Abstract
Peripheral neuropathy is one of the major side effects of cisplatin; however, effective treatments are lacking. Curcumin is a polyphenol found in the root of Curcuma longa and has been shown neuroprotective against several neurological diseases. Nevertheless, its effects on cisplatin neuropathy remain unclear. This study aimed to clarify this issue by inducing neuropathy in the rats with intraperitoneal injection of cisplatin 2 mg/kg twice a week for 5 consecutive weeks. Curcumin 200 mg/kg/day was given by gavage to a group of cisplatin-treated rats during these five weeks. The results showed that cisplatin induced thermal hypoalgesia in the 5th week which could be prevented by curcumin. In the 5th and 8th weeks, sciatic motor nerve conduction velocity was reduced in the cisplatin compared with the control groups. Curcumin significantly attenuated this deficit. Morphometric analysis of L4 dorsal root ganglia from the cisplatin group revealed nuclear and nucleolar atrophy including loss of neurons in the 8th week. These alterations were significantly blocked by curcumin. Moreover, curcumin also ameliorated the reduced myelin thickness in the sciatic nerve of cisplatin-treated rats. Taken together, our findings suggest the favorable effects of curcumin on both functional and structural abnormalities in cisplatin neuropathy. Future studies are needed to clarify the exact underlying mechanisms.
Keywords: cisplatin, curcumin, neuropathy, sciatic nerve, dorsal root ganglion
INTRODUCTION
Cisplatin has been used to treat cancers of various organs, including lung, urinary bladder, testis and ovary [1]. However, peripheral neuropathy is one of its major side effects which can lead to cessation of treatment and poor quality of life. Sensory perception and nerve conduction deficits are similarly observed in both patients and animals treated with cisplatin [2,3,4,5,6,7]. In addition, structural alterations including loss of dorsal root ganglion (DRG) neurons and atrophy of neuronal nucleus and nucleolus have also been reported [5,7,8,9,10].
Curcumin is a polyphenol found in the root of Curcuma longa and possesses antioxidant, anti-inflammatory and antineoplastic properties [11]. Its beneficial effects on various neurological diseases have also been shown [12]. However, the data regarding its effect on cisplatin neuropathy remain lacking. Mendonca and co-workers reported that curcumin could reduce the toxicity of cisplatin on neurite outgrowth [13]. In cisplatin-treated rats, curcumin had some favorable effects on biochemical and histological alterations of sciatic nerve [14]. Despite these data, stronger evidence is still needed to prove the efficacy of curcumin. In this study, we showed the novel findings that curcumin could attenuate the functional and structural abnormalities in the rats receiving cisplatin.
MATERIALS AND METHODS
Twenty-four female Wistar rats weighing 200~250 g were housed on a 12 hour light-dark cycle with access to food and water ad libitum. This experiment was approved by the institutional ethics committee, Faculty of Medicine, Chulalongkorn University and was carried out in accordance with the guidelines of the National Research Council of Thailand. All efforts were done to minimize pain and discomfort.
Drug administration
The animals were divided into 3 groups: control, cisplatin and cisplatin+curcumin (n=8 each). The cisplatin and cisplatin+curcumin groups received cisplatin (Pfizer) 2 mg/kg intraperitoneally twice a week for five consecutive weeks (20 mg/kg cumulative dose). Cisplatin was diluted in normal saline to the final concentration of 0.5 mg/ml. The dilution was done to give excess fluid to prevent nephrotoxicity. Functional and structural abnormalities of peripheral nerve were induced with this dosage regimen of cisplatin [4]. Administration of curcumin (Cayman Chemical) 200 mg/kg dissolved in 1% sodium carboxy methyl cellulose (SCMC) was given by gavage to the cisplatin+curcumin group once daily for five weeks. A group of curcumin alone was not included since the results from our pilot study (unpublished data) showed no significant effects. The cisplatin group also received the vehicle for curcumin (1% SCMC) while the control group received the vehicles for cisplatin (normal saline) and curcumin. After 5 weeks of treatments, all the animals were left untreated until sacrificed at the 8th week since this was the appropriate time-point to observe the structural abnormalities [10].
