Abstract
Purpose
Rheumatoid arthritis (RA) is an inflammatory joint disorder, the progression of which leads to the destruction of cartilage and bone. Chemokines are involved in RA pathogenesis. In this study, we investigated the chemokine signaling pathway associated with CCL2 in peripheral blood (PB) and synovial tissues (ST) of RA patients based on our previous work about chemokine signaling pathway involved in the activation of CCL2 production in collagen-induced arthritis rat ST.
Materials and Methods
Total RNA was isolated from PB leukocytes and synovium of the knee joint in both RA patients and control populations. Real-time polymerase chain reaction was used to determine CCL4, CCR5, c-Jun, c-Fos, and CCL2 expressions. Serum level of CCL2 was assessed by enzyme-linked immunosorbent assay, and the production of CCL2 in ST was analyzed immunohistochemically.
Results
The expressions of CCL4, CCR5, c-Jun, c-Fos, and CCL2 messenger RNA in RA patients were significantly higher than those in healthy controls, both in ST and on PB leukocyte. Serum CCL2 levels were elevated in RA patients. Histological examination of rheumatoid joints revealed extensive CCL2 expression in RA ST.
Conclusion
CCL2, CCL4, c-Jun, c-Fos, and CCR5 may play an important role in the recruitment of PB leukocytes into the RA joints. These data provide evidence that the chemokine signaling pathway is involved in CCL2 expression in RA patient tissues, which may contribute to chronic inflammation associated with RA. Targeting this signaling pathway may provide a novel therapeutic avenue in RA.
Keywords: CCL2, leukocytes, rheumatoid, arthritis, synovial membrane
INTRODUCTION
Rheumatoid arthritis (RA) is a complex autoimmune disease of the synovial joints. The typical features for RA include the chronic inflammatory of synovial membrane and formation of a pannus, which leads to joint destruction finally.1 Although the pathogenesis of RA is largely unknown, it is recognized that leukocyte migration, mediated in part by chemokines and chemokine receptors, plays an important role in the perpetuation of inflammation in rheumatoid synovium.2 For example, monocyte chemoattractant protein 1/CCL2, macrophage inflammatory protein 1β/CCL4 and CCR5 are all key molecules involved in rheumatoid synovium.3 A vicious cycle of altered chemokine and signal transduction pathways contribute to cartilage and bone destruction by RA synoviocytes and osteoclasts.4
CCL2 is chemotactic for monocyte/macrophage, B-cell, and T-lymphocyte, and belongs to the CC subfamily of chemokines.2 Moreover, CCL2 is highly expressed in the RA plasma, synovial fluid, and the synovial tissue reported in previous literature.5 Injection of CCL2 into rabbit joints resulted in marked macrophage infiltration in the affected joint,5 and CCL2 antagonist treatment was found to ameliorate disease severity in adjuvant-induced arthritis by decreasing macrophage infiltration.6 CCL4 is expressed at high levels in collagen-induced arthritis (CIA) synovial tissue and is produced by synovial fibroblasts; it also preferentially attracts CD4+ T cells.7 The CD4+/45RO+ T cells are prevalent in the RA synovium. CCL4 may have a central role in the trafficking and activation of lymphocytes and monocytes involved in the inflammatory processes in RA. Th17 and regulatory T cells are believed to be the predominant effecter T cells, and their balance is thought to be critical in maintaining inflammation of RA.8 Th17 cells and regulatory T cells express CCR5, which has a major role in their recruitment to the site of inflammation.9 In addition to T cells, monocytes in RA synovial tissue also predominantly express CCR5.10 Studies have found CCR5 immunostaining on a majority of RA synovial tissues (ST) macrophages, fibroblasts, vascular smooth muscle cells, and perivascular lymphocytes, indicating that CCR5 may play a role in monocyte migration into the synovial tissue.
Chemokines and their receptors play a fundamental role in the trafficking and activation of monocytes and lymphocytes at sites of inflammation. However, the mechanism of interaction of these molecules in an inflammatory milieu, which helps perpetuate local inflammation in the joint of RA patient, is not fully understood. The present study was undertaken to investigate the chemokine signaling pathway associated with CCL2, examine RA patient tissues for constitutive gene expression for CCL4, CCR5, c-Jun, c-Fos, and CCL2 on leukocyte and in the knee joint. RA peripheral blood (PB) and ST samples were analysed.
MATERIALS AND METHODS
Patients and specimens
A total of 34 individuals were recruited in the study. All 17 patients fulfilled the American College of Rheumatology (formerly, the American Rheumatism Association) criteria for RA.11 Among the patients, 100% were rheumatoid factor (RF) positive. The mean±standard deviation (SD) erythrocyte sedimentation rate (ESR) was 40.0±14.7 mm/hour, the mean±SD serum level of C-reactive protein (CRP) was 2.12±1.83 mg/dL, the mean±SD serum level of RFs was 853±1317 IU/mL, and the mean±SD serum level of anticyclic citrullinated peptide (anti-CCP) was 844±404 U/mL. Selected demographic, clinical, and laboratory characteristics of RA patients are summarized in Table 1. All patients gave informed consent, and the Medical Ethics Committee of the Tianjin Medical University approved the study protocol.
Table 1. Demographic, Clinical, and Laboratory Characteristics of Rheumatoid Arthritis Patients.
| Characteristics | Value | n |
|---|---|---|
| Age (yrs) | 55.6 (20.2-82.1) | 17 |
| Sex (woman) | 112 (77.8)* | 17 |
| Disease duration (yrs) | 11.0 (2.0-50.2) | 17 |
| Erythrocyte sedimentation rate | 40.0±14.7 mm/hr | 17 |
| C-reactive protein | 2.12±1.83 mg/dL | 17 |
| Rheumatoid factors | 853±1317 IU/mL | 17 |
| Anticyclic citrullinated peptide | 40.0±14.7 mm/hr | 17 |
Values are given as median (range).
