Abstract
Backgroundg:
Root canal irrigation plays a pivotal role in endodontics. Constant increase in antibiotic resistance and side effects caused by synthetic irrigants has shifted the research toward developing herbal alternatives. The current study aims to assess the ex vivo effectiveness of an indigenously prepared herbal extract “EndoPam” and compare it with the conventional endodontic irrigants for disinfection of root canals infected with Enterococcus faecalis.
Materials and Methods:
As a preliminary study of the antimicrobial efficacy of the test irrigants, an Agar diffusion study was conducted, and zone of inhibition measured. Forty extracted mandibular premolars with straight root canals were selected and standardized to 12 ± 1 mm in length. Root canals were prepared using rotary ProTaper system until F3 instrument and were infected with the culture of E. faecalis for three weeks. Specimens were divided into four groups (n = 10). Group 1: EndoPam (
Ingredients:
Syzigium aromaticum, Eucalyptus globulus, Cinnamomum zeylanicum and Mentha piperita), Group 2: 2% chlorhexidine, Group 3: 5.25% Sodium hypochlorite, Group 4: Normal Saline. Irrigation was performed for each group. Samples were inoculated and incubated for 24 h at 37°C for qualitative analysis qualitative analysis.
Results:
In the preliminary Agar diffusion study, EndoPam exhibited a zone of inhibition comparable to that of sodium hypochrorite. The diameter of the inhibition zone was in the following order: 2% chlorhexidine gluconate > EndoPam > 5.25% NaOCl > Normal Saline. The qualitative assay done by culturing the bacteria after a period of 3 weeks showed no bacterial growth in any of the tested irrigants, except in normal saline.
Conclusion:
It was found that the experimental product was as effective as conventional irrigants in reducing the microbial count.
Keywords: Chlorhexidine gluconate, EndoPam, Enterococcus faecalis, herbal, irriagants, rootcanal treatment, sodium hypoclorite
Introduction
The goal of endodontic therapy is the removal of all vital or necrotic tissue, microorganisms, and microbial by-products from the root canal system.1 Instrumentation of the root canal system should be supported by irrigation, which is capable of removing necrotic debris, as well as microorganisms. Without irrigation, accumulation of this debris causes instruments to become rapidly ineffective. Irrigating solutions are constantly modified and further developed to improve their properties. However, there is currently no unique irrigant that meets all of the requirements for an optimal irrigating solution.1,2
Constant increase in antibiotic resistance and side effects caused by synthetic irrigants shifted the research towards developing herbal alternatives. Several natural extracts have been evaluated for endodontic purpose, with conflicting results.3,4
In the ancient science of Ayurveda, many herbs with potent therapeutic effects including antibacterial, antifungal, analgesic and anti-inflammatory properties have been demonstrated such as S. aromaticum,5 C. zeylanicum,6 E. globulus7 and M. piperita.8 These have been widely used for several medicinal purposes, however their role in endodontics have not been evaluated, to date. Hence, in the present study, these were combined to prepare an indigenous product, to achieve a potential additive or synergistic effect, as an endodontic irrigant.
Hence, the purpose of this study is to assess the ex vivo effectiveness of an indigenously prepared herbal extract and compare it with the conventional endodontic irrigants for disinfection of root canals infected with Enterococcus faecalis.
Materials and Methods
The in vitro study was done at the Departments of Conservative Dentistry & Endodontics and Department of Microbiology, Mar Baselios Dental College, Kothamangalam, Kerala. The extracted teeth for the study were obtained from the Department of Oral and Maxillofacial Surgery of the same college.
The ingredients of the herbal product used in this study are S. aromaticum, C. zeylanicum, E. globulus and M. piperita. The ethanolic extracts of the individual ingredients were obtained (Kancor Flavours and Extracts Limited, Angamaly, Kerala, India) and they were combined in an equal ratio to obtain the final product, EndoPam (PamLabs India Pvt. Ltd., Kerala, India).
A pure culture of the test strain, E. faecalis ATCC 29212 (Microbial Type Culture Collection, IMTECH, Chandigarh), was prepared in sterile brain heart infusion (BHI) broth (HiMedia Laboratory Private Limited, Mumbai, India) and adjusted spectrophotometrically (Shimadzu UV 2400 PC, Tokyo, Japan) to an optical density of 560 nm corresponding to match the turbidity of McFarland 0.5 scale (1.5 ×108 CFU/mL) and incubated at 37°C for 24 h.
