Abstract
Background and Aims Intra-population variation in seed dormancy is an advantage for population persistence in unpredictable environments. The important role played by physically dormant species in these habitats makes understanding the level of variation in their dormancy a key ecological question. Heat produced in the soil is the major dormancy-breaking stimulus and, in fire prone ecosystems, soil temperatures generated by fire may vary spatially and over time. While many studies have investigated variation in initial dormancy, a measure that is of little value in fire-prone ecosystems, where initial dormancy levels are uniformly high, intra-population variation in dormancy-breaking temperature thresholds has never been quantified. This study predicted that species would display variation in dormancy-breaking temperature thresholds within populations, and investigated whether this variation occurred between individual plants from the same maternal environment.
Methods The intra-population variation in dormancy-breaking thresholds of five common physically dormant shrub species (family Fabaceae) from fire-prone vegetation in south-eastern Australia was assessed using heat treatments and germination trials. Replicate batches of seeds from each of four maternal plants of Dillwynia floribunda, Viminaria juncea, Bossiaea heterophylla, Aotus ericoides and Acacia linifolia were treated at 40, 60, 80, 100 and 120 °C.
Key Results Dormancy-breaking response to heat treatments varied significantly among individual plants for all species, with some individuals able to germinate after heating at low temperatures and others restricting germination to temperatures that only occur as a result of high-severity fires. Germination rate (T50) varied among individuals of three species.
Conclusions Variation detected among individuals that were in close proximity to each other indicates that strong differences in dormancy-breaking temperature thresholds occur throughout the broader population. Differences found at the individual plant level could contribute to subsequent variation within the seed bank, providing a bet-hedging strategy, and represent a mechanism for increasing the probability of population persistence in the face of fire regime variability.
Keywords: Seed germination, bet-hedging strategy, coexistence, dormancy-breaking thresholds, Fabaceae, fire, intra-population, germination, hard seeds, population persistence, seed bank.
INTRODUCTION
Within plant species, intra-population variation in seed dormancy has been described as an advantage for the survival of populations in unpredictable environments (Fenner, 1991; Beckstead et al., 1996; Andersson and Milberg, 1998; Gutterman, 2000). Examples of such variation in seed dormancy and germination response have been presented primarily from arid or water-limited ecosystems (e.g. Quinlivan, 1971; Benech Arnold et al., 1992; Baskin and Baskin 1998; Baloch et al., 2001). However, fire-driven vegetation is one of the primary disturbance-prone environments in the world, where recruitment from seed is a critical life-history stage (Keeley, 1991; Bond and van Wilgen, 1996), yet very little is known about how dormancy, and more specifically the degree of dormancy, varies in these ecosystems (Ooi, 2007; Ooi et al., 2014).
For physically dormant species, where germination is prevented by an impermeable seed coat, the majority of studies investigating variation in dormancy have focused primarily on measuring the proportion of dormant seeds that occurs among populations, although several studies have also looked within populations, including both between and within individual plants (e.g. Pérez-García, 1997; Norman et al., 2002; Lacerda et al., 2004; Masaka and Yamada, 2009). However, in fire-prone regions, dormancy of seed lots of such species usually exceeds 75 %, and can often be 100 % (e.g. Jeffery et al., 1988; Auld and O’Connell, 1991; Herranz et al., 1998; Ooi et al., 2014). Assessing the proportion of dormant seeds produced in these habitats therefore provides little useful information when trying to determine variation among or within populations (Hudson et al., 2015). Heat produced by the passage of fire provides the main dormancy-breaking cue and the levels of response of dormant seeds to different fire-related temperature treatments would provide a useful and much more relevant measure of dormancy variation in fire-prone flora (Auld and O’Connell, 1991; Ooi et al., 2009, 2012). The important role played by physically dormant species in these regions, where they are often the dominant component of the flora, as well as the increase in anthropogenic fire management (Ooi, 2007; Moreira and Pausas, 2012; Ooi et al., 2014), makes understanding the variation in their germination response a key ecological question to be addressed.
Maintaining variation in seed germination response in fire-prone regions is important because of the inherent variability of heat produced by fire in the soil (Auld, 1986; Bradstock et al., 1992; Govender et al., 2006; Penman and Towerton, 2008; Ruprecht et al., 2013). Soil temperatures during any particular fire incident can vary over space due to available fuel moisture and distribution of fuel load, and by the subsequent varying fire intensity produced (Govender et al., 2006). Additionally, fire-induced heating of the soil decreases with depth (Auld, 1986). As a result, species would ideally need to maintain seed germination strategies that allow some portion of seeds to germinate under either high or low soil temperatures. This would ensure that at least some seedling emergence, and potentially recruitment, will occur in response to naturally variable components of the fire regime. For physically dormant species in fire-prone regions, it is likely that temperature thresholds have been shaped in response to the selective pressure of fire (Keeley et al., 2011), and that variation in dormancy breaking temperature thresholds at both the inter- and the intra-specific scale is a key driver of population persistence and community coexistence (Ooi et al., 2014).
Variation in the degree of dormancy within a population can provide species with a mechanism to overcome the risk of failed recruitment. In fire-prone regions, two consecutive fires within a short period of time can negatively affect population persistence because the second fire kills the seedlings produced after the first fire before they reach maturity and contribute to replenishment of the seed bank (Whelan, 1995; Odion and Tyler, 2002). Germination variability within a population would ensure that germination of some portion of seeds from the seed bank could occur after a single fire, while a portion remains in the seed bank and is able to germinate after subsequent fires (Baskin and Baskin, 1998; Ayre et al., 2009; Ooi et al., 2014). Variation in the dormancy loss provides a bet-hedging mechanism to buffer populations against such losses (Thanos and Georghiou, 1988; Gutterman, 2000; Ooi et al., 2009). Furthermore, differences found in dormancy and germination responses within and between individuals, especially where maternal plants have grown in similar microclimates and the seeds produced during similar time periods, may suggest that it is not only parent plant environmental effects but also genetic factors that can play a role in driving variation both within and between plants (Baloch et al., 2001; Donohue et al., 2005; Narbona et al., 2006).
