Abstract
Background and Aims Root hemiparasites from the rhinanthoid clade of Orobanchaceae possess metabolically active glandular trichomes that have been suggested to function as hydathode trichomes actively secreting water, a process that may facilitate resource acquisition from the host plant’s root xylem. However, no direct evidence relating the trichomes to water secretion exists, and carbon budgets associated with this energy-demanding process have not been determined.
Methods Macro- and microscopic observations of the leaves of hemiparasitic Rhinanthus alectorolophus were conducted and night-time gas exchange was measured. Correlations were examined among the intensity of guttation, respiration and transpiration, and analysis of these correlations allowed the carbon budget of the trichome activity to be quantified. We examined the intensity of guttation, respiration and transpiration, correlations among which indicate active water secretion.
Key Results Guttation was observed on the leaves of 50 % of the young, non-flowering plants that were examined, and microscopic observations revealed water secretion from the glandular trichomes present on the abaxial leaf side. Night-time rates of respiration and transpiration and the presence of guttation drops were positively correlated, which is a clear indicator of hydathode trichome activity. Subsequent physiological measurements on older, flowering plants indicated neither intense guttation nor the presence of correlations, which suggests that the peak activity of hydathodes is in the juvenile stage.
Conclusions This study provides the first unequivocal evidence for the physiological role of the hydathode trichomes in active water secretion in the rhinanthoid Orobanchaceae. Depending on the concentration of organic elements calculated to be in the host xylem sap, the direct effect of water secretion on carbon balance ranges from close to neutral to positive. However, it is likely to be positive in the xylem-only feeding holoparasites of the genus Lathraea, which is closely related to Rhinanthus. Thus, water secretion by the hydathodes might be viewed as a physiological pre-adaptation in the evolution of holoparasitism in the rhinanthoid lineage of Orobanchaceae.
Keywords: Ecophysiology, holoparasite, hydathode trichome, Lathraea, parasitic plant, respiration, Rhinanthus alectorolophus, rhinanthoid Orobanchaceae, orobanche, root hemiparasite, transpiration, Triticum aestivum, water regime, water secretion, xylem
INTRODUCTION
About 1 % of flowering plants corresponding to 4500 species parasitize other plants by specialized organs called haustoria to acquire essential resources (Heide-Jørgensen, 2008). The majority of parasitic plant species are hemiparasites, green photosynthetic plants acquiring water, mineral nutrients and a certain amount of heterotrophic carbon from the host xylem (Press, 1989; Irving and Cameron, 2009; Těšitel et al., 2010a; Heide-Jørgensen, 2013). In contrast, holoparasites completely lack photosynthetic ability and thus acquire all essential resources heterotrophically from the host (Hibberd and Jeschke, 2001; Irving and Cameron, 2009).
Holoparasites are generally thought to have evolved repeatedly from hemiparasites (Westwood et al., 2010; McNeal et al., 2013; Naumann et al., 2013), but such an evolutionary transition can rarely be documented or studied due to the extinction of assumed hemiparasitic ancestors (Nickrent and Duff, 1996; Nickrent et al., 1998; Naumann et al., 2013). However, the family Orobanchaceae provides an opportunity to study the macroevolutionary transition between the trophic strategies of parasitic plants as it encompasses closely related non-parasitic, hemiparasitic and holoparasitic species (Bennett and Mathews, 2006; Heide-Jørgensen, 2008; Westwood et al., 2010; McNeal et al., 2013; Naumann et al., 2013). This is the case of the sister genera Rhinanthus and Lathraea, and closely related Rhynchocorys which form a separate sub-clade within the Rhinanthoid clade of Orobanchaceae (Těšitel et al., 2010c). Moreover, Tozzia alpina, another related Rhinanthoid species, displays a parallel evolutionary tendency towards holoparasitism (Těšitel et al., 2010c).
Rhinanthus species are hemiparasitic annuals possessing a highly efficient resource acquisition strategy based on an open vascular connection with the host xylem (Cameron et al., 2006) and a high transpiration rate directing the xylem stream from the host (Klaren and Janssen, 1978; Stewart and Press, 1990; Jiang et al., 2010). Despite the acquisition of substantial amount of carbon from the host in the form of xylem-mobile organic elements (Těšitel et al., 2010a, 2011), the hemiparasite’s own photosynthesis plays a crucial role in realization of its fitness (Těšitel et al., 2015). Most of the species of the Rhinanthoid clade are in principal physiologically similar to Rhinanthus, i.e. they are photosynthetic root hemiparasites acquiring resources from the host root xylem (Těšitel et al., 2010a; McNeal et al., 2013).
In contrast, Lathraea, T. alpina and the perennial species Rhynchocorys are holoparasitic, at least in early ontogenic stages of underground individuals, but unlike most other holoparasitic species (Irving and Cameron, 2009) they do not feature a connection to the host phloem in their haustoria. Lathraea species are characterized by extensive perennial underground rhizomes covered by fleshy scales of leaf origin (Ziegler, 1955; Renaudin, 1966). Shoots are short lived and their only function is flowering and seed production. The third genus of the sub-clade, Rhynchocorys, contains both species which are morphologically similar to Lathraea (rhizomes with scales, e.g. R. elephas), but retain photosynthetic activity in their green above-ground shoots (Kubat and Weber, 1987), and annual species which are closely similar to Rhinanthus (e.g. R. orientalis) (Těšitel et al., 2010c). The plant architecture and physiological functioning of the more distantly related T. alpina are closely similar to those of perennial Rhynchocorys species and the species is also known to have only a xylem connection in its haustoria (Weber, 1973). As a result of the underground growth habit, these species cannot transpire to discharge excess water taken up from the host xylem, which requires an alternative mechanism of water secretion for their physiological functioning.
