Abstract
Background:
Microorganisms and their by-products in pulpal and periapical diseases are to be considered as the primary etiological agents of the pulpal necrosis and apical periodontitis. Enterococcus faecalis, which is the most common organism isolated from failed root canals, is a Gram-positive facultative anaerobe. Yeasts can be detected in 7-18% of infected root canals.
Materials and Methods:
Designed to evaluate the antimicrobial and antifungal efficacy of chlorhexidine gutta-percha (CHX-GP), and calcium hydroxide GP points against E. faecalis and Candida albicans. The test materials used are divided into 3 groups; Group A - Control, Regular GP, Group B – CHX-GP, Group C - Calcium hydroxide GP. Detail Method is explained in the article.
Results:
There was a significant difference in the inhibition of E. faecalis, in different materials at each time interval period (P < 0.05). Similarly, the inhibition of C. albicans in the different materials at each time period was found to be statistically significant (P < 0.05). Calcium hydroxide GP did not exhibit any antimicrobial effect on any of tested microorganisms for all the time periods.
Conclusion:
CHX-GP had the maximum effect on the test microorganisms, with the maximum efficacy on E. faecalis followed by C. albicans. Regular GP also had a significant efficacy on test microorganisms, with the maximum efficacy on E. faecalis followed by C. albicans. Calcium hydroxide GP did not have any effect on any test microorganisms during the entire test periods.
Keywords: Candida albicans, endodontics, Enterococcus faecalis, periapical diseases, pulpal diseases
Introduction
The main aim and purpose of endodontic therapy is to eliminate the microorganisms from the root canal system and to prevent the subsequent reinfection. Most of the treatment failures are caused by microorganisms surviving the treatment procedures and causing re-infection of the root canal system. Although the major numbers of bacteria are always eliminated by the biomechanical preparation of root canal space, but a few microorganisms sometimes still survive. These challenges by the residing of microorganisms in an anatomical complexities of the root canals, which include cementum crypts, secondary root canals, dentin tubules, and deltas. In these locations, most of the time the bacteria may be unaffected by chemo-mechanical preparation of root canals of the tooth, and so the use of intracanal medication with the use of the filling materials with antimicrobial, and the sealing properties are of essentially important, to avoid the growth of bacteria and other microorganism. The endodontic microflora is typically a polymicrobial flora of Gram-positive and Gram-negative bacteria, dominated by obligate anaerobes. Staphylococcus aureus and Streptococcus mutans are the most common organisms associated with dental caries, and are involved in initial pulpal infection. Enterococcus faecalis, which is a Gram-positive facultative anaerobe is the most common organism isolated from failed root canals.1,2 It can survive extreme environment challenges and is particularly resistant to many conventional antimicrobial agents used routinely.2,3 An inter-appointment antimicrobial and antifungal medication is therefore recommended even after careful instrumentation and debridement of the root canal system in order to prevent recovery and multiplication of remnant microorganisms. Different attempts are been made to improve the antimicrobial and antifungal efficacy of the gutta-percha (GP) points which are used exclusively, as an inter-appointment intracanal dressing by corporating Calcium hydroxide, chlorhexidine, and tetracycline. This study was designed to evaluate the antimicrobial and antifungal efficacy of chlorhexidine GP (CHX-GP), and calcium hydroxide GP points against E. faecalis and Candida albicans.
Aim and objective
The main purpose of this study was to evaluate antimicrobial and antifungal efficacy of CHX-GP and calcium hydroxide GP used as inter appointment intracanal medicament against E. faecalis and C. albicans in vitro.
Materials and Methods
Test materials used:
Regular GP (RGP). (Dentsply, Maillefer)
CHX-GP (Active points by Reoko Company Germany) which contains chlorhexidine diacetate, GP, ZnO, BaSO4, Coloring agent.
Calcium hydroxide GP (Calcium hydroxide plus points by Roeko, Germany) containing GP, calcium hydroxide, sodium chloride, surfactant, coloring agent.
Microorganisms
Standard strains of E. faecalis MTCC 439 and C. albicans MTCC 227 were obtained from Institute of Microbial Technology, Chandigarh, were used for the study.
