the FH enhancement by four of the Fabs and by the serum anti-FHbp antibodies of a fourth subject informed us that the mechanism by which these human anti-FHbp antibodies confer protection is different from that seen with immunized wild-type mice.
Because of a polymorphism in macaque FH, some animals have FH that binds strongly to FHbp whereas other animals have FH that binds weakly (14). Serum antibodies from 4CMenB-vaccinated macaques with FH that bound weakly to the vaccine elicited greater activation of the classical complement pathway and higher bactericidal activity than were seen with macaques whose FH bound strongly to the FHbp antigen (18). These data, taken together with the results from immunized humans reported here, have important implications for improving FHbp vaccines. We have shown that introduction of single amino acid substitutions in FHbp can decrease FH binding by more than 100-fold (15, 40). In human FH transgenic mice, these mutant vaccines elicited serum anti-FHbp antibodies that blocked FH binding (15, 17), and the anti-FHbp antibodies had higher serum bactericidal activity than the anti-FHbp antibodies elicited by control FHbp vaccines that bound FH (15, 41–43). Thus, although the two licensed serogroup B vaccines elicit serum bactericidal activity in humans, it is likely that the protection elicited can be improved by incorporating mutations that decrease FH binding. The mutant antigens also may be safer, with less risk of eliciting autoantibodies to human FH (observed in two human FH transgenic mice immunized with the Novartis 4CMenB vaccine) (17).
MATERIALS AND METHODS
Production of anti-FHbp Fabs.
Three human subjects were immunized in Krakow, Poland, in a study sponsored by Novartis Vaccine, using two doses of multicomponent serogroup B meningococcal vaccines containing recombinant FHbp ID 1 (9, 11). The protocol was approved by the Bioethics Committee of the District Medical Doctors’ Chamber in Krakow, and written informed consent was obtained from each of the subjects. Eight days after the second dose, plasmablast cells were isolated from peripheral blood and sorted individually into the wells of a microtiter plate. The plates were frozen and sent on dry ice to the Children’s Hospital Oakland Research Institute (Oakland, CA, USA), where VH and VL genes of single cells were amplified separately and then joined by overlap extension PCR (L. Liu and A. H. Lucas, unpublished data). Products from the wells were pooled, cloned into pET22, and expressed in E. coli. Bacterial lysates were screened for binding to FHbp ID 1 by ELISA. The V region genes of positive Fabs (optical density at 405 nm [OD405] greater than 0.1) were sequenced. Thirteen Fab clones encoded by unique germ line gene rearrangements were expressed and purified by Ni2+-affinity chromatography, quantified by capture ELISA, and stored at 4°C. Of the 13 Fabs, 10 were obtained in sufficient quantity for further analysis. The GenBank accession numbers of the immunoglobulin V region genes encoding the 10 Fabs are provided (Table 2). As controls for the functional assays, we prepared two chimeric human-mouse anti-FHbp Fabs from previously described V region genes encoding murine MAbs JAR 5 (20) and 502 (19). The respective mouse V region gene segments were cloned into the same vector described above, using constant regions of the CH1 region from human IgG1 and human kappa L chain.
Recombinant FHbp.
FHbp amino acid sequence variants were expressed as soluble proteins in E. coli using T7 expression plasmid pET21b (Novagen) as previously described (8). The recombinant FHbp was purified by Ni2+-affinity chromatography using an Akta Purifier liquid chromatography system and HITRAP Chelating HP columns (GE Life Sciences) (5 ml) and buffer solutions recommended by the column manufacturer. Fractions containing FHbp were pooled, dialyzed against phosphate-buffered saline (PBS), and stored at 4°C. The concentrations of FHbp were determined by UV absorbance using the molar extinction coefficient calculated from the respective amino acid sequences and ProtParam (44).
Binding of Fabs to FHbp by ELISA.
The method was previously described (45) with the exception that bound Fab was detected by (Fab-specific) goat anti-human IgG conjugated to alkaline phosphatase (Sigma) (1:5,000 in PBS-Tween 20 [PBST]–bovine serum albumin [BSA]).
Cross-reactivity of Fabs with FHbp.