Hind-paw thermal threshold
The hot plate test was used to evaluate the thermal perception and the procedure used here was able to detect the cisplatin-induced thermal hypoalgesia in the previous study [10]. Briefly, the rats were allowed to familiarize with the test procedure and apparatus prior to the measurement. The test was done at baseline and the 3rd, 5th and 8th weeks after the start of cisplatin injection. Each rat was placed on the hot plate analgesia meter (Harvard Apparatus, UK) maintained at 55℃. When the rat licked its hind paw on either side, elapsed time was recorded as latency. The cut-off duration of 35 s was employed to avoid skin injury. If the latency was over this limit, 35 s was used for further analysis. The test was repeated at least 3 times with an interval of 15 min and mean latency was obtained for each rat.
Sciatic motor nerve conduction velocity
The motor nerve conduction velocity (MNCV) was measured at baseline and the 5th and 8th weeks. The rat was anesthetized using isoflurane and rectal temperature was maintained at 37±0.3℃ using a heating pad and digital rectal thermometer. The stimulating and recording needle electrodes were inserted at the sciatic notch and the second interosseous muscle of the hind foot, respectively. The ground electrode was placed at the laletal side of the hind foot. These electrodes were connected to the oscilloscope (Neurostar, Oxford Instrument). The sciatic nerve was stimulated with a supramaximal stimulus and compound muscle action potential (CMAP) was recorded. Latency1 (L1) was measured from the stimulation artifact to the positive peak of M wave. The average L1 was derived from at least five stimulations. Then, the stimulating electrode was moved to the side of Achilles' tendon and the procedure was repeated to determine the average latency2 (L2). The MNCV was calculated by dividing the distance between the two stimulation points by the latency difference (L1-L2).
Tissue collection
At the end of the 8th week, all rats were sacrificed by overdose anesthetics and then transcardially perfused with 200 ml of normal saline followed by 400 ml of 4% paraformaldehyde. L4 DRG and sciatic nerves were post-fixed in 3% glutaraldehyde for 6 hours and embedded in epoxy resin. These specimens were used for structural analysis.
Nerve morphometry
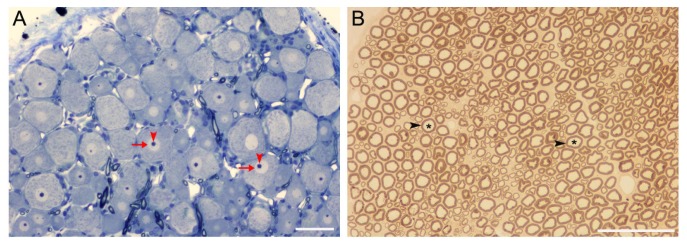
Transverse 1 µm-thick sections of the sciatic nerve were cut, mounted on slides, and stained with paraphenylenediamine. Representative image is shown in Fig. 1. The sections were examined under a light microscope and the cross-sectional areas were chosen using the three-window sampling method. Details of this sampling technique were described elsewhere [15]. Briefly, under 40× objective lens, three windows of 0.012 mm2 were randomly placed, one in the middle and the other two in the periphery of fascicle. Images of these windows were imported into the microcomputer via a digital camera. Morphometric analysis was done to obtain the number of myelinated fibers, axon diameter, myelinated fiber density, myelin thickness and g ratio using the Image-Pro Plus software. The values derived from the three windows were extrapolated to the whole nerve.
Fig. 1. Representative images of dorsal root ganglion (A) and sciatic nerve (B) used for morphometric analyses. Since the pathological changes could not be seen with eyes and images from different groups looked similar, only representative images from the control group are shown. The sections of ganglion and nerve were stained with toluidine blue and paraphenylenediamine, respectively. In A, arrows and arrowheads indicate nuclei and nucleoli of neurons, respectively. In B, asterisks and arrowheads indicate axons and myelin sheath, respectively. Scale bars represent 50 µm.