Procedure
The two standard strains were activated in Brain heart infusion broth and inoculated on blood agar and sabouraud dextrose agar, respectively. Incubation was done at 37°C for 24 h for both the strains. Turbidity of the broth suspension was adjusted to No1 McFarland standard (3 × 108 cells/ml). Antimicrobial activity was determined using the agar diffusion method.
The test materials used are divided into 3 groups;
Group distribution:
Group A - Control, RGP.
Group B - CHX-GP.
Group C - Calcium hydroxide GP.
A 50 µl of broth suspension, containing aliquots of E. faecalis was spread on the Petri dish, which contained Mueller-Hilton agar medium. C. albicans was separately spread onto Sabouraud Dextrose agar plates using a sterile spreader. Then the content from the swab was distributed uniformly in the surface of the plate of the agar Mueller-Hinton for E. faecalis and Sabouraud’s dextrose agar for C. albicans. Before the test, each GP cones were soaked by complete immersing them in 2ml of sterile water in the test tube for 1 h. After the soaking procedure, the wet GP cones were dried with the help of sterile paper. Finally, the sterile swabs were made to touch all around the edge of the agar. It was left to dry and the GP were applied manually using sterile tweezers. After its placement, the GP was pressed carefully against the agar surface. They were placed 20 mm beyond the edge of the plate and were distributed by avoiding the overlap halos of inhibition. Petri plates of 90 mm were used and 3 GP were placed in each plate. After 15 min, the plates were turned and placed into an incubator at 35-37°C for 24, 48, and 72 h, respectively. Inhibition zones of tested materials for different time periods for E. faecalis and C. albicans was measured and the average was recorded. Inhibition of each bacterium with the different materials at each time interval was compared using Student’s t-test. The data were subjected to statistical analysis.
Results
Inhibition of each bacterium with the different materials at each time interval was compared using Student’s t-test, results are tabulated in Tables 1 and 2. It was noticed that there was a significant difference in the inhibition of E. faecalis, in different materials at each time interval period (P < 0.05). Similarly, the inhibition of C. albicans in the different materials at each time period was found to be statistically significant (P < 0.05). Calcium hydroxide GP did not exhibit any antimicrobial effect on any of tested microorganisms for all the time periods as shown in Tables 1 and 2, Graphs 1 and 2.
Table 1.
Comparison of the effect of test materials on the inhibition of E. faecalis at each time period.
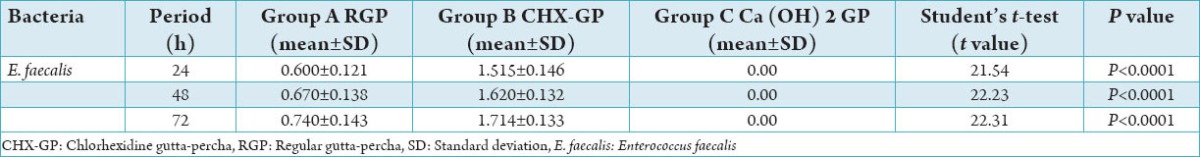
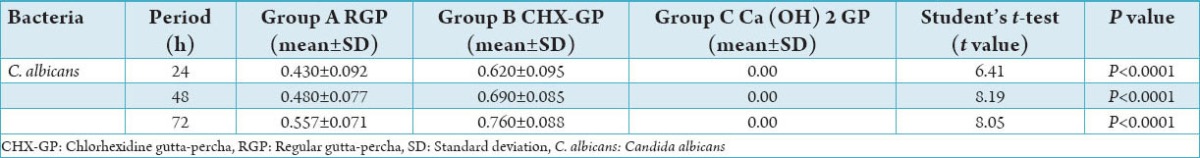
Table 2.
Comparison of the effect of test materials on the inhibition of C. albicans at each time period.
Graph 1.
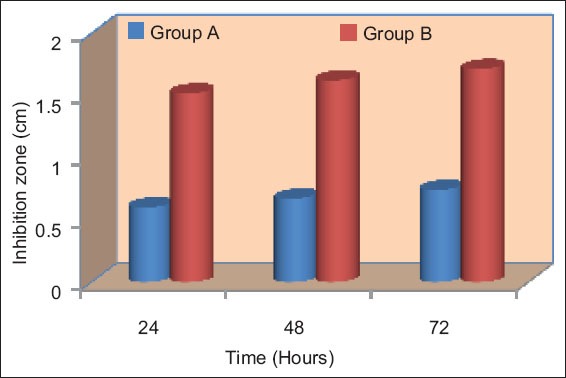
Mean inhibition of different groups at different time intervals for Enterococcus faecalis.