We used an inhibition ELISA with eight diverse recombinant FHbp amino acid sequence variants to measure Fab cross-reactivity. The sequence variants used as inhibitors included five from variant group 1 (ID 1, 4, 13, 74, and 55), two from variant group 2 (ID 22 and 77), and one from variant group 3 (ID 28). The wells of microtiter plates were coated with FHbp ID 1, blocked, and washed as described above for the direct anti-FHbp binding ELISA. Serial dilutions of each of the individual soluble FHbp inhibitors were added with a constant concentration of the Fab (approximately equal to that needed to yield an optical density of 2.0 in the absence of the inhibitor). The plate was incubated at 4°C overnight. After washing was performed, bound Fab was detected as described above. Percent inhibition by the heterologous FHbp soluble inhibitors was expressed as the percentage of Fab binding in the presence of soluble FHbp compared to Fab binding in the absence of inhibitor.
Fab binding and kinetic analysis by surface plasmon resonance.
Binding and kinetic analyses were performed with 200 to 400 response units of purified recombinant FHbp immobilized to a biosensor chip using amine coupling (CM5 chip and amine coupling kit; GE Life Sciences). The running buffer was HBS-EP (10 mM HEPES [pH 7.4], 150 mM NaCl, 3 mM EDTA, 0.05% surfactant P-20 [GE Life Sciences]); the regeneration buffer was 100 mM glycine (pH 2.0)–3 M NaCl or 10 mM glycine (pH 1.7). For multiple-cycle kinetic experiments, the injection times with the Fabs were 3 min for association, 5 to 10 min for dissociation, and 1 to 2 min for regeneration and the flow rate was 30 µl/min. Data were collected on a Biacore X100 Plus instrument and analyzed with X100 Evaluation software (GE Life Sciences) using a 1:1 binding model.
Bacterial growth.
We used serogroup B strain H44/76 (B:15:P1.7,16; ST-32) that expresses FHbp ID 1 (matched to the FHbp variant group 1 antigen in the vaccines). This strain is mismatched for all of the other antigens in the Novartis 4CMenB vaccine (9, 17). In some experiments, we used a previously described mutant of H44/76 with lower FHbp expression than the wild-type strain (23). The bacteria were grown in regular Franz medium (46) containing 0.02 mM CMP–N-acetylneuraminic acid (CMP-NANA) to the mid-log phase (OD600 = 0.6) as previously described (21).
Binding of Fabs to the surface of live bacteria and inhibition of FH binding.
Binding of Fabs to live meningococci was determined by flow cytometry as previously described (21). Bound Fabs were detected using biotinylated goat anti-human Fab (Rockland Immunochemicals) (1.5 µg/ml), followed by streptavidin-phycoerythrin (eBioscience) (1:300).
Flow cytometry also was used to measure the ability of the Fabs to inhibit binding of FH to live bacteria (21). The cells were grown and washed as described above, and a mixture of purified human FH (2 µg/ml final concentration) and Fab (0 or 10 µg/ml final concentration) was added. The reaction mixtures were incubated for 1 h at room temperature. Bound FH was detected using polyclonal sheep anti-human FH (Abcam) followed by washing and incubation with donkey anti-sheep IgG antibody conjugated with Alexa Fluor 488.
Bactericidal activity.
The test sera (see below) were heated for 30 min at 56°C to inactivate complement. The exogenous human complement was human serum depleted of IgG with a protein G column (HITRAP Protein G HP; GE Healthcare) (5 ml) (15).
Classical and alternative complement pathway activity elicited by anti-FHbp antibodies.
Flow cytometry was used to measure the deposition of human C3b or C4b on the surfaces of live bacteria of the H44/76 wild-type strain (21). In brief, bacteria were grown and harvested as described above and resuspended in Dulbecco’s PBS containing 1% BSA to a density of 108 CFU/ml. The bacteria were incubated with human complement (5% IgG-depleted human serum) and different concentrations of antibodies. After 10 to 15 min of incubation at room temperature, bound human C3b or C4b was detected with 1:100 dilutions of fluorescein isothiocyanate-conjugated anti-human C3c or C4b (Abcam). In some experiments, to inhibit alternative complement pathway activation, we added 100 µg/ml of a mouse anti-Bb MAb (Quidel Corp.) to the reaction mixture as previously described (22). Zymosan is a potent activator of the alternative pathway (47). By flow cytometry, this concentration of the anti-Bb MAb completely inhibited C3b deposition on zymosan particles when incubated with 15% IgG-depleted human serum (complement) under conditions that blocked the classical pathway but were permissive for the alternative pathway (chelation of Ca2+ but not Mg2+ by the addition of 10 mM EGTA and 10 mM MgCl2) (data not shown).