Several studies have identified variation in dormancy-breaking thresholds between populations by seeds subjected to the same heat treatments (Auld and O’Connell, 1991; Ooi et al., 2012), but variation among individuals within the same population, using a range of temperatures to ascertain temperature thresholds required to break dormancy, has not previously been the subject of any study. In this study, we predicted that species would display a range of fire-related dormancy-breaking temperature thresholds within populations, which can result from seeds produced by different neighbouring individual plants. We also set out to investigate whether the range of variation in dormancy-breaking requirements among individuals differed between species. This would allow us to predict whether some species would have a greater ability to respond to variation in dormancy-breaking cues than other species, and therefore potentially have a greater ability for population persistence in unpredictable environments under both current conditions and future climatic conditions. More specifically, we addressed the following main questions: (1) Do seed characteristics, including weight, initial viability, initial dormancy, dormancy-breaking temperature thresholds and germination rate, vary among individuals within populations in physically dormant species from fire-prone environments? (2) Does the level of dormancy broken differ between individuals in response to the same temperature treatment? (3) Do some species maintain greater variation in dormancy-breaking thresholds among individuals than others, and how could this affect their ability to persist?
METHODS
Study species and site
We selected a group of common species from coastal fire-prone heath and woodland to gain both an understanding of variation in dormancy between individuals within each species, and how this variation differed among the species themselves. The five species studied were all from the Fabaceae: Dillwynia floribunda, Viminaria juncea, Bossiaea heterophylla, Aotus ericoides and Acacia linifolia. All species are native perennial shrubs from south-eastern Australia, which have physical dormancy (Ooi et al., 2014). The physically dormant Fabaceae family is a major component of the understorey vegetation in Australia (Auld, 1986). The majority of adult plants of D. floribunda, A. linifolia and V. juncea are killed by fire, while B. heterophylla and A. ericoides adults are often killed by fire but maintain some capacity to resprout (Auld and O’Connell, 1991; Kubiak, 2009). The study sites were located in fire-prone vegetation in the Heathcote (34°07ʹ S, 150°58ʹ E) and Royal (34°03ʹ S, 151°03ʹ E) national parks, both at the southern end of the Sydney sandstone basin in south-eastern Australia. All species produce mature fruits between October and January.
Seed collection
Mature fruits were collected from four individual plants for each species within around 10 m proximity, with seeds of each individual kept in separate seed lots throughout the experiments. Viminaria juncea, B. heterophylla, A. ericoides and A. linifolia fruits were collected between October and November 2013, while D. floribunda fruits were collected in October 2012. The collected fruits were opened and seeds were extracted in the laboratory. Healthy and intact seeds were identified visually and stored in sealed paper bags at room temperature until temperature treatments were commenced in December of the same year for each seed collection.
Initial seed weight, viability and dormancy levels for individual plants
To compare initial seed characteristics among individuals within each species, seed mass, viability and dormancy level were assessed within 30 d of seed collection. Fifty seeds from each individual plant were weighed individually to the nearest 0·01 mg and mean seed weight was calculated. Three replicates of 15–20 seeds were placed on moistened filter paper in 9 cm Petri dishes and incubated at 25/18 °C temperature and 12/12 h light/dark conditions. During incubation, Petri dishes were sealed with plastic to prevent water loss and filter paper was remoistened whenever necessary. Seeds were checked every second day for germination, which was scored on radicle emergence. After 6 weeks of observation, the seeds that did not germinate were checked for viability. Imbibed seeds were tested for viability by pressing them gently using forceps, while the remaining hard seeds were manually scarified using a scalpel and allowed to germinate in the same incubation conditions to determine the total viable seed number. The number of seeds that remained ungerminated prior to scarification was used to determine the dormancy level for each individual.
Dormancy-breaking temperature thresholds of seeds from individual plants
Temperature treatments ranging from 40 to 120 °C were applied for 10 minutes to determine the dormancy-breaking temperature thresholds for individual plants from each species. These treatments represent the soil temperatures and durations that commonly occur in these and other fire-prone habitats (Auld, 1986; Bradstock et al., 1992; Penman and Towerton, 2008; Ooi et al., 2014). Lower temperatures are indicative of cooler fires with low amounts of fine leaf litter (Bradstock and Auld, 1995), while higher temperatures can result from higher-severity fires. Four sets of three replicates of between 15 and 20 seeds were treated for each individual plant for V. juncea, A. ericoides and A. linifolia at 40, 60, 80 and 100 °C in an oven for 10 min. Seeds of individuals from D. floribunda and B. heterophylla were treated at 40, 60 and 80 °C only, due to limited seed numbers. Sufficient seeds for V. juncea and A. linifolia allowed an additional treatment of 120 °C to be applied. Following the heat treatments, seeds were allowed to cool and then placed on moistened filter papers in 9-cm Petri dishes. Seeds were incubated at 25/18 °C and light/dark conditions on a 12/12-h cycle. Three replicates of untreated seeds for each individual were included as the control. Germination and viability were assessed following the same procedure as for the initial viability test.
To calculate the mean germination percentages for each individual, we divided the number of germinated seeds by the corrected total viable seed number determined after each treatment, for each replicate. However, when mortality was >90 % as a result of the experimental treatments, data were excluded due to insufficient surviving numbers of seeds. The mean percentage mortality was calculated for each individual for each temperature treatment. The number of days to reach 50 % of the final seed germination (T50) was calculated after the 80 °C treatment, which is the temperature found to produce the most germination across species (Auld and O’Connell, 1991; Ooi et al., 2014). To calculate T50, cumulative germination was plotted against the number of days for each replicate and a polynomial model fitted to the data. The time taken to reach 50 % of the final germination was calculated from the regression equation and the mean T50 taken from the three replicates. Note that the T50 values were calculated at 100 °C for V. juncea individuals due to insufficient germination after the 80 °C treatment. Finally, we also calculated the minimum temperatures required to achieve 20 % (G20), 50 % (G50) and maximum (Gmax) germination of viable seeds for each individual, and compared the level of variation in germination response between species using the coefficient of variation (CV). The CV was calculated using germination data recorded from the maximum temperature tested for each species, except A. linifolia, where we used data from the 80 °C treatment due to high seed mortality at 100 °C.