DRG morphometry
The DRG were serially cut into 2 µm-thick sections and stained with toluidine blue. Representative image is shown in Fig. 1. The estimation for total number of neurons in each ganglion was done using the physical dissector method modified from those reported by Tredici et al. [9] and Schenker et al. [16]. Details of the procedures were described elsewhere [17]. In brief, every 20th section was selected and the number of neurons with prominent nucleus and nucleolus was counted. Then, this number was extrapolated to the total number for the whole DRG. Moreover, at least 300 neurons in each DRG were randomly analyzed for areas of the nucleus and nucleolus using the Image-Pro Plus software.
Statistical analysis
One-way ANOVA was used to compare mean body weight, thermal latencies, MNCV and morphometric parameters between the experimental groups at each time point. This test was done using SPSS for Windows version 13. Statistically significant differences were considered when p<0.05.
RESULTS
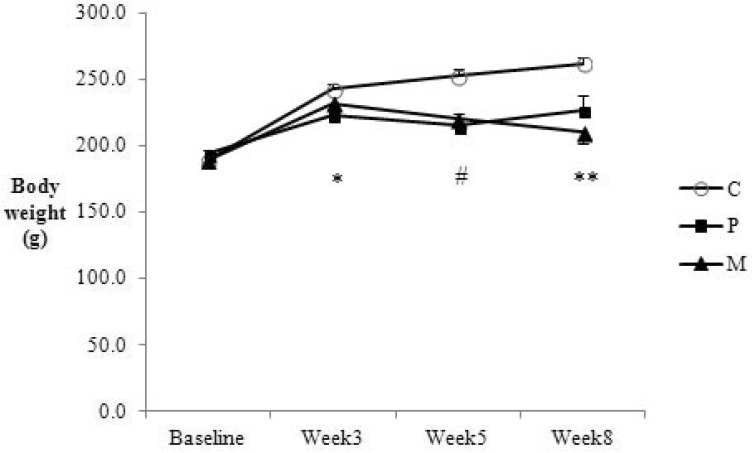
Control rats showed continuous weight gain over the study period (Fig. 2). In contrast, body weight of the cisplatin and cisplatin+curcumin groups started to decrease significantly compared with that of the control group in the 3rd week and remained lower until the end of study. There was no significant difference between the cisplatin and cisplatin+curcumin groups at any time point.
Fig. 2. Changes in the average body weight of the control (C), cisplatin (P) and cisplatin+curcumin (M) groups. The graph shows means and SEM. *p<0.05 C vs. P, #p<0.01 C vs. P & M, **p<0.05 C vs. P & p<0.01 C vs. M.
Hind-paw thermal threshold
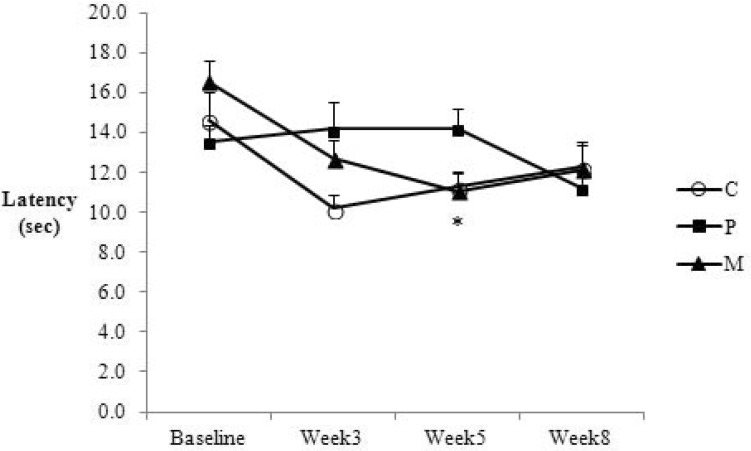
Since heat latencies were highly variable, comparisons were made only between groups at each time point. The values were significantly higher in the cisplatin compared with the control and cisplatin+curcumin groups in the 5th week, indicating thermal hypoalgesia (Fig. 3, p<0.05). At other time points, the latencies were not significantly different between groups.
