Abstract
Intracerebral hemorrhage (ICH) is one of the devastating types of stroke. Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) have potential benefits in recovery from brain damage following ICH. This study aimed to identify the beneficial effects of hUCB-MSCs and investigate whether they have anti-inflammatory effects on the ICH brain via neurotrophic factors or cytokines. hUCB-MSCs were transplanted into a collagenase-induced ICH rat model. At 2, 9, 16, and 30 days after ICH, rotarod and limb placement tests were performed to measure behavioral outcomes. ICH rats were sacrificed to evaluate the volume of lesion using H&E staining. Immunostaining was performed to investigate neurogenesis, angiogenesis, and anti-apoptosis at 4 weeks after transplantation. Inflammatory factors (TNF-α, COX-2, microglia, and neutrophils) were analyzed by immunofluorescence staining, RT-PCR, and Western blot at 3 days after transplantation. hUCB-MSCs were associated with neurological benefits and reduction in lesion volume. The hUCB-MSCs-treated group tended to reveal high levels of neurogenesis, angiogenesis, and anti-apoptosis (significant for angiogenesis). The expression levels of inflammatory factors tended to be reduced in the hUCB-MSCs-treated group compared with the controls. Our study suggests that hUCB-MSCs may improve neurological outcomes and modulate inflammation-associated immune cells and cytokines in ICH-induced inflammatory responses.
Keywords: intracerebral hemorrhage, hUCB-MSCs, neurogenesis, angiogenesis, apoptosis, inflammatory factor
INTRODUCTION
Intracerebral hemorrhage (ICH) is one of the devastating types of stroke carrying a high risk of mortality and a morbidity rate of about 40% at 1 month post-stroke [1]. Management of ICH may include medical and surgical treatments to stop the bleeding, remove the clot, and reduce the pressure on the brain [2]. Despite the standard therapy and ongoing clinical trials to improve outcome in ICH, the prognosis is poor and the patients who survive have neurological deterioration [3].
Recent studies have investigated the therapeutic effect of stem cells [4]. Of the types of stem cells that may be useful for treatment of ICH, human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) have been regarded as an ideal source. Besides self-renewal and multilineage differentiation properties, MSCs isolated from umbilical cord blood are associated with few ethical concerns and low immune rejection response and tumor formation [5].
It was reported that transplantation of MSCs could improve recovery from brain damage following ICH. In an ICH rat model, MSCs promoted neurogenesis, functional recovery, and reduced hemorrhage volume and apoptosis [6]. In addition, MSCs could promote angiogenesis and inhibit inflammation [7]. Recent studies revealed that inflammation has an important role in brain recovery after ICH [8]. Furthermore, MSCs may modulate inflammation-associated immune cells and cytokines in the cerebral inflammatory response [9]. However, the underlying mechanisms of the interaction between hUCB-MSCs and inflammation remain largely unknown.
In previous studies, we observed that hUCB-MSCs have a therapeutic effect in rat ischemia models induced by middle cerebral artery occlusion (MCAO). In this study, we examined whether transplantation of hUCB-MSCs in an ICH rat model has beneficial effects on neuropathological and behavioral deficits. We then investigated whether hUCB-MSCs have anti-inflammatory properties by modulating neurotrophic factors or cytokines in the ICH brain.
MATERIALS AND METHODS
Preparation of hUCB-MSCs
hUCB-MSCs were provided by MEDIPOST Co., Ltd. (Seoul, Korea). This study was approved by the Institutional Review Boards of the Seoul National University Hospital and MEDIPOST Co., Ltd. Umbilical cord blood was collected from the umbilical veins after neonatal delivery with informed consent of the pregnant mother. MSCs were isolated from mononuclear cells (MNCs) using a Ficoll-Hypaque solution (Sigma). Following transfer to α-minimum essential medium (α-MEM; Gibco) supplemented with fetal bovine serum (FBS; Gibco), MSCs were seeded in culture flasks at 5×105 cells/cm2. Cells maintained in humidified 5% CO2 at 37℃ formed colonies of spindle-shaped cells. At 50% confluence, cells were harvested after treatment with 0.25% trypsin-EDTA (Gibco) and were reseeded for expansion.
Animal Procedures
Adult male Sprague-Dawley rats (250~300 g; Orient, Korea) were housed in an animal care facility under a 12 hours light/dark cycle. Animal care and surgical procedures were performed in accordance with guidelines approved by the Experimental Animals Committee of Seoul National University Hospital for the ethical use of animals. All efforts were made to minimize the suffering and number of animals used in experiments.
Collagenase-Induced ICH model
Rats were anesthetized using an intramuscular (i.m.) injection of 1% zoletil (20 mg/kg) and xylazine hydrochloride (5 mg/kg). In brief, 1 µl (0.2 µl/min) saline containing 0.2 units of bacterial collagenase (type VII; Sigma) was injected stereotaxically into the striatum at coordinates of 3.0 mm lateral to bregma and 5.0 mm ventral to the cortical surface. After injection, the Hamilton syringe was left in place for 5 minutes. The needle was slowly removed after an additional 10 minutes to prevent backflow. The hole was sealed with bone wax and the wound was sutured.
