Abstract
Intracranial germ cell tumors (ICGCT) occur in 2-11% of children with brain tumors between 0-19 years of age. For treatment of germinoma, relatively low radiation doses with or without chemotherapy show excellent 10 year survival rate of 80-100%. Past studies showed that neoadjuvant chemotherapy combined with focal radiotherapy resulted in unacceptably high rates of periventricular tumor recurrence. The use of generous radiation volume which covers the whole ventricular space with later boost treatment to primary site is considered as standard treatment of intracranial germinomas. For non-germinomatous germ cell tumors (NGGCT), 10-year overall survival rate is still much inferior than that of intracranial germinoma despite intensive chemotherapy and high-dose radiotherapy. Craniospinal radiotherapy combined with cisplatin-based chemotherapy provides the best treatment outcome for NGGCT; 60-70% of overall survival rate. There is a debate on the surgical role whether surgery can contribute to improved treatment outcome of NGGCT when added to combined chemoradiotherapy. Because higher dose of radiotherapy is required for treatment of NGGCT than for germinoma, it is tested whether whole ventricular irradiation can replace craniospinal irradiation in intermediate risk group of NGGCT to minimize radiation-related late toxicity in the recent studies. To minimize the treatment-related neural deficit and late sequelae while maintaining long-term survival rate of ICGCT patients, optimized administration of chemotherapy and radiotherapy should be selected. Use of technically upgraded radiotherapy modalities such as intensity-modulated radiotherapy or proton beam therapy is expected to bring an improved neurocognitive outcome with longitudinal assessment of the patients.
Keywords: Intracranial germ cell tumor, ICGCT, Radiotherapy, Proton beam therapy, Biology
INTRODUCTION
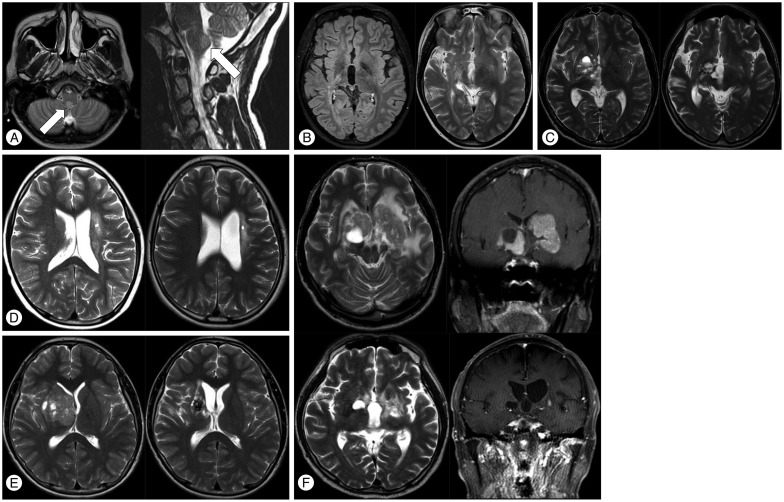
Intracranial germ cell tumors (ICGCT) are rare in North America and Europe, occurring in 2-4% of children with brain tumors between birth and 19 years of age6,44,65). By contrast, ICGCT is the most common supratentorial brain tumor in Japan, Taiwan, and Korea, accounting for 11-15% of brain tumors in children and adolescents14,30,49,67). ICGCT occurs as midline lesions around the third ventricle, most commonly involving the pineal gland and the neurohypophyseal region of the brain, which includes the infundibulum and/or pituitary stalk. Less commonly, tumors occur in the basal ganglia or thalamic nuclei and, rarely, in the floor of the fourth ventricle at the medulla (Fig. 1A). ICGCT arising in the basal ganglia (Fig. 1B-F) is worthy of mention because of its clinical characteristics, especially male predominance, and the difficulties in diagnosing and evaluating the response to treatment (Fig. 1C-F). Hemiparesis is the most common symptom, and it rarely resolves even with long-term disease control. It affects 4-15% of patients with ICGCT and predominantly occurs in boys47,59,61,66). Up to 20% of ICGCT present as multiple midline tumors. It is unclear whether multicentric tumors are present from tumor initiation or whether microscopic tumors infiltrate into the subependymal periventricular area from a single tumor. Bifocal tumors are more common in germinomas than in mixed ICGCT29). However, it has been reported that a small number of patients with normal or slightly elevated levels of α-fetoprotein (AFP) and β-human chorionic gonadotropin (β-HCG) have tumors with a malignant germ cell component on pathologic examination1). The natural course of bifocal germinomas is not significantly different from that of localized intracranial germinoma, which can be controlled with an extended focal radiotherapy field64). However, 23-55% of bifocal tumors were shown to harbor metastatic tumors when closely evaluated by imaging or cytology imaging studies or cytologic examinations were performed48,64). Therefore, careful evaluation of brain and spinal cerebrospinal space metastases is critical at initial presentation.
Fig. 1. Unusual sites of germ cell tumors in the brain. A : T2-axial (left) and T2-sagittal (right) magnetic resonance images show a nodular mass (arrow) in the posterior aspect of medulla. B : Pre-treatment images of a subtle patch streaky lesion involving the right internal capsule. Left : T2 image, Right : nodular contrast enhancement of the right basal ganglia. C : A solid and cystic mass in the right lenticular nucleus area before treatment (left) and 5 years after radiotherapy (right). D : Germinoma of the left basal ganglia. Subtle patchy lesion visible mainly in T2-weighted and fluid-attenuated inversion recovery images with no contrast enhancement in the left basal ganglia before treatment (left) and 2 years after treatment (right). The biopsy site is seen at the left basal ganglia in both scans. E : A huge mass in right basal ganglia with mass effect. The mass did not show any contrast enhancement before treatment (left) or 2 years after treatment (right). F : Huge germinoma involving bilateral basal ganglia, which shows contrast enhancement and peritumor edema. Top left and top right : pre-treatment axial T2 and coronal T1 images with contrast enhancement, Bottom left : axial T2 image 3 years after proton beam therapy, Bottom right : coronal T1 image with contrast enhancement. Magnetic resonance imaging 3 years after treatment shows a residual cystic lesion in the right basal ganglia, with white matter changes in the left frontal periventricular area.