Agar diffusion test
As a preliminary study of the antimicrobial efficacy of the test irrigants, an Agar diffusion study was conducted. Pre-sterilized Whatman paper discs of 6 mm diameter were soaked in 20 ml of each one of the test solutions. These discs were then placed on Mueller-Hinton agar plates and incubated at 37°C for 24 h. Zones of inhibition were measured across the diameter with a pair of Vernier calipers.
Ex vivo study on extracted teeth
Forty recently extracted human permanent single-rooted, fully formed mandibular premolars without dental caries, root fractures and root resorption were selected for the study. The teeth were cleaned of superficial debris and tissue tags and is stored in normal saline to prevent dehydration. Each tooth was radiographically assessed to confirm the presence of a single patent canal. Using rotary diamond disc teeth was decoronated below the cement-enamel junction and the length is standardized to 12 ± 1 mm. The root canals were then instrumented using the crown-down technique with rotary instrument (ProTaper, Dentsply Maillefer, Ballaigues, Switzerland), and the canals were enlarged to an apical size F3 ProTaper. 2 ml of 3% NaOCl was used between each instrument during the procedure, which is followed by a final rinse with 17% EDTA. All the specimens were sterilized by autoclaving and stored aseptically in 100% humidity at 30°C until use.
A volume of 10 µL of suspension is inoculated into each tooth and incubated in 2 ml BHI medium for 3 weeks, for the establishment of E. faecalis biofilm. The medium was replenished every 3 days.
The tooth specimens were then divided into four groups (1-4) of 10 samples each, according to the irrigant used.
Group 1: EndoPam (Ingredients: S. aromaticum, E. globulus, C. zeylanicum and M. piperita) (n = 10)
Group 2: 5.25% NaOCl (n = 10)
Group 3: 2% Chlorhexidine gluconate (CHX) (n = 10)
Group 4: Normal Saline (control) (n = 10)
The specimens were handled using flamed tweezers, to prevent contamination. Canals in each group were filled with 2 ml of the corresponding irrigant and were allowed to remain for 10 minutes. After that the root canals were rinsed with 5 ml saline for 1 min. Sterile paper points (DentsplyMaillefer, Ballaigues, Switzerland) were left in the wet canal for 1 min and then transferred to tubes containing 5 ml of BHI broth, which were vortexed for 5 min and then incubated at 37°C for 48 h. The samples were then inoculated on Mueller-Hinton agar plates and incubated for 24 h at 37°C for qualitative analysis.
Results
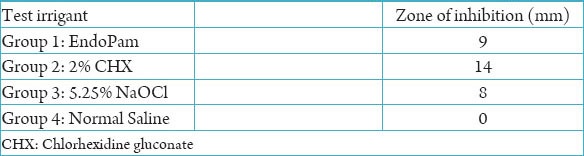
In the preliminary Agar diffusion study, chlorhexidine had the maximum zone of inhibition (14 mm) and saline had no zone of inhibition (Figure 1). Endopam exhibited a zone of inhibition comparable to that of sodium hypochrorite. The diameter of the inhibition zone was in the following order: 2% CHX > EndoPam > 5.25% NaOCl > Normal Saline (Table 1).
Figure 1.
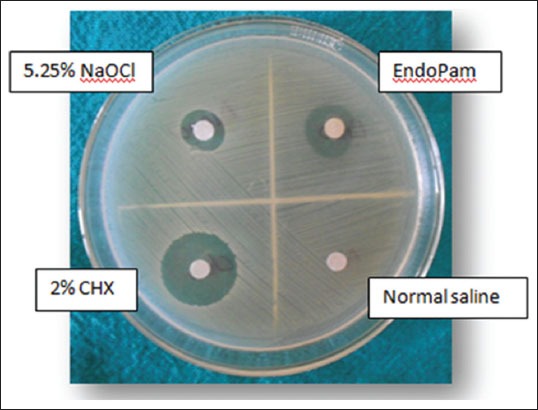
Agar diffusion study.
Table 1.
Zones of inhibition from the Agar diffusion study.
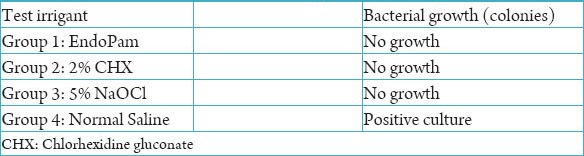
The qualitative assay done by culturing the bacteria after a period of 3 weeks showed no bacterial growth (Figure 2) in any of the tested irrigants, except in normal saline, which was the negative control group (Table 2).
Figure 2.