Transplantation of hUCB-MSCs
Two days after injection of collagenase, we performed behavioral tests and divided the rats into 2 groups. Rats in the control group were injected with 5 µl of 10 X PBS. For transplantation in the experimental group, 5×105 MSCs in 5 µl of PBS were stereotactically injected into the left lateral ventricle of anesthetized animals at 3.0 mm lateral to bregma and 5.0 mm ventral to the cortical surface. Animals were not given any prophylactic immunosuppressant.
Behavioral Test
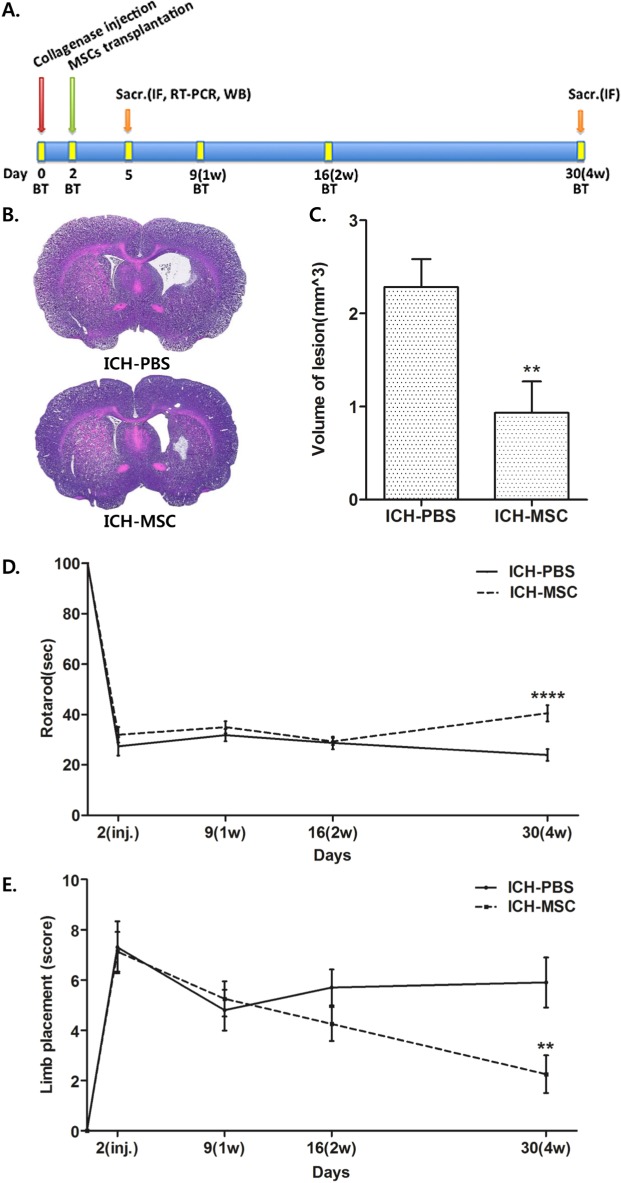
Behavioral tests were performed and evaluated by the rotarod and limb placement tests 1 day before ICH and at 2, 9, 16, 30 days after ICH (Fig. 1A).
Fig. 1. Transplantation of hUCB-MSCs reduces lesion volume and improves the behavioral recovery of ICH rats. (A) Schematic timeline of the experimental procedures. MSCs indicates mesenchymal stem cells; Sacr, sacrifice; IF, immunofluorescence staining; WB, western blot; and BT, behavior test. (B) H&E staining of coronal sections for characterization of unilateral brain injury at 30 days after ICH. (C) The volume of lesion in the hUCB-MSCs-treated rats (n=8) was significantly smaller than in the controls (n=10) at 4 weeks after transplantation. **p<0.01. The lesion volume was determined by summation of the lesion area and analyzed by the Mann-Whitney U test. Data are shown as mean and SEM. (D) Results of the rotarod test. The hUCB-MSCs-treated rats (n=8) showed significantly better performance than the controls (n=10) at 4 weeks after transplantation. ****p<0.0001, repeated-measured analysis of variance (ANOVA). (E) Results of the limb placement test. The hUCB-MSCs-treated group (n=8) showed better performance than the controls (n=10) at 4 weeks after transplantation. **p<0.01, repeated-measured ANOVA. All data are shown as mean and SEM.
In the rotarod test, rats were trained at a constant speed of 16 rpm (600 seconds) for 3 days before ICH. The rats were placed on an accelerating rod (speed from 3.5 to 35 rpm) and the duration (s) the rats remained on the rotarod was measured three times, 1 day before surgery. Rotarod test data were presented as percentiles of the maximal duration compared with the baseline control (before ICH).
The limb placement test had three tasks that assessed motor integration of the forelimbs and hindlimbs by checking responses to tactile and proprioceptive stimulations. 'Visual forward' was the observation of forelimb flexion when the tail was held up. The stretch of the forelimbs towards the table was evaluated as normal stretch (0 point) and abnormal flexion (1 point). 'Visual lateral' was the observation of the forelimb stretch by stimulating the rat's whiskers while the examiner held the rat's trunk. The evaluations were: normal lifting, 0 point; abnormal lifting, 1, 2, or 3 points according to the number of normal stretches. 'Proprioception' was the observation of stepping up of the forelimbs and hindlimbs on the table after a pull-down of the forelimb and hindlimbs below the level of the table (normal lifting, 0 point; abnormal lifting, 1, 2, or 3 points according to the number of normal stretches). The authors defined the severity of injury by scoring on a scale of 0 to 10 (normal score 0, maximal deficit score 10).