Human sera.
A microbiologist had been vaccinated 6 years earlier with two doses of an investigational serogroup B vaccine containing FHbp ID 1 as part of a European clinical trial sponsored by Novartis Vaccines. The subject received a third dose in Europe, where the Novartis 4CMenB vaccine is licensed. Serum samples obtained 2 weeks before and 3 weeks after the booster dose were assayed for IgG antibody titers for FHbp and their ability to inhibit or enhance FH binding to live bacteria. Informed written consent was obtained from the subject, and the studies were approved by the UCSF Benioff Children’s Hospital Oakland Institutional Review Board.
Purification of anti-FHbp antibodies.
Purified recombinant FHbp ID 1 R41S protein was coupled to activated cyanogen bromide Sepharose as previously described (48). The human serum sample (500 µl diluted 1:2 in PBS) was incubated with the immunoadsorbent for 1 h at room temperature. Efficiency of serum adsorption of anti-FHbp antibody (>98%) was determined by ELISA. Specificity of the serum antibody adsorption was confirmed by lack of adsorption of antibodies to OMV and to NHba, two other antigens in the 4CMenB vaccine (data not shown). There was no detectable binding by the affinity-purified antibody fraction measured by ELISA against two negative-control antigens, OMV and NHba.
Fab sequences.
The GenBank accession numbers for the human Fabs are listed in Table 2. The V region sequences for the control Fabs 502 and JAR 5 were reported previously (19, 20).
SUPPLEMENTAL MATERIAL
Immunoglobulin gene usage in recombinant human anti-FHbp Fab clones.
Results of studies investigating the effect of anti-FHbp antibody on binding of human FH to FHbp.
ACKNOWLEDGMENTS
The work was supported by grants R01 AI046464 (D.M.G.), R01 AI099125 (P.T.B.), and R01 AI114701 (D.M.G. and P.T.B.) from the National Institute of Allergy and Infectious Diseases, NIH. Isolation of the Fabs was supported by a research contract sponsored by Novartis Vaccines and UCSF Benioff Children’s Hospital Oakland (to A.H.L.). The work was performed in a facility funded by the Research Facilities Improvement Program (grant C06 RR016226) from the National Center for Research Resources, NIH.
We are grateful to Liliana Alleri (Novartis Vaccines, Siena, Italy) who performed the B cell sorting. We thank Elias Tsadik, Nolan Meghrouni-Brown, Heather Stefek, and Kelsey Sharkey for technical assistance and Sanjay Ram for helpful comments on the manuscript.
Footnotes
Citation Beernink PT, Giuntini S, Costa I, Lucas AH, Granoff DM. 2015. Functional analysis of the human antibody response to meningococcal factor H binding protein. mBio 6(3):e00842-15. doi:10.1128/mBio.00842-15.