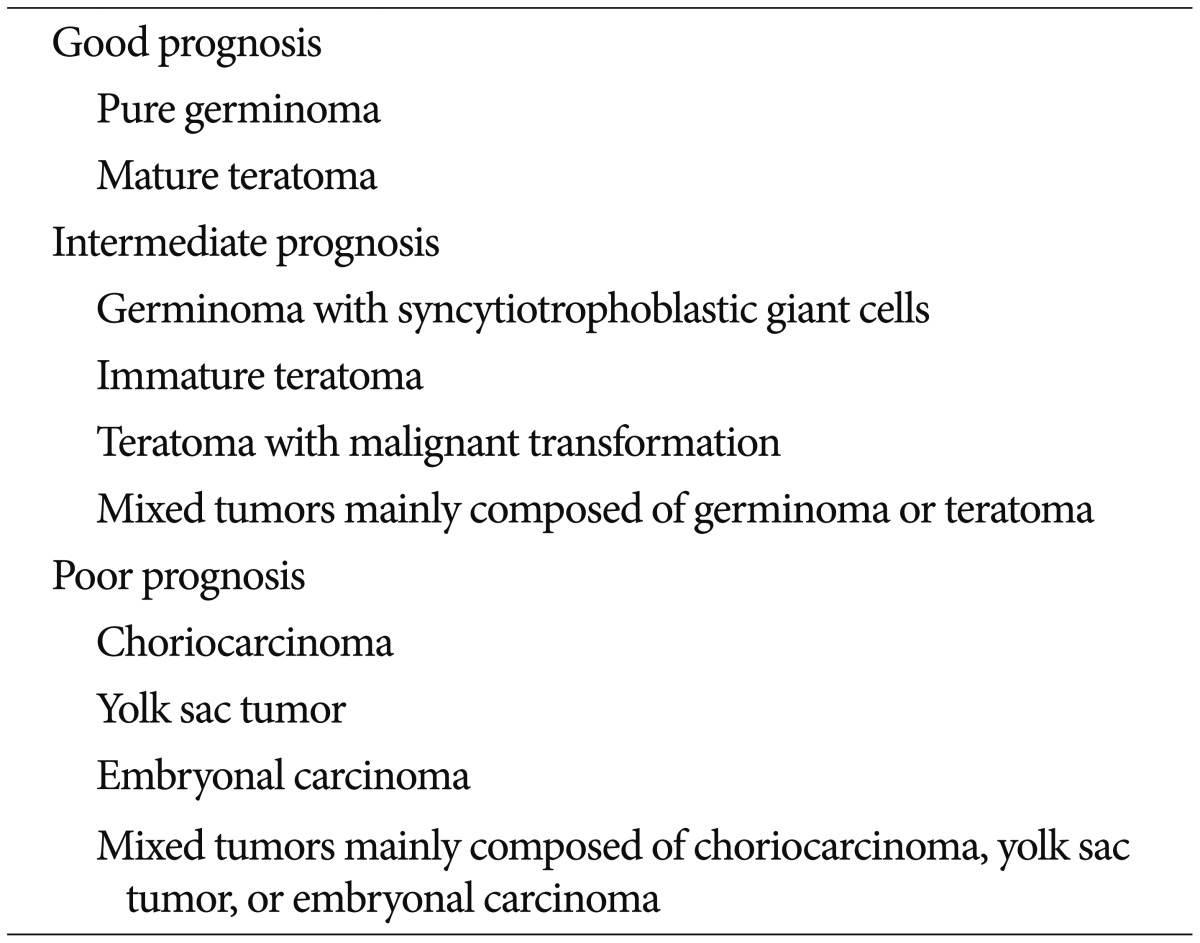
The survival rate of patients with pure germinoma appears to be very high, reaching a 10-year overall survival rate of 90%13,24,32,37,56), whereas that of patients with non-germinomatous GCT (NGGCT) tends to be 30-80%27,34,35). A Japanese study group examined 153 patients with ICGCT, of which 147 patients whose tumors were surgically resected were assessed to examine the correlation between histologic examination and survival outcome. According to their study, three groups of patients showed significantly different survival rates of 94.1%, 70%, and 9.3%. The three therapeutic groups were classified as showing good, intermediate, and poor prognosis, respectively (Table 1)20,29).
Table 1. Classification of intracranial germ cell tumors by prognosis33).
NGGCT is diagnosed as ICGCT with any malignant germ cell component and/or any tumor that secretes AFP or high levels of serum human chorionic gonadotropin. High β-HCG refers to a level greater than 50-100 IU; however, the cut-off value for the diagnosis of NGGCT varies between institutes or study groups. Tumors with high β-HCG concentrations were considered to be associated with worse prognosis by some researchers4,51,60). However, many other studies have shown that an elevated β-HCG level is not particularly associated with poor prognosis23,26,28,34,42,43) when there is no evidence of dissemination through the cerebrospinal fluid (CSF). It is uncertain what level of β-HCG should be regarded as high, because the cut-off level of elevated β-HCG differs in each study. In a study of 58 patients with a newly diagnosed intracranial germinoma who underwent histologic confirmation by biopsy and complete staging work-up, 60% had normal β-HCG levels in both serum and CSF, 34.5% had a normal serum level and an elevated CSF level, 3.5% had elevated levels in both serum and CSF, and 2% had an elevated serum level and a normal CSF level3). Although a β-HCG cut-off value of ≥50 mIU/mL in either serum or CSF was arbitrarily selected by several cooperative groups, the absolute β-HCG ceiling consistent with a pure central nervous system (CNS) germinoma has not been established. Previously, the Children's Oncology Group study ACNS0232 used serum or CSF β-HCG levels of 50 mIU/mL as the cut-off value for NGGCT diagnosis, and the recently started ACNS1123 study includes patients with β-HCG levels ranging from 50 to 100 mIU/mL. By contrast, the Japanese CNS GCT study group uses a much higher β-HCG cut-off level of 2000 mIU/mL for the diagnosis of NGGCT in patients with a poor prognosis, and includes patients with a histological diagnosis of choriocarcinoma, embryonal carcinoma, and yolk sac tumors. The difference between the current treatment strategy of the Japanese CNS GCT study group and other groups is that the former strongly advocates surgical biopsy for all ICGCTs. Therefore, the diagnosis is less dependent on serum and CSF tumor markers (personal communication, Masao Matsutani, Saitama International Medical Center, Hidaka, Japan). Currently, there is a debate about the role of surgery regarding its contribution to a superior treatment outcome when given before35,51) or after radiotherapy combined with chemotherapy50).