Hemiparasites of the Rhinanthoid clade of Orobanchaceae were shown to have glandular trichomes on the abaxial side of their leaves (Fedorowicz, 1915; Kaplan and Inceoglu, 2003; Těšitel and Tesařová, 2013), frequently located close to leaf veins (Govier et al., 1968). Anatomically identical trichomes were also revealed on the scales of the below-ground rhizomes of Lathraea and Rhynchocorys (Groom, 1897; Ziegler, 1955; Renaudin, 1966; Kubat and Weber, 1987). The ultrastructure of these trichomes revealed numerous mitochondria, labyrinthine cell walls and plasmodesmata, structures suggesting their high metabolic activity (Schnepf, 1964; Renaudin and Garrigues, 1967; Těšitel and Tesařová, 2013). Govier et al. (1968) suggested a function of the trichomes as hydathode trichomes actively secreting water based on their observation of guttation from the leaves of hemiparasitic Odontites vernus Dumort. and a radioisotope tracing experiment. Moreover, extensive water secretion was also observed from the underground scale-like leaves of Lathraea. First reported by Darwin (1880), the secretion was later suggested to be associated with the glandular trichomes (Renaudin and Garrigues, 1967). To sum up, there is convincing evidence of the presence of metabolically active glandular trichomes in the Rhinanthoid Orobanchaceae and of an intense water secretion from the leaves of these parasitic plants. However, direct evidence relating the trichomes to water secretion and the carbon budget of the assumed, energy-demanding water secretion is yet to be revealed.
In this study, we aim to present conclusive direct evidence on the physiological role of the assumed hydathode trichomes and integrate their function into the physiology of hemiparasites. Macroscopic and microscopic observations were combined with gas exchange measurements to capture the physiological activity of the trichomes on the leaves of hemiparasitic Rhinanthus alectorolophus. Using the gas exchange measurements, we were able to estimate the carbon budget of the hydathode trichome activity. Moreover, our experimental set-up allowed testing of the effects of the hemiparasite developmental stage and availability of below-ground abiotic resources on the hydathode trichome activity.
MATERIALS AND METHODS
Plant material
Seeds of Rhinanthus alectorolophus (Scop.) Pollich were collected from the natural population near Zechovice, Czech Republic (49 °09′28″N, 13 °52′13″E; 510 m a.s.l.). Seeds of wheat (Triticum aestivum L.) used as a host species were obtained from the school farm of the Faculty of Agriculture, University of South Bohemia.
Experimental design and conditions
The experiment was carried out in a growth chamber at the Faculty of Science, University of South Bohemia from December 2013 to March 2014. Three-day-old seedlings of wheat germinated on a Petri dish with moist filter paper were sown to 0·8 L pots (one seedling per pot) filled with a mixture of sand and peat (1:1, v/v ratio). Half of the pots received 1 g of Osmocote Exact Standard 5–6 M fertilizer (Scotts Miracle-Gro Company, UK) per litre of substrate (high nutrient treatment, N+). According to the manufacturer’s specifications, the fertilizer contains 150 mg N g–1, 90 mg P g–1 and 120 mg K g–1. The other half of the pots did not receive any additional nutrients (low nutrient treatment, N–). All pots (n = 98) were well watered and maintained in the growth chamber with a 12 h light/12 h dark cycle and temperature regime of 20–22 °C (light):17–18 °C (dark). The photosynthetically active radiation (PAR) intensity during the day period was from 400 to 500 μmol m–2 s–1. The pots were randomized once a week to filter out possible heterogeneity in non-treatment cultivation conditions (mainly PAR intensity). Seedlings of R. alectorolophus, pre-germinated on moist filter paper at 4 °C after approx. 8 weeks, were added to the pots (two seedlings per pot) 1 d after wheat sowing. The hemiparasite seedlings were thinned to one per pot, and two contrasting water regimes were established 27 d after Rhinanthus sowing (DAS). High irrigation pots (W+) and low irrigation pots (W–) received 150 and 100 mL of tap water every fourth day, respectively. The nutrient and watering treatments were established in a full factorial design. The purpose of the nutrient and water treatments was to create certain environmental variability since hemiparasite physiology is known to be profoundly affected by the availability of these abiotic resources (Těšitel et al., 2015). However, the length of the simulated environmental gradients was much shorter than in the study of Těšitel et al. (2015) and was not of primary interest in our study.
Two sets consisting of 20 plants (i.e. five individual plants per each treatment combination, Supplementary Data Table S1) were selected for observations and physiological measurements conducted before and during the peak flowering period (55 and 73 DAS, Supplementary Data Fig. S1A, B). The plants were watered (following the watering protocol) several hours before the measurements. Repeated measurements on individual plants usually could not be performed due to frequent mortality of plants that had been subjected to the first measurement. Elevated plant mortality was probably caused by accidental mechanic damage.
Macroscopic and microscopic observations
The leaf surface of plants to be measured by gas exchange (see ‘Gas exchange measurements’) was examined for the density and size of guttation drops immediately before the measurements. Drops were classified on an ordinal scale (0, no drops; 0·5, small drops, i.e. <25 % leaf area covered by guttation drops; and 1, large drops, i. e. >25 % leaf area covered by guttation drops; Fig. 1). Leaves of R. alectorolophus were detached from some of the young non-flowering plants cultivated under each treatment combination and cut with a razor blade into thin sections. These sections were placed in either water or mineral oil as mounting media and subsequently subjected to light microscopy using an Olympus CX41 Microscope (Olympus Imaging America Inc., Center Valley, PA, USA) and INFINITY1-3 C 3.1 MP CMOS Color Camera (Lumenera Corp., Ottawa, Canada).
Fig. 1.