Histological Examination
When behavioral tests were completed at 30 days after ICH, the animals were anesthetized and perfused intracardially with saline followed by ice-cold 4% paraformaldehyde in PBS (pH 7.4) for 10 min. Brains were removed, post-fixed for one day at 4℃, and then cryoprotected overnight in the same fixative supplemented with 25% sucrose. The tissues were embedded in OCT compound (Sakura Finetek, Inc., Tokyo, Japan) and frozen at -70℃ with dry ice. Coronal sections (14 µm thick; 28 µm apart) were cut with a cryostat (Leica Microsystems, Nussloch, Germany). Sections were stained with Hematoxylin and Eosin (H&E) and the volume of lesion (e.g., cavity, cellular debris) was calculated using ImageJ 1.38× (National Institutes of Health) as:
Volume of lesion (mm3)=area of lesion (mm2)×thickness of slice (mm/slice)×sampling interval (slice)
Immunofluorescence Staining
The sections were permeabilized with 0.1% saponin for 30 min and blocked with 5% normal goat serum (NGS) for 30 min. The sections were also permeabilized for 30 min with 0.1% Triton X. Thy were then incubated overnight at 4℃ with primary antibody followed by incubation in fluorescently labeled secondary antibody and mounted with Vectashield medium containing DAPI (Vector Laboratories, Burlingame, CA). The primary antibodies used in the experiments were: anti-Human Nuclei (1:100; Chemicon), anti-laminin (1:200; Sigma, Inc.), anti-neuron specific beta III tubulin (Tuj1) (1:100; abcam), anti-COX2 (1:100; abcam), anti-TNF alpha (1:100; abcam), anti-CD11b (1:100; BD Pharmingen), and anti-myeloperoxidase (1:100; abcam). All secondary antibodies, Alexa 488, Alexa 594 goat anti-mouse IgG, and goat anti-rabbit IgG were purchased from Molecular Probes (Invitrogen, Co.). The In Situ Cell Death Detection Kit, TMR red (Roche), was used for indirect the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeled (TUNEL) staining.
Fluorescently immunolabeled sections were imaged by confocal laser scanning microscope (Leica TCS SP8; Leica Microsystems, Mannheim, Germany). Tile scanning was performed (10×, 20× magnification) following which the images ware merged to obtain the whole brain image. To evaluate the labeling, confocal scanning was performed at 40× magnification. Staining density was analyzed using Image-Pro Plus 4.5 (Media Cybernetics, Silver Spring, MD, USA).
RT-PCR
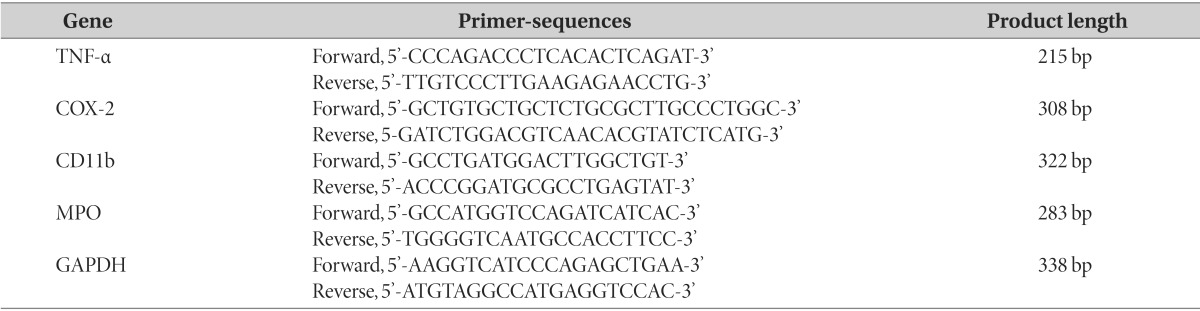
RNA was isolated at day 5 after ICH using the RNeasy Lipid Tissue Mini Kit (QIAGEN, Hilden, Germany). First-strand cDNA synthesis was carried out by random priming of the total RNA using a random primer mixture (Invitrogen) and reverse transcriptase with superscript 3 (Invitrogen). The sequences of the primers and the cycles of PCR are given in Table 1. The PCR products were resolved in 1% agarose gels. The band density from gel images was determined by Gel Doc XR System (Bio-Rad, Milan, Italy). Rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control.
Table 1. Primers used for RT-PCR.
Western Blot Analysis
Animals were sacrificed to extract the brain at 5 days after ICH and whole brains were divided into ipsilateral (infarcted) and contralateral hemispheres, which were frozen in liquid nitrogen. Brain tissues from each group were homogenized in Neuronal Protein Extraction Reagent (Pierce), which included 1× protease and phosphatase inhibitor (Pierce). Protein concentrations were determined using BCA Protein Assay Kit (Pierce). The samples were subjected to 10% SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride (PVDF) membranes (Amersham) that were blocked in 5% non-fat dried milk (NFDM) and incubated with anti-COX2 (1:1000; abcam), anti-TNF alpha (1:1000; abcam), anti-CD11b (1:500; BD Pharmingen), and anti-myeloperoxidase (1: 1000; abcam) and β-actin. Membranes were incubated for 1 hour with a secondary antibody and the intensity of all protein bands was quantified by densitometry using the Bio-image Processing System (GenDix). Densitometric values were normalized to those of beta-actin, used as an internal control.