BIOLOGY OF ICGCT
The origin of ICGCT is considered to be primordial germ cells of the embryo20). Human primordial germ cells from the yolk sac endoderm migrate along the dorsal mesentery of the hindgut to the genital ridges for incorporation into developing gonads during week 4 of embryogenesis. Failure to initiate migration, abnormal migration, abnormal termination of migration, and entrapment during the migration, observed in midline extragonadal areas in chick, human, and mouse embryos, appears to coincide with the occurrence of extragonadal GCT, including CNS GCT20). Studies of the molecular and cytogenetic characteristics of ICGCT are rare because of the infrequency of surgical biopsy and the small size of specimen even if biopsies are performed. As shown in methylation and comparative genomic hybridization studies, both germinoma and NGGCT originate from primordial germ cells52,57). DNA microarray analysis and hierarchal cluster analysis for ovarian dysgerminomas versus ovarian endodermal sinus tumors showed a differential expression of the genes in the Wnt/β-catenin signaling pathway. β-catenin expression was observed in all of endodermal sinus tumors and immature teratomas whereas there was no expression in other types of ICGCT. Especially, nuclear expression of β-catenin were observed in 50-70% endodermal sinus tumor and immature teratoma compared to those in dysgerminoma, embryonal carcinoma, and choriocarcinoma16), suggesting activation of Wnt/β-catenin signaling pathway in these tumors. In one DNA methylation study, yolk sac tumors had a greater number of methylated tumor suppressor genes than seminomas and teratomas18). Oct-4, a transcription factor encoded by the POU5F1 gene is involved in the initiation, maintenance, and differentiation of pluripotent and germline cells during normal development. It is expressed in mouse and human embryonic stem and germ cells but is absent in all differentiated somatic cell types. Oct-4 is considered to be a highly sensitive and specific and immunohistochemical marker for primary ICGCT12,22).
Recently, genome-wide analyses of DNA copy number alterations were published17,59,62). Analysis of 62 ICGCT samples from three countries revealed that the overall pattern of genomic aberration was similar in germinoma and mixed GCT59). There were frequent aberrations of CCND2 (12p13) and RB1 (13q14), suggestive of Cyclin/CDK-RB-E2F pathway abnormalities in the pathogenesis of ICGCT. Additionally, the frequent gain of PRDM14 (8q13) suggested an abnormality in the transcriptional regulation of primordial germ cells. Somatic mutations in KIT and RAS have been reported in ICGCT and extracranial GCT. A recent targeted sequencing study confirmed that activating mutations in KIT and RAS were frequent and mutually exclusive in pure germinoma, suggesting that changes in the KIT signaling pathway play an important role in the development of germinomas17). A whole-exome sequencing study also detected mutations in components of the KIT/RAS signaling pathway in >50% of ICGCTs that included recurrent somatic mutations in KIT and KRAS as well as in NRAS and its negative regulator, CBL63). In addition, somatic alterations in the AKT/mTOR pathway were discovered, and these alterations increase the copy numbers of the AKT1 locus at 14q32.33. Global microRNA profiling studies of both ICGCT and other GCT have shown distinct expression profiles in germinoma and NGGCT19,39,45). These studies also revealed a high expression of miR-371-373 and miR-302 clusters in all ICGCT histological subtypes. The microRNA from these two clusters was present at high levels in the patients' sera at the time of extracranial malignant GCT diagnosis. A decrease in the level of miR-372 following treatment suggested a diagnostic value of these markers in patients with marker-negative disease19,45).
The biological factors responsible for the different ethnic incidence of ICGCT are poorly understood. Nevertheless, the whole-exome sequencing study of Japanese ICGCT patients detected significant enrichment of novel and rare germline variants in JMJD1C, which encodes a histone demethylase, a coactivator of the androgen receptor63). This finding could indicate a strong association between JMJD1C variants and the risk of developing ICGCT, but further international genome-wide association studies are needed to confirm the genetic and ethnic risk factors for the development of ICGCT.
RADIOTHERAPY OF INTRACRANIAL GERMINOMA
Based on retrospective studies, craniospinal low-dose radiotherapy plus boost results in a 96-100% relapse-free survival rate in patients with ICGCT. The cure rate of germinoma is more strongly influenced by a radiotherapy volume that generously covers the cerebrospinal space than the radiation dose itself. The accumulating long-term treatment results show excellent control rates with reduced radiation doses with or without chemotherapy11,13,40,55,56). Neoadjuvant chemotherapy has been given to reduce the volume and dose of radiation reaching the gross tumor; however, many retrospective and prospective studies have shown that additional chemotherapy does not prevent periventricular tumor recurrence when only a small radiation field is used2,4,9,15,55). Currently, a radiation volume that covers at least the whole ventricular space is the standard for radiation therapy of ICGCT. Whole-ventricular radiation plus a boost, with or without chemotherapy, achieved reasonably good relapse-free survival rates of 88-96%. The relapse-free survival rate of patients with ICGCT reaches 90-100% with low-dose radiotherapy alone. Recent prospective studies showed that radiotherapy alone or chemotherapy followed by radiotherapy had similar treatment outcomes9,56). The superiority of chemotherapy combined with radiotherapy would be difficult to prove unless the combined therapy substantially reduced the side-effects of radiotherapy. Accordingly, the role of neoadjuvant chemotherapy should be examined in a well-designed clinical trial in which the end-points include late sequelae, quality of life, and survival outcomes53). Preexisting endocrinopathies, growth problems, and the patient's neurocognitive status due to both the tumor itself and surgical complications observed in the majority of ICGCT patients should be considered as baseline data in the assessment of any treatment-related morbidity.
Historically, a radiation dose up to 45-60 Gy has been used for various radiotherapy volumes in patients with ICGCT. However, published results from various institutes worldwide show that much lower radiation doses in the range of 30-39 Gy for gross tumors and 19.5-25 Gy for microscopic tumors are sufficient for good disease-control rates11,13,21,41,68). It has been suggested that the radiation dose needed to control germinomas ranges from 36-45 Gy, depending on the volume of the tumor54). Although ICGCT mostly affects patients in late childhood and adolescence and the effect of radiation therapy on neurocognitive function is relatively mild31,37), recent Japanese data have shown a decrease in the social function of survivors of ICGCT24). The optimal radiation dose and volume should be selected to minimize late sequelae while maintaining long-term survival. The median time to recurrence ranges from 30 to 50 months25,46). Late recurrence after 8-15 years of age is not uncommon, suggesting the need for an observation period of ≥10 years2,13,23,25,46).