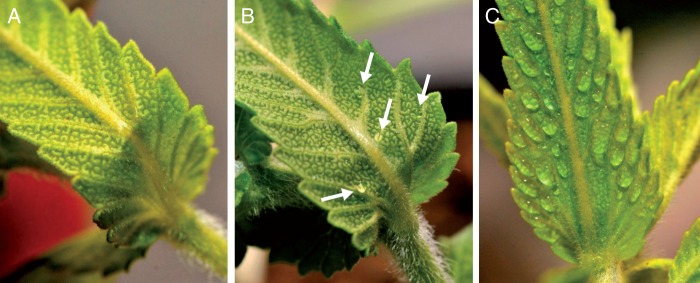
The density and size of drops on the leaves of Rhinanthus alectorolophus (55 d after sowing) classified on an ordinal scale: (A) no drops (0), (B) small drops (0·5), (C) large drops (1). The plant was cultivated under (A) low irrigation and nutrient treatment, (B) low irrigation and high nutrient treatment, and (C) high irrigation and nutrient treatment. Images were taken immediately before the physiological measurement.
Gas exchange measurements
Night-time rates of respiration (μmol CO2 m–2 s–1) and transpiration (mmol H2O m–2 s–1) were measured on intact leaves with a Li-6400 Portable Photosynthetic System (Li-Cor, Lincoln, NE, USA) coupled to a 2 cm2 circular leaf chamber. Each measurement was done between 0200 and 0900 h at ambient temperature and an air relative humidity of 65–70 % in the dark. Air relative humidity inside the measurement chamber and ambient CO2 concentration were controlled at 60–75 % and 400 μmol mol–1, respectively. The surface of the leaves subjected to measurements had been dried by filter paper prior to the gas exchange measurements. Dark respiration and transpiration rates were recorded in 5 s intervals for approx. 3 min after a steady-state gas exchange rate was achieved. The surface of the measured leaves was dry before and after the gas exchange measurements. Mean values of these measurement series were then used in the data analysis as respiration and transpiration rates of the corresponding plants.
In addition, the relative water content (RWC) of substrate was measured in the pots used in the gas exchange measurements with an HH2 Moisture Meter with an SM200 sensor (Delta-T Devices Ltd, Cambridge, UK).
Carbon budget calculations
Gas exchange measurements allowed us to estimate the concentration of organic carbon in the xylem sap of the hemiparasite necessary to compensate the carbon loss through respiration. Since no studies on the efficiency of carbon filtering from the xylem sap of hemiparasites were available, we assumed only the concentration of organic carbon in the xylem sap (i.e. filtering efficiency of 100 %) in the calculation of the carbon budget of the hydathode trichome activity (Supplementary Data Methods). Therefore, our carbon budget calculation indicates the maximal possible carbon acquisition from the xylem sap. In reality this might be lower, which is reflected in the discussion.
Data analysis
Linear (LM) and generalized linear models (GLM) were used to analyse the effect of developmental stage and water and nutrient treatments on the physiological parameters of Rhinanthus plants. Respiration and transpiration rates were analysed by LMs, while binomial GLM was used to analyse the presence and size of guttation drops, which was allowed by the quasi-binomial coding. The correlation between night-time transpiration and respiration rates was analysed as a linear regression (respiration–transpiration), which produces numerical results identical to Pearson correlation. All analyses were conducted in R, version 3.0.1 (R Core Team, 2013). The relationships among all treatments and parameters monitored were summarized by principal component analyses (one analysis for each of the two developmental stages) included as Supplementary Data Fig. S3. These analyses were based on the variables centred by mean subtraction and standardized by dividing by the standard deviation, and were performed in Canoco for Windows, version 5 (ter Braak and Šmilauer, 2012).
RESULTS
Macroscopic and microscopic observations
Guttation drops were observed on the abaxial leaf surface of 50 % of non-flowering plants (55 DAS) and 15 % of flowering plants (73 DAS). The presence and size of drops were significantly (P < 0·05) affected by the developmental stage of a plant and nutrient treatment (Table 1). The presence of large drops was significantly higher under the N+ treatment (z = 2·076, P = 0·038) and lower in flowering plants (z = –2·311, P = 0·021). No large drops were found on flowering plants (Supplementary Data Table S1). Both stalked and sessile hydathode trichomes were observed on the abaxial leaf surface of examined plants of all treatments. They were omnipresent on the abaxial surface, but sporadically occurred also on the adaxial surface. Microscopic observation in mineral oil revealed drops of liquid secreted from both trichome types (Figs 2A–D and 3A–F). No drops of liquid were observed in water as the mounting medium (Supplementary Data Fig. S2).
Table 1.
Summary of (generalized) linear models testing the effects of developmental stage, water and nutrient treatment on the presence and size of guttation drops, respiration and transpiration rates in R. alectoroplophus
| Drops |
Respiration |
Transpiration |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | d.f. | Deviance | P | Sum Sq. | F | P | Sum Sq. | F | P |
| Nutrients | 1 | 4·39 | 0·0362 | 3·17 | 10·23 | 0·0031 | 0·36 | 0·51 | 0·48 |
| Water | 1 | 0·04 | 0·84 | 0·03 | 0·10 | 0·76 | 0·60 | 0·84 | 0·37 |
| Stage | 1 | 7·08 | 0·0078 | 0·0002 | 0·0005 | 0·98 | 9·28 | 13·02 | 0·0010 |
| Nutrients × Water | 1 | 0·65 | 0·42 | 0·16 | 0·52 | 0·48 | 0·76 | 1·07 | 0·31 |
| Nutrients × Stage | 1 | 0·50 | 0·48 | 0·29 | 0·95 | 0·34 | 0·01 | 0·01 | 0·93 |
| Water × Stage | 1 | 0·13 | 0·72 | 1·02 | 3·30 | 0·08 | 1·99 | 2·79 | 0·10 |
| Nutrients × Water × Stage | 1 | 0·00 | 1·00 | 0·56 | 1·79 | 0·19 | 0·50 | 0·70 | 0·41 |
| Residuals | 32 | 21·37 | 9·92 | 22·80 | |||||
Statistically significant results (P < 0·05) are highlighted in bold.
Non-significant terms (P > 0·05) were omitted from the final models.
Fig. 2.