Statistical Analysis
Data are presented as mean±SEM. The significance of differences between test conditions was assessed using the Mann-Whitney U test. Comparisons of continuous variables between the two groups were performed by a two-way ANOVA. p<0.05 was considered statistically significant. The GraphPad Prism 5 software (GraphPad Prism, Sandiego, California, USA) was used for the statistical tests.
RESULTS
Volume of lesions in hUCB-MSCs transplanted Rats
Hemorrhagic lesions mainly appeared in the striatum as observed by H&E staining at 4 weeks after transplantation. Ventricles were expanded in the ipsilateral hemisphere more than in the contralateral (Fig. 1B). The volume of lesion in the hUCB-MSCs-treated group was significantly smaller than the controls (Fig. 1C, **p<0.01). These results indicate that hUCB-MSCs reduce the volume of lesion after ICH.
Neurological function in rats with transplanted hUCB-MSCs
To evaluate behavioral outcome, the rotarod and limb placement tests were performed (Fig. 1A). The hUCB-MSCs-treated group could remain on the rotarod for a significantly longer duration than the controls at 4 weeks after transplantation (Fig. 1D, ****p<0.001). Additionally, at 4 weeks after transplantation, the limb test score revealed a significant neurological benefit in the hUCB-MSCs-treated group compared with the controls (Fig. 1E, **p<0.01). These results indicate that hUCB-MSCs promote functional recovery after ICH.
Neurogenesis, angiogenesis, and apoptosis after transplantation of hUCB-MSCs
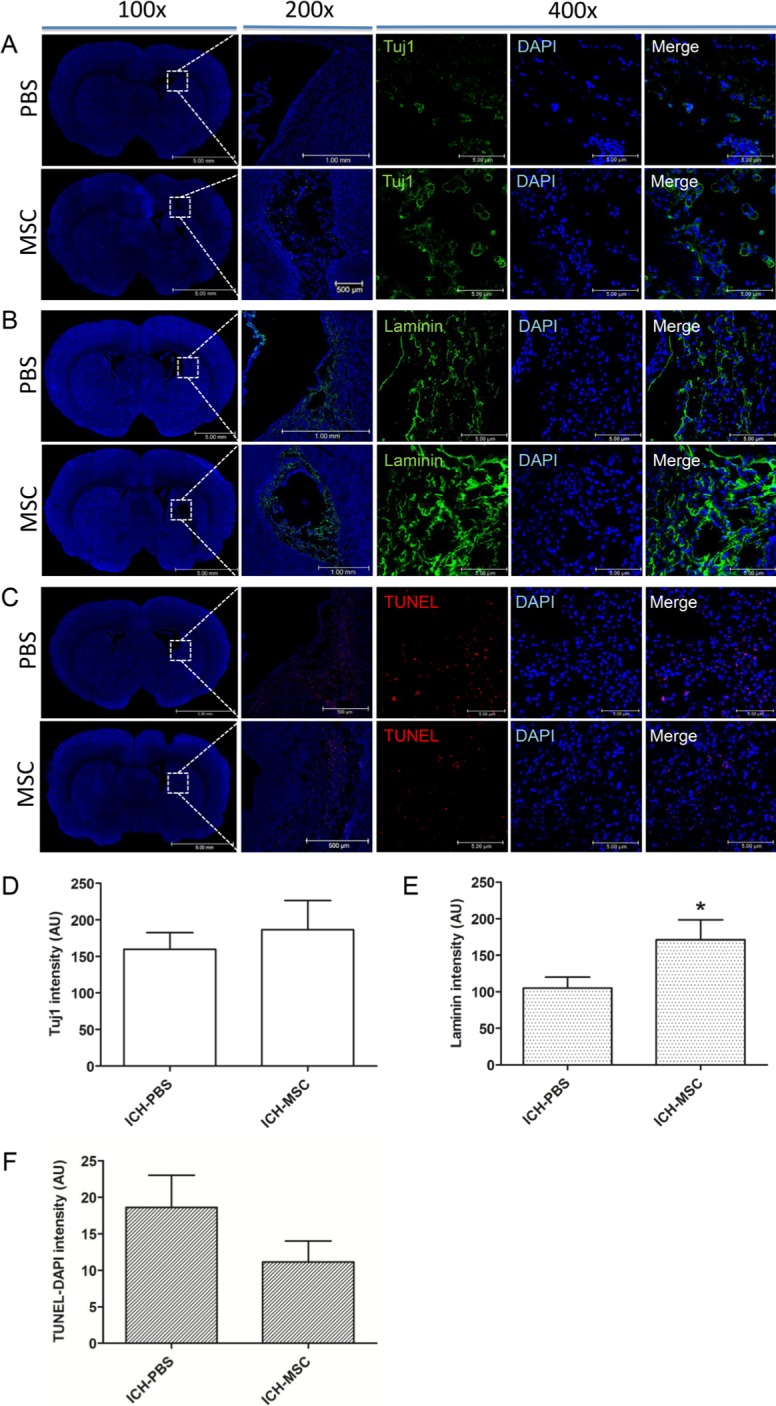
Previously, we observed neurogenesis, angiogenesis, and anti-apoptosis effects of hUCB-MSCs in rat ischemia models. To determine if transplantation of hUCB-MSCs might have a similar impact on in the post-hemorrhagic brain, we performed immunofluorescence staining for specific markers: Tuj1 for neurons and Laminin for blood vessels, and TUNEL staining for apoptotic cells in the rats at 4 weeks after transplantation. We found Tuj1-positive cells (green) in the subventricular zone (SVZ) or in the hemorrhage area (Fig. 2A). Statistical analysis indicated an increase, although not significant, in the number of Tuj1-positive cells in the hUCB-MSCs-treated group than in the controls (Fig. 2D). Laminin-positive cells (green) were observed in the hemorrhage site and were present in large numbers in the peri-infarct regions (Fig. 2B). The number of laminin-positive cells was significantly higher in the hUCB-MSCs-treated group than in the controls (Fig. 2E, *p<0.05). These results indicate that hUCB-MSCs promote angiogenesis after ICH. TUNEL-positive cells (red) were found within the hemorrhage lesion as well as in the surrounding periphery in the hUCB-MSCs-treated rats and the controls (Fig. 2C). Although the number of TUNEL-positive cells was lower in the hUCB-MSCs-treated group than in the controls, the difference was not significant (Fig. 2F).
