Abstract
Objective
The main causes of spinal cord ischemia are a variety of vascular pathologies causing acute arterial occlusions. We investigated neuroprotective effects of kefir on spinal cord ischemia injury in rats.
Methods
Rats were divided into three groups : 1) sham operated control rats; 2) spinal cord ischemia group fed on a standard diet without kefir pretreatment; and 3) spinal cord ischemia group fed on a standard diet plus kefir. Spinal cord ischemia was performed by the infrarenal aorta cross-clamping model. The spinal cord was removed after the procedure. The biochemical and histopathological changes were observed within the samples. Functional assessment was performed for neurological deficit scores.
Results
The kefir group was compared with the ischemia group, a significant decrease in malondialdehyde levels was observed (p<0.05). Catalase and superoxide dismutase levels of the kefir group were significantly higher than ischemia group (p<0.05). In histopathological samples, the kefir group is compared with ischemia group, there was a significant decrease in numbers of dead and degenerated neurons (p<0.05). In immunohistochemical staining, hipoxia-inducible factor-1α and caspase 3 immunopositive neurons were significantly decreased in kefir group compared with ischemia group (p<0.05). The neurological deficit scores of kefir group were significantly higher than ischemia group at 24 h (p<0.05).
Conclusion
Our study revealed that kefir pretreatment in spinal cord ischemia/reperfusion reduced oxidative stress and neuronal degeneration as a neuroprotective agent. Ultrastructural studies are required in order for kefir to be developed as a promising therapeutic agent to be utilized for human spinal cord ischemia in the future.
Keywords: Spinal cord ischemia, Cultured milk products, Reperfusion injury
INTRODUCTION
Spinal cord ischemia and reperfusion (SCIR) may develop in a variety of situations. The main event of situations with reduced perfusion are a variety of vascular pathologies causing acute arterial occlusion, surgical interventions requiring clamping, trauma causing ischemia, transplantation and shock. Many complications may develop after ischemia and reperfusion damage from paraparesis to death.
Reversible or irreversible cell damage of ischemia is linked to insufficient blood flow perfusing organs or tissues. Ischemia disrupts oxidative phosphorylation in the cell causing reduced intercellular adenosine triphosphate (ATP) and phosphocreatin synthesis. This situation disrupts the ionic pump function related to ATP in the cell membrane and results in more calcium, sodium and water entering the cell. During ischemia, degradation of adenin nucleotides increases. This causes increased accumulation of hypoxanthine, the precursor of reactive oxygen species (ROS), within the cell. After ischemia the reperfusion of the region and the renewed presence of molecular oxygen in the cell creates ROS6). It is thought that the ROS linked to high levels of malondialdehyde (MDA) causes lipid peroxidation. Lipid peroxidation results in damage to cell membranes. ROS is cleared from the cell by superoxide dismutase (SOD) and catalase (CAT) enzymes11).
Nutritional sources of probiotics are fermented yogurts, cheeses, pickles, raw sausage, bread, beer, wine, kumis and kefir using lactobacilli, bifidobacteria, enterococcus, and streptococcus2). Kefir is a cultured milk product produced using kefir grains, kefir culture or kefir starter culture. It is a slightly acidic, alcoholic and foamy drink. In kefir ethyl alcohol and lactic acid fermentation occur simultaneously21). A various of biological activity has been reported in kefir. Kefir is known to have antibiotic and antifungal activity against yeast and acetic acid bacteria in intestinal microflora8,27). Kefir has been reported to have anti-mutagenic, immunostimulant, anti-inflammatory and anti-oxidant activity and anti-diabetic effects in animals10,13,15,18,20,26).
In our study the anti-oxidant and protective effects of kefir on SCIR damage in rats was researched. With this aim the effects of kefir on SOD and CAT activity and MDA levels after SCIR were studied. Additionally, the dead neurons and the immunopositive cells were counted in spinal cord histological samples. Toluidine-blue staining was used to evaluate general histological examination, Hipoxia-inducible factor-1α (HIF1-α) and caspase 3 primary antibodies were used to label and evaluate these proteins immunohistochemically.