Fig. 3. Changes in the heat latency of the control (C), cisplatin (P) and cisplatin+curcumin (M) groups. The graph shows means and SEM. *p<0.05 P vs. C & M.
Sciatic motor nerve conduction velocity
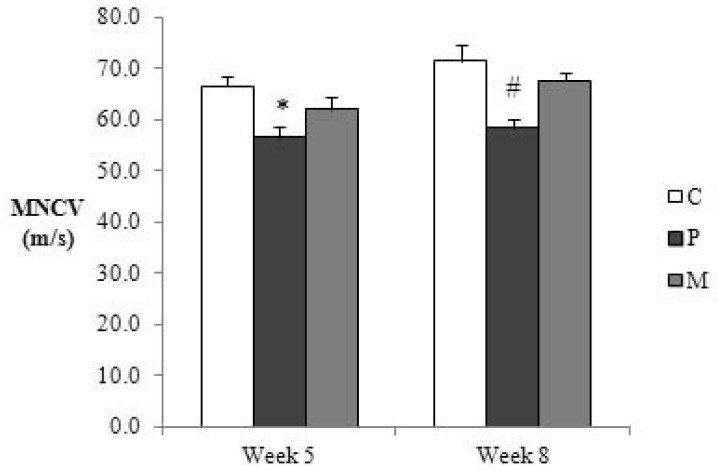
The MNCV of the cisplatin-treated rats was significantly lower than that of the controls in the 5th week (14.8% reduction, p<0.05) (Fig. 4). The velocity of the cisplatin+curcumin group was between those of the two groups with no significant differences. In the 8th week, the MNCV of the cisplatin group remained significantly lower than that of the control group (18.3% reduction, p<0.05) (Fig. 4). Moreover, the value of the cisplatin+curcumin group was comparable to that of the control group with significant difference from that of the cisplatin group (p<0.05).
Fig. 4. Changes in the sciatic motor nerve conduction velocity (MNCV) of the control (C), cisplatin (P) and cisplatin+curcumin (M) groups. The graph shows means and SEM. *p<0.05 P vs. C, #p<0.05 P vs. C & M.
Nerve morphometry
No significant change was observed in any morphometric parameter of sciatic nerve (Table 1). However, it is worth mentioning that myelin thickness was slightly decreased in the cisplatin compared with the control groups. In the cisplatin+curcumin group, the thickness was similar to that of the controls.
Table 1. Morphometric data of sciatic nerve.
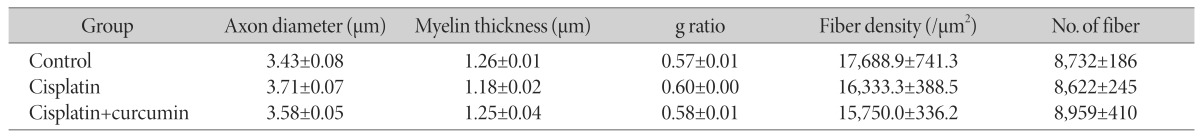
Data are means±SEM.
DRG morphometry
The cisplatin group had significantly decreased nuclear and nucleolar areas compared with the control group, p<0.05 and p<0.01, respectively (Table 2). The nuclear area of the cisplatin+curcumin group was in between those of the two groups. In contrast, the cisplatin+curcumin group had the significantly higher nucleolar area than the cisplatin group (p<0.01) and the value was similar to that of the controls. Regarding the number of L4 DRG neurons, the significant loss of neurons was observed in the cisplatin group (29.1% reduction, p<0.05) (Table 2). The number in the cisplatin+curcumin group was higher than that of the cisplatin group but remained lower than that of the control group.
Table 2. Morphometric data of L4 DRG.