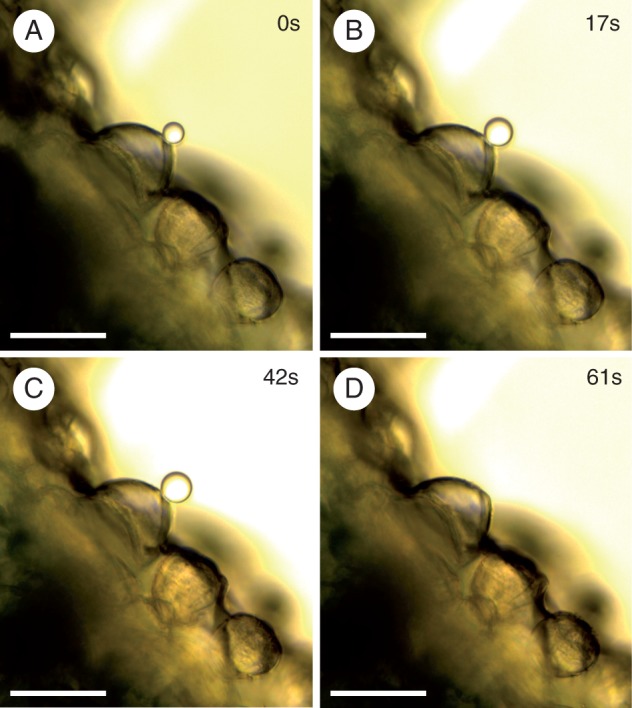
Micrographs showing secretion from sessile hydathode trichomes on the abaxial leaf surface of Rhinanthus alectorolophus. The secretion was observed in oil shortly after immersion of the sample (0s, A) and in the time series as indicated (B–D). The drop of liquid finally detached from the trichome and moved out of view (D). The scale bars indicate 50 μm.
Fig. 3.
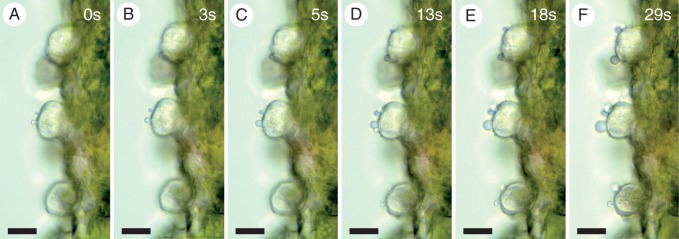
Micrographs showing secretion from stalked hydathode trichomes on the abaxial leaf surface of Rhinanthus alectorolophus. The secretion was observed in oil shortly after immersion of the sample (0s, A) and in the time series as indicated (B–F). The scale bars indicate 25 μm.
Gas exchange measurements
Dark respiration and transpiration rates were affected by the nutrient treatment and developmental stage, respectively (Table 1). Flowering Rhinanthus plants had lower transpiration rates than those measured before flowering (t38 = –3·613, P < 0·001). Rhinanthus cultivated under the N+ treatment displayed a higher dark respiration rate (t38 = 3·172, P = 0·003). Regardless of the significant effect of the water treatment on the RWC in pots (Welch two sample t-test: t32.3 = 3·005, P = 0·005), it did not have any significant effect on the gas exchange parameters (Table 1).
The gas exchange measurements revealed a strong positive relationship between night-time respiration and transpiration rates in non-flowering R. alectorolophus (Fig. 4A). The regression slope estimate was 0·55, which corresponds to 0·55 μmol respired carbon for the release of 1 mmol water in the form of guttation drops and stomatal transpiration. Moreover, both processes were also positively associated with the presence and size of guttation drops (Figs 4A and 5). The positive correlation among transpiration, respiration and size of the guttation drops is also demonstrated by the principal component analysis (Supplementary Data Fig. S3). In contrast, flowering hemiparasites exhibited no such relationship between the gas exchange physiological processes (Fig. 4B; Table S1; Fig. S3).
Fig. 4.
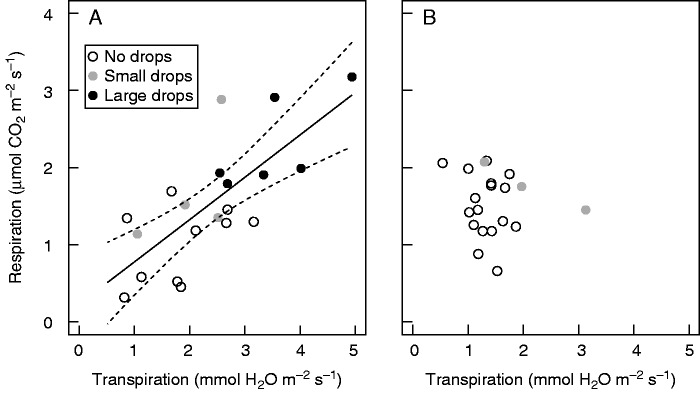
The relationship between the night-time rates of respiration and transpiration in (A) non-flowering and (B) flowering Rhinanthus alectorolophus. Each circle relates to one individual plant. The size of drops observed on the leaves of examined plants immediately before the physiological measurement is indicated in the key. Linear regression (r2 = 0·55, F1,18 = 22·31, P < 0·001) and the 95 % confidence interval are presented by solid and dashed lines, respectively. No large drops were observed on the leaves of flowering plants.
Fig. 5.