MATERIALS AND METHODS
Animals
The methods used for animal experiments were in accordance with the international guiding principles for biomedical research involving animals recommended by the World Health Organization. Permission was granted by Canakkale Onsekiz Mart University Animal Experiments Local Ethics Committee (Protocol number : 2014/03-02, date of approval : 21/03/2014). This study was conducted in Canakkale Onsekiz Mart University Experimental Research Center.
In this study, we used 24 male Sprague-Dawley rats weighing 250-350 g. All animals were fed ad libitum with 7-8 mm pellet rat food (Bil-Yem Ltd., Ankara, Turkey) and tap water. To provide 12 hour darkness, 12 hour light environment photoperiodic white fluorescent light was used and the temperature and humidity were held at 21±2℃ and 55-60%. Before the operation no rat was found to have any abnormalities.
Rats were randomly divided into three equal groups (consisting of eight rats each) : 1) sham operated control group. Laparotomy and infrarenal abdominal aorta dissection was completed but occlusion was not performed; 2) SCIR (ischemia) group fed on a standard diet without kefir pretreatment; and 3) kefir group fed on a standard diet plus kefir (NBL Probiotic ATP, Cell Biotech Co., Ltd., Seoul, Korea) (10 cc/kg/day; 2×109 cfu/kg/day) by gastric gavage during 1 month before SCIR.
Dosage
The dosage to reveal the beneficial effects of kefir was determined as 10 cc/kg/day body weight (2×109 cfu/kg/day) based on preliminary studies with various doses (3×108, 9×109, 1.8×1010 cfu/kg bw)14,23).
Surgical procedure
SCIR was induced as reported by Lafci et al.16). A Biopac MP36 (BIOPAC Systems, Inc., Goleta, CA, USA) device was used as a monitor. Mean arterial pulse was 375 per minute during surgery. Body temperature was monitored with a rectal probe and was adjusted to 37.1 to 37.4℃ with a heating pad during surgery. Rats were given premedication with intraperitoneal ketamine (50 mg/kg) and xylazine (5 mg/kg). Anesthesia was continued with ketamine injections at intervals without intubation or mechanical ventilation. Surgical approach was supine position. After the operating field was prepared in sterile fashion, laparotomy was performed with a standard midline incision. After retracting the intestines laterally, the retroperitoneum was opened and was reached to the abdominal aorta. The spinal cord ischemia was induced by cross-clamping the aorta with mini aneurysm clip between just below the left renal artery and just proximal to the aortic bifurcation. Loss of aortic pulse was confirmed by palpation. The duration of ischemia was set at 45 minutes and later the cross clamps were removed and distal reperfusion was observed visually. At the end of the procedure, the abdominal wall was closed with 5/0 prolene sutures. Animals in sham group underwent a surgical procedure similar to the other groups but the aorta was not clamped. This group of animals was used for eliciting the effects of anesthesia and operation on results and also determining the biochemical parameters studied in the normal spinal cord tissue. Animals fed on a standard diet and water ad libitum in their cages after surgery. At 24th hour all animals were anesthetized with penthobarbital (20 mg/kg) and sacrificed. The lumbar spinal cord was harvested immediately via posterior approach. Each spinal cord was longitudinally divided into 2 equal parts with a fine scalpel. Half of the specimen taken for histopathological investigation and it was fixed in formalin for 7 days. The other half was stored in a freezer at -80℃ for biochemical estimations.
Evaluation of neurological status
Neurological status of animals was assessed blindly by a neurologist at 1, 12, and 24th hour after SCIR. To assess the motor function of rats after SCIR, rats were scored by a modified Tarlov's scale3) as follows : 0, no lower extremity movement; 1, lower extremity motion without gravity; 2, lower extremity motion against gravity; 3, able to stand with assistance; 4, able to walk with assistance; 5, normal.