Fig. 2. Representative results showing neurogenesis, angiogenesis, and apoptosis following the transplantation of hUCB-MSCs into ICH rats. The rats at 4 weeks after transplantation were analyzed for the presence of Tuj1, Laminin, and TUNEL using immunofluorescence staining. (A) Tuj1-positive cells (green) were detected in the SVZ. (B) Laminin-positive cells (green) were present in large numbers in the peri-infarct regions. (C) TUNEL-positive cells (red) were detected in peri-infarct regions. (D) Tuj1-positive cells increased in the hUCB-MSCs-treated group (n=8) compared with the controls (n=10) (not significant). (E) Laminin-positive cells increased significantly the hUCB-MSCs-treated rats (n=8) compared with the controls (n=10). (F) TUNEL-positive cells decreased in the hUCB-MSCs-treated group (n=8) compared with the controls (n=10) (not significant). Nuclei were counterstained with DAPI (blue). Values are shown as mean and SEM. *p<0.05 compared with the controls.
Anti-inflammatory effects of transplanted hUCB-MSCs
To confirm the existence of transplanted hUCB-MSCs in the rat brains, we performed immunofluorescence staining with anti-human nuclei antibody (HuNu). HuNu-positive hUCB-MSCs (red) survived and majority of the cells were distributed close to the hemorrhagic boundary zone at 3 days after transplantation (Fig. 3A). However, HuNu-positive cells were not detected in both groups at 4 weeks after transplantation (data not shown).
Fig. 3. Typical presence of hUCB-MSCs and expression of inflammatory factors following the transplantation of hUCB-MSCs into ICH rats. (A) Three days after the transplantation, hUCB-MSCs were identified by staining with human nuclei antibody (HuNu, red). Confocal micrograph of a brain section show the HuNu-positive cells in peri-infarct regions. The expressions of TNF-α (green) (B), COX-2 (green) (C), CD11b (green) (D), and MPO (green) (E) were detected in the perihemorrhagic regions. The number of TNF-α-positive cells (F), COX-2 positive cells (G), CD11b-reactive microglia (H), and MPO-reactive neutrophils (I) decreased in the hUCB-MSCs-treated group compared with the controls (no significant differences). Nuclei were counterstained with DAPI (blue). Values are shown as mean and SEM; n=3 per group.