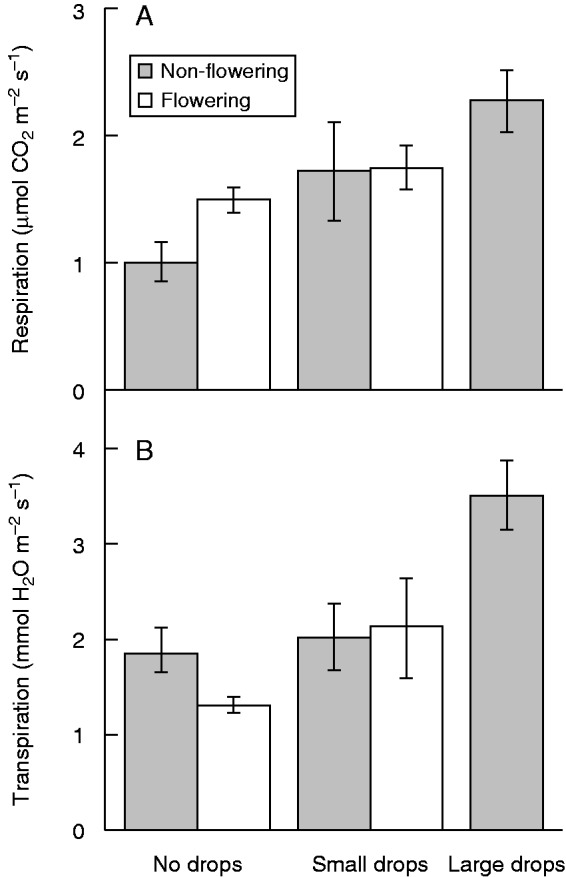
Rates of respiration (A) and transpiration (B) measured on the leaves of Rhinanthus alectorolophus with various sizes of water drops at the two developmental stages of the plants. Means and standard errors are presented. Non-flowering plants (55 d after sowing) and flowering plants (73 d after sowing) are indicated in the key. No flowering plants with large drops were recorded.
DISCUSSION
The combination of macroscopic and microscopic observations with the gas exchange measurements of Rhinanthus leaves provided the first unequivocal direct evidence on the physiological role of hydathode trichomes in water secretion in the Rhinanthoid Orobanchaceae. Their role is further supported by their ultrastructure (Schnepf, 1964; Renaudin and Garrigues, 1967; Těšitel and Tesařová, 2013) and explains earlier field measurements documenting an elevated night-time respiration and its correlation with night-time transpiration in multiple young hemiparasitic species (Press et al., 1988; Press, 1989). A similar relationship was found here in young leaves of R. alectorolophus and it was correlated with the presence and size of guttation drops secreted from hydathode trichomes.
The observed effects of developmental stage (young vs. flowering plants) and nutrient availability on the hydathode trichome activity provide a partial explanation of the high variability in the respiration rate and net photosynthesis reported in the Rhinanthoid hemiparasites (Press et al., 1988; Press, 1989; Seel and Press, 1993; Lechowski, 1996; Těšitel et al., 2011). The other part of the explanation lies in well-known effects of host species and nutrient availability on the photosynthetic efficiency and growth of hemiparasites (van Hulst et al., 1987; Seel et al., 1993; Cameron and Seel, 2007; Mudrák and Lepš, 2010; Těšitel et al., 2013, 2015). Thus, the physiological functioning of attached hemiparasites is highly plastic, depending not only on the host quality and environmental conditions, but also on the developmental stage. This should be considered in all ecophysiological studies focusing on the Rhinanthoid hemiparasites as it is unlikely to capture the activity of hydathode trichomes during standard photosynthetic measurements (e.g. light response curves) of flowering specimens.
Resource acquisition from the host is driven by the water potential difference between the host and parasites in xylem-feeding parasitic plants (Ehleringer and Marshall, 1995; Seel and Jeschke, 1999; Hibberd and Jeschke, 2001). A strongly negative water potential is maintained by the high content of osmotically active compounds (such as sugar alcohols) and the elevated transpiration rate, physiological traits shared by many Rhinanthoid Orobanchaceae (Hodgson, 1973; Press et al., 1988; Ehleringer and Marshall, 1995; Jiang et al., 2003; Phoenix and Press, 2004). Stomata of some hemiparasitic species including Rhinanthus spp. are insensitive to abscisic acid and remain open even at night or under water stress (Smith and Stewart, 1990; Jiang et al., 2003). Still, the hemiparasite’s night transpiration rate is very low due to high ambient relative air humidity. Driving the xylem stream during night-time independently of air humidity, the active water secretion by hydathode trichomes can play a crucial role of an additional mechanism decreasing the water potential. The hemiparasite does not compete with the host shoot for the host xylem stream under these conditions, which results in an exclusive flow of the xylem sap to the hemiparasite strongly facilitating resource acquisition. Such a role for hydathode trichomes in plant mineral nutrition and water balance is not unique to the (hemi)parasitic plants discussed here. These structures were suggested to play a similar role in young leaves of some non-parasitic plants, in particular under the conditions when transpiration is low (Frey-Wyssling, 1941; Höhn 1950; Klepper and Kaufmann, 1966; Heide-Jørgensen, 1980). The mechanism of active water secretion from hydathode trichomes, when water is transported through the cell wall against its osmotic potential, is not known yet. Nevertheless, recent studies suggest that water secretion may be driven by a co-transport of water and ions through specialized protein co-transporters (Zeuthen and MacAulay, 2012; Wegner, 2014).
Despite requiring energy, the water secretion from the hydathode trichomes is highly efficient according to our gas exchange measurements (1 mmol water release per the loss of 0·55 μmol C) (Fig. 4A). The effect of water secretion on the carbon balance of hemiparasites depends on the concentration of carbon in the xylem sap (Těšitel et al., 2010b, 2011; Bell and Adams, 2011) and the efficiency of its filtering from the sap on its way to the guttation fluid (Govier et al., 1968). The organic carbon is contained in the xylem sap mostly in the form of organic acids, amino acids and sugars (Canny and McCully, 1988). The concentration of organic carbon (in terms of organic C atoms) in the xylem sap necessary to compensate the carbon loss through respiration is 31 mm (Supplementary Data Methods). Taking this concentration into account and considering the filtering efficiency of <100 %, we expect that the direct effect of water secretion on carbon balance would be close to neutral (Govier et al., 1967; Seel and Jeschke, 1999; Alvarez et al., 2008) to positive (Canny and McCully, 1988) in hemiparasites growing on grass species. Although the amount of organic carbon in the xylem sap of trees varied significantly between seasons, the effect of water secretion on carbon balance in holoparasitic Lathraea growing on tree species would be positive [Schill et al., 1996; Heizmann et al., 2001; Escher et al., 2004; but not in all cases, see Furukawa et al. (2011); Supplementary Data Methods]. The positive carbon balance of the active water secretion by hydathode trichomes might be crucial for the evolution of the xylem-only feeding holoparasitic strategy of Lathraea (Ziegler, 1955) and early developmental stages of Rhynchocorys and Tozzia species (Weber, 1973; Kubat and Weber, 1987), which would not be able to compensate the negative carbon balance of the active water secretion by their own photosynthesis.