Biochemical estimations of spinal cord tissue
For biochemical investigation, MDA levels, CAT, and SOD activities from each supernatant were measured in duplicate with highly sensitive ELISA spectrophotometry, respectively. The protein concentrations were determined by the Lowry method19) using commercial protein standards (Sigma Aldrich, Inc., St. Louis, MO, USA; Total protein kit-TP0300-1KT).
Tissue MDA levels
Tissue MDA levels were determined according to Buege's method5). MDA levels were analyzed for lipid peroxidation products in spinal cord tissue using the thiobarbituric acid reactive substance test (Cell Biolabs, Inc., San Diego, CA, USA; STA-330 MDA Assay Kit). The results were expressed as nmol/mg protein.
Tissue CAT activity
Tissue CAT activity was determined according to Aebi's method1). Decomposition of H2O2 in the presence of CAT was followed at 540 nm (Cell Biolabs, Inc., San Diego, CA, USA; STA-341 Catalase Activity Assay Kit). CAT activity was reported as U/mg protein.
Tissue SOD activity
Tissue SOD activity was measured with a modified spectrophotometric method at 560 nm as described by Sun et al.29). SOD activity was reported as U/mg protein.
Histopathological investigation
The spinal cord samples were fixed with 10% neutral formalin and then histologically processed and embedded in paraffin. Five micron-thick sections were mounted onto slides for histochemistry. Toluidine-blue staining was used to evaluate general histological examination. In the evaluation of toluidine blue-stained sections the dead neurons which were characterized by karyolitic, karyorectic nucleus and vacuolated cytoplasm were counted in 6 different random areas under ×20 objective magnification. All the sections were evaluated under light microscope (Eclipse E-600, Nikon, Tokyo, Japan). The data were statistically analyzed.
Immunohistochemistry
The spinal cord samples were fixed with 10% neutral formalin and then histologically processed and embedded in paraffin. Five micron-thick sections were mounted onto poly-l-lysine coated slides for immunohistochemistry. Tissue samples were deparaffinized and hydrated and immunohistochemically stained with HIF1-α and caspase 3 primary antibodies. Citrate buffer (pH=6.0) was used for antigen retrieval. Endogenous peroxidase activity was blocked by 3% hydrogen peroxidase in methanol. Then primary antibodies were dropped and incubated overnight for both in dilution 1 : 50. After incubation, HRP secondary antibody kit was used as a secondary antibody and AEC kit for chromogen. Finally, all the slides were counter-stained with Mayers hematoxylin and mounted with water-based mounting medium. All the chemicals were purchased from Labvision corp (Fremont, CA, USA). HIF1-α and caspase 3 primary antibodies were used to label and evaluate these proteins immunohistochemically. The immunopositive cells in slides were counted in 6 different random areas under ×20 objective magnification. Immunopositive cells were counted by Image Analysis Software (NIS Elements, Nikon, Tokyo, Japan). The data were statistically analyzed.
Statistical analysis
Results were subjected to one-way analysis of variance using the Statistical Package for the Social Sciences (SPSS 19.0, SPSS Inc., Chicago, IL, USA) software. Differences among the groups were obtained using the Bonferonni's test option and were considered to be significant at p<0.05. All data was expressed as mean±standard deviation in each group. Tarlov scale results were analyzed by the Kruskal-Wallis followed by the Mann-Whitney U test. Difference was considered significant when p<0.05.
RESULTS
Neurological examination results
Fourty-five minutes of ischemia resulted in severe motor deficit in the hind limbs of ischemia groups, while all sham group animals maintained normal motor behavior (score of 5), as assessed by the motor deficit score. Pre-treatment with kefir did not prevent the development of paraparesis. Most of animals in the kefir group exhibited score 3 or score 4 motor function at 24 h. The scores in kefir group were significantly higher than ischemia group at 24 h (3.25±0.37 vs. 1.37±0.46; p<0.05) (Table 1).
Table 1. Tarlov scores at 1, 12, and 24 hours after surgery.