Data analysis
Generalized linear models with a binomial error structure and logit link function were used to compare final germination percentages of individuals of each species at each temperature separately. When the final germination proportions among individuals were significant, Tukey’s test was used for multiple comparisons (Hothorn et al., 2008). The same analyses were applied to final mortality and viability for each individual. A generalized linear model with a Poisson error structure and log link function were used to compare seed mass and T50 at 80 °C among individuals, followed by Tukey’s test when differences were significant. All graphs were plotted using untransformed data. All analyses were conduct using the R statistical platform (R Core Development Team, 2014).
RESULTS
Initial seed weight, viability and dormancy levels for individual plants
The seed characteristics tested did not vary significantly among individual plants for any of the study species, except for mean seed weight. Dormancy levels and initial viabilities were close to 100 % for all individual plants except A. linifolia, which displayed considerable variation in dormancy level, and B. heterophylla and D. floribunda, which displayed variation in viability (Table 1). Seeds of one A. linifolia individual plant displayed an almost 65 % non-dormant seed portion. Mean seed weights differed significantly among individuals for all the tested species (Table 1), and varied the most among individuals of A. linifolia and the least for B. heterophylla, with the percentage difference between the lowest and highest individuals calculated as 48·2 and 15·5 % for A. linifolia and B. heterophylla, respectively.
Table 1.
Mean seed weight (W), initial viability (V), initial dormancy (D), time taken to 50 % seed germination (T50) and temperatures required for 20 % (G20), 50 % (G50) and maximum (Gmax) germination within the temperature ranges used for individuals of all studied species. The dash (–) indicates insufficient germinations to represent temperatures. Different letters between individuals within each species section indicate significant differences between means
| Species | Individual | W (mg) | V (%) | D (%) | T50 (days) | G20 (°C) | G50 (°C) | Gmax (°C) |
|---|---|---|---|---|---|---|---|---|
| Acacia linifolia | Individual 1 | 26·88a | 93·15a | 70·73a | 16·66 ± 2·39a | Control | – | 40 |
| Individual 2 | 35·58b | 100a | 60·0ab | 42·13 ± 13·36b | Control | – | Control | |
| Individual 3 | 31·46c | 100a | 61·67ab | 18·56 ± 0·6a | Control | 100 | 100 | |
| Individual 4 | 39·84d | 100a | 35·65b | 21·38 ± 2·54a | Control | Control | 80 | |
| Viminaria juncea | Individual 1 | 7·09a | 100a | 93·33a | 10·83 ± 1·52a | 100 | 100 | 100 |
| Individual 2 | 5·89b | 100a | 95·0a | 11·74 ± 0·93a | 100 | 100 | 100 | |
| Individual 3 | 5·92b | 100a | 98·33a | 12·75 ± 0·98a | 80 | 100 | 100 | |
| Individual 4 | 5·36c | 100a | 86·67a | 11·12 ± 0·94a | 80 | 100 | 100 | |
| Aotus ericoides | Individual 1 | 4·20a | 95·56a | 97·62a | 6·83 ± 0·20a | 60 | 60 | 100 |
| Individual 2 | 4·94b | 95·24a | 80·20a | 10·11 ± 1·23b | 40 | 60 | 100 | |
| Individual 3 | 4·05a | 100a | 97·78a | 9·50 ± 0·86b | 60 | 60 | 100 | |
| Individual 4 | 4·23a | 100a | 100a | 5·88 ± 0·20a | 60 | 60 | 100 | |
| Dillwynia floribunda | Individual 1 | 1·54a | 48a | 100a | 15·42 ± 0·37a | 60 | 60 | 80 |
| Individual 2 | 1·89b | 78b | 94·12a | 27·29 ± 3·04b | 60 | 80 | 80 | |
| Individual 3 | 2·10cd | 87bc | 90·68a | 20·48 ± 1·22c | 60 | 80 | 80 | |
| Individual 4 | 1·96bd | 98·3dc | 100a | 18·80 ± 1·31ac | 60 | 60 | 80 | |
| Bossiaea heterophylla | Individual 1 | 14·64a | 72·22a | 82·23a | 6·70 ± 1·27a | 40 | – | 80 |
| Individual 2 | 15·57a | 85·93a | 83·07a | 5·86 ± 0·45a | 60 | 60 | 60 | |
| Individual 3 | 14·73a | 65·08a | 86·60a | 8·96 ± 1·62a | 60 | – | 60 | |
| Individual 4 | 16·91b | 100a | 86·67a | 11·73 ± 3·74a | – | – | 80 |
Dormancy-breaking temperature thresholds of seeds from individual plants
All species produced different levels of germination among individuals in response to one or several temperature treatments after 6 weeks of incubation (Fig. 1), meaning that dormancy-breaking thresholds differed between individuals. Germination significantly increased above that of the control after heat treatments for all individuals within all species except for A. linifolia (Fig. 1A). In contrast to the germination responses of the other species tested, three A. linifolia individuals did not show heat-dependent germination (Fig. 1A). Non-dormancy produced high levels of germination in the control seeds, and a lack of germination after heat treatments was only partially explained by treatment-induced mortality (Fig. 2A). Only Individual 3 of A. linifolia produced a notably high germination response after heating compared with the control, with an increase in germination of ∼59 % after the 100 °C treatment. The highest heat treatment (120 °C) caused 100 % mortality for all A. linifolia individuals. Three of the individuals treated at 100 °C suffered >90 % mortality, whilst the remaining Individual 3 produced its highest germination (97 %). There was also large variation between A. linifolia individuals in T50 values. Individual 2 took almost 3 weeks longer than the other three individuals to reach 50 % of its final maximum germination (Table 1).
Fig. 1.
