To the Editor:
We have read with great interest the review published in your journal titled "Hypothesis: Somatic Mosaicism and Parkinson Disease (Exp Neurobiol. 2014 Dec;23:271-276)," which discusses the "potential" existence of mosaicisms in Parkinson's disease.
We find it essential to clarify some concepts that we consider inadequate/incorrect because they are presented as a new hypothesis when in fact our group has already published two papers including cases reporting the presence of Mosaicisms of Alpha-Synuclein rearrangements associated to Early Onset Parkinson Disease (EOPD) cases [1,2].
The paper by Han-Joon Kim and Beom S. Jeon published in Dec. 2014 speculates about the possibility that "a somatic mutation affecting cells in the central nervous system, including substantia nigra dopaminergic neurons could lead to the development of PD through the same pathomechanisms of genetic PD even in the absence of a germ-line mutation". Furthermore, the manuscript mentions in two sections (in its abstract and in section "PD and somatic mosaicism") that "The study of somatic mosaicism in Parkinson disease (PD) is only in its infancy, and a case with somatic mutation has not yet been described."
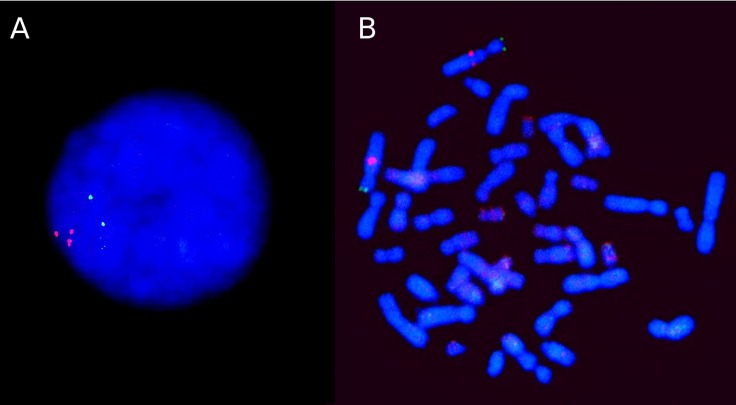
In our first paper, available online as early as May 2014 [1], we described the clinical phenotype and the genetic and immunohistochemical findings in two unrelated cases with early-onset PD due to Alpha-Synuclein (SNCA) mosaicism. Although Fluorescent In- Situ Hybridization (FISH) results indicated few or no rearrangements (i.e. ≤4 FISH probe signals in >20% of interphase cells scored) in peripheral leucocytes from either case, 75% of oral mucosal cells showed triplication and duplication of SNCA in case 1 and 43% showed duplication in case 2. This duplication occurs in the original chromosome (Fig. 1).
Fig. 1. FISH analysis for Alpha-Synuclein gene. Panel A presents an interphase of a lymphocyte showing the presence of three DNA regions hybridized with probes obtained from BAC (Bacterial Artificial Chromosomes) containing the Alpha-Synuclein gene. Panel B, on the right, shows a metaphase where the chromosomes carrying the Alpha-Synuclein gene can be observed. One of the chromosomes can be seen to be more intense and have a larger region than its homologous pair. Telomere markers (green) do not present noticeable differences. These results suggest that the duplication presents in the same chromosome, near the region where the gene is located.
In the "Testing the hypothesis" section, Han-Joon Kim and Beom S. Jeon [3], present the need to investigate other tissues other than peripheral blood lymphocytes. They suggest evaluating oral mucosa cells, something that was conducted and described for the first time by us in our above-mentioned paper. Our paper also describes the technique used to identify the ectodermal origin of the buccal mucosa cells evaluated in both patients (for further details, see Fig. 2 in [1]).
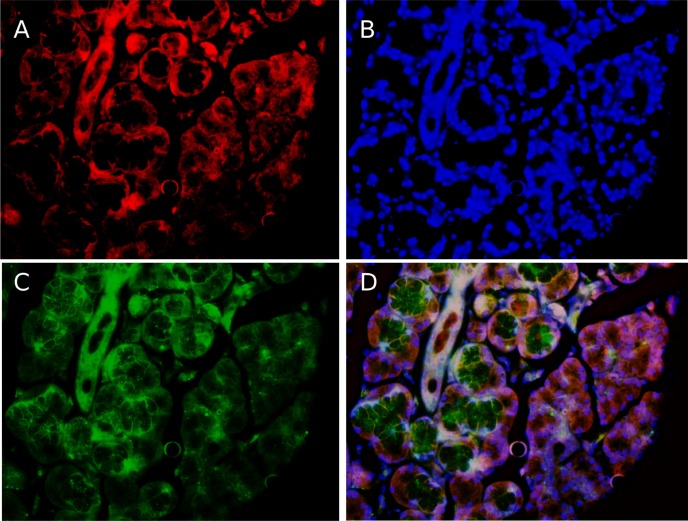
Fig. 2. Immunohistochemical assays for the detection of alpha-synuclein protein. The figure shows the section of a salivary gland dyed with red fluorescence for neurofilament protein (A), the fluorescent DAPI with the genomic DNA in cell nuclei appears in blue (B) and the alpha-synuclein protein is detected by a monoclonal antibody and developed in green (C). The overlapping of images (D) highlights that the relative intensity of alpha-synuclein is larger on the left side of the gland in the image. The increase is not homogeneous in patients with the amplification of alpha-synuclein in mosaic. A:200×.