Data are expressed as means±standard deviation. Statistical analysis was by means of the Kruskal-Wallis test. p-value defines the significant difference (p<0.05)
Biochemical estimation results
MDA levels were found to be significantly higher in the ischemia group compared to sham group (p<0.05). Also it was determined that spinal cord SOD and CAT activities decreased in the ischemia group (p<0.05). When the kefir group was compared with ischemia group, a significant decrease in MDA levels of spinal cord tissues was observed (p<0.05). CAT and SOD levels in spinal cord tissues of the kefir group were significantly higher than ischemia group (p<0.05) (Table 2).
Table 2. Effects of kefir on changes in lipid peroxidation levels (MDA), catalase (CAT) and enzymatic activity of superoxide dismutase (SOD) in spinal cord tissues.

Results were given as mean±standard deviation. Means in the same line by the same letter are significantly different in each group to the one-way analysis of variance-Bonferroni test. p<0.05 : defines the significant difference
Histopathological results
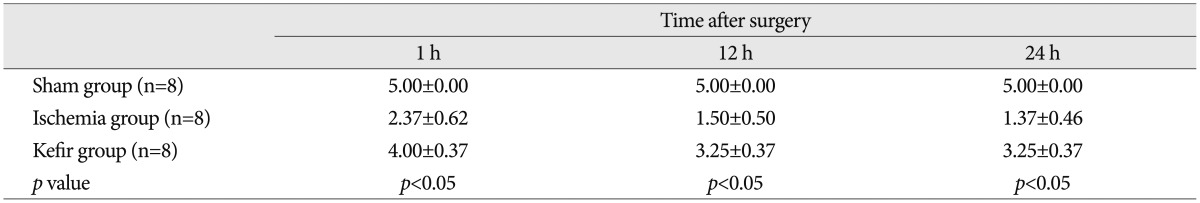
Histopathological examination of toluidine blue staining in the sham group showed normal morphology. Ischemia group showed many dead and degenerated neurons with karyolitic, karyorectic nucleus and vacuolated cytoplasm. Comparing the ischemia group with sham group, there was a significant increase in the number of dead and degenerated neurons in ischemia group (p<0.05). Comparing the ischemia and kefir group with the sham group, the number of the dead and degenerated neurons were significantly higher than sham group (p<0.05). When the kefir group is compared with ischemia group there was a significant decrease in numbers of dead and degenerated neurons in kefir group (p<0.05) (Fig. 1).
Fig. 1. Representative photomicrographs showing dead and degenerated neurons stained with toluidine blue in the ischemic spinal cord area of rats. A : Sham group showing normal architecture. B : Ischemia group showing degenerated neurons (arrowheads). C : Kefir group showing degenerated neurons (arrowhead). Toluidine blue, ×400.
Immunohistochemical analysis
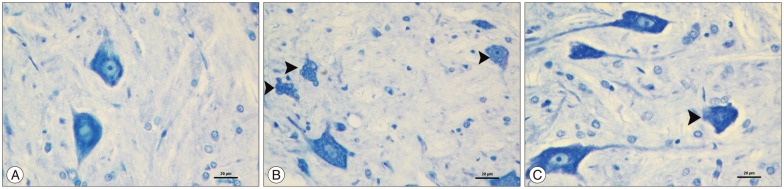
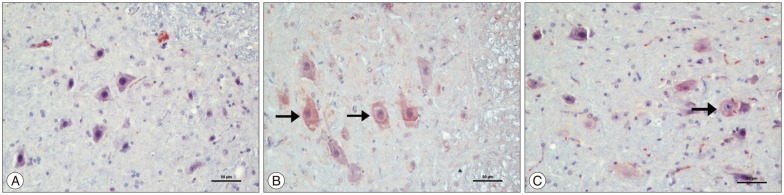
Immunohistochemical results showed that cytoplasmic positivity was found in neuron cells with caspase 3 immunostaining. Sham group showed few caspase 3 immunopositive neurons. Ischemia group showed many caspase 3 immunopositive neurons compared with the sham group and caspase 3 immunopositive neurons significantly increased in the ischemia group (p<0.05). Kefir group showed fewer caspase 3 immunopositive neurons than ischemia group and compared with ischemia group caspase 3 immunopositive neurons were significantly decreased in the kefir group (p<0.05) (Fig. 2).