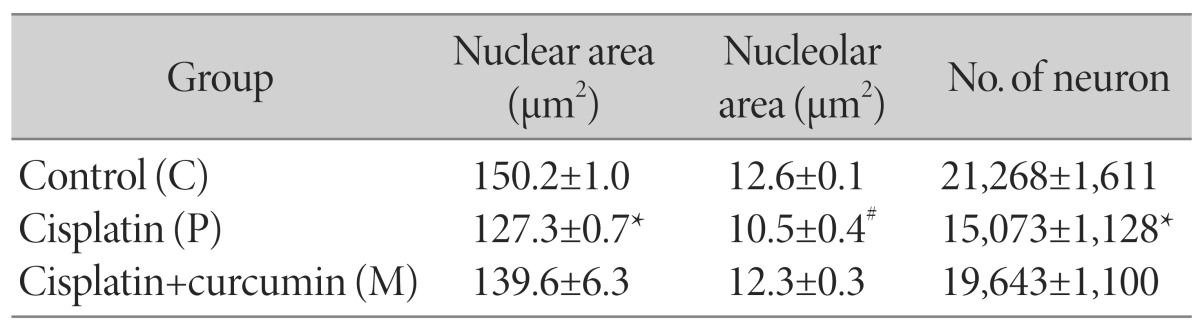
Data are means±SEM, *p<0.05 vs. C, #p<0.01 vs. C&M.
DISCUSSION
Administration of cisplatin induced significant weight loss consistent with other studies using similar dosage regimens [4,7,10,14,17]. Curcumin had no significant effect on this general toxicity as shown by this and the previous studies [14]. Transient thermal hypoalgesia in the 5th week was also observed. It appeared that there was a trend toward reduced latency in both control & curcumin groups during the study period. This is unlikely hyperalgesia as it was also observed in the control group. The reason remains unknown, but it might be an adaptation due to learning. The similar finding was also observed in our previous work [10]. Besides the learning behavior, high variations in the data might also be partly responsible for these temporal changes. Due to these issues, comparisons must be made among groups within each time point, not between the different time points. Therefore, the longer latency of the cisplatin group compared with those of other groups in the 5th week likely suggests the heat hypoalgesia. Thermal hypoalgesia after cisplatin treatment was previously reported [4,7,10,17]. Curcumin could prevent this sensory deficit in the 5th week. In contrast, the study by Al Moundhri and colleagues could not demonstrate its efficacy, likely due to the highly variable data [14]. Mechanical hyperalgesia could also develop after cisplatin treatment [4]. However, in our previous report using the same dose regimen of cisplatin, the mechanical hyperalgesia was not found [10]. Therefore, the mechanical perception test was not included in this study.
The sciatic MNCV was significantly lower in the cisplatin group in the 5th and 8th weeks relative to the controls. It appears that the MNCV deficit is dose-dependent since high doses of cisplatin caused the slow MNCV [10,17,18] but low doses did not [6,19]. Curcumin was able to improve this abnormality in the 5th week and prevented it in the 8th week. Regarding the above data, our findings indicate the favorable effects of curcumin on functional deficits caused by cisplatin.
Morphometric analysis of DRG neurons from the cisplatin-treated rats in the 8th week demonstrated significant atrophy of nucleus and nucleolus. The similar findings were also reported by the previous studies [5,7,8,9,10,17]. Furthermore, we found the significant reduction in the number of DRG neurons in the cisplatin compared with the control groups. This was consistent with a trend toward reduced number of neurons after cisplatin treatment in other studies [9,10,17]. The nucleolar atrophy was completely prevented by curcumin, whereas the nuclear atrophy was partially blocked. Moreover, curcumin could partially attenuate the loss of neurons. Mendonca et al., 2013, however, showed that curcumin did not affect the viability of PC12 cells reduced by cisplatin [13]. More data are needed to verify if curcumin could attenuate neuronal loss after cisplatin treatment.
Morphometric study of the sciatic nerve revealed no statistically significant changes among the three groups. Nevertheless, it is worth noting that there was a trend toward thinner myelin sheath in the cisplatin relative to the other groups. Reduction in the myelin thickness after cisplatin treatment was reported in the previous studies [10,17]. This abnormality might be due to apoptosis of Schwann cells induced by cisplatin [20]. Possible demyelination seen in this study could explain the slow MNCV in the cisplatin group and improvement in the velocity with increased myelin thickness observed in the curcumin group. These data further support the findings in Al Moundhri et al., 2013 that curcumin improved the cisplatin-induced myelin loss by qualitative examination of nerve sections [14].