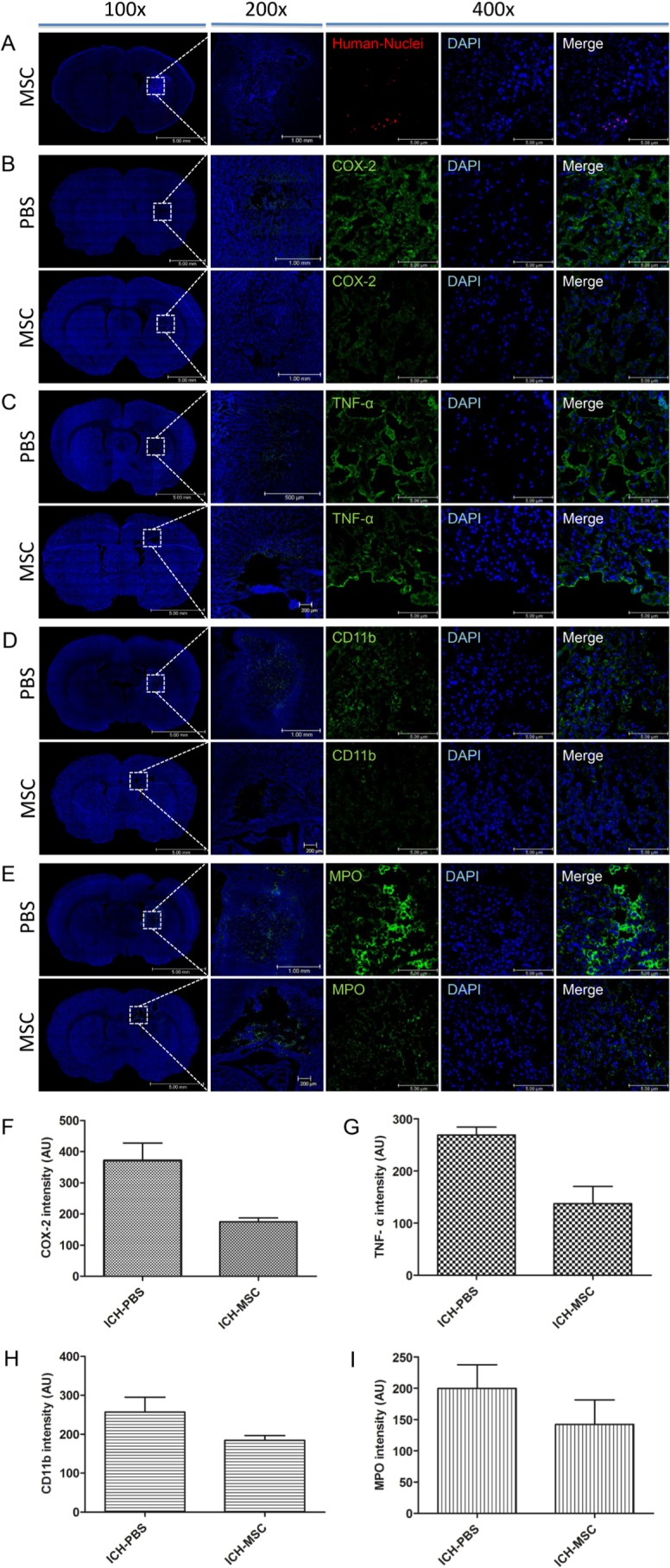
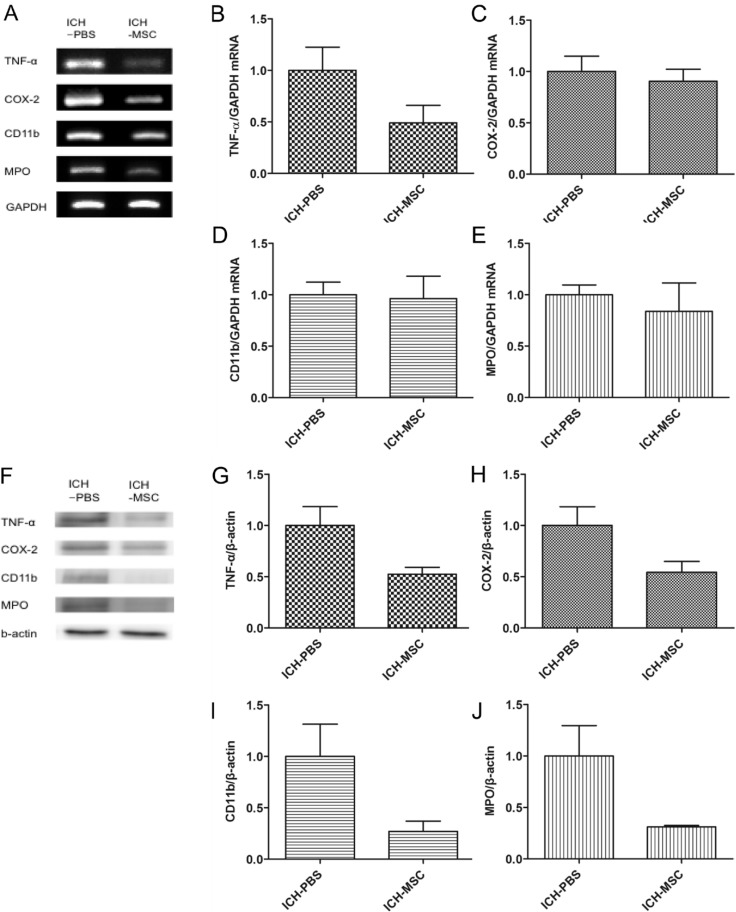
To investigate the effect of hUCB-MSCs in the inflammatory response, the expressions of inflammatory factors (tumor necrosis factor alpha [TNF-α], cyclooxygenase [COX]-2, CD11b positive to microglia, and myeloperoxidase [MPO] positive to neutrophils were examined at 3 days after transplantation.
Confocal micrographs of brain sections showed the expression of TNF-α, COX-2, CD11b, and MPO in peri-hemorrhagic regions (Fig. 3B~E). The expression levels of these inflammatory factors were lower in the hUCB-MSCs-treated rats than in the controls. However, there was no significant difference between the two groups (Fig. 3F~I).
mRNA and protein expression of the inflammatory factors in transplanted hUCB-MSCs
The mRNA levels of TNF-α, COX-2, microglia, and neutrophils were detected by RT-PCR (Fig. 4A). The expression levels of these inflammatory factors were down-regulated in the hUCB-MSCs-treated rats compared with the controls. However, there was no significant difference between the two groups (Fig. 4B~E).
Fig. 4. RT-PCR and western blot analysis of inflammatory factors. The mRNA and protein levels were detected in whole brain tissue of ICH rats at 3 days after transplantation. (A) mRNA levels of inflammatory factors (TNF-α, COX-2, CD11b, and MPO) by RT-PCR. (B~E) Quantification of the mRNA expression of inflammatory factors in the hUCB-MSCs-treated group compared with the controls. (F) Representative western blots of the four inflammatory factors (G~J). Quantification (protein expression) of inflammatory factors in the hUCB-MSCs-treated group compared with the controls. mRNA and protein levels of inflammatory factors were lower in the hUCB-MSCs-treated group than in the controls (not significant). Densitometric analysis was performed using the Image J software by normalizing to GAPDH for RT-PCR and β-actin for western blot as the loading control; n=3 per group.