Fig. 2. The representative example of caspase 3 immunohistochemical images in ischemic spinal cord section. A : Sham group showing lower caspase 3 immunopositive neurons. B : Ischemia group showing many caspase 3 immunopositive neurons (thick arrows). C : Sham and kefir group showing fewer caspase 3 immunopositive neurons than ischemia group (thick arrows). Caspase 3 primary antibody, ×200.
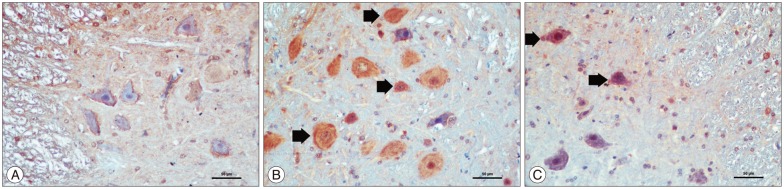
In staining with HIF1-α, positive staining in cytoplasm and cytoplasmic membrane was observed. Sham group showed few immunopositive neurons. Ischemia group showed many HIF1-α immunopositive neurons and HIF1-α immunopositive neurons were significantly increased compared with the sham group (p<0.05). The kefir group showed fewer HIF1-α immunopositive neurons than ischemia group and compared with the ischemia group the HIF1-α immunopositive neurons were significantly decreased in the kefir group (p<0.05) (Table 3, Fig. 3).
Table 3. Toluidine blue, caspase 3, and HIF1-α staining results for all groups.
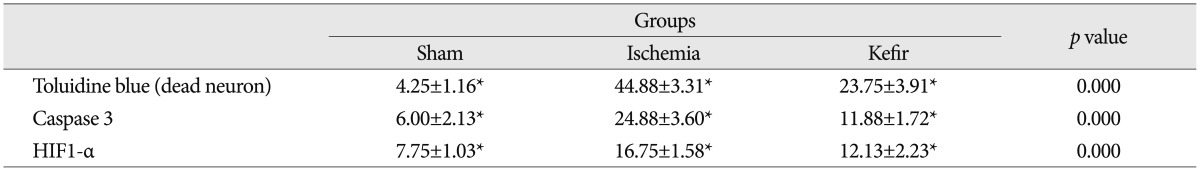
One way analysis of variance and post hoc analysis with Bonferroni test. Data are expressed as mean±standard deviation. *p<0.05 : defines the significant difference
Fig. 3. The representative example of HIF1-α immunohistochemical images in ischemic spinal cord section. A : Sham group showing no HIF1-α immunopositive neuron. B : Ischemia group showing many HIF1-α immunopositive neurons (thin arrows). C : Kefir group showing lower HIF1-α immunopositive neurons than ischemia group (thin arrow). HIF1-α, ×200.
DISCUSSION
Neural tissue is very sensitive to ischemia. Cross-clamping the aorta during the surgical treatment of descending thoracic and thoracoabdominal aortic disease inevitably results in temporary or permanent ischemia of the spinal cord. Several strategies have been implemented to maintain spinal cord blood flow (distal aortic perfusion, intrathecal vasodilators, reattachment of intercostal and lumbar vessels, decreasing cerebrospinal fluid pressure), to increase spinal cord tolerance to ischemia (hypothermia, anesthetic agents, calcium-channel blockers, excitatory amino acid antagonists), and to decrease reperfusion injury (free radical scavengers, immune system modulation, adenosine)22). Mauney et al.22) reported that SCIR injury was prevented in animals receiving methylprednisolone intravenously. The protective effect of corticosteroids were thought to be related to their ability to stabilize membranes, modulate the immune system, and scavenge for free radicals. The 21-amino-steroids are potent scavengers of superoxide and lipid peroxyl radicals. However, paraplegia remains an uncommon but devastating complication of aortic diseases.