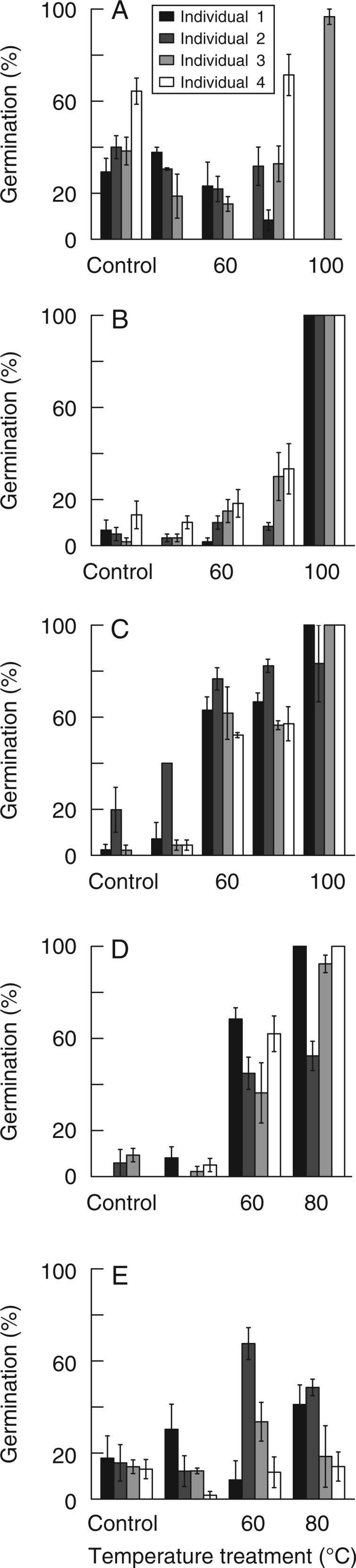
Mean (± s.e.) germination percentages of seeds from four individuals after different temperature treatments. (A) Acacia linifolia; (B) Viminaria juncea; (C) Aotus ericoides; (D) Dillwynia floribunda; (E) Bossiaea heterophylla.
Fig. 2.
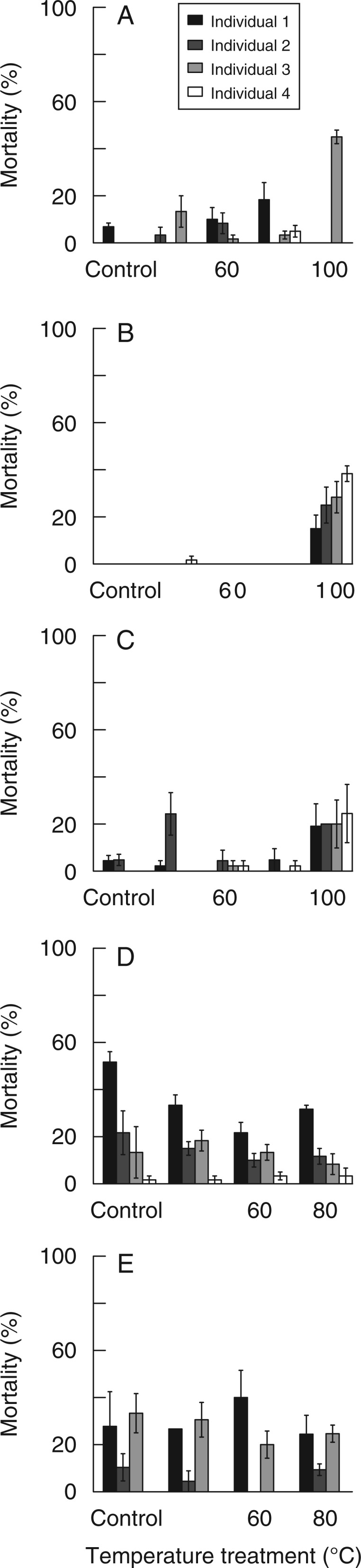
Mean (± s.e.) mortality percentages of the seeds from four individuals after different temperature treatments. (A) Acacia linifolia; (B) Viminaria juncea; (C) Aotus ericoides; (D) Dillwynia floribunda; (E) Bossiaea heterophylla.
Germination response of V. juncea at lower temperatures was relatively small (<20 % at 40 and 60 °C). Nevertheless, seeds from individuals displayed two broadly different response patterns. Seed germination percentages of Individuals 3 and 4 gradually increased with increasing temperature treatments, whereas Individuals 1 and 2 showed a sudden increase in germination (100 %) after the 100 °C treatment, compared with low (<10 %) germination after lower temperature treatments (Fig. 1B). This showed a strong high-temperature threshold requirement to break dormancy and therefore a strong bond to fire-related dormancy-breaking cues for all individuals; this was particularly strong for Individuals 1 and 2. Increasing mortality was initiated at the 100 °C treatment for all the individuals of V. juncea.(Fig. 2B). For all the individuals of V. juncea, the 120 °C treatment caused 100 % seed mortality.
Germination percentages for all A. ericoides individuals gradually increased with increasing temperature treatments (Fig. 1C), although Individual 2 significantly increased germination to 40 % after the low-temperature 40 °C treatment, while the other three individuals needed a 60 °C treatment for a significant germination increase. Maximum germination of the seeds from the latter three individuals was clearly reached at 100 °C, whereas the peak germination produced by Individual 2 did not differ significantly for seeds treated at 60, 80 or 100 °C. Mortality for all individuals gradually increased with increasing temperature treatments (Fig. 2C). The T50 value was significantly slower only for Individuals 1 and 4 compared with Individuals 2 and 3 (Table 1).
The seeds of individuals of D. floribunda showed a gradual increase in germination with increasing temperature treatment, except for Individual 2, which did not significantly increase germination after the 60 °C treatment. Almost half (48 %) of Individual 2’s viable seed lot remained dormant after treatment at 80 °C (Fig. 1D). Even though mortality differed significantly among individuals for each of the temperature treatments, there was no trend of increased mortality with increasing temperature for any of the four individuals (Fig. 2D). The T50 values varied among all individuals at 80 °C, and Individual 2 took ∼1 week or longer to germinate than other three (Table 1).