The authors Han-Joon Kim and Beom S. Jeon [3], also suggest evaluating salivary gland cells in order to increase the chance of finding somatic mosaicisms in PD. In connection with this suggestion, we consider it relevant to point out that our publication included the evaluation of cells from a biopsy of a minor salivary gland, but with the purpose of evaluating the presence of immunoreactive augment as a consequence of an increase in the alpha-synuclein protein (immunohistochemical assays), rather than to detect rearrangements of the Alpha-Synuclein gene. We decided not to use minor salivary gland cells to evaluate gene rearrangements taking into account that salivary glands have a mixed endodermal-ectodermal embryological origin. It is also worth pointing out that, in patients with Alpha-Synuclein mosaicisms, the studies performed on samples from the minor salivary glands using a rabbit polyclonal SNCA antibody [4] showed intense SNCA immunoreactive profiles (Fig. 2).
We agree with the authors' statement concerning the fact that the technology used in the detection of mosaicisms is key. However, we consider it relevant to stress that precisely one of the techniques suggested by the authors, "High Resolution Melting PCR" based on the findings of Prourakis et al. [5], is not the most suitable for detection of Copy-Number Variations (CNVs). We would like to mention that the de novo mutation rate for CNV can be two to four orders of magnitude higher than for Single Nucleotide Variations (SNVs) [6], with CNVs arising in mitosis, explaining the increasing evidence for CNV mosaicisms in healthy humans, including the brain. In our experience, the mosaicism of CNVs in SNCA is one of the most important targets when searching for PD somatic mosaicism. Therefore, in our paper we postulate that techniques such as FISH (low cost, high reproducibility, possibility to perform individual tests cell by cell) should be taken into account at present, at least until single cell sequencing becomes optimized and generally available.
In conclusion, somatic mosaicism has already been confirmed as an etiological factor for Parkinson's disease.
In our experience, the keys for detection of somatic mosaicism in PD are:
a) Diagnostic suspicion based on the clinical characteristics of patients who present autonomic dysfunction and cognitive impairment in addition to EOPD
b) The evaluation of multiple tissues of ectodermal origin that are accessible in patients
c) The use of techniques that guarantee individual cell analysis as well as the detection of low-level mosaicisms.
Given all of the above, we deem the publication of this letter important in order to shed some light about the existence of somatic mosaicisms as a relevant (although relatively unexplored) etiologic mechanism, not only in PD but in other neurological diseases.
References
- 1.Perandones C, Giugni JC, Calvo DS, Raina GB, De Jorge Lopez L, Volpini V, Zabetian CP, Mata IF, Caputo M, Corach D, Radrizzani M, Micheli FE. Mosaicism of alpha-synuclein gene rearrangements: report of two unrelated cases of early-onset parkinsonism. Parkinsonism Relat Disord. 2014;20:558–561. doi: 10.1016/j.parkreldis.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perandones C, Aráoz Olivos N, Raina GB, Pellene LA, Giugni JC, Calvo DS, Radrizzani M, Piedimonte F, Micheli FE. Successful GPi stimulation in genetic Parkinson's disease caused by mosaicism of alpha-synuclein gene duplication: first description. J Neurol. 2015;262:222–223. doi: 10.1007/s00415-014-7576-4. [DOI] [PubMed] [Google Scholar]
- 3.Kim HJ, Jeon BS. Hypothesis: somatic mosaicism and Parkinson disease. Exp Neurobiol. 2014;23:271–276. doi: 10.5607/en.2014.23.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cersósimo MG, Perandones C, Micheli FE, Raina GB, Beron AM, Nasswetter G, Radrizzani M, Benarroch EE. Alpha-synuclein immunoreactivity in minor salivary gland biopsies of Parkinson's disease patients. Mov Disord. 2011;26:188–190. doi: 10.1002/mds.23344. [DOI] [PubMed] [Google Scholar]
- 5.Proukakis C, Houlden H, Schapira AH. Somatic alpha-synuclein mutations in Parkinson's disease: hypothesis and preliminary data. Mov Disord. 2013;28:705–712. doi: 10.1002/mds.25502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lupski JR. Brain copy number variants and neuropsychiatric traits. Biol Psychiatry. 2012;72:617–619. doi: 10.1016/j.biopsych.2012.08.007. [DOI] [PubMed] [Google Scholar]