Graph 2.
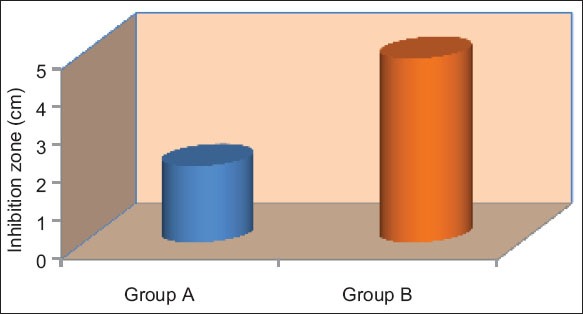
Mean inhibition of different groups on an average for Enterococcus faecalis.
CHX-GP cones could inhibit both the tested bacterial strains for 24 h. Regardless of the time period or pathogen strain, CHX-GP was statistically more effective than other test materials (P < 0.05) as shown in Tables 1 and 2. The largest mean inhibition zone with CHX GP occurred with E. faecalis with a mean diameter of 15.2 mm, 16.2 mm, and 17.1 mm in 24 h, 48 h, and 72 h, respectively followed by C. albicans with a mean diameter of 6.2 mm, 6.9 mm, and 7.6 mm in 24 h, 48 h, and 72 h, respectively. RGP also showed inhibition zone with E. faecalis with mean diameter of 6 mm, 6.7 mm, and 7.4 mm in 24 h, 48 h, and 72 h, respectively, followed by C. albicans with a mean diameter of 4.3 mm, 4.8 mm, and 5.5 mm in 24 h,48 h, and 72 h, respectively.
Tables 3 and 4, Graphs 3 and 4 shows that there was a significant differences in the inhibitor of both (E. faecalis and C. albicans) in different materials on an average (P < 0.05).
Table 3.
Comparison of the effect of test materials on the inhibition of E. faecalis on an average.

Table 4.
Comparison of the effect of test materials on the inhibition of C. albicans on an average.

Graph 3.
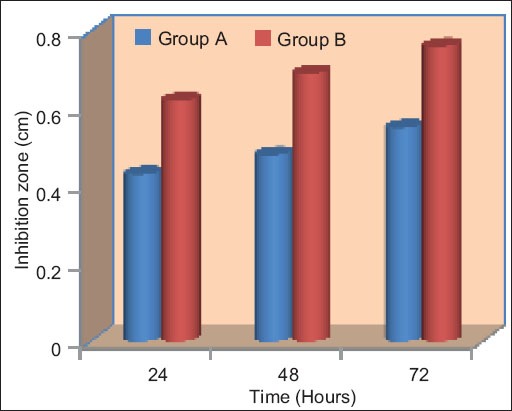
Mean inhibition of different groups at different time intervals for Candida albicans.
Graph 4.
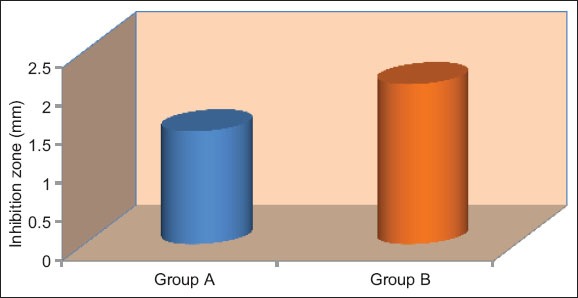
Mean inhibition of different groups on an average Candida albicans.
Discussion
The practice of endodontics is constantly evolving specialty. Most pathosis found in dental pulp and the periapical tissues are either directly or indirectly related to bacteria and other microorganisms.4,5 Thorough debridement of the root canal system is considered to be the most important step in endodontic therapy. Ideally, all bacteria should be eradicated prior to obturation; however, bacteria may persist in the root canal system despite debridement and disinfection by residing in accessory canals, dentinal tubules, where bacteria are unaffected by routine chemo-mechanical preparation procedures. These residual microorganisms in the root canal system following cleaning and shaping or microbial contamination of the root canal system between appointments are of great concern.