The increased activity of the hemiparasite hydathode trichomes under the N+ conditions probably reflects a generally better physiological performance of hemiparasitic plants. However, the host may also perform better under the N+ conditions and its competitive ability (in terms of competition for light) may increase. This can reduce the fitness of hemiparasites which are in general poor competitors (Matthies, 1995; Lepš, 1999; Mudrák and Lepš, 2010; Fibich et al., 2010; Těšitel et al., 2013) and decrease the effect of parasitism (Těšitel et al., 2015). The increased activity of the hydathode trichomes might thus partially compensate this negative effect by facilitating host-derived carbon acquisition and also inflicting more harm to the host. Both of these effects would decrease the competitive ability of the host and shift the hemiparasite–host fitness balance in favour of the hemiparasite.
Conclusion
Hydathode trichomes might be seen as an evolutionary innovation facilitating the resource acquisition of hemiparasitic Rhinanthoid Orobanchaceae and decreasing the adverse effects of the competitive pressure from the host community. Given their ubiquity among the Rhinanthoid Orobanchaceae (Fedorowicz, 1915; Kaplan and Inceoglu, 2003), they might also be considered a physiological pre-adaptation allowing the evolution of the xylem-only feeding holoparasitic strategy. This xylem-only feeding holoparasitic strategy evolved two or three times independently within the Rhinanthoid clade, and the incomplete and complete transitions from hemiparasitism to holoparasitism in the Rhinanthoid clade represent relatively recent evolutionary events (Těšitel et al., 2010c; Scheunert et al., 2012; McNeal et al., 2013). The knowledge of the evolutionary mechanism of these transitions together with well-resolved phylogenetic relationships thus make the Rhinanthoid clade an ideal model group for studying the macroevolution of trophic strategies in parasitic plants.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournaljournal.org and consist of the following. Figure S1: images of hemiparasitic Rhinanthus alectorolophus before and during the peak flowering period. Figure S2: image of stalked and sessile hydathode trichomes on the abaxial leaf surface of R. alectorolophus in water as mounting medium. Figure S3: ordination diagrams correlating response data and environmental variables in non-flowering and flowering plants. Table S1: guttation, respiration, transpiration and relative water content data recorded in the study. Methods: carbon budget calculations regarding the activity of hydathode trichomes.
ACKNOWLEDGEMENTS
The study was supported by the Czech Science Foundation (project no. P505/12/1390) and by a long-term research development project of the Institute of Botany, Czech Academy of Sciences (RVO 67985939). We thank two anonymous reviewers for their valuable comments on a previous version of the manuscript.
LITERATURE CITED
- Alvarez S, Marsh EL, Schroeder SG, Schachtman DP. 2008. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant, Cell and Environment 31: 325–340. [DOI] [PubMed] [Google Scholar]
- Bell T, Adams M. 2011. Attack on all fronts: functional relationships between aerial and root parasitic plants and their woody hosts and consequences for ecosystems. Tree Physiology 31: 3–15. [DOI] [PubMed] [Google Scholar]
- Bennett J, Mathews S. 2006. Phylogeny of the parasitic plant family Orobanchaceae inferred from phytochrome A. Americal Journal of Botany 93: 1039–1051. [DOI] [PubMed] [Google Scholar]
- ter Braak CJF, Šmilauer P. 2012. Canoco 5, Windows release (5.00) . [Software for canonical community ordination]. Microcomputer Power, Ithaca, NY. [Google Scholar]
- Cameron DD, Seel WE. 2007. Functional anatomy of haustoria formed by Rhinanthus minor: linking evidence from histology and isotope tracing. New Phytologist 174: 412–419. [DOI] [PubMed] [Google Scholar]
- Cameron DD, Coats AM, Seel WE. 2006. Differential resistance among host and non-host species underlies the variable success of the hemi-parasitic plant Rhinanthus minor. Annals of Botany 98: 1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canny M, McCully M. 1988. The xylem sap of maize roots: its collection, composition and formation. Australian Journal of Plant Physiology 15: 557–566. [Google Scholar]
- Darwin C. 1880. General considerations on the movements and growth of seedling plants. In: The power of movements in plants . London: John Murray, 67–128. [Google Scholar]
- Ehleringer J, Marshall J. 1995. Water relations. In: Press MC, Graves JD, eds. Parasitic plants. London: Chapman & Hall, 125–140. [Google Scholar]
- Escher P, Eiblmeier M, Hetzger I, Rennenberg H. 2004. Seasonal and spatial variation of carbohydrates in mistletoes (Viscum album) and the xylem sap of its hosts (Populus × euamericana and Abies alba). Physiologia Plantarum 120: 212–219. [DOI] [PubMed] [Google Scholar]
- Fedorowicz MS. 1915. Die Drüsenformen der Rhinanthoidae-Rhinanthae. Bulletin International de l’Académie des Sciences de Cracovie, Classe de Sciences Mathématiques et Naturelles: 286–322. [Google Scholar]
- Fibich P, Lepš J, Berec L. 2010. Modelling the population dynamics of root hemiparasitic plants along a productivity gradient. Folia Geobotanica 45: 425–442. [Google Scholar]