RADIOTHERAPY OF NON-GERMINOMATOUS GERM CELL TUMOR
NGGCT is diagnosed by the presence of any of the following germ cell types, such as embryonal carcinoma, endodermal sinus tumor, choriocarcinoma, or teratoma. Despite intensive chemotherapy and high-dose radiotherapy, 10-year overall survival rates range from 30% to 70%, with worst survival rate for patients in the poor prognosis group defined by Japanese prognostic classification. Currently, there is a debate on the contribution of surgery to a superior treatment outcome when performed before35,51) or after radiotherapy combined with chemotherapy50). Radiotherapy combined with cisplatin-based chemotherapy provides the best treatment outcome, increasing the survival rate of patients with NGGCT to 60-70%. In a retrospective analysis of 32 patients with NGGCT, the relapse-free and overall survival rates were 77.6% and 74.6%, respectively. In this study, the most important prognostic factors were the implementation of craniospinal radiotherapy and the Japanese risk-group classification27). However, it has not yet been determined whether the whole craniospinal axis must be included in the radiation field for all patients with NGGCT. The International Society of Pediatric Oncology GCT phase II study defined the high-risk group as patients with a serum AFP level >1000 ng/mL and age <6 years. This study utilized focal radiotherapy with radiation doses of 54 Gy for the standard-risk group. By contrast, craniospinal radiotherapy with a radiation dose of 30 Gy plus an additional 24 Gy to the gross tumor was used to treat the high-risk group. The Japanese CNS GCT study group set an AFP level >2000 ng/mL and/or a serum β-HCG level >2000 mIU/mL as cut-off values for their phase II single-arm prospective study of concomitant radiochemotherapy for NGGCT33). Craniospinal radiotherapy was applied at a dose of 30.6 Gy for patients with poor prognosis and 23.4-27 Gy of whole-ventricular radiation for patients with intermediate prognosis, yielding a final dose to the focal tumors of 50.4 Gy and 61.2 Gy, respectively. The North American protocol COG ACNS1123 is underway in which the dose and volume of radiotherapy is adapted according to the tumor response to six cycles of chemotherapy, regardless of prognosis or tumor markers. This study is also testing whether the volume of irradiation can be safely restricted to the whole ventricle. The study is using a radiation dose of 30.6 Gy for whole-ventricular irradiation with a 23.4 Gy primary site boost for patients with localized NGGCT. This treatment regimen will only be used in patients confirmed by magnetic resonance imaging (MRI) to have a complete response to induction chemotherapy. In addition, serum and CSF tumor markers must be in the normal range for this therapy. The same treatment scheme can also be applied to patients who show a partial response to chemotherapy and to those who have undergone second-look surgery revealing mature teratomas.
PROTON BEAM THERAPY IN TREATING ICGCT
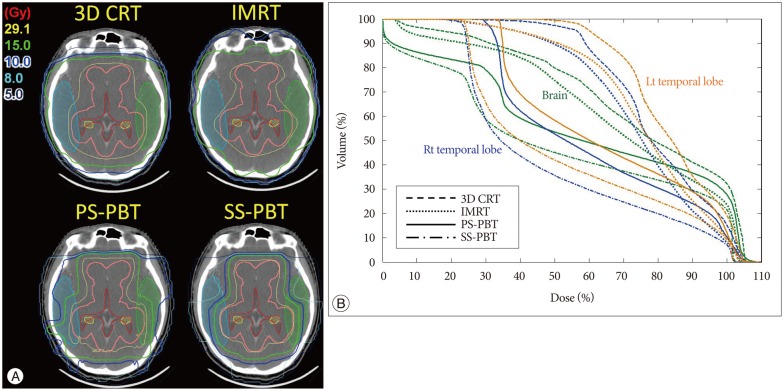
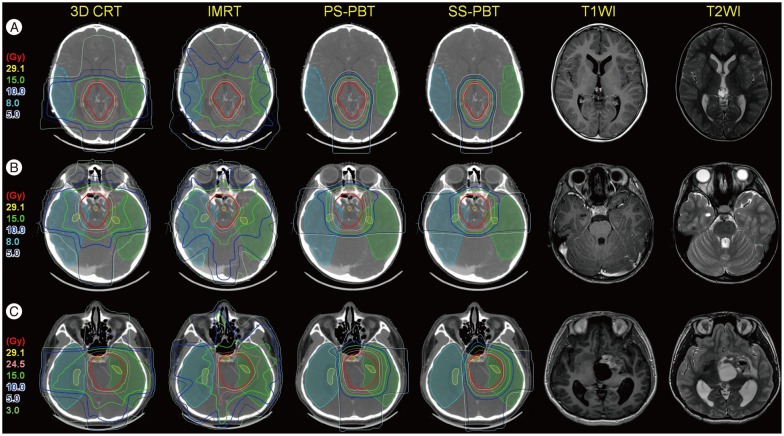
Proton beam therapy (PBT) has dosimetric benefit in reducing radiation dose to the normal tissues while concentrating radiation dose to the tumor because of its inherent physical characteristics of absence of exit radiation dose, i.e., Bragg peak. This characteristic have been considered to be particularly useful when treating children with brain tumors because it reduces radiation dose to the various substructures of brain which are critical in intellectual functioning and hence preserving neural functions in brain tumor survivors. Both germinomas and mixed GCTs require a generous radiotherapy volume that covers the whole ventricle, if not the whole craniospinal space. Comparison of the dose-volume histograms of intensity-modulated radiotherapy (IMRT) and three dimensional-conformal radiotherapy (3D-CRT) for whole-ventricular irradiation showed that IMRT reduced the irradiated volume receiving radiation doses of 20 Gy, 30 Gy, and 40 Gy by 7.5%, 12.2%, and 9.0%, respectively, compared with 3D-CRT. IMRT was associated with a statistically significant reduction of the median irradiated volumes at all dose levels (p<0.002) compared with 3D-CRT; however, with IMRT the radiation dose was 1.9 times higher than 3D-CRT and had greater effects on peripheral areas of the body compared with 3D-CRT10). PBT significantly reduces the incidence of early and late toxicities of radiotherapy, and is expected to help improve the therapeutic ratio because of its physical characteristics involves absence of radiation dose beyond target tissue. The use of PBT for whole craniospinal irradiation also significantly reduces hematological toxicity7,8,58) as well as decreases the risk of secondary malignancy (Fig. 2)38,69). In addition, whole-ventricular PBT brings the volume of the cerebral cortex receiving ≤10 Gy and ≤15 Gy down to two-third to a half of those of 3D-CRT and IMRT (Fig. 3). In tumors arising in the pineal gland, suprasellar area, or basal ganglia, PBT also delivers lower mean radiation doses to the cochlea, pituitary gland, and temporal lobes (Fig. 4). When combined with a focal boost radiotherapy field, IMRT and PBT significantly differed in the volume of brain parenchyma that received doses of 5-20 Gy, and the volume of temporal lobes that received doses of 5-30 Gy when a total of 30.6 Gy was delivered to the primary tumor. The benefit of PBT increased when the pencil beam scanning (or spot scanning) technology of PBT was used for the same target volume, because numerous small proton beams from thousands directions paint the target area in this technique, resulting in greater conformity to the irregularly shaped tumors. When radiation doses of ≥25 Gy were delivered to the whole brain, dose-cognitive effect modeling revealed that the radiation dose to the supratentorial and infratentorial brain parenchyma, temporal lobes, and the hippocampus was linearly associated with a decline in late neurocognitive function5,36). It is expected that this dosimetric benefit of PBT can be translated into clinical benefit.