In an effort to sample microorganisms that were obligate and facultative anaerobes in root canals, several investigators examined the flora of intact teeth with necrotic pulps, found minor differences in the number of microorganisms isolated from such teeth. Gram-positive organisms were found in approximately 75% of the samples; with the most predominant species were streptococci 28%, staphylococci 15%, corynebacteria 10%, yeast 12%, and others.6-8 In the present study, E. faecalis was chosen because of its implication as the possible microbial factor in therapy-resistant apical periodontitis. It is Gram-positive, facultative anaerobe, catalase-negative, and able to grow in 6.5% sodium chloride, at temperatures ranging from 10 to 45°C, and they survive 30 min at 60°C and pH with 9.6 and can survive the extreme environmental challenges.7 It is the most common organism isolated from failed root canals as it is particularly resistant to many conventional antimicrobial agents used routinely.8
Although genus enterococci, make up only a small proportion of initial flora of the untreated teeth with necrotic pulp, enterococci, particularly E. faecalis, have been frequently found in the obturated root canals exhibiting the signs of chronic apical periodontitis, and is isolated in 23-70% of the positive cultures. A recognized pathogen in post-treatment endodontic infections that is E. faecalis is frequently isolated from both in mixed flora and in monocultures. It is also apparent from the dental literature that resistant strains of E. faecalis such as E. faecalis ATCC 29212, 35550, 33012, 33186, 19433, etc. are often difficult to eradicate from the root canal system with current intracanal medications (Figures 1 and 2).4,7,9,10
Figure 1.

Enterococcus faecalis (colonies).
Figure 2.
Enterococcus faecalis (microscopic view).
Fungi, sometimes have been found in the primary root canal infections, but they seems to occur more often in root canals of obturated teeth with failed treatment. With the ability of the C. albicans to invade dentinal tubules and form a resistance to commonly used Figuresd intracanal medicaments it may help to explain why C. albicans has been associated with cases of persistent root canal infections.11-13 In the present study, standard strains E. faecalis MTCC 439 (Microbial type culture collection) and C. albicans MTCC 227 were used (Figures 3 and 4). The agar diffusion test used in the study is the most frequently used methods for the assessment of the antimicrobial activity of endodontic materials. It’s ease to allows the direct comparisons of the filling materials against test microorganisms, indicating which of the material has the potential to eliminate bacteria in the local microenvironment of the tooth’s root canal system.4,10,14 Other factors that may limit the dynamics and variability of agar diffusion tests includes control and standardization of the inoculum density, moments at which the results are read, choice of agar, incubation temperature of the plates, and reading of inhibition zone.13,15,16 Another relevant factor concerning the methodology used in this study was the optimization of the culture media following the incubation period by adding aliquots of 10 ml of triphenyltetrazolium chloride (0.05% TTC). This procedure helps to distinguish inhibition haloes from bacterial growth which is often interpreted as a diffusion halo of materials and this procedure facilitated the observation of the zones of inhibition.16
Figure 3.
Candida albicans (Colonies).
Figure 4.
Candida albicans (microscopic view).
Organisms were incubated on specific selective media, as growths of the test organisms were found to be favorable with these selective media; Mueller Hilton agar medium for E. faecalis and sabouraud dextrose agar medium for C. albicans. Microorganisms were incubated at 37°C, which is the normal human body temperature and also the optimum temperature for bacterial and fungal growth. Calcium hydroxide is the most commonly utilized and studied root canal medication. Calcium hydroxide has alkaline pH, ionic activity, diffusion through dentinal tubules, influence on apical micro-leakage and placement within the root canal are examples on how this material has been evaluated since its introduction.15,17,18 Conventionally, calcium hydroxide is prepared by mixing the powder with a liquid and inserted into the root canal by means of an injection, root canal instrument, GP or paper points. Sterile water or glycerin are recommended as vehicles to make a paste with Ca(OH)2 powder. The ideal vehicle should allow gradual and slow release of calcium and hydroxyl ions.18
In addition to the placement, removal of calcium hydroxide from the root canals without leaving any residues behind is also a time-consuming and cumbersome procedure. These shortcomings have prompted the development of GP points containing calcium hydroxide and could be easily placed in the root canal, especially in the periapical area. The benefits of these materials are that there is no mixing procedure, easy to apply, leaves no residue, and the root canals could be filled from periapex to the coronal area. In the present study, a new formulation of calcium hydroxide containing GP with composition of calcium hydroxide 52-54%, GP 35-37%, sodium chloride, surfactant, and the coloring agents was assessed for antimicrobial activity.15,19
However, no observation was made for antimicrobial activity for the GP containing calcium hydroxide (Table 1 and Graph 1). The results, with calcium hydroxide GP points in the study coincides with the results of the previous studies conducted on the same material. Success of calcium hydroxide reported with earlier studies were due to its increased quantity and its pure form in those formulations, whereas when integrated with GP there was decrease in the quantity of actual calcium hydroxide available for ionization. Furthermore, it has been demonstrated in a study that the liberation of calcium ion and hydroxyl ions was faster and more significant, when it was used as calcium hydroxide and distilled water paste.