- Frey-Wyssling A. 1941. Die Guttation als allgemeine Erscheinung. Bericht der Schweizerischen Botanischen Gesellschaft 51: 321–325. [Google Scholar]
- Furukawa J, Abe Y, Mizuno H, et al. 2011. Seasonal fluctuation of organic and inorganic components in xylem sap of Populus nigra. Plant Root 5: 56–62. [Google Scholar]
- Govier R, Nelson M, Pate J. 1967. Hemiparasitic nutrition in angiosperms I. The transfer of organic compounds from host to Odontites verna (Bell.) Dum. (Scrophulariaceae). New Phytologist 66: 285–297. [Google Scholar]
- Govier R, Brown JGS, Pate JS. 1968. Hemiparasitic nutrition in angiosperms II. Root haustoria and leaf glands of Odontites verna (Bell.) Dum. and their relevance to the abstraction of solutes from the host. New Phytologist 67: 963–972. [Google Scholar]
- Groom P. 1897. On the leaves of Lathraea squamaria and of some allied Scrophulariaceae. Annals of Botany 11: 385–398. [Google Scholar]
- Heide-Jørgensen HS. 1980. The xeromorphic leaves of Hakea suaveolens R.Br. III. Ontogeny, structure and function of the T-shaped trichomes. Botanisk Tidsskrift 75: 181–196. [Google Scholar]
- Heide-Jørgensen HS. 2008. Parasitic flowering plants. Leiden: Brill. [Google Scholar]
- Heide-Jørgensen HS. 2013. Introduction: the parasitic syndrome in higher plants. In: Joel D, Gressel J, Musselman L, eds. Parasitic Orobanchaceae: parasitic mechanisms and control strategies . Berlin: Springer-Verlag, 1–14. [Google Scholar]
- Heizmann U, Kreuzwieser J, Schnitzler J-P, Brüggemann N, Rennenberg H. 2001. Assimilate transport in the xylem sap of pedunculate oak (Quercus robur) saplings. Plant Biology 3: 132–138. [Google Scholar]
- Hibberd JM, Jeschke WD. 2001. Solute flux into parasitic plants. Journal of Experimental Botany 52: 2043–2049. [DOI] [PubMed] [Google Scholar]
- Hodgson JF. 1973. Aspects of the carbon nutrition of angiospermous parasites. PhD thesis, University of Sheffield, Sheffield, UK. [Google Scholar]
- Höhn K. 1950. Untersuchungen über hydathoden und function. Akademie der Wissenschaften und der Literatur, Abhandlungen der Mathematisch-Naturwissenschaftlichen Klasse 2: 9–42. [Google Scholar]
- van Hulst R, Shipley B, Theriault A. 1987. Why is Rhinanthus minor (Scrophulariaceae) such a good invader? Canadian Journal of Botany 65: 2373–2379. [Google Scholar]
- Irving LJ, Cameron DD. 2009. You are what you eat: interactions between root parasitic plants and their hosts. Advances in Botanical Research 50: 87–138. [Google Scholar]
- Jiang F, Jeschke WD, Hartung W. 2003. Water flows in the parasitic association Rhinanthus minor/Hordeum vulgare. Journal of Experimental Botany 54: 1985–1993. [DOI] [PubMed] [Google Scholar]
- Jiang F, Jeschke WD, Hartung W, Cameron DD. 2010. Interactions between Rhinanthus minor and its hosts: a review of water, mineral nutrient and hormone flows and exchanges in the hemiparasitic association. Folia Geobotanica 45: 369–385. [Google Scholar]
- Kaplan A, Inceoglu Ö. 2003. Leaf anatomy and morphology of 14 species belonging to the Turkish Rhinantheae (Scrophulariaceae) tribe. Israel Journal of Plant Sciences 51: 297–305. [Google Scholar]
- Klaren CH, Janssen G. 1978. Physiological changes in the hemiparasite Rhinanthus serotinus before and after attachment. Physiologia Plantarum 42: 151–155. [Google Scholar]
- Klepper B, Kaufmann MR. 1966. Removal of salt from xylem sap by leaves and stems of guttating plants. Plant Physiology 41: 1743–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubat R, Weber HC. 1987. Zur Biologie von Rhynchocorys elephas (L.) Griseb. (Scrophulariaceae). Beiträge zur Biologie der Pflanzen 62: 239–250. [Google Scholar]
- Lechowski Z. 1996. Gas exchange in leaves of the root hemiparasite Melampyrum arvense L. before and after attachment to the host plant. Biologia Plantarum 38: 85–93. [Google Scholar]
- Lepš J. 1999. Nutrient status, disturbance and competition: an experimental test of relationships in a wet meadow. Journal of Vegetation Science 10: 219–230. [Google Scholar]
- Matthies D. 1995. Parasitic and competitive interactions between the hemiparasites Rhinanthus serotinus and Odontites rubra and their host Medicago sativa. Journal of Ecology 83: 245–251. [Google Scholar]
- McNeal JR, Bennett JR, Wolfe AD, Mathews S. 2013. Phylogeny and origins of holoparasitism in Orobanchaceae. American Journal of Botany 100: 971–983. [DOI] [PubMed] [Google Scholar]
- Mudrák O, Lepš J. 2010. Interactions of the hemiparasitic species Rhinanthus minor with its host plant community at two nutrient levels. Folia Geobotanica 45: 407–424. [Google Scholar]
- Naumann J, Salomo K, Der JP, et al. 2013. Single-copy nuclear genes place haustorial Hydnoraceae within Piperales and reveal a Cretaceous origin of multiple parasitic Angiosperm lineages. PLoS One 8: e79204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickrent DL, Duff RJ. 1996. Molecular studies of parasitic plants using ribosomal RNA. In: Moreno MT, Cubero JI, Berner D, Joel D, Musselman LJ, Parker C, eds. Advances in parasitic plant research . Cordoba, Spain: Junta de Andalucia, Dirección General de Investigación Agraria, 28–52. [Google Scholar]
- Nickrent DL, Duff RJ, Colwell AE, et al. 1998. Molecular phylogenetic and evolutionary studies of parasitic plants. In: Soltis D, Soltis P, Doyle J, eds. Molecular systematics of plants II. DNA sequencing . Boston: Kluwer Academic Publishers, 211–241. [Google Scholar]