For B. heterophylla, germination was highly variable among individuals across all temperature treatments (Fig. 1E). Individual 1 displayed a general increase in germination response with increasing temperature (albeit with a decline at the 60 °C treatment), peaking after the 80 °C treatment, the highest temperature used for this species. Individuals 2 and 3 reached their maximum germination after treatment at 60 °C, with Individual 2 showing a particularly strong response, reaching 80 % germination (significantly greater than any other treatment). The subsequent decline in germination after the 80 °C treatment was not caused by increasing mortality (Fig. 2E). Individuals 3 and 4 both maintained a high threshold temperature requirement (>80 °C) to break dormancy, with >66 and 88 % of their viable seed lots, respectively, remaining dormant after the 80 °C treatment.
Overall comparison between species showed that the dormancy-breaking threshold variation was relatively high for all species except V. juncea. All four V. juncea individuals reached 50 % (G50) or maximum (Gmax) seed germination at the same temperature treatment of 100 °C (Table 1), with no individual producing germination >30 % at lower temperatures. This is reflected by the CV values, measuring the level of variability in germination response between species, where V. juncea had the lowest CV. Ranking of species by CV showed A. linifolia (CV = 0·69) > B. heterophylla (CV = 0·58) > D. floribunda (CV = 0·26) > A. ericoides (CV = 0·08) > V. juncea (CV = 0).
DISCUSSION
Variation in dormancy-breaking temperature thresholds is rarely studied within populations but can contribute to our understanding of the strategies involved in plant population dynamics (Ooi et al., 2014). In this study, we found highly significant variation in dormancy-breaking temperature thresholds among individual plants for all five of the study species from fire-prone vegetation, while T50 for most of the species also varied. This variation is likely to occur throughout the broader populations of the species studied because strong differences were detectable, even among four individuals that were in relatively close proximity to each other. These findings help to explain how differences in dormancy and germination characteristics at the individual plant level can contribute to subsequent variation within the seed bank. This finding is novel, highlighting a level of variation that cannot be obtained from studies focused on dormancy variation among species or populations (e.g. Auld and O’Connell, 1991; Ooi et al., 2012). This study has implications both for understanding the strategies that species have developed for population persistence and for how well species may respond to variation in fire severity, a key aspect of the fire regime, which is subject to change as a result of increased management and climatic changes (Ooi et al., 2014).
The type of variation usually considered in germination strategies, allowing species to regenerate in a range of conditions to overcome the unpredictability of disturbances, is the difference in initial levels of dormancy of the seed lot (Cohen, 1966; Philippi and Seger, 1989; Philippi, 1993; Venable, 2007). However, variation of initial dormancy among individuals is not characteristic of our study species, and indeed is not characteristic of many species from fire-prone environments generally (Jeffery et al., 1988; Auld and O’Connell, 1991; Herranz et al., 1998; Ooi et al., 2014). Instead of having variation in initial dormancy, it is clear that these species maintain variation in germination within a population by having different dormancy-breaking temperature thresholds. Differences in seed dormancy thresholds between species have been proposed as a mechanism by which species co-exist (Trabaud and Oustric, 1989; Moreno and Oechel, 1991; Herranz et al., 1998; Ooi et al., 2014). We suggest that intra-population variation in dormancy-breaking threshold temperature is also a mechanism for ensuring population persistence, a strategy likely to be maintained by many species from fire-prone environments.
The variation in dormancy-breaking temperature thresholds within a population can be considered as a form of bet-hedging, although rather than betting against failed recruitment it provides insurance against variability in soil heating. For example, seeds entering the seed bank from plants with a low temperature dormancy-breaking threshold ensure that a portion of seeds germinate, even after a low-severity fire (which potentially produces low levels of soil heating), while the remaining portion of higher temperature-threshold seeds can contribute to a residual soil seed bank. Seeds are then available to germinate after subsequent fires, and supplement seeds produced by newly recruited individuals.
Variation of the fire regime can affect recruitment within a population due to the related variability of the resulting dormancy-breaking or germination cues (Thanos and Georghiou, 1988; Whelan, 1995). For example, variation in the level of heat produced in the soil depends on the fuel type and decreases with soil depth (Bradstock et al., 1992; Auld and Bradstock, 1996). Maintenance of only a single dormancy-breaking threshold for any species within a population would increase risk, particularly for obligate seeders, by limiting the capacity to produce a sufficient germination response for subsequent regeneration. However, having dormancy-breaking temperature thresholds ranging from low to high within a population ensures that a portion of seeds can respond, even with variation in fire-related cues. In our study, all species except V. juncea displayed a significant germination response to a range of dormancy-breaking temperatures. Viminaria juncea only responded to very high temperature treatments, suggesting it would have the least ability to recruit in the absence of high-severity fires (and subsequent high soil temperatures) or cope with a series of low-severity burns, unless seeds undergo a change in dormancy-breaking thresholds over time. Species that maintain similar dormancy profiles may be more susceptible to decline as a result of variation in the current fire regime, particularly to an increase in low-intensity fires. Identifying such species would ensure that increasing levels of fire management, often consisting of low-severity controlled burns with corresponding low soil temperatures (Penman and Towerton, 2008), do not have a negative impact on recruitment and subsequent biodiversity.
Another key finding from our study is the level of response shown by some individuals at relatively low dormancy-breaking temperatures. Individuals with high dormancy-breaking temperature thresholds (>80 °C) show a strong pyrogenic dormancy-breaking relationship, because such temperatures occur only during the passage of fire in these environments (Auld and Bradstock, 1996; Penman and Towerton 2008; Ooi et al., 2014). However, while lower temperatures of 40 or 60 °C regularly occur during a fire, they can also occur in vegetation gaps during hot months in the inter-fire period (Santana et al., 2013). Individuals with lower dormancy-breaking temperature thresholds can therefore potentially respond to vegetation gaps, providing an opportunity for plant regeneration both after fire and during the inter-fire period (Hanley et al., 2003; Tavsanoglu and Çatav, 2012; Ooi et al., 2012, 2014). While it is understood that the primary recruitment period occurs after fire, some inter-fire recruitment may contribute to persistence, particularly in long unburnt vegetation, and our results show that this could be related to differences in seeds produced at the individual plant level (Ooi et al., 2012, 2014).