The protein levels of the inflammatory factors were detected by western blot (Fig. 4F). The protein expression levels were down-regulated in the hUCB-MSCs-treated rats compared with the controls. However, there was no significant difference between the two groups (Fig. 4G~J).
DISCUSSION
The primary brain injury caused by ICH occurs at the time of the hemorrhage. Secondary injury after ICH can be caused by primary injury and results in severe neurological deficits and death of patients with ICH [10]. Recent studies have focused on therapies for secondary brain injury and showed that inflammation is the key contributor to ICH-induced secondary injury [11]. These evidences indicate that reduction in levels of inflammatory factors may improve neurological recovery after ICH.
The activation of microglia plays a role in the secondary injury after ICH and results in their production of pro-inflammatory cytokines and chemokines [12]. In ICH animal models, the inhibition of microglial activation by tuftsin fragment 1-3 reduced the hemorrhage size and improved the neurobehavioral deficits [13]. Neutrophils infiltrate into hematoma after ICH and the infiltrating neutrophils can also damage brain tissue by generating reactive oxygen species (ROS) and secreting pro-inflammatory proteases [14]. In ICH mouse models, neutrophil depletion results in improved functional outcomes at day 3 post-hemorrhage [15]. TNF-α, a pro-inflammatory cytokine, is synthesized and released by astrocytes and some neurons after injury [16]. TNF-α antagonism by a murine infliximab improves neurological recovery after ICH [17]. COX-2 is expressed in several cell types in response to growth factors, cytokines, and pro-inflammatory molecules [18]. In animal models, COX-2 inhibition by celecoxib improves functional outcome and reduces inflammation after ICH [19]. Our results suggest that hUCB-MSCs tend to have an anti-inflammatory effect after ICH.
The early stage after ICH involves the immediate infiltration of blood components. Levels of reactive microglia peak at 3 to 7 days and those of neutrophils peak at 3 days post-ICH [20]. Therefore, early management of patients with ICH is important. Moreover, hUCB-MSCs might be neuroprotective if the cells are transplanted early after an insult [21]. In a previous study using a rat brain ischemia-model, we observed that transplantation of hUCB-MSCs 2 days after MCAO could reduce infract volume and improve neurological deficits. Therefore, in this study, hUCB-MSCs were transplanted 2 days after ICH.
Recent studies demonstrate that MSCs secrete immunomodulatory cytokines in response to inflammatory molecules. MSCs crosstalk with components of the immune system and they reveal the mechanisms of both anti-and pro-inflammatory effects [22]. In response to inflammatory molecules, MSCs secrete the immunomodulatory cytokines including, prostaglandin 2, transforming growth factor-beta 1, hepatocyte growth factor, stromal cell-derived factor 1, nitrous oxide, indoleamine 2,3-dioxygenase, IL-4, IL-6, IL-10, IL-1 receptor antagonist and soluble tumor necrosis factor-α receptor [23]. In this study, we obtained the cytokine array data of hUCB-MSCs from MEDIPOST Inc. [24]. We identified six immunomodulatory cytokines associated with neuroinflammation: brain-derived neuroptophic factor (BDNF), ciliary neurotrophic factor (CNTF), intracellular adhesion molecule-5 (ICAM-5), interleukin-1 receptor antagonist (IL-1 ra), macrophage colony-stimulating factor (M-CSF), and oncostatin M (OSM). These immunomodulatory foctors are up-regulated in an inflammatory environment [25,26,27,28,29,30]. These data suggest that the immunomodulators secreted by hUCB-MSCs may reduce inflammatory factors and, in turn, improve recovery from neurological deficits.
Recently, MSCs have been shown to have multilateral paracrine effects and therapeutic effects during injury [31]. In this study, we also observed that the transplantation of hUCB-MSCs tended to improve neurological recovery after ICH. It is likely that hUCB-MSCs secrete anti-inflammatory factors, which may modulate the inflammatory environment of ICH. Therefore, to completely understand the paracrine effects of hUCB-MSCs in ICH, it is necessary to investigate the mechanisms of the inflammatory modulators.
ACKNOWLEDGEMENTS
This study was supported by the Korea Institute of Planning & Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries, Republic of Korea (311011-05-3-SB020), by the Korea Healthcare Technology R&D Project (HI10C14110400, HI12C02050101, HI11C21100200) funded by Ministry of Health & Welfare, Republic of Korea, and by the Technology Innovation Program (10050154, Business Model Development for Personalized Medicine Based on Integrated Genome and Clinical Information) funded By the Ministry of Trade, industry & Energy (MI, Korea).