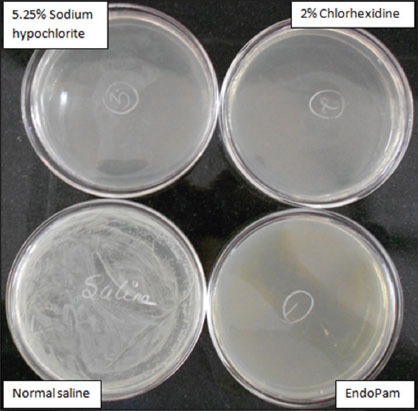
The bacterial culture plates.
Table 2.
Results of the qualitative assay.
Discussion
Indian traditional medicine was dependent on herbs and herbal products, and recently it has gained importance. The major advantages of herbal alternatives are easily available, inexpensive, less side-effects and lack of microbial resistance.8
Several natural extracts with proven antimicrobial efficacy against E. faecalis like Arctium lappa,9 triphala, green tea polyphenols,10 liquorice11 etc. have been tested as endodontic irrigants, with conflicting results.
In the present study, four potent antimicrobial herbs were used as a combination to be tested as a root canal irrigant. S. aromaticum which is commonly known as clove, is found in the Maluku islands in east Indonesia. This plant is widely used in pharmaceutical, cosmetic, food and agricultural applications since it is rich in pharmacologically active compounds such as eugenol, eugenol acetate and gallic acid.12
Compounds such as 1,8-cineole, citronellal, citronellol, citronellyl acetate, p-cymene, eucamalol, limonene, linalool,13 β- pinene, γ-terpinene, α-terpinol, alloocimene and aromadendrene14 give eucalyptus extracts their antibacterial activity. Recent studies have shown many potentially beneficial health effects of cinnamon such as anti-inflammatory properties, anti-microbial activity, blood glucose control, reducing cardiovascular disease, boosting cognitive function, and reducing risk of colonic cancer.15 M. piperita extract has been reported to have antioxidant16 and antimicrobial properties.17
E. faecalis was chosen as one of the test microorganisms in this experiment due to the following reasons: (i) In endodontically treated teeth, with persistent apical periodontics18 it is recognized as the associated pathogen (ii) it is resistant to NaOCl19 (iii) entire width of dentinal tubules can be colonized by it (iv) and it grows rapidly.20
The clinical efficacy of sodium hypochlorite (NaOCL) for the irrigation of root canal space in endodontic therapy makes it the gold standard.13 It has a unique property of dissolving necrotic as well as vital tissues.14 However, there are some disadvantages such as toxicity,15 disagreeable smell and taste,16 and potential for allergic reactions.17
CHX has been suggested as an endodontic irrigant with its advantages including antibacterial effects, lower cytotoxicity than NaOCl, existence of substantivity and efficient clinical performance.18 However, it has disadvantages like tissue irritability and the reactive oxygen species formation.19
The antibacterial studies on agar plates are very common tests, but the microbial inhibition zone depends on the test substance’s solubility and ability to diffuse through agar, so it may not express its full effective potential.20 Therefore, the information obtained from agar diffusion studies does not reliably reflect their in vitro or in vivo antimicrobial activity. So the agar diffusion test was conducted as a preliminary study to assess the efficacy of the indigenous herbal irrigant, EndoPam. As it demonstrated promising results, with an inhibition zone comparable to sodium hypochlorite, the assay was further extended to 3 weeks.
The results of the bacterial culture after biofilm formation on the tooth substrate also showed that the experimental irrigant had antimicrobial efficacy comparable to the convention chemical irrigants. Thus, the present study showed that all the three irrigants tested had higher antibacterial action than the negative control group, i.e. saline. Normal saline was shown to be totally ineffective in eliminating E. faecalis.
There are several imitations for this study. The toxicity, allergic potential and tooth discoloration potential of the experimental irrigant was not taken into consideration. The possible interactions between the ingredients of the product also need to be taken into account. Further studies like quantitative real time-PCR and confocal microscopic examination of the biofilms will provide better insight into the herbal irrigant.