Germination rates varied significantly among individuals in our study, a strategy maintained by species as a form of temporal bet-hedging (Venable, 1989; Fenner and Thompson, 2005; Wang et al., 2009). Differences in germination rate spread germination over time, ensuring that some seeds can respond quickly to a rainfall event after dormancy has broken, while others require longer to initiate germination and therefore enable maintenance of a seed bank that can respond to subsequent rainfall events, even if the initial cohort has failed (Aŕan et al., 2013; Chamorro et al., 2013). While all individuals of some study species, such as D. floribunda and A. ericoides, showed similar patterns of higher germination at higher temperature treatments, variation in germination rates among individuals were maintained. This form of bet-hedging is rarely acknowledged in fire-prone environments, even though low levels of recruitment have been observed during 9 months after fire (e.g. Auld and Tozer, 1995).
Variation in germination rate is also a mechanism that can reduce intra-specific seedling competition (Venable, 1989; Fenner and Thompson, 2005). The flush of post-fire seedling emergence shows that conditions immediately after fire are favourable for seedling establishment (van Wilgen and Forsyth, 1992; Carrington and Keeley, 1999; Fenner and Thompson, 2005). However, competition caused by the high density of seedlings is a major reason for seedling mortality after disturbance (Taylor and Aarssen, 1989; Fenner and Thompson, 2005). The differences in germination rates that we found among individuals provides evidence for a mechanism contributing to successful seedling recruitment within a population after fire, by staggering the timing of emergence and reducing seedling–seedling competition.
Treatment-induced seed mortality may also enable a reduction of seedling–seedling competition, and potentially contribute to the persistence of a mixture of high- and low-threshold seeds within a population. For two of our study species, seeds produced from individuals with a low dormancy-breaking temperature threshold suffered greater mortality than their high-threshold counterparts. Individual plants that produce seeds with low thresholds have seeds that can germinate after low-severity fires or, potentially, within canopy gaps during the inter-fire period due to the relatively low soil temperatures generated under these conditions. On the other hand, high-severity fires generate soil temperatures that could promote germination of both low- and high-threshold seeds. This means that there would be an increase in intra-specific seedling competition after high-severity fires. However, the high temperature treatment-induced mortality of seeds from low-threshold V. juncea and A. ericoides individuals suggests that the level of intra-specific competition after high-severity fires can be offset by seed mortality. The inherent small-scale patchiness of many fires (Ooi et al., 2006; Penman and Towerton, 2008) would mean that areas within the same fire event could be dominated by individuals with either high- or low-threshold seeds, depending on the soil temperatures reached.
While it is clear that there is strong selective pressure for maintaining dormancy threshold variation within a plant population, or even within individuals, it is much less clear whether this variation is a result of genetic differences among individuals or of variation in the microclimatic environments that maternal plants experience during seed development. The impacts of environmental factors, including temperature, moisture, light level, nutrients and grazing, on flowering, seed development, dormancy and germination have been observed in many species from a wide range of environments (Taylor and Palmer, 1979; Lacey et al., 1997; Gutterman, 2000; Galloway, 2001; Norman et al., 2002; Bischoff and Müller-Schärer, 2010; Hernandez-Verdugo et al., 2010), highlighting that maternal temperature can influence seed dormancy, even within the same maternal plant. For example, a meta-analysis of such studies has identified a general trend that increasing maternal temperature decreases levels of (physiological) dormancy (Wood et al., 1980; Fenner, 1991; Fenner and Thompson, 2005), and that drought can produce seeds with thicker seed coats and increased dormancy in physically dormant seeds (Nooden el al., 1985; Fenner and Thompson, 2005). However, a number of studies have also shown variation in dormancy of seeds produced from plants grown under similar environmental conditions, suggesting that there could be a genetic influence in determining variation in seed and dormancy characters (Galloway, 2001; Donohue et al., 2005; Narbona et al., 2006; Bischoff and Müller-Schärer, 2010). The results from our study, where individuals grown under very similar maternal environment conditions produced seeds with different responses, suggest that genetic differentiation can, in part, play a role in determining the dormancy-breaking thresholds of physically dormant seeds. However, the relative influence of the mother plant environment and the mother plant genotype is yet to be fully understood.
ACKNOWLEDGEMENTS
We thank David Ayre for his comments on the manuscript and Isuru Koongahagoda, Alice Hudson and Juana Correa-Hernandez for help with seed collection and experiments. G.S.L. is supported by University of Wollongong IPTA and UPA scholarships. This work was conducted under an Australian Research Council Linkage Project grant awarded to M.K.J.O. (grant number LP110100527).