- Phoenix GK, Press MC. 2004. Linking physiological traits to impacts on community structure and function: the role of root hemiparasitic Orobanchaceae (ex-Scrophulariaceae). Journal of Ecology 93: 67–78. [Google Scholar]
- Press M. 1989. Autotrophy and heterotrophy in root hemiparasites. Trends in Ecology and Evolution 4: 258–263. [DOI] [PubMed] [Google Scholar]
- Press M, Graves J, Stewart G. 1988. Transpiration and carbon acquisition in root hemiparasitic angiosperms. Journal of Experimental Botany 39: 1009–1014. [Google Scholar]
- R Core Team. 2013. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; URL http://www.R-project.org/. [Google Scholar]
- Renaudin S. 1966. Sur les glandes de Lathraea clandestina L. Bulletin de la Société Botanique de France 113: 379–385. [Google Scholar]
- Renaudin S, Garrigues R. 1967. Sur l’ultrastructure des glandes en bouclier de Lathraea clandestina L. et leur rôle physiologique. Comptes Rendus de’l Académie de Sciences, Paris 264: 1984–1987. [Google Scholar]
- Scheunert A, Fleischmann A, Olano-Marín C, Bräuchler C, Heubl G. 2012. Phylogeny of tribe Rhinantheae (Orobanchaceae) with a focus on biogeography, cytology and re-examination of generic concepts. Taxon 61: 1269–1285. [Google Scholar]
- Schill V, Hartung W, Orthen B, Weisenseel MH. 1996. The xylem sap of maple (Acer platanoides) trees—sap obtained by a novel method shows changes with season and height. Journal of Experimental Botany 47: 123–133. [Google Scholar]
- Schnepf E. 1964. Über Zellwandstrukturen bei den Köpfchendrüsen der Schuppenblätter von Lathraea clandestina L. Planta 60: 473–482. [Google Scholar]
- Seel WE, Jeschke WD. 1999. Simultaneous collection of xylem sap from Rhinanthus minor and the hosts Hordeum and Trifolium: hydraulic properties, xylem sap composition and effects of attachment. New Phytologist 143: 281–298. [Google Scholar]
- Seel WE, Press MC. 1993. Influence of the host on three sub-Arctic annual facultative root hemiparasites. I. Growth, mineral accumulation and above-ground dry-matter partitioning. New Phytologist 125: 131–138. [DOI] [PubMed] [Google Scholar]
- Seel WE, Cooper RE, Press MC. 1993. Growth, gas exchange and water use efficiency of the facultative hemiparasite Rhinanthus minor associated with hosts differring in foliar nitrogen concentration. Physiologia Plantarum 89: 64–70. [Google Scholar]
- Smith S, Stewart GR. 1990. Effect of potassium levels on the stomatal behavior of the hemi-parasite Striga hermonthica. Plant Physiology 94: 1472–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GR, Press MC. 1990. The physiology and biochemistry of parasitic angiosperms. Annual Review of Plant Physiology and Molecular Biology 41: 127–151. [Google Scholar]
- Těšitel J, Tesařová M. 2013. Ultrastructure of hydathode trichomes of hemiparasitic Rhinanthus alectorolophus and Odontites vernus: how important is their role in physiology and evolution of parasitism in Orobanchaceae? Plant Biology 15: 119–125. [DOI] [PubMed] [Google Scholar]
- Těšitel J, Plavcová L, Cameron DD. 2010a. Heterotrophic carbon gain by the root hemiparasites, Rhinanthus minor and Euphrasia rostkoviana (Orobanchaceae). Planta 231: 1137–1144. [DOI] [PubMed] [Google Scholar]
- Těšitel J, Plavcová L, Cameron DD. 2010b. Interactions between hemiparasitic plants and their hosts: the importance of organic carbon transfer. Plant Signaling and Behavior 5: 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Těšitel J, Říha P, Svobodová Š, Malinová T, Štech M. 2010c. Phylogeny, life history evolution and biogeography of the Rhinanthoid Orobanchaceae. Folia Geobotanica 45: 347–367. [Google Scholar]
- Těšitel J, Lepš J, Vráblová M, Cameron DD. 2011. The role of heterotrophic carbon acquisition by the hemiparasitic plant Rhinanthus alectorolophus in seedling establishment in natural communities: a physiological perspective. New Phytologist 192: 188–199. [DOI] [PubMed] [Google Scholar]
- Těšitel J, Hejcman M, Lepš J, Cameron DD. 2013. How does elevated grassland productivity influence populations of root hemiparasites? Commentary on Borowicz and Armstrong (Oecologia 2012). Oecologia 172: 933–936. [DOI] [PubMed] [Google Scholar]
- Těšitel J, Těšitelová T, Fisher JP, Lepš J, Cameron DD. 2015. Integrating ecology and physiology of root-hemiparasitic interaction: interactive effects of abiotic resources shape the interplay between parasitism and autotrophy. New Phytologist 205: 350–360. [DOI] [PubMed] [Google Scholar]
- Weber HC. 1973. Zur Biologie von Tozzia alpina L. (Standort, Wirtspflanzen, Entwicklung und Parasitismus). Beiträge zur Biologie der Pflanzen 49: 237–249. [Google Scholar]
- Wegner LH. 2014. Root pressure and beyond: energetically uphill water transport into xylem vessels? Journal of Experimental Botany 65: 381–393. [DOI] [PubMed] [Google Scholar]
- Westwood JH, Yoder JI, Timko MP, dePamphilis CW. 2010. The evolution of parasitism in plants. Trends in Plant Science 15: 227–235. [DOI] [PubMed] [Google Scholar]
- Zeuthen T, MacAulay N. 2012. Transport of water against its concentration gradient: fact or fiction? WIREs Membrane Transport and Signaling 1: 373–381. [Google Scholar]
- Ziegler H. 1955. Lathraea, ein Blutungssaft-schmarotzer. Berichte der Deutschen Botanischen Gesellschaft 68: 311–318. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