Fig. 2. Sample treatment plan for craniospinal irradiation showing dose distributions. A : Sagittal view of 3-dimensional conformal radiation therapy (3D-CRT). B : Axial view of 3D-CRT. C : Sagittal view of proton beam treatment (PBT). D : Axial view of PBT. Yoon M, Shin DH, Kim J, Kim JW, Kim DW, Park SY, et al. Int J Radiat Oncol Biol Phys 81 : 637-646, 201169) with permission.
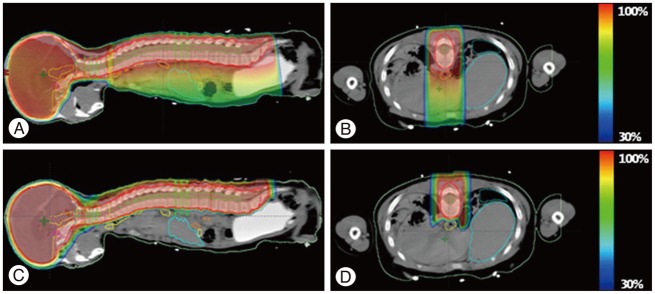
Fig. 3. A : Radiation dose distribution for whole ventricle (WV) irradiation. Comparison of 3D-conformal radiotherapy (3D-CRT), intensity-modulated radiotherapy (IMRT) passive scattering proton beam therapy (PS-PBT), and spot scanning proton beam therapy (SS-PBT). Thick pink lines are planning target volume (PTV) for the WV. The blue, green, and yellow lines represent the 10 Gy, 15 Gy, and 29.1 Gy isodose lines, respectively. B : Dose-volume histogram of the normal brain and both temporal lobes for WV irradiation, including the pineal gland tumor bed. The prescribed (100%) doses are 30.6 Gy for 95% volume of the WV PTV.
Fig. 4. Dose distribution of primary site irradiation to tumors arising in the pineal gland (A), suprasellar (B), and basal ganglia (C), with their pre-chemotherapy magnetic resonance imaging. The thick red lines represent planning target volumes (PTV) to the primary tumor sites. The thick blue and green lines represent 10 Gy and 15 Gy isodose lines, respectively. The prescribed doses are 30.6 Gy at 95% volume of the PTV.
CONCLUSION
ICGCT affects includes heterogeneous disease entities which have variable prognosis. Radiotherapy and chemotherapy can be used in various combinations, resulting in 80-100% and 60-70% survival rates, respectively for germinoma and NGGCT. However, most of the patients who survive the disease are at risk for cognitive impairments and poor quality of life secondary to disease itself and treatment related factors. To achieve high long-term disease control rate and good quality of life of the survivors, carefully selected multimodality treatment needs to be combined with following systemic assessment of late tumor- and treatment-related functional outcome.
Acknowledgements
This work was supported by a grant from the National Cancer Center, Korea (No. 1310080). We are grateful to Dr. Keita Terashima of Children's Cancer Center, National Center for Child Health and Development, Tokyo, for helping with the section of biology of intracranial germ cell tumor.
References
- 1.Aizer AA, Sethi RV, Hedley-Whyte ET, Ebb D, Tarbell NJ, Yock TI, et al. Bifocal intracranial tumors of nongerminomatous germ cell etiology : diagnostic and therapeutic implications. Neuro Oncol. 2013;15:955–960. doi: 10.1093/neuonc/not050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alapetite C, Brisse H, Patte C, Raquin MA, Gaboriaud G, Carrie C, et al. Pattern of relapse and outcome of non-metastatic germinoma patients treated with chemotherapy and limited field radiation : the SFOP experience. Neuro Oncol. 2010;12:1318–1325. doi: 10.1093/neuonc/noq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen J, Chacko J, Donahue B, Dhall G, Kretschmar C, Jakacki R, et al. Diagnostic sensitivity of serum and lumbar CSF bHCG in newly diagnosed CNS germinoma. Pediatr Blood Cancer. 2012;59:1180–1182. doi: 10.1002/pbc.24097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoyama H, Shirato H, Ikeda J, Fujieda K, Miyasaka K, Sawamura Y. Induction chemotherapy followed by low-dose involved-field radiotherapy for intracranial germ cell tumors. J Clin Oncol. 2002;20:857–865. doi: 10.1200/JCO.2002.20.3.857. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong GT, Jain N, Liu W, Merchant TE, Stovall M, Srivastava DK, et al. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro Oncol. 2010;12:1173–1186. doi: 10.1093/neuonc/noq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arora RS, Alston RD, Eden TO, Estlin EJ, Moran A, Birch JM. Age-incidence patterns of primary CNS tumors in children, adolescents, and adults in England. Neuro Oncol. 2009;11:403–413. doi: 10.1215/15228517-2008-097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barney CL, Brown AP, Grosshans DR, McAleer MF, de Groot JF, Puduvalli V, et al. Technique, outcomes, and acute toxicities in adults treated with proton beam craniospinal irradiation. Neuro Oncol. 2014;16:303–309. doi: 10.1093/neuonc/not155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown AP, Barney CL, Grosshans DR, McAleer MF, de Groot JF, Puduvalli VK, et al. Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int J Radiat Oncol Biol Phys. 2013;86:277–284. doi: 10.1016/j.ijrobp.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calaminus G, Kortmann R, Worch J, Nicholson JC, Alapetite C, Garrè ML, et al. SIOP CNS GCT 96 : final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol. 2013;15:788–796. doi: 10.1093/neuonc/not019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen MJ, Santos Ada S, Sakuraba RK, Lopes CP, Gonçalves VD, Weltman E, et al. Intensity-modulated and 3D-conformal radiotherapy for whole-ventricular irradiation as compared with conventional whole-brain irradiation in the management of localized central nervous system germ cell tumors. Int J Radiat Oncol Biol Phys. 2010;76:608–614. doi: 10.1016/j.ijrobp.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 11.Chen YW, Huang PI, Ho DM, Hu YW, Chang KP, Chiou SH, et al. Change in treatment strategy for intracranial germinoma : long-term follow-up experience at a single institute. Cancer. 2012;118:2752–2762. doi: 10.1002/cncr.26564. [DOI] [PubMed] [Google Scholar]