References
- 1.van Asch CJ, Luitse MJ, Rinkel GJ, van der, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 2.Morgenstern LB, Hemphill JC, 3rd, Anderson C, Becker K, Broderick JP, Connolly ES, Jr, Greenberg SM, Huang JN, MacDonald RL, Messe SR, Mitchell PH, Selim M, Tamargo RJ American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–2129. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzales NR. Ongoing clinical trials in intracerebral hemorrhage. Stroke. 2013;44:S70–S73. doi: 10.1161/STROKEAHA.111.000563. [DOI] [PubMed] [Google Scholar]
- 4.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 5.Dalous J, Larghero J, Baud O. Transplantation of umbilical cord-derived mesenchymal stem cells as a novel strategy to protect the central nervous system: technical aspects, preclinical studies, and clinical perspectives. Pediatr Res. 2012;71:482–490. doi: 10.1038/pr.2011.67. [DOI] [PubMed] [Google Scholar]
- 6.Wang SP, Wang ZH, Peng DY, Li SM, Wang H, Wang XH. Therapeutic effect of mesenchymal stem cells in rats with intracerebral hemorrhage: reduced apoptosis and enhanced neuroprotection. Mol Med Rep. 2012;6:848–854. doi: 10.3892/mmr.2012.997. [DOI] [PubMed] [Google Scholar]
- 7.Liao W, Zhong J, Yu J, Xie J, Liu Y, Du L, Yang S, Liu P, Xu J, Wang J, Han Z, Han ZC. Therapeutic benefit of human umbilical cord derived mesenchymal stromal cells in intracerebral hemorrhage rat: implications of anti-inflammation and angiogenesis. Cell Physiol Biochem. 2009;24:307–316. doi: 10.1159/000233255. [DOI] [PubMed] [Google Scholar]
- 8.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42:1781–1786. doi: 10.1161/STROKEAHA.110.596718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang R, Liu Y, Yan K, Chen L, Chen XR, Li P, Chen FF, Jiang XD. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10:106. doi: 10.1186/1742-2094-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11:720–731. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014;115:25–44. doi: 10.1016/j.pneurobio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol. 2013;2013:746068. doi: 10.1155/2013/746068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Rogove AD, Tsirka AE, Tsirka SE. Protective role of tuftsin fragment 1-3 in an animal model of intracerebral hemorrhage. Ann Neurol. 2003;54:655–664. doi: 10.1002/ana.10750. [DOI] [PubMed] [Google Scholar]
- 14.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92:463–477. doi: 10.1016/j.pneurobio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sansing LH, Harris TH, Kasner SE, Hunter CA, Kariko K. Neutrophil depletion diminishes monocyte infiltration and improves functional outcome after experimental intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:173–178. doi: 10.1007/978-3-7091-0693-8_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figiel I. Pro-inflammatory cytokine TNF-alpha as a neuroprotective agent in the brain. Acta Neurobiol Exp (Wars) 2008;68:526–534. doi: 10.55782/ane-2008-1720. [DOI] [PubMed] [Google Scholar]
- 17.Lei B, Dawson HN, Roulhac-Wilson B, Wang H, Laskowitz DT, James ML. Tumor necrosis factor α antagonism improves neurological recovery in murine intracerebral hemorrhage. J Neuroinflammation. 2013;10:103. doi: 10.1186/1742-2094-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–910. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- 19.Chu K, Jeong SW, Jung KH, Han SY, Lee ST, Kim M, Roh JK. Celecoxib induces functional recovery after intracerebral hemorrhage with reduction of brain edema and perihematomal cell death. J Cereb Blood Flow Metab. 2004;24:926–933. doi: 10.1097/01.WCB.0000130866.25040.7D. [DOI] [PubMed] [Google Scholar]
- 20.Ziai WC. Hematology and inflammatory signaling of intracerebral hemorrhage. Stroke. 2013;44:S74–S78. doi: 10.1161/STROKEAHA.111.000662. [DOI] [PubMed] [Google Scholar]
- 21.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong SY, Kim DH, Ha J, Jin HJ, Kwon SJ, Chang JW, Choi SJ, Oh W, Yang YS, Kim G, Kim JS, Yoon JR, Cho DH, Jeon HB. Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells. 2013;31:2136–2148. doi: 10.1002/stem.1471. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y, Wei N, Zhu J, Lu T, Chen Z, Xu G, Liu X. Effects of brain-derived neurotrophic factor on local inflammation in experimental stroke of rat. Mediators Inflamm. 2010;2010:372423. doi: 10.1155/2010/372423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linker RA, Mäurer M, Gaupp S, Martini R, Holtmann B, Giess R, Rieckmann P, Lassmann H, Toyka KV, Sendtner M, Gold R. CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nat Med. 2002;8:620–624. doi: 10.1038/nm0602-620. [DOI] [PubMed] [Google Scholar]
- 27.Tian L, Lappalainen J, Autero M, Hänninen S, Rauvala H, Gahmberg CG. Shedded neuronal ICAM-5 suppresses T-cell activation. Blood. 2008;111:3615–3625. doi: 10.1182/blood-2007-09-111179. [DOI] [PubMed] [Google Scholar]
- 28.Greenhalgh AD, Brough D, Robinson EM, Girard S, Rothwell NJ, Allan SM. Interleukin-1 receptor antagonist is beneficial after subarachnoid haemorrhage in rat by blocking haem-driven inflammatory pathology. Dis Model Mech. 2012;5:823–833. doi: 10.1242/dmm.008557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith AM, Gibbons HM, Oldfield RL, Bergin PM, Mee EW, Curtis MA, Faull RL, Dragunow M. M-CSF increases proliferation and phagocytosis while modulating receptor and transcription factor expression in adult human microglia. J Neuroinflammation. 2013;10:85. doi: 10.1186/1742-2094-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruprecht K, Kuhlmann T, Seif F, Hummel V, Kruse N, Brück W, Rieckmann P. Effects of oncostatin M on human cerebral endothelial cells and expression in inflammatory brain lesions. J Neuropathol Exp Neurol. 2001;60:1087–1098. doi: 10.1093/jnen/60.11.1087. [DOI] [PubMed] [Google Scholar]
- 31.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]