REFERENCES
- 1.Pace D, Pollard AJ, Messonier NE. 2009. Quadrivalent meningococcal conjugate vaccines. Vaccine 27(Suppl 2):B30–B41. doi: 10.1016/j.vaccine.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Finne J, Leinonen M, Mäkelä PH. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355–357. [DOI] [PubMed]
- 3.Giuliani MM, Adu-Bobie J, Comanducci M, Aricò B, Savino S, Santini L, Brunelli B, Bambini S, Biolchi A, Capecchi B, Cartocci E, Ciucchi L, Di Marcello F, Ferlicca F, Galli B, Luzzi E, Masignani V, Serruto D, Veggi D, Contorni M, Morandi M, Bartalesi A, Cinotti V, Mannucci D, Titta F, Ovidi E, Welsch JA, Granoff D, Rappuoli R, Pizza M. 2006. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A 103:10834–10839. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang HQ, Hoiseth SK, Harris SL, Mcneil LK, Zhu D, Tan C, Scott AA, Alexander K, Mason K, Miller L, Dasilva I, Mack M, Zhao XJ, Pride MW, Andrew L, Murphy E, Hagen M, French R, Arora A, Jones TR, Jansen KU, Zlotnick GW, Anderson AS. 2010. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine 28:6086–6093. doi: 10.1016/j.vaccine.2010.06.083. [DOI] [PubMed] [Google Scholar]
- 5.Madico G, Welsch JA, Lewis LA, Mcnaughton A, Perlman DH, Costello CE, Ngampasutadol J, Vogel U, Granoff DM, Ram S. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol 177:501–510. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welsch JA, Ram S, Koeberling O, Granoff DM. 2008. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J Infect Dis 197:1053–1061. doi: 10.1086/528994. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, Ooi P, Smith RP, Weise P, Wetherell M, Xie X, Zagursky R, Zhang Y, Zlotnick GW. 2004. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun 72:2088–2100. doi: 10.1128/IAI.72.4.2088-2100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, Brunelli B, Pieri A, Santini L, Savino S, Serruto D, Litt D, Kroll S, Welsch JA, Granoff DM, Rappuoli R, Pizza M. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med 197:789–799. doi: 10.1084/jem.20021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, Evans A, Telford KL, Ypma E, Toneatto D, Oster P, Miller E, Pollard AJ. 2010. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis 51:1127–1137. doi: 10.1086/656741. [DOI] [PubMed] [Google Scholar]
- 10.Marshall HS, Richmond PC, Nissen MD, Jiang Q, Anderson AS, Jansen KU, Reynolds G, Ziegler JB, Harris SL, Jones TR, Perez JL. 2012. Safety and immunogenicity of a meningococcal B bivalent rLP2086 vaccine in healthy toddlers aged 18–36 months: a phase 1 randomized-controlled clinical trial. Pediatr Infect Dis J 31:1061–1068. doi: 10.1097/INF.0b013e31826327e4. [DOI] [PubMed] [Google Scholar]
- 11.Snape MD, Dawson T, Oster P, Evans A, John TM, Ohene-Kena B, Findlow J, Yu LM, Borrow R, Ypma E, Toneatto D, Pollard AJ. 2010. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr Infect Dis J 29:e71–e79. doi: 10.1097/INF.0b013e3181f59f6d. [DOI] [PubMed] [Google Scholar]
- 12.Konar M, Granoff DM, Beernink PT. 2013. Importance of inhibition of binding of complement factor H for serum bactericidal antibody responses to meningococcal factor H-binding protein vaccines. J Infect Dis 208:627–636. doi: 10.1093/infdis/jit239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granoff DM, Welsch JA, Ram S. 2009. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect Immun 77:764–769. doi: 10.1128/IAI.01191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beernink PT, Shaughnessy J, Stefek H, Ram S, Granoff DM. 2014. Heterogeneity in rhesus macaque complement factor H binding to meningococcal factor H binding protein (FHbp) informs selection of primates to assess immunogenicity of FHbp-based vaccines. Clin Vaccine Immunol 21:1505–1511. doi: 10.1128/CVI.00517-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, Granoff DM. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol 186:3606–3614. doi: 10.4049/jimmunol.1003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granoff DM, Ram S, Beernink PT. 2013. Does binding of complement factor H to the meningococcal vaccine antigen, factor H binding protein, decrease protective serum antibody responses? Clin Vaccine Immunol 20:1099–1107. doi: 10.1128/cvi.00260-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa I, Pajon R, Granoff DM. 2014. Human factor H (FH) impairs protective meningococcal anti-FHbp antibody responses and the antibodies enhance FH binding. mBio 5:e01625-14. doi: 10.1128/mBio.01625-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granoff