- 12.Cheng L, Sung MT, Cossu-Rocca P, Jones TD, MacLennan GT, De Jong J, et al. OCT4 : biological functions and clinical applications as a marker of germ cell neoplasia. J Pathol. 2007;211:1–9. doi: 10.1002/path.2105. [DOI] [PubMed] [Google Scholar]
- 13.Cho J, Choi JU, Kim DS, Suh CO. Low-dose craniospinal irradiation as a definitive treatment for intracranial germinoma. Radiother Oncol. 2009;91:75–79. doi: 10.1016/j.radonc.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Committee of Brain Tumor Registry of Japan. Report of Brain Tumor Registry of Japan (1969-1996) Neurol Med Chir (Tokyo) 2003;43(Suppl):i–vii. 1–111. [PubMed] [Google Scholar]
- 15.Eom KY, Kim IH, Park CI, Kim HJ, Kim JH, Kim K, et al. Upfront chemotherapy and involved-field radiotherapy results in more relapses than extended radiotherapy for intracranial germinomas : modification in radiotherapy volume might be needed. Int J Radiat Oncol Biol Phys. 2008;71:667–671. doi: 10.1016/j.ijrobp.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 16.Fritsch MK, Schneider DT, Schuster AE, Murdoch FE, Perlman EJ. Activation of Wnt/beta-catenin signaling in distinct histologic subtypes of human germ cell tumors. Pediatr Dev Pathol. 2006;9:115–131. doi: 10.2350/08-05-0097.1. [DOI] [PubMed] [Google Scholar]
- 17.Fukushima S, Otsuka A, Suzuki T, Yanagisawa T, Mishima K, Mukasa A, et al. Mutually exclusive mutations of KIT and RAS are associated with KIT mRNA expression and chromosomal instability in primary intracranial pure germinomas. Acta Neuropathol. 2014;127:911–925. doi: 10.1007/s00401-014-1247-5. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa S, Haruta M, Arai Y, Honda S, Ohshima J, Sugawara W, et al. Yolk sac tumor but not seminoma or teratoma is associated with abnormal epigenetic reprogramming pathway and shows frequent hypermethylation of various tumor suppressor genes. Cancer Sci. 2009;100:698–708. doi: 10.1111/j.1349-7006.2009.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillis AJ, Stoop HJ, Hersmus R, Oosterhuis JW, Sun Y, Chen C, et al. High-throughput microRNAome analysis in human germ cell tumours. J Pathol. 2007;213:319–328. doi: 10.1002/path.2230. [DOI] [PubMed] [Google Scholar]
- 20.Glenn OA, Barkovich AJ. Intracranial germ cell tumors : a comprehensive review of proposed embryologic derivation. Pediatr Neurosurg. 1996;24:242–251. doi: 10.1159/000121046. [DOI] [PubMed] [Google Scholar]
- 21.Hardenbergh PH, Golden J, Billet A, Scott RM, Shrieve DC, Silver B, et al. Intracranial germinoma : the case for lower dose radiation therapy. Int J Radiat Oncol Biol Phys. 1997;39:419–426. doi: 10.1016/s0360-3016(97)00330-1. [DOI] [PubMed] [Google Scholar]
- 22.Hattab EM, Tu PH, Wilson JD, Cheng L. OCT4 immunohistochemistry is superior to placental alkaline phosphatase (PLAP) in the diagnosis of central nervous system germinoma. Am J Surg Pathol. 2005;29:368–371. doi: 10.1097/01.pas.0000149709.19958.a7. [DOI] [PubMed] [Google Scholar]
- 23.Jensen AW, Laack NN, Buckner JC, Schomberg PJ, Wetmore CJ, Brown PD. Long-term follow-up of dose-adapted and reduced-field radiotherapy with or without chemotherapy for central nervous system germinoma. Int J Radiat Oncol Biol Phys. 2010;77:1449–1456. doi: 10.1016/j.ijrobp.2009.06.077. [DOI] [PubMed] [Google Scholar]
- 24.Jinguji S, Yoshimura J, Nishiyama K, Aoki H, Nagasaki K, Natsumeda M, et al. Factors affecting functional outcomes in long-term survivors of intracranial germinomas : a 20-year experience in a single institution. J Neurosurg Pediatr. 2013;11:454–463. doi: 10.3171/2012.12.PEDS12336. [DOI] [PubMed] [Google Scholar]
- 25.Kamoshima Y, Sawamura Y, Ikeda J, Shirato H, Aoyama H. Late recurrence and salvage therapy of CNS germinomas. J Neurooncol. 2008;90:205–211. doi: 10.1007/s11060-008-9649-7. [DOI] [PubMed] [Google Scholar]
- 26.Kawabata Y, Takahashi JA, Arakawa Y, Shirahata M, Hashimoto N. Long term outcomes in patients with intracranial germinomas : a single institution experience of irradiation with or without chemotherapy. J Neurooncol. 2008;88:161–167. doi: 10.1007/s11060-008-9542-4. [DOI] [PubMed] [Google Scholar]
- 27.Kim JW, Kim WC, Cho JH, Kim DS, Shim KW, Lyu CJ, et al. A multimodal approach including craniospinal irradiation improves the treatment outcome of high-risk intracranial nongerminomatous germ cell tumors. Int J Radiat Oncol Biol Phys. 2012;84:625–631. doi: 10.1016/j.ijrobp.2011.12.077. [DOI] [PubMed] [Google Scholar]
- 28.Kim JY. The Treatment Result of Intracranial Germ Cell Tumors in Korea; KSPNO G081 Multicenter trial, KSPNO-G052/082 Trial : New Horizon of the Germ Cell Therapy. Seoul: The Korean Society for Pediatric Neuro-Oncology; 2013. p. 42.p. 49. [Google Scholar]
- 29.Kun LE, MacDonald S, Tarbell NJ. Supratentorial Brain Tumors. In: Halperin EC, Constine LS, Tarbell NJ, Kun LE, editors. Pediatric Radiation Oncology. Philadelphia: Lippincott Williams & Wilkins; 2011. p. 44. [Google Scholar]