Conclusion
This was a preliminary study of the antimicrobial efficacy of an indigenously prepared herbal irrigant against E. faecalis. The results indicate that, within the limitations of this study, EndoPam has the potential to be used as an herbal alternative in root canal irrigation.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Zehnder M. Root canal irrigants. J Endod. 2006;32(5):389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Haapasalo M, Shen Y, Qian W, Gao Y. Irrigation in endodontics. Dent Clin North Am. 2010;54(2):291–312. doi: 10.1016/j.cden.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Prabhakar J, Senthilkumar M, Priya MS, Mahalakshmi K, Sehgal PK, Sukumaran VG. Evaluation of antimicrobial efficacy of herbal alternatives (Triphala and green tea polyphenols), MTAD, and 5% sodium hypochlorite against Enterococcus faecalis biofilm formed on tooth substrate:an in vitro study. J Endod. 2010;36(1):83–6. doi: 10.1016/j.joen.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 4.Alemdar S, Agaoglu S. Investigation of in vitro antimicrobial activity of aloe vera juice. J Anim Vet Adv. 2009;8:99–102. [Google Scholar]
- 5.Assiri AM, Hassanien MF. Bioactive lipids, radical scavenging potential, and antimicrobial properties of cold pressed clove (Syzygium aromaticum) oil. J Med Food. 2013;16(11):1046–56. doi: 10.1089/jmf.2012.0288. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh V, Saranya S, Mukherjee A, Chandrasekaran N. Cinnamon oil nanoemulsion formulation by ultrasonic emulsification: investigation of its bactericidal activity. J Nanosci Nanotechnol. 2013;13(1):114–22. doi: 10.1166/jnn.2013.6701. [DOI] [PubMed] [Google Scholar]
- 7.Bachir RG, Benali M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus . Asian Pac J Trop Biomed. 2012;2(9):739–42. doi: 10.1016/S2221-1691(12)60220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behnam S, Farzaneh M, Ahmadzadeh M, Tehrani AS. Composition and antifungal activity of essential oils of Mentha piperita and Lavendula angustifolia on post-harvest phytopathogens. Commun Agric Appl Biol Sci. 2006;71:1321–6. [PubMed] [Google Scholar]
- 9.Chan YS, Cheng LN, Wu JH, Chan E, Kwan YW, Lee SM, et al. A review of the pharmacological effects of Arctium lappa (burdock) Inflammopharmacology. 2011;19(5):245–54. doi: 10.1007/s10787-010-0062-4. [DOI] [PubMed] [Google Scholar]
- 10.Shakouie S, Eskandarinezhad M, Gasemi N, Milani AS, Samiei M, Golizadeh S. An in vitro comparison of the antibacterial efficacy of triphala with different concentrations of sodium hypochlorite. Iran Endod J. 2014;9(4):287–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Badr AE, Omar N, Badria FA. A laboratory evaluation of the antibacterial and cytotoxic effect of Liquorice when used as root canal medicament. Int Endod J. 2011;44(1):51–8. doi: 10.1111/j.1365-2591.2010.01794.x. [DOI] [PubMed] [Google Scholar]
- 12.Cortés-Rojas DF, de Souza CR, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014;4:90–6. doi: 10.1016/S2221-1691(14)60215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung GS, Stock CJ. In vitro cleaning ability of root canal irrigants with and without endosonics. Int Endod J. 1993;26(6):334–43. doi: 10.1111/j.1365-2591.1993.tb00766.x. [DOI] [PubMed] [Google Scholar]
- 14.Jeansonne MJ, White RR. A comparison of 2.0& chlorhexidine gluconate and 5.25& sodium hypochlorite as antimicrobial endodontic irrigants. J Endod. 1994;20(6):276–8. doi: 10.1016/s0099-2399(06)80815-0. [DOI] [PubMed] [Google Scholar]
- 15.Spangberg L, Pascon EA. The importance of material preparation for the expression of cytotoxicity during in vitro evaluation of biomaterials. J Endod. 1988;14(5):247–50. doi: 10.1016/S0099-2399(88)80178-X. [DOI] [PubMed] [Google Scholar]
- 16.Busslinger A, Sener B, Barbakow F. Effects of sodium hypochlorite on nickel-titanium Lightspeed instruments. Int Endod J. 1998;31(4):290–4. doi: 10.1046/j.1365-2591.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman AY, Keila S. Hypersensitivity to sodium hypochlorite. J Endod. 1989;15(5):224–6. doi: 10.1016/S0099-2399(89)80241-9. [DOI] [PubMed] [Google Scholar]
- 18.Leonardo MR, Tanomaru Filho M, Silva LA, Nelson Filho P, Bonifácio KC, Ito IY. In vivo antimicrobial activity of 2& chlorhexidine used as a root canal irrigating solution. J Endod. 1999;25(3):167–71. doi: 10.1016/s0099-2399(99)80135-6. [DOI] [PubMed] [Google Scholar]
- 19.Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997;24(5):287–96. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 20.Mayrhofer S, Domig KJ, Mair C, Zitz U, Huys G, Kneifel W. Comparison of broth microdilution, Etest, and agar disk diffusion methods for antimicrobial susceptibility testing of Lactobacillus acidophilus members . Appl Environ Microbiol. 2008;74(12):3745–8. doi: 10.1128/AEM.02849-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