DM, Costa I, Konar M, Giuntini S, Van Rompay KK, Beernink PT. 12 February 2015. Binding of complement factor H decreases protective anti-FHbp antibody responses of infant rhesus macaques immunized with a meningococcal serogroup B vaccine. J Infect Dis doi: 10.1093/infdis/jiv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giuliani MM, Santini L, Brunelli B, Biolchi A, Aricò B, Di Marcello F, Cartocci E, Comanducci M, Masignani V, Lozzi L, Savino S, Scarselli M, Rappuoli R, Pizza M. 2005. The region comprising amino acids 100 to 255 of Neisseria meningitidis lipoprotein GNA 1870 elicits bactericidal antibodies. Infect Immun 73:1151–1160. doi: 10.1128/IAI.73.2.1151-1160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsch JA, Rossi R, Comanducci M, Granoff DM. 2004. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J Immunol 172:5606–5615. doi: 10.4049/jimmunol.172.9.5606. [DOI] [PubMed] [Google Scholar]
- 21.Giuntini S, Reason DC, Granoff DM. 2011. Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infect Immun 79:3751–3759. doi: 10.1128/IAI.05182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giuntini S, Reason DC, Granoff DM. 2012. Combined roles of human IgG subclass, alternative complement pathway activation, and epitope density in the bactericidal activity of antibodies to meningococcal factor H binding protein. Infect Immun 80:187–194. doi: 10.1128/IAI.05956-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pajon R, Fergus AM, Koeberling O, Caugant DA, Granoff DM. 2011. Meningococcal factor H binding proteins in epidemic strains from Africa: implications for vaccine development. PLoS Negl Trop Dis 5:e1302. doi: 10.1371/journal.pntd.0001302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michaelsen TE, Ihle Ø, Beckstrøm KJ, Herstad TK, Kolberg J, Høiby EA, Aase A. 2003. Construction and functional activities of chimeric mouse-human immunoglobulin G and immunoglobulin M antibodies against the Neisseria meningitidis PorA P1.7 and P1.16 epitopes. Infect Immun 71:5714–5723. doi: 10.1128/IAI.71.10.5714-5723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams JN, Skipp PJ, Humphries HE, Christodoulides M, O’Connor CD, Heckels JE. 2007. Proteomic analysis of outer membranes and vesicles from wild-type serogroup B Neisseria meningitidis and a lipopolysaccharide-deficient mutant. Infect Immun 75:1364–1372. doi: 10.1128/IAI.01424-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song J, Minetti CA, Blake MS, Colombini M. 1999. Meningococcal PorA/C1, a channel that combines high conductance and high selectivity. Biophys J 76:804–813. doi: 10.1016/S0006-3495(99)77244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucas AH, Moulton KD, Reason DC. 1998. Role of kappa II-A2 light chain CDR-3 junctional residues in human antibody binding to the Haemophilus influenzae type b polysaccharide. J Immunol 161:3776–3780. [PubMed] [Google Scholar]
- 28.Reason DC, Wagner TC, Tang VR, Moulton KD, Lucas AH. 1999. Polysaccharide binding potential of the human A2 or A18 kappa light chain homologues. Infect Immun 67:994–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Lucas AH. 2003. IGH V3-23*01 and its allele V3-23*03 differ in their capacity to form the canonical human antibody combining site specific for the capsular polysaccharide of Haemophilus influenzae type b. Immunogenetics 55:336–338. doi: 10.1007/s00251-003-0583-8. [DOI] [PubMed] [Google Scholar]
- 30.Beernink PT, Welsch JA, Bar-Lev M, Koeberling O, Comanducci M, Granoff DM. 2008. Fine antigenic specificity and cooperative bactericidal activity of monoclonal antibodies directed at the meningococcal vaccine candidate factor H-binding protein. Infect Immun 76:4232–4240. doi: 10.1128/IAI.00367-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis LA, Carter M, Ram S. 2012. The relative roles of factor H binding protein, neisserial surface protein a, and lipooligosaccharide sialylation in regulation of the alternative pathway of complement on meningococci. J Immunol 188:5063–5072. doi: 10.4049/jimmunol.1103748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beernink PT, Lopasso C, Angiolillo A, Felici F, Granoff D. 2009. A region of the N-terminal domain of meningococcal factor H-binding protein that elicits bactericidal antibody across antigenic variant groups. Mol Immunol 46:1647–1653. doi: 10.1016/j.molimm.