Although the mechanisms underlying cisplatin neuropathy are not fully understood, current evidence suggests oxidative stress as a major culprit. Increased generation of reactive oxygen species (ROS) was observed in neurons exposed to cisplatin [21,22]. Furthermore, cisplatin also induces mitochondrial impairments in DRG neurons [23]. Elevated ROS levels and mitochondrial dysfunction together cause oxidative stress. Since cisplatin preferentially accumulates in the DRG [24] and can induce neuronal apoptosis [25,26], morphological changes and loss of DRG neurons including sensory impairment ensue. Besides neurons, cisplatin toxicity on Schwann cells likely resulted in reduced myelin thickness and MNCV. Therefore, it is conceivable to assume that the favorable effects of curcumin on these abnormalities in cisplatin neuropathy are likely via reduction in oxidative stress. Nevertheless, direct evaluation of oxidative stress in the nervous tissues should be done to prove this hypothesis.
In fact, several anti-oxidants have been tested and demonstrated promising results in experimental cisplatin neuropathy [27,28]. However, clinical trials so far yielded inconclusive results [29]. Newer and more potent anti-oxidants are, thus, needed to improve the efficacy. Curcumin possesses anti-oxidant property and is effective against several neurological diseases in which oxidative stress is known to play a role, for example, Alzheimer's disease [30], Parkinson's disease [31], stroke [32], brain injury [33] and diabetic neuropathy [34]. Regarding cisplatin neuropathy, curcumin starts to show favorable effects as shown in this and the previous studies [14]. However, there is a concern that concomitant administration of curcumin might reduce anticancer activity of cisplatin. Recent reports have shown that curcumin did not decrease but, instead, enhanced the antitumor activity of cisplatin, at least in hepatic cancer cells [13,35]. Another advantage of curcumin is that it is proved non-toxic and tolerable even at high doses in humans [36]. The only major problem is its poor absorption and thus bioavailability, but, many approaches are being developed to solve this [37]. Taken together, curcumin is very promising as the safe and effective therapeutic agent against cisplatin neuropathy.
In conclusion, this study showed that curcumin could ameliorate the functional and structural abnormalities observed in experimental cisplatin neuropathy. Future studies are needed to elucidate the mechanisms underlying the neuroprotective effects of curcumin.
ACKNOWLEDGEMENT
This work was supported by Rachadaphiseksomphot Fund 2010 and 2014 from the Faculty of Medicine, Chulalongkorn University. The authors would like to thank Dr. Tulaporn Wongtawatchai and Ms. Sompit Piroh for their assistance during the animal experiment and collection of samples.