Previously, it has been demonstrated in various studies, that the calcium hydroxide GP were unable to alter pH and the calcium release was lower in calcium hydroxide GP. The GP matrix probably gets binded with the hydroxyl ions and blocked their release at the site where it is applied. This could be the possible reason for which calcium hydroxide GP points did show negative results or no inhibition zone in the present study. In recent years, chlorhexidine gluconate has emerged as an effective disinfecting agent in endodontic therapy. Chlorhexidine, a cationic biguanide with the ability to adsorb onto the dentin is considered a broad-spectrum antimicrobial agent. It acts by adsorbing onto the microbial cell wall and causing intracellular component leakage.17,20,21 It also causes precipitation of cytoplasmic content so that mitochondria do not produce energy, disturbing glycolytic enzyme, which eventually decreases acid production and later cell death.22
Results showed that regardless of the time period or the pathogen strain, CHX-GP was found to be statistically more effective than the other test materials (P < 0.05) as shown in Tables 1 and 2 and Graphs 1 and 3, followed by the calcium hydroxide GP, which had no effect on test bacteria at all time periods. The RGP which acted as a control had an antimicrobial effect on E. faecalis and C. albicans with inhibition zone observed for at all time periods. CHX-GP showed the best results under the conditions of the present study, however before they are used in the clinical situations further in vivo studies are required to prove their efficacy.
Conclusion
With the conditions of the present study, following conclusion can be drawn;
CHX-GP had the maximum effect on the test microorganisms, with the maximum efficacy on E. faecalis followed by C. albicans.
RGP also had a significant efficacy on test microorganisms, with the maximum efficacy on E. faecalis followed by C. albicans
Calcium hydroxide GP had no effect on any test microorganisms during the entire test periods.
Footnotes
Conflicts of Interest: None
Source of Support: Nil
References
- 1.Grossman LI, Oliet S, Rio CE. 11th ed. India: Varghese Publishing House; 1988. Endodontic Practice; pp. 234–41. [Google Scholar]
- 2.Baron EJ, Peterson LR, Finegold SM. 9th ed. St. Louis, Baltimore, Philadelphia: Mosby; 1994. Bailey and Scott's Diagnostic Microbiology; pp. 168–92. [Google Scholar]
- 3.Holland R, Murata SS, Dezan E, Garlipp O. Apical leakage after root canal filling with an experimental calcium hydroxide gutta-percha point. J Endod. 1996;22(2):71–3. doi: 10.1016/S0099-2399(96)80275-5. [DOI] [PubMed] [Google Scholar]
- 4.Podbielski A, Boeckh C, Haller B. Growth inhibitory activity of gutta-percha points containing root canal medications on common endodontic bacterial pathogens as determined by an optimized quantitative in vitro assay. J Endod. 2000;26(7):398–403. doi: 10.1097/00004770-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Melker KB, Vertucci FJ, Rojas MF, Progulske-Fox A, Bélanger M. Antimicrobial efficacy of medicated root canal filling materials. J Endod. 2006;32(2):148–51. doi: 10.1016/j.joen.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Lui JN, Sae-Lim V, Song KP, Chen NN. In vitro antimicrobial effect of chlorhexidine-impregnated gutta percha points on Enterococcus faecalis . Int Endod J. 2004;37(2):105–13. doi: 10.1111/j.0143-2885.2004.00734.x. [DOI] [PubMed] [Google Scholar]