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giuntini S, Beernink PT, Reason DC, Granoff DM. 2012. Monoclonal antibodies to meningococcal factor H binding protein with overlapping epitopes and discordant functional activity. PLoS One 7:e34272. doi: 10.1371/journal.pone.0034272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scarselli M, Cantini F, Santini L, Veggi D, Dragonetti S, Donati C, Savino S, Giuliani MM, Comanducci M, Di Marcello F, Romagnoli G, Pizza M, Banci L, Rappuoli R. 2009. Epitope mapping of a bactericidal monoclonal antibody against the factor H binding protein of Neisseria meningitidis. J Mol Biol 386:97–108. doi: 10.1016/j.jmb.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Schneider MC, Prosser BE, Caesar JJ, Kugelberg E, Li S, Zhang Q, Quoraishi S, Lovett JE, Deane JE, Sim RB, Roversi P, Johnson S, Tang CM, Lea SM. 2009. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature 458:890–893. doi: 10.1038/nature07769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pajon R, Beernink PT, Granoff DM. 2012. Design of meningococcal factor H binding protein mutant vaccines that do not bind human complement factor H. Infect Immun 80:2667–2677. doi: 10.1128/IAI.00103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahu A, Pangburn MK. 1994. Covalent attachment of human complement C3 to IgG. Identification of the amino acid residue involved in ester linkage formation. J Biol Chem 269:28997–29002. [PubMed] [Google Scholar]
- 38.Fries LF, Gaither TA, Hammer CH, Frank MM. 1984. C3b covalently bound to IgG demonstrates a reduced rate of inactivation by factors H and I. J Exp Med 160:1640–1655. doi: 10.1084/jem.160.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jelezarova E, Vogt A, Lutz HU. 2000. Interaction of C3b(2)—IgG complexes with complement proteins properdin, factor B and factor H: implications for amplification. Biochem J 349:217–223. doi: 10.1042/0264-6021:3490217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konar M, Rossi R, Walter H, Pajon R, Beernink PT. 2015. A mutant library approach to identify improved meningococcal factor H binding protein vaccine antigens. PLoS One 10:e0128185. doi: 10.1371/journal.pone.0128185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Der Veen S, Johnson S, Jongerius I, Malik T, Genovese A, Santini L, Staunton D, Ufret-Vincenty RL, Pickering MC, Lea SM, Tang CM. 2014. Nonfunctional variant 3 factor H binding proteins as meningococcal vaccine candidates. Infect Immun 82:1157–1163. doi: 10.1128/IAI.01183-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi R, Granoff DM, Beernink PT. 2013. Meningococcal factor H-binding protein vaccines with decreased binding to human complement factor H have enhanced immunogenicity in human factor H transgenic mice. Vaccine 31:5451–5457. doi: 10.1016/j.vaccine.2013.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beernink PT, Shaughnessy J, Pajon R, Braga EM, Ram S, Granoff DM. 2012. The effect of human factor H on immunogenicity of meningococcal native outer membrane vesicle vaccines with over-expressed factor H binding protein. PLoS Pathog 8:e1002688. doi: 10.1371/journal.ppat.1002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. 2003. Expasy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beernink PT, Granoff DM. 2008. Bactericidal antibody responses induced by meningococcal recombinant chimeric factor H-binding protein vaccines. Infect Immun 76:2568–2575. doi: 10.1128/IAI.00033-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frasch CE, Van Alphen L, Holst J, Poolman JT, Rosenqvist E. 2001. Outer membrane protein vesicle vaccines for meningococcal disease, p 81–107. In Pollard AJ, Maiden MC (ed), Meningococcal vaccines: methods and protocols. Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 47.Bisno AL. 1979. Alternate complement pathway activation by group A streptococci: role of M-protein. Infect Immun 26:1172–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vu DM, Wong TT, Granoff DM. 2011. Cooperative serum bactericidal activity between human antibodies to meningococcal factor H binding protein and neisserial heparin binding antigen. Vaccine 29:1968–1973. doi: 10.1016/j.vaccine.2010.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kask L, Villoutreix BO, Steen M, Ramesh B, Dahlbäck B, Blom AM. 2004. Structural stability and heat-induced conformational change of two complement inhibitors: C4b-binding protein and factor H. Protein Sci 13:1356–1364. doi: 10.1110/ps.03516504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunoglobulin gene usage in recombinant human anti-FHbp Fab clones.
Results of studies investigating the effect of anti-FHbp antibody on binding of human FH to FHbp.