LITRATURE CITED
- Andersson L, Milberg P. 1998. Variation in seed dormancy among mother plants, populations and years of seed collection. Seed Science Research 8: 29–38. [Google Scholar]
- Arán D, Garcia-Duro J, Reyes O, Casal M. 2013. Fire and invasive species: modifications in the germination potential of Acacia melanoxylon, Conyza canadensis and Eucalyptus globulus. Forest Ecology And Management 302: 7–13. [Google Scholar]
- Auld TD. 1986. Population dynamics of the shrub Acacia suaveolens (SM) Willd.: fire and the transition to seedlings. Australian Journal of Ecology 11: 373–385. [Google Scholar]
- Auld TD, Bradstock RA. 1996. Soil temperatures after the passage of a fire: do they influence the germination of buried seeds? Australian Journal of Ecology 21: 106–109. [Google Scholar]
- Auld TD, O’Connell MA. 1991. Predicting patterns of post-fire germination in 35 eastern Australian Fabaceae. Australian Journal of Ecology 16: 53–70. [Google Scholar]
- Auld TD, Tozer MG. 1995. Patterns of emergence of Acacia and Grevillea seedlings after fire. Proceedings of the Linnean Society of New South Wales 115: 5–15. [Google Scholar]
- Ayre DJ, Ottewell KM, Krauss SL, Whelan RJ. 2009. Genetic structure of seedling cohorts following repeated wildfires in the fire-sensitive shrub Persoonia mollis ssp. nectens. Journal of Ecology 97: 752–760. [Google Scholar]
- Baloch HA, DiTommaso A, Watson AK. 2001. Intrapopulation variation in Abutilon theophrasti seed mass and its relationship to seed germinability. Seed Science Research 11: 335–343. [Google Scholar]
- Baskin CC, Baskin JM. 1998. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press. [Google Scholar]
- Beckstead J, Meyer SE, Allen PS. 1996. Bromus tectorum seed germination: between-population and between year variation. Canadian Journal of Botany 74: 875–882. [Google Scholar]
- Benech Arnold RL, Fenner M, Edwards PJ. 1992. Changes in dormancy levels in Sorghum halepense (L) Pers. Seeds induced by water stress during seed development. Functional Ecology 6: 596–605. [Google Scholar]
- Bischoff A, Müller-Schärer H. 2010. Testing population differentiation in plant species – how important are environmental maternal effects. Oikos 119: 445–454. [Google Scholar]
- Bond WJ, van Wilgen BW. 1996. Fire and plants. New York: Springer. [Google Scholar]
- Bradstock RA, Auld TD. 1995. Soil temperatures during experimental bushfires in relation to fire intensity: consequences for legume and fire management in south-eastern Australia. Journal of Applied Ecology 32: 76–84. [Google Scholar]
- Bradstock RA, Auld TD, Ellis ME, Cohn JS. 1992. Soil temperatures during bushfire in semi-arid, mallee shrublands. Australian Journal of Ecology 17: 433–440. [Google Scholar]
- Carrington ME, Keeley JE. 1999. Comparison of post-fire seedling establishment between scrub communities in Mediterranean and non-Mediterranean climate ecosystems. Journal of Ecology 87: 1025–1036. [Google Scholar]
- Chamorro D, Luna B, Moreno JM. 2013. Germination response to various temperature regimes of four Mediterranean seeder shrubs across a range of altitudes. Plant Ecology 214: 1431–1441. [Google Scholar]
- Cohen D. 1966. Optimizing reproduction in a randomly varying environment. Journal of Theoretical Biology 12: 119–129. [DOI] [PubMed] [Google Scholar]
- Donohue K, Dorn L, Griffith C, et al. 2005. Environmental and genetic influences on the germination of Arabidopsis thaliana in the field. Evolution 59: 740–757. [PubMed] [Google Scholar]
- Fenner M. 1991. The effects of the parent environment on seed germinability. Seed Science Research 1: 75–84. [Google Scholar]
- Fenner M, Thompson K. 2005. The ecology of seeds. Cambridge: Cambridge University Press. [Google Scholar]
- Galloway LF. 2001. The effect of maternal and paternal environments on seed character in the herbaceous plant Campanula americana (Campanulaceae). American Journal of Botany 8: 832–840. [PubMed] [Google Scholar]
- Govender N, Trollope WSW, van Wilgen BW. 2006. The effect of fire season, fire frequency, rainfall and management on fire intensity in savanna vegetation in South Africa. Journal of Applied Ecology 43: 748–758. [Google Scholar]
- Gutterman Y. 2000. Maternal effects on seed during development. In: Fenner M. ed. Seeds: the ecology of regeneration in plant communities. Wallingford, UK: CABI Publishing, 59–84. [Google Scholar]
- Hanley ME, Unna JE, Darvill B. 2003. Seed size and germination response: a relationship for fire-following plant species exposed to thermal shock. Oecologia 134: 18–22. [DOI] [PubMed] [Google Scholar]
- Hernandez-Verdugo S, Lopez-Espana RG, Porras F, Parra-Terraza S, Villarreal-Romero M, Osuna-Enciso T. 2010. Variation in germination among populations and plants of wild chili pepper. Agrociencia 44: 667–677. [Google Scholar]
- Herranz JM, Ferrandis P, Martinez-Sanchez JJ. 1998. Influence of heat on seed germination of seven Mediterranean Leguminosae species. Plant Ecology 136: 95–103. [Google Scholar]
- Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometrical Journal 50: 346–363. [DOI] [PubMed] [Google Scholar]
- Hudson AR, Ayre DJ, Ooi MKJ. 2015. Physical dormancy in a changing climate. Seed Science Research, doi:10.1017/S0960258514000403. [Google Scholar]
- Jeffery DJ, Holmes PM, Rebelo AG. 1988. Effects of dry heat on seed-germination in selected indigenous and alien legume species in South-Africa. South African Journal of Botany 54: 28–34. [Google Scholar]
- Keeley JE. 1991. Seed-germination and life-history syndromes in the California Chaparral. Botanical Review 57: 81–116. [Google Scholar]
- Keeley JE, Pausas JG, Rundel PW, Bond WJ, Bradstock RA. 2011. Fire as an evolutionary pressure shaping plant traits. Trends in Plant Science 16: 406–411. [DOI] [PubMed] [Google Scholar]
- Kubiak PJ. 2009. Fire responses of bushland plants after the January 1994 wildfires in northern Sydney. Cunninghamia 11: 131–165. [Google Scholar]
- Lacerda DR, Lemos JP, Goulart MF, Ribeiro RA, Lovato MB. 2004. Seed-dormancy variation in natural populations of two tropical leguminous tree species: Senna multijuga (Caesalpinoideae) and Plathymenia reticulata (Mimosoideae). Seed Science Research 14: 127–135. [Google Scholar]