- 30.Lee CH, Jung KW, Yoo H, Park S, Lee SH. Epidemiology of primary brain and central nervous system tumors in Korea. J Korean Neurosurg Soc. 2010;48:145–152. doi: 10.3340/jkns.2010.48.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mabbott DJ, Monsalves E, Spiegler BJ, Bartels U, Janzen L, Guger S, et al. Longitudinal evaluation of neurocognitive function after treatment for central nervous system germ cell tumors in childhood. Cancer. 2011;117:5402–5411. doi: 10.1002/cncr.26127. [DOI] [PubMed] [Google Scholar]
- 32.Maity A, Shu HK, Janss A, Belasco JB, Rorke L, Phillips PC, et al. Craniospinal radiation in the treatment of biopsy-proven intracranial germinomas : twenty-five years' experience in a single center. Int J Radiat Oncol Biol Phys. 2004;58:1165–1170. doi: 10.1016/j.ijrobp.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 33.Matsutani M. Treatment of intracranial Germ Cell Tumor in Japan : New Horizon of the Germ Cell Therapy. Seoul: The Korean Society for Pediatric Neuro-Oncology; 2013. p. 8. [Google Scholar]
- 34.Matsutani M Japanese Pediatric Brain Tumor Study Group. Combined chemotherapy and radiation therapy for CNS germ cell tumors--the Japanese experience. J Neurooncol. 2001;54:311–316. doi: 10.1023/a:1012743707883. [DOI] [PubMed] [Google Scholar]
- 35.Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O, Funata N, et al. Primary intracranial germ cell tumors : a clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86:446–455. doi: 10.3171/jns.1997.86.3.0446. [DOI] [PubMed] [Google Scholar]
- 36.Merchant TE, Kiehna EN, Li C, Shukla H, Sengupta S, Xiong X, et al. Modeling radiation dosimetry to predict cognitive outcomes in pediatric patients with CNS embryonal tumors including medulloblastoma. Int J Radiat Oncol Biol Phys. 2006;65:210–221. doi: 10.1016/j.ijrobp.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 37.Merchant TE, Sherwood SH, Mulhern RK, Rose SR, Thompson SJ, Sanford RA, et al. CNS germinoma : disease control and long-term functional outcome for 12 children treated with craniospinal irradiation. Int J Radiat Oncol Biol Phys. 2000;46:1171–1176. doi: 10.1016/s0360-3016(99)00375-2. [DOI] [PubMed] [Google Scholar]
- 38.Miralbell R, Lomax A, Cella L, Schneider U. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys. 2002;54:824–829. doi: 10.1016/s0360-3016(02)02982-6. [DOI] [PubMed] [Google Scholar]
- 39.Murray MJ, Halsall DJ, Hook CE, Williams DM, Nicholson JC, Coleman N. Identification of microRNAs From the miR-371~373 and miR-302 clusters as potential serum biomarkers of malignant germ cell tumors. Am J Clin Pathol. 2011;135:119–125. doi: 10.1309/AJCPOE11KEYZCJHT. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen QN, Chang EL, Allen PK, Maor MH, Ater JL, Mahajan A, et al. Focal and craniospinal irradiation for patients with intracranial germinoma and patterns of failure. Cancer. 2006;107:2228–2236. doi: 10.1002/cncr.22246. [DOI] [PubMed] [Google Scholar]
- 41.Odagiri K, Omura M, Hata M, Aida N, Niwa T, Ogino I, et al. Treatment outcomes, growth height, and neuroendocrine functions in patients with intracranial germ cell tumors treated with chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:632–638. doi: 10.1016/j.ijrobp.2011.12.084. [DOI] [PubMed] [Google Scholar]
- 42.Ogawa K, Shikama N, Toita T, Nakamura K, Uno T, Onishi H, et al. Long-term results of radiotherapy for intracranial germinoma : a multi-institutional retrospective review of 126 patients. Int J Radiat Oncol Biol Phys. 2004;58:705–713. doi: 10.1016/j.ijrobp.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Ogino H, Shibamoto Y, Takanaka T, Suzuki K, Ishihara S, Yamada T, et al. CNS germinoma with elevated serum human chorionic gonadotropin level : clinical characteristics and treatment outcome. Int J Radiat Oncol Biol Phys. 2005;62:803–808. doi: 10.1016/j.ijrobp.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 44.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, et al. CBTRUS statistical report : primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palmer RD, Murray MJ, Saini HK, van Dongen S, Abreu-Goodger C, Muralidhar B, et al. Malignant germ cell tumors display common microRNA profiles resulting in global changes in expression of messenger RNA targets. Cancer Res. 2010;70:2911–2923. doi: 10.1158/0008-5472.CAN-09-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paximadis P, Hallock A, Bhambhani K, Chu R, Sood S, Wang Z, et al. Patterns of failure in patients with primary intracranial germinoma treated with neoadjuvant chemotherapy and radiotherapy. Pediatr Neurol. 2012;47:162–166. doi: 10.1016/j.pediatrneurol.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 47.Phi JH, Cho BK, Kim SK, Paeng JC, Kim IO, Kim IH, et al. Germinomas in the basal ganglia : magnetic resonance imaging classification and the prognosis. J Neurooncol. 2010;99:227–236. doi: 10.1007/s11060-010-0119-7. [DOI] [PubMed] [Google Scholar]
- 48.Phi JH, Kim SK, Lee J, Park CK, Kim IH, Ahn HS, et al. The enigma of bifocal germ cell tumors in the suprasellar and pineal regions : synchronous lesions or metastasis? J Neurosurg Pediatr. 2013;11:107–114. doi: 10.3171/2012.10.PEDS11487. [DOI] [PubMed] [Google Scholar]
- 49.Report of Brain Tumor Registry of Japan (1984-2000) Neurol Med Chir (Tokyo) 2009;49:PS1–PS96. [PubMed] [Google Scholar]