References
- 1.Boulikas T, Vougiouka M. Recent clinical trials using cisplatin, carboplatin and their combination chemotherapy drugs (review) Oncol Rep. 2004;11:559–595. [PubMed] [Google Scholar]
- 2.Krarup-Hansen A, Fugleholm K, Helweg-Larsen S, Hauge EN, Schmalbruch H, Trojaborg W, Krarup C. Examination of distal involvement in cisplatin-induced neuropathy in man. An electrophysiological and histological study with particular reference to touch receptor function. Brain. 1993;116:1017–1041. doi: 10.1093/brain/116.5.1017. [DOI] [PubMed] [Google Scholar]
- 3.Krarup-Hansen A, Helweg-Larsen S, Schmalbruch H, Rørth M, Krarup C. Neuronal involvement in cisplatin neuropathy: prospective clinical and neurophysiological studies. Brain. 2007;130:1076–1088. doi: 10.1093/brain/awl356. [DOI] [PubMed] [Google Scholar]
- 4.Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. An animal model of nociceptive peripheral neuropathy following repeated cisplatin injections. Exp Neurol. 2003;182:12–20. doi: 10.1016/s0014-4886(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 5.Barajon I, Bersani M, Quartu M, Del Fiacco M, Cavaletti G, Holst JJ, Tredici G. Neuropeptides and morphological changes in cisplatin-induced dorsal root ganglion neuronopathy. Exp Neurol. 1996;138:93–104. doi: 10.1006/exnr.1996.0050. [DOI] [PubMed] [Google Scholar]
- 6.Bárdos G, Móricz K, Jaszlits L, Rabloczky G, Tory K, Rácz I, Bernáth S, Sümegi B, Farkas B, Literáti-Nagy B, Literáti-Nagy P. BGP-15, a hydroximic acid derivative, protects against cisplatin- or taxol-induced peripheral neuropathy in rats. Toxicol Appl Pharmacol. 2003;190:9–16. doi: 10.1016/s0041-008x(03)00155-8. [DOI] [PubMed] [Google Scholar]
- 7.Oztürk G, Erdoğan E, Anlar O, Kösem M, Taşpinar M. Effect of leukemia inhibitory factor in experimental cisplatin neuropathy in mice. Cytokine. 2005;29:31–41. doi: 10.1016/j.cyto.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Cavaletti G, Tredici G, Marmiroli P, Petruccioli MG, Barajon I, Fabbrica D. Morphometric study of the sensory neuron and peripheral nerve changes induced by chronic cisplatin (DDP) administration in rats. Acta Neuropathol. 1992;84:364–371. doi: 10.1007/BF00227662. [DOI] [PubMed] [Google Scholar]
- 9.Tredici G, Braga M, Nicolini G, Miloso M, Marmiroli P, Schenone A, Nobbio L, Frattola L, Cavaletti G. Effect of recombinant human nerve growth factor on cisplatin neurotoxicity in rats. Exp Neurol. 1999;159:551–558. doi: 10.1006/exnr.1999.7174. [DOI] [PubMed] [Google Scholar]
- 10.Wongtawatchai T, Agthong S, Kaewsema A, Chentanez V. Altered phosphorylation of mitogen-activated protein kinases in dorsal root ganglia and sciatic nerve of rats with cisplatin-induced neuropathy. Asian Biomed (Res Rev News) 2012;6:397–411. [Google Scholar]
- 11.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Adv Exp Med Biol. 2007;595:197–212. doi: 10.1007/978-0-387-46401-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendonça LM, da Silva Machado C, Teixeira CC, de Freitas LA, Bianchi Mde L, Antunes LM. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology. 2013;34:205–211. doi: 10.1016/j.neuro.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Al Moundhri MS, Al-Salam S, Al Mahrouqee A, Beegam S, Ali BH. The effect of curcumin on oxaliplatin and cisplatin neurotoxicity in rats: some behavioral, biochemical, and histopathological studies. J Med Toxicol. 2013;9:25–33. doi: 10.1007/s13181-012-0239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chentanez V, Cha-oumphol P, Kaewsema A, Agthong S, Huanmanop T. Accuracy of the three-window sampling method in morphometric analysis of human sural nerve. J Neurosci Methods. 2006;157:154–157. doi: 10.1016/j.jneumeth.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Schenker M, Kraftsik R, Glauser L, Kuntzer T, Bogousslavsky J, Barakat-Walter I. Thyroid hormone reduces the loss of axotomized sensory neurons in dorsal root ganglia after sciatic nerve transection in adult rat. Exp Neurol. 2003;184:225–236. doi: 10.1016/s0014-4886(03)00255-3. [DOI] [PubMed] [Google Scholar]
- 17.Wongtawatchai T, Agthong S, Kaewsema A, Chentanez V. Sex-related differences in cisplatin-induced neuropathy in rats. J Med Assoc Thai. 2009;92:1485–1491. [PubMed] [Google Scholar]