- Lacey EP, Smith S, Case AL. 1997. Parental effects on seed mass: seed coat but not embryo/endosperm effects. American Journal of Botany 84: 1617–1620. [PubMed] [Google Scholar]
- Masaka K, Yamada K. 2009. Variation in germination character of Robinia pseudoacacia L. (Leguminosae) seeds at individual tree level. Journal of Forest Research 14:167–177. [Google Scholar]
- Moreira B, Pausas JG. 2012. Tanned or burned: the role of fire in shaping physical seed dormancy. PLoS ONE 7: e51523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JM, Oechel WC. 1991. Fire intensity effects on germination of shrubs and herbs in southern California chaparral. Ecology 72: 1993–2004. [Google Scholar]
- Narbona E, Ortizb PL, Aristab M. 2006. Germination variability and the effect of various pre-treatment on germination in the perennial spurge Euphorbia nicaeensis All. Flora 201: 633–641. [Google Scholar]
- Nooden LD, Blakey KA, Grzybowski JM. 1985. Control of seed coat thickness and permeability in soybean; a possible adaptation to stress. Plant Physiology 79: 543–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman HC, Galwey NW, Cocks PS. 2002. Hardseededness in annual clovers: variation within populations and subsequent shifts due to environmental changes. Australian Journal of Agricultural Research 53: 831–836. [Google Scholar]
- Odion D, Tyler C. 2002. Are long fire-free periods needed to maintain the endangered, fire-recruiting shrub Arctostaphylos morroensis (Ericaceae)? Conservation Ecology 6: 4 (online): http://www.consecol.org/vol6/iss2/art4. [Google Scholar]
- Ooi MKJ. 2007. Dormancy classification and potential dormancy-breaking cues for shrub species from fire-prone south-eastern Australia. In: Adkins SW, Ashmore S, Navie SC. eds. Seed: biology, development and ecology. Wallingford, UK: CABI Publishing, 205–216. [Google Scholar]
- Ooi MKJ, Whelan RJ, Auld TD. 2006. Persistence of obligate-seeding species at the population scale: the effects of fire intensity, fire patchiness and long fire-free intervals. International Journal of Wildlife Fire 15: 261–269. [Google Scholar]
- Ooi MKJ, Auld TD, Denham AJ. 2009. Climate change and bet-hedging: interactions between increased soil temperatures and seed bank persistence. Global Change Biology 15: 2375–2386. [Google Scholar]
- Ooi MKJ, Auld TD, Denham AJ. 2012. Projected soil temperature increase and seed dormancy response along an altitudinal gradient: implications for seed bank persistence under climate change. Plant and Soil 353: 289–303. [Google Scholar]
- Ooi MKJ, Denham AJ, Santana VM, Auld TD. 2014. Temperature thresholds of physically dormant seeds and plant functional response to fire: variation among species and relative impact of climate change. Ecology and Evolution 4: 656–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman TD, Towerton AL. 2008. Soil temperatures during autumn prescribed burning: implications for the germination of fire responsive species? International Journal of Wildland Fire 5: 572–578. [Google Scholar]
- Pérez-García F. 1997. Germination of Cistus ladanifer seeds in relation to parent material. Plant Ecology 133: 57–62. [Google Scholar]
- Philippi T. 1993. Bet-hedging germination of desert annuals-variation among populations and maternal effects in Lepidium lasiocarpum. American Naturalist 142: 488–507. [DOI] [PubMed] [Google Scholar]
- Philippi T, Seger J. 1989. Hedging ones evolutionary bets, revisited. Trends in Ecology & Evolution 4: 41–44. [DOI] [PubMed] [Google Scholar]
- Quinlivan BJ. 1971. Seed coat impermeabilty in legumes. Journal of the Australian Institute of Agricultural Science 37: 283–295. [Google Scholar]
- R Core Development Team. 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/. [Google Scholar]
- Ruprecht E, Fenesi A, Fodor AI, Kuhn T. 2013. Prescribed burning as an alternative management in grasslands of temperate Europe: the impact on seeds. Basic and Applied Ecology 14: 642–650. [Google Scholar]
- Santana VM, Baeza MJ, Blanes MC. 2013. Clarifying the role of heat and daily temperature fluctuations as germination cues for Mediterranean Basin obligate seeders. Annals of Botany 111: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavsanoglu C, Catav SS. 2012. Seed size explains within-population variability in post-fire germination of Cistus salvifolius. Annales Botanici Fennici 49: 331–340. [Google Scholar]
- Taylor GB, Palmer MJ. 1979. The effect of some environmental conditions on seed development and hard-seededness in subterranean clover (Trifolium subterraneum L.). Australian Journal of Agricultural Research 30: 65–76. [Google Scholar]
- Taylor KM, Aarssen LW. 1989. Neighbor effects in mast year seedlings of Acer saccharum. American Journal of Botany 76: 546–554. [Google Scholar]
- Thanos CA, Georghiou K. 1988. Ecophysiology of fire-stimulated seed germination in Cistus incanus ssp. creticus (L.) Heywood and C. salvifolius L. Plant, Cell and Environment 11: 841–849. [Google Scholar]
- Trabaud L, Oustric J. 1989. Heat requirements for seed germination of three Cistus species in the garrigue of southern France. Flora 183: 321–325. [Google Scholar]
- van Wilgen BW, Forsyth GG. 1992. Regeneration strategies in fynbos plants and their influence on the stability of community boundaries after fire. In: Billings WD, Golley F, Lange OL, Olson JS, Remmert H, eds. Fire in South African mountain fynbos. New York: Springer, 54–80. [Google Scholar]
- Venable DL. 1989. Modelling the evolutionary ecology of seed banks. In: Leck MA, Parker VT, Simpson RL. eds. Ecology of soil seed banks. San Diego: Academic Press, 67–87. [Google Scholar]
- Venable DL. 2007. Bet hedging in a guild of desert annuals. Ecology 88: 1086–1090. [DOI] [PubMed] [Google Scholar]
- Wang JH, Baskin CC, Cui XL, Du GZ. 2009. Effect of phylogeny, life history and habitat correlates on seed germination of 69 arid and semi-arid zone species from northwest China. Evolutionary Ecology 23:827–846. [Google Scholar]
- Whelen RJ. 1995. The ecology of fire. London: Cambridge University Press. [Google Scholar]
- Wood DW, Scott RK, Longden PC. 1980. The effects of mother-plant temperature on seed quality in Beta vulgaris L. (sugar beet) . In: Hebblethwaite PD. ed. Seed production. London: Butterworth, 257–270. [Google Scholar]