- 50.Robertson PL, DaRosso RC, Allen JC. Improved prognosis of intracranial non-germinoma germ cell tumors with multimodality therapy. J Neurooncol. 1997;32:71–80. doi: 10.1023/a:1005732105727. [DOI] [PubMed] [Google Scholar]
- 51.Sawamura Y, Ikeda J, Shirato H, Tada M, Abe H. Germ cell tumours of the central nervous system : treatment consideration based on 111 cases and their long-term clinical outcomes. Eur J Cancer. 1998;34:104–110. doi: 10.1016/s0959-8049(97)10045-4. [DOI] [PubMed] [Google Scholar]
- 52.Schneider DT, Schuster AE, Fritsch MK, Hu J, Olson T, Lauer S, et al. Multipoint imprinting analysis indicates a common precursor cell for gonadal and nongonadal pediatric germ cell tumors. Cancer Res. 2001;61:7268–7276. [PubMed] [Google Scholar]
- 53.Shibamoto Y. Management of central nervous system germinoma : proposal for a modern strategy. Prog Neurol Surg. 2009;23:119–129. doi: 10.1159/000210058. [DOI] [PubMed] [Google Scholar]
- 54.Shibamoto Y, Sasai K, Oya N, Hiraoka M. Intracranial germinoma : radiation therapy with tumor volume-based dose selection. Radiology. 2001;218:452–456. doi: 10.1148/radiology.218.2.r01ja08452. [DOI] [PubMed] [Google Scholar]
- 55.Shim KW, Kim TG, Suh CO, Cho JH, Yoo CJ, Choi JU, et al. Treatment failure in intracranial primary germinomas. Childs Nerv Syst. 2007;23:1155–1161. doi: 10.1007/s00381-007-0394-6. [DOI] [PubMed] [Google Scholar]
- 56.Shim KW, Park EK, Lee YH, Suh CO, Cho J, Choi JU, et al. Treatment strategy for intracranial primary pure germinoma. Childs Nerv Syst. 2013;29:239–248. doi: 10.1007/s00381-012-1902-x. [DOI] [PubMed] [Google Scholar]
- 57.Sievers S, Alemazkour K, Zahn S, Perlman EJ, Gillis AJ, Looijenga LH, et al. IGF2/H19 imprinting analysis of human germ cell tumors (GCTs) using the methylation-sensitive single-nucleotide primer extension method reflects the origin of GCTs in different stages of primordial germ cell development. Genes Chromosomes Cancer. 2005;44:256–264. doi: 10.1002/gcc.20237. [DOI] [PubMed] [Google Scholar]
- 58.Song S, Park HJ, Yoon JH, Kim DW, Park J, Shin D, et al. Proton beam therapy reduces the incidence of acute haematological and gastrointestinal toxicities associated with craniospinal irradiation in pediatric brain tumors. Acta Oncol. 2014;53:1158–1164. doi: 10.3109/0284186X.2014.887225. [DOI] [PubMed] [Google Scholar]
- 59.Terashima K, Yu A, Chow WY, Hsu WC, Chen P, Wong S, et al. Genome-wide analysis of DNA copy number alterations and loss of heterozygosity in intracranial germ cell tumors. Pediatr Blood Cancer. 2014;61:593–600. doi: 10.1002/pbc.24833. [DOI] [PubMed] [Google Scholar]
- 60.Uematsu Y, Tsuura Y, Miyamoto K, Itakura T, Hayashi S, Komai N. The recurrence of primary intracranial germinomas. Special reference to germinoma with STGC (syncytiotrophoblastic giant cell) J Neurooncol. 1992;13:247–256. doi: 10.1007/BF00172477. [DOI] [PubMed] [Google Scholar]
- 61.Villani A, Bouffet E, Blaser S, Millar BA, Hawkins C, Bartels U. Inherent diagnostic and treatment challenges in germinoma of the basal ganglia : a case report and review of the literature. J Neurooncol. 2008;88:309–314. doi: 10.1007/s11060-008-9568-7. [DOI] [PubMed] [Google Scholar]
- 62.Wang HW, Wu YH, Hsieh JY, Liang ML, Chao ME, Liu DJ, et al. Pediatric primary central nervous system germ cell tumors of different prognosis groups show characteristic miRNome traits and chromosome copy number variations. BMC Genomics. 2010;11:132. doi: 10.1186/1471-2164-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L, Yamaguchi S, Burstein MD, Terashima K, Chang K, Ng HK, et al. Novel somatic and germline mutations in intracranial germ cell tumours. Nature. 2014;511:241–245. doi: 10.1038/nature13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weksberg DC, Shibamoto Y, Paulino AC. Bifocal intracranial germinoma : a retrospective analysis of treatment outcomes in 20 patients and review of the literature. Int J Radiat Oncol Biol Phys. 2012;82:1341–1351. doi: 10.1016/j.ijrobp.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 65.Wöhrer A, Waldhör T, Heinzl H, Hackl M, Feichtinger J, Gruber-Mösenbacher U, et al. The Austrian Brain Tumour Registry : a cooperative way to establish a population-based brain tumour registry. J Neurooncol. 2009;95:401–411. doi: 10.1007/s11060-009-9938-9. [DOI] [PubMed] [Google Scholar]
- 66.Wong TT, Chen YW, Guo WY, Chang KP, Ho DM, Yen SH. Germinoma involving the basal ganglia in children. Childs Nerv Syst. 2008;24:71–78. doi: 10.1007/s00381-007-0495-2. [DOI] [PubMed] [Google Scholar]
- 67.Wong TT, Ho DM, Chang KP, Yen SH, Guo WY, Chang FC, et al. Primary pediatric brain tumors : statistics of Taipei VGH, Taiwan (1975-2004) Cancer. 2005;104:2156–2167. doi: 10.1002/cncr.21430. [DOI] [PubMed] [Google Scholar]
- 68.Yen SH, Chen YW, Huang PI, Wong TT, Ho DM, Chang KP, et al. Optimal treatment for intracranial germinoma : can we lower radiation dose without chemotherapy? Int J Radiat Oncol Biol Phys. 2010;77:980–987. doi: 10.1016/j.ijrobp.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 69.Yoon M, Shin DH, Kim J, Kim JW, Kim DW, Park SY, et al. Craniospinal irradiation techniques : a dosimetric comparison of proton beams with standard and advanced photon radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81:637–646. doi: 10.1016/j.ijrobp.2010.06.039. [DOI] [PubMed] [Google Scholar]