In our study a cultured milk product, kefir, which can be obtained naturally was used.
Kefir is a traditional drink obtained via fermentation of milk by "kefir grains". Kefir grains, which are complex mixtures of bacteria, yeast, and the polysaccharides produced by this microflora. Kefir, which is believed to be a "functional food" due to its health benefits and disease prevention properties beyond its basic nutritional value, is becoming increasingly popular throughout the world10). Understanding the bacterial community in the kefir grain is important for use of kefir as functional food. The most possible gradients in kefir are Lactobacillus kefiranofaciens, Lactobacillus buchneri, and Lactobacillus helveticus8,10).
In the SCIR model in rats, kefir was shown to have a protective effect on the ischemic spinal cord histopathologically. In addition after treatment MDA levels were decreased and SOD and CAT activities were increased in biochemical tests, showing a reduction in oxidative stress forming after ischemia.
ROS developing in oxidative stress during SCIR, affects membrane lipids, cellular proteins and DNA. These processes trigger lipid peroxidation. Kefir may make neural tissues more sensitive to lipid peroxidation, thus anti-oxidant enzymes are induced and causes a beneficial effect26). In our study, kefir pre-treatment significantly decreased MDA. It is known that the bioactive peptides released during fermentation by proteolytic lactic acid bacteria can scavenge ROS and inhibit MDA26).
All peroxides, especially H2O2, can be metabolized by CAT enzymes. Additionally cellular membranes, membrane and cellular proteins and DNA are protected from lipid peroxidation. However CAT works as an anti-oxidant in the cell during oxidative stress. CAT activity increases during oxidative stres12). But if oxidative stress lasts a long time and overwhelms the capacity of CAT activity, CAT activity can reduce24). In our study the CAT activity of the ischemia group can be compared with the sham and kefir groups. These results were admitted as the scavenger effect of CAT due to high levels of ROS in the ischemia group. Compared to the ischemia group, application of kefir had a significant increase in CAT activity.
To protect neural tissue from the unwanted effects of ROS, cells have a large number of enzymatic and nonenzymatic anti-oxidants. SOD activity protects the proteins from the metal-catalyzed reaction between O2 and H2O2. Oxidative stress developing after ischemia/reperfusion causes an increase in SOD activity18). But if oxidative stress lasts a long time and overwhelms the capacity of SOD activity, SOD activity can reduce24). In our study compared to the ischemia group, the SOD activity in the kefir group was significantly increased. This may be due to the rats in the kefir group being protected from ROS.
In ischemia apoptosis may begin by two paths; intrinsic and extrinsic. Cytochrome c and caspase 3 stimulation released by mitochondria forms the intrinsic pathway25). Caspase 3 is known to be important in apoptosis caused by ischemia of neuronal cells. Studies of apoptotic neuronal cells have found that caspase 3 may be activated by both intrinsic and extrinsic signal pathways and as a result supported the idea that caspase 3 plays a key role in ischemic apoptosis25). Some studies have shown a relationship between apoptosis and development of ischemia17,31). Li et al.17), in a spinal cord ischemia model in rats, showed that caspase 3 activity clearly increased compared to the control group. In our study, kefir group showed fewer caspase 3 immunopositive neurons than ischemia group and compared with ischemia group caspase 3 immunopositive neurons were significantly decreased in kefir group (Fig. 2).
HIF-1 is a nuclear protein and plays an important role in the cell in hypoxia homeostasis28). The HIF-1α expression increases linked to reduced oxygen concentration. To rapidly respond to hypoxia, cells regularly and continuously synthesize and eliminate HIF-1α in non-hypoxic conditions. In the hypoxic environment HIF-1α degradation is inhibited and the amount of this protein increases30). The mechanisms of effect of this protein can be listed as regulating the transcription of many genes, cell dedifferentiation, vascularization, production of autocrine growth factor, proliferation, invasion and metastasis, and metabolic reprogramming7). Linked to oxygen concentration changes, arrangements of transcription of genes regulated by HIF-1 occur4). In accordance with the literature, in our study the HIF-1α expression of the ischemia group was increased, while this level was statistically significantly reduced in the kefir group.