- 7.Portenier I, Waltimo T, Ørstavik D, Haapasalo M. The susceptibility of starved, stationary phase, and growing cells of Enterococcus faecalis to endodontic medicaments. J Endod. 2005;31(5):380–6. doi: 10.1097/01.don.0000145421.84121.c8. [DOI] [PubMed] [Google Scholar]
- 8.Mathew S, Boopathy T. Enterococcus faecalis – An endodontic challenge JPBS. 2012;4:294–8. [Google Scholar]
- 9.Oztan MD, Kiyan M, Gerçeker D. Antimicrobial effect in vitro of gutta-percha points containing root canal medications against yeasts and Enterococcus faecalis . Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(3):410–6. doi: 10.1016/j.tripleo.2005.10.073. [DOI] [PubMed] [Google Scholar]
- 10.Smadi L, Mahafzah A, Khraisat A. An in vitro evaluation of the antimicrobial activity of nine root canal sealers. J Contemp Dent Pract. 2008;9(5):60–7. [PubMed] [Google Scholar]
- 11.Delgado RJ, Gasparoto TH, Sipert CR, Pinheiro CR, de Moraes IG, Garcia RB, et al. Antimicrobial activity of calcium hydroxide and chlorhexidine on intratubular Candida albicans . Int J Oral Sci. 2013;5(1):32–6. doi: 10.1038/ijos.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siqueira JF, Jr, Sen BH. Fungi in endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(5):632–41. doi: 10.1016/S1079210404000046. [DOI] [PubMed] [Google Scholar]
- 13.Waltimo T, Haapasalo M, Zehnder M, Meyer J. Clinical aspects related to endodontic yeast infections. Endod Top. 2004;9(1):66–78. [Google Scholar]
- 14.Pirani C, Bertacci A, Cavrini F, Foschi F, Acquaviva GL, Prati C, et al. Recovery of Enterococcus faecalis in root canal lumen of patients with primary and secondary endodontic lesions. New Microbiol. 2008;31(2):235–40. [PubMed] [Google Scholar]
- 15.Ballal V, Kundabala M, Acharya S, Ballal M. Antimicrobial action of calcium hydroxide, chlorhexidine and their combination on endodontic pathogens. Aust Dent J. 2007;52(2):118–21. doi: 10.1111/j.1834-7819.2007.tb00475.x. [DOI] [PubMed] [Google Scholar]
- 16.Sipert CR, Hussne RP, Nishiyama CK, Torres SA. In vitro antimicrobial activity of Fill Canal, Sealapex, Mineral Trioxide Aggregate, Portland cement and EndoRez. Int Endod J. 2005;38(8):539–43. doi: 10.1111/j.1365-2591.2005.00984.x. [DOI] [PubMed] [Google Scholar]
- 17.Podbielski A, Spahr A, Haller B. Additive antimicrobial activity of calcium hydroxide and chlorhexidine on common endodontic bacterial pathogens. J Endod. 2003;29(5):340–5. doi: 10.1097/00004770-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Pezelj-Ribaric S, Brekalo I, Abram M, Doric M, Miletic I, Karlovic Z. Influence of calcium hydroxide root-canal sealer on microbial growth in vitro. Folia Microbiol (Praha) 2002;47(4):458–60. doi: 10.1007/BF02818709. [DOI] [PubMed] [Google Scholar]
- 19.Desai S, Chandler N. Calcium hydroxide-based root canal sealers: A review. J Endod. 2009;35(4):475–80. doi: 10.1016/j.joen.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Lin S, Zuckerman O, Weiss EI, Mazor Y, Fuss Z. Antibacterial efficacy of a new chlorhexidine slow release device to disinfect dentinal tubules. J Endod. 2003;29(6):416–8. doi: 10.1097/00004770-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Lin S, Levin L, Weiss EI, Peled M, Fuss Z. In vitro antibacterial efficacy of a new chlorhexidine slow-release device. Quintessence Int. 2006;37(5):391–4. [PubMed] [Google Scholar]
- 22.Rathke A, Meisohle D, Bokelmann J, Haller B. Antibacterial activity of calcium hydroxide and chlorhexidine containing points against Fusobacterium nucleatum and Parvimonas micra. Eur J Dent. 2012;6(4):434–9. [PMC free article] [PubMed] [Google Scholar]