- 18.Verdú E, Vilches JJ, Rodríguez FJ, Ceballos D, Valero A, Navarro X. Physiological and immunohistochemical characterization of cisplatin-induced neuropathy in mice. Muscle Nerve. 1999;22:329–340. doi: 10.1002/(sici)1097-4598(199903)22:3<329::aid-mus5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Müller LJ, Gerritsen van der Hoop R, Moorer-van Delft CM, Gispen WH, Roubos EW. Morphological and electrophysiological study of the effects of cisplatin and ORG.2766 on rat spinal ganglion neurons. Cancer Res. 1990;50:2437–2442. [PubMed] [Google Scholar]
- 20.Jirsova K, Mandys V, Gispen WH, Bär PR. Cisplatin-induced apoptosis in cultures of human Schwann cells. Neurosci Lett. 2006;392:22–26. doi: 10.1016/j.neulet.2005.08.068. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, Guo C, Vasko MR, Kelley MR. Implications of apurinic/apyrimidinic endonuclease in reactive oxygen signaling response after cisplatin treatment of dorsal root ganglion neurons. Cancer Res. 2008;68:6425–6434. doi: 10.1158/0008-5472.CAN-08-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291:1–9. doi: 10.1016/j.tox.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Melli G, Taiana M, Camozzi F, Triolo D, Podini P, Quattrini A, Taroni F, Lauria G. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp Neurol. 2008;214:276–284. doi: 10.1016/j.expneurol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 24.McDonald ES, Randon KR, Knight A, Windebank AJ. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: a potential mechanism for neurotoxicity. Neurobiol Dis. 2005;18:305–313. doi: 10.1016/j.nbd.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Gill JS, Windebank AJ. Cisplatin-induced apoptosis in rat dorsal root ganglion neurons is associated with attempted entry into the cell cycle. J Clin Invest. 1998;101:2842–2850. doi: 10.1172/JCI1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer SJ, McDonald ES, Gross L, Windebank AJ. Alterations in cell cycle regulation underlie cisplatin induced apoptosis of dorsal root ganglion neurons in vivo. Neurobiol Dis. 2001;8:1027–1035. doi: 10.1006/nbdi.2001.0426. [DOI] [PubMed] [Google Scholar]
- 27.Tredici G, Cavaletti G, Petruccioli MG, Fabbrica D, Tedeschi M, Venturino P. Low-dose glutathione administration in the prevention of cisplatin-induced peripheral neuropathy in rats. Neurotoxicology. 1994;15:701–704. [PubMed] [Google Scholar]
- 28.Leonetti C, Biroccio A, Gabellini C, Scarsella M, Maresca V, Flori E, Bove L, Pace A, Stoppacciaro A, Zupi G, Cognetti F, Picardo M. Alpha-tocopherol protects against cisplatin-induced toxicity without interfering with antitumor efficacy. Int J Cancer. 2003;104:243–250. doi: 10.1002/ijc.10933. [DOI] [PubMed] [Google Scholar]
- 29.Albers JW, Chaudhry V, Cavaletti G, Donehower RC. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst Rev. 2011;(2):CD005228. doi: 10.1002/14651858.CD005228.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi T, Yamashita H, Nakamura T, Nagano Y, Nakamura S. Tyrosine 125 of alpha-synuclein plays a critical role for dimerization following nitrative stress. Brain Res. 2002;938:73–80. doi: 10.1016/s0006-8993(02)02498-8. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q, Sun AY, Simonyi A, Jensen MD, Shelat PB, Rottinghaus GE, MacDonald RS, Miller DK, Lubahn DE, Weisman GA, Sun GY. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res. 2005;82:138–148. doi: 10.1002/jnr.20610. [DOI] [PubMed] [Google Scholar]
- 33.Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Murugan P, Pari L. Antioxidant effect of tetrahydrocurcumin in streptozotocin-nicotinamide induced diabetic rats. Life Sci. 2006;79:1720–1728. doi: 10.1016/j.lfs.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Notarbartolo M, Poma P, Perri D, Dusonchet L, Cervello M, D'Alessandro N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005;224:53–65. doi: 10.1016/j.canlet.2004.10.051. [DOI] [PubMed] [Google Scholar]
- 36.Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr. 2010;103:1545–1557. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 37.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]