In our study, all animals of the kefir and ischemia groups experienced severe hind limb motor deficit. This means that a strategy focusing only on the attenuation of the SCIR injury alone (i.e., anti-oxidant agents) is not likely to prevent severe spinal cord injury. Spinal cord hypoperfusion needs to be avoided during aortic surgery. Ege et al.9) induced a SCIR model and achieved a significant improvement in limb function over 72 hours. Their results are consistent with our study. Pre-treatment with kefir significantly improved the Tarlov scores of the animals compared with the sham group. The ranges of Tarlov scores were significantly higher in the kefir group than ischemia group.
CONCLUSION
This study proposes that prophylactic administration of kefir can reduce spinal cord ischemia in an aortic occlusion model of rats. In this manner, dietary supplementation with kefir may be beneficial to preserve or ameliorate spinal cord ischemia. In conclusion; kefir has neuroprotective and anti-oxidant effects on biochemical and histopathological parameters of spinal cord ischemia and reperfusion injury.
Acknowledgements
The authors thank to experimental research center of Canakkale Onsekiz Mart University.
References
- 1.Aebi H, Wyss SR, Scherz B, Skvaril F. Heterogeneity of erythrocyte catalase II. Isolation and characterization of normal and variant erythrocyte catalase and their subunits. Eur J Biochem. 1974;48:137–145. doi: 10.1111/j.1432-1033.1974.tb03751.x. [DOI] [PubMed] [Google Scholar]
- 2.Akman SA, Yagci RV. Prebiotics and probiotics. Cocuk Sagligi ve Hastaliklari Dergisi. 2002;45:337–344. [Google Scholar]
- 3.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 4.Berchner-Pfannschmidt U, Frede S, Wotzlaw C, Fandrey J. Imaging of the hypoxia-inducible factor pathway : insights into oxygen sensing. Eur Respir J. 2008;32:210–217. doi: 10.1183/09031936.00013408. [DOI] [PubMed] [Google Scholar]
- 5.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 6.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Endler A, Shibasaki F. Hypoxia and angiogenesis : regulation of hypoxia-inducible factors via novel binding factors. Exp Mol Med. 2009;41:849–857. doi: 10.3858/emm.2009.41.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen TH, Wang SY, Chen KN, Liu JR, Chen MJ. Microbiological and chemical properties of kefir manufactured by entrapped microorganisms isolated from kefir grains. J Dairy Sci. 2009;92:3002–3013. doi: 10.3168/jds.2008-1669. [DOI] [PubMed] [Google Scholar]
- 9.Ege E, Ilhan A, Gurel A, Akyol O, Ozen S. Erdosteine ameliorates neurological outcome and oxidative stress due to ischemia/reperfusion injury in rabbit spinal cord. Eur J Vasc Endovasc Surg. 2004;28:379–386. doi: 10.1016/j.ejvs.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Farnworth ER. Handbook of Fermented Functional Foods. ed 1. Boca Raton, FL, USA: CRC Press; 2003. pp. 59–75. [Google Scholar]
- 11.Ginsberg MD. Review : Neuroprotection in brain ischemia : an update (part I ) Neuroscientist. 1995;1:95–103. [Google Scholar]
- 12.Güven A, Güven A, Gülmez M. The effect of kefir on the activities of GSH-Px, GST, CAT, GSH and LPO levels in carbon tetrachloride-induced mice tissues. J Vet Med B Infect Dis Vet Public Health. 2003;50:412–416. doi: 10.1046/j.1439-0450.2003.00693.x. [DOI] [PubMed] [Google Scholar]
- 13.Guzel-Seydim ZB, Kok-Tas T, Greene AK, Seydim AC. Review : functional properties of kefir. Crit Rev Food Sci Nutr. 2011;51:261–268. doi: 10.1080/10408390903579029. [DOI] [PubMed] [Google Scholar]
- 14.Hong WS, Chen YP, Chen MJ. The antiallergic effect of kefir Lactobacilli. J Food Sci. 2010;75:H244–H253. doi: 10.1111/j.1750-3841.2010.01787.x. [DOI] [PubMed] [Google Scholar]
- 15.Kanbak G, Uzuner K, Kuşat Ol K, Oğlakçı A, Kartkaya K, Şentürk H. Effect of kefir and low-dose aspirin on arterial blood pressure measurements and renal apoptosis in unhypertensive rats with 4 weeks salt diet. Clin Exp Hypertens. 2014;36:1–8. doi: 10.3109/10641963.2013.783046. [DOI] [PubMed] [Google Scholar]
- 16.Lafci G, Gedik HS, Korkmaz K, Erdem H, Cicek OF, Nacar OA, et al. Efficacy of iloprost and montelukast combination on spinal cord ischemia/reperfusion injury in a rat model. J Cardiothorac Surg. 2013;8:64. doi: 10.1186/1749-8090-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 18.Liu JR, Chen MJ, Lin CW. Antimutagenic and antioxidant properties of milk-kefir and soymilk-kefir. J Agric Food Chem. 2005;53:2467–2474. doi: 10.1021/jf048934k. [DOI] [PubMed] [Google Scholar]
- 19.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Maeda H, Zhu X, Omura K, Suzuki S, Kitamura S. Effects of an exopolysaccharide (kefiran) on lipids, blood pressure, blood glucose, and constipation. Biofactors. 2004;22:197–200. doi: 10.1002/biof.5520220141. [DOI] [PubMed] [Google Scholar]
- 21.Mainville I, Robert N, Lee B, Farnworth ER. Polyphasic characterization of the lactic acid bacteria in kefir. Syst Appl Microbiol. 2006;29:59–68. doi: 10.1016/j.syapm.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Mauney MC, Blackbourne LH, Langenburg SE, Buchanan SA, Kron IL, Tribble CG. Prevention of spinal cord injury after repair of the thoracic or thoracoabdominal aorta. Ann Thorac Surg. 1995;59:245–252. doi: 10.1016/0003-4975(94)00815-O. [DOI] [PubMed] [Google Scholar]
- 23.Owaga EE, Chen MJ, Chen WY, Chen CW, Hsieh RH. Oral toxicity evaluation of kefir-isolated Lactobacillus kefiranofaciens M1 in Sprague-Dawley rats. Food Chem Toxicol. 2014;70:157–162. doi: 10.1016/j.fct.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Ozen OA, Cosar M, Sahin O, Fidan H, Eser O, Mollaoglu H, et al. The protective effect of fish n-3 fatty acids on cerebral ischemia in rat prefrontal cortex. Neurol Sci. 2008;29:147–152. doi: 10.1007/s10072-008-0926-1. [DOI] [PubMed] [Google Scholar]
- 25.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 26.Punaro GR, Maciel FR, Rodrigues AM, Rogero MM, Bogsan CS, Oliveira MN, et al. Kefir administration reduced progression of renal injury in STZ-diabetic rats by lowering oxidative stress. Nitric Oxide. 2014;37:53–60. doi: 10.1016/j.niox.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues KL, Caputo LR, Carvalho JC, Evangelista J, Schneedorf JM. Antimicrobial and healing activity of kefir and kefiran extract. Int J Antimicrob Agents. 2005;25:404–408. doi: 10.1016/j.ijantimicag.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 30.Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15:621–627. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 31.Yamauchi T, Sawa Y, Sakurai M, Hiroshi T, Matsumiya G, Abe K, et al. ONO-5046 attenuation of delayed motor neuron death and effect on the induction of brain-derived neurotrophic factor, phosphorylated extracellular signal-regulated kinase, and caspase3 after spinal cord ischemia in rabbits. J Thorac Cardiovasc Surg. 2006;131:644–650. doi: 10.1016/j.jtcvs.2005.06.041. [DOI] [PubMed] [Google Scholar]