*Number (percentage).
Blood samples were obtained from 11 patients at the time of clinical examination for the determination of ESR, CRP, RFs, and anti-CCP. For ten of the eleven corresponding paired samples, an additional set of PB leukocytes was used for analyses of gene expression. Control PB leukocytes and control sera were obtained from a group of 11 healthy individuals who were matched with the RA patient group for gender and mean age. At the time of sample collection, patients had not received immunosuppressive or immunomodulatory drugs for at least 2 months.
Synovial tissue was obtained from six patients undergoing total joint replacements who met the American College of Rheumatology criteria for RA.11 Six subjects without inflammatory joint disease and meniscus pathology served as control subjects. The ST from all six control patients with meniscus pathology was obtained by surgical synovectomy or arthroscopy with 5.0 mm grasping forceps before the meniscectomy. Half of the ST samples from one knee joint was stored in liquid nitrogen and used for quantitative real-time polymerase chain reaction (PCR). In parallel, half of the corresponding matched ST samples from the same knee joint were fixed in formalin, subsequently embedded in paraffin and then used for immunohistochemistry and histological examination. The mean ages were similar for patients and controls.
Quantitative real-time polymerase chain reaction (PCR)
The leukocytes were prepared from 10 mL of EDTA anticoagulated PB and lysed with TRIZOL LS reagent. The total cellular RNA was purified according to the instruction manual. Total RNA was isolated from synovial tissue using the TRIZOL reagent (10296010; Invitrogen, Carlsbad, CA, USA). The reverse transcription reaction was performed with Superscript III first-strand kit (Invitrogen, 18080-051) according to the manufacturer's instructions. Messenger RNA (mRNA) expression was determined by real-time PCR using SYBR Green Master Mix under standard thermocycler conditions (4367659; Applied Biosystems, Foster City, CA, USA) on an ABI Prism 7900 Sequence Detection System (Applied Biosystems). A human GAPDH gene was used as an endogenous control for sample normalization. Results are presented as the fold expression relative to that of GAPDH. Values were calculated as means±SD. Differences between groups were evaluated using Student's t-test. PCR primers were as follows: for human GAPDH, forward 5'-ATTGCCCTCAACGACCACT-3' and reverse 5'-AT GAGGTCCACCACCCTGT-3'; for human CCL2, forward 5'-CTTCTGTGCCTGCTGCTCAT-3' and reverse 5'-CG GAGTTTGGGTTTGCTTGTC-3'; for human CCL4, forward 5'-CTGTGCTGATCCCAGTGAATC-3' and reverse 5'-TCAGTTCAGTTCCAGGTCATACA-3'; for human CCR5, forward 5'-TTCTGGGCTCCCTACAACATT-3' and reverse 5'-TTGGTCCAACCTGTTAGAGCTA-3'; for human c-Jun, forward 5'-CGCCCCTGTCCCCCATCG-3' and reverse 5'-TGTGCCACCTGTTCCCTG-3', and for human c-Fos, forward 5'-GGGGCAAGGTGGAACAGT TAT-3' and reverse 5'-CCGCTTGGAGTGTATCAGTCA-3'.
Enzyme-linked immunosorbent assays (ELISAs)
Serum level of CCL2 was determined by enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN, USA), according to the manufacturer's instructions. Values were calculated as means±SD. Differences between groups were evaluated using Student's t-test.
Immunohistochemical analysis
Tissue specimens were processed for paraffin wax embedding and multiple 4-µm sections were prepared from each block for routine histological and subsequent immunohistochemical examination. Immunohistochemical staining was performed with a polyclonal rabbit antibody against CCL2 (LifeSpan BioSciences, Inc., Seattle, WA, USA), as described previously.9
RESULTS
Quantification of CCL2, CCL4, CCR5, c-Jun, and c-Fos genes expressions in ST and PB of the RA patients using real-time PCR
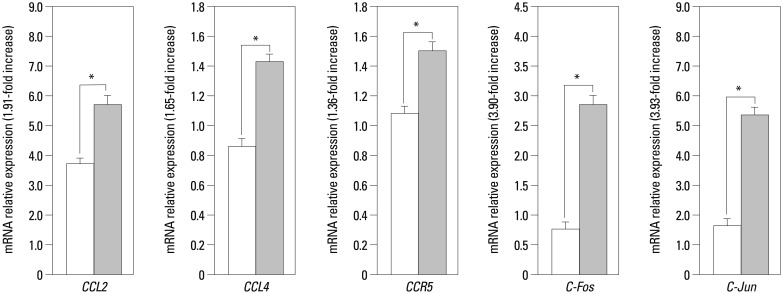
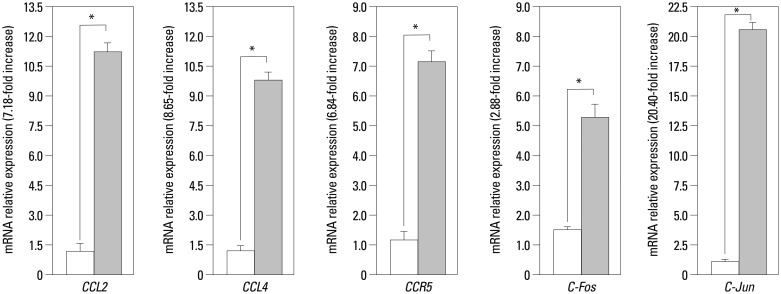
Real-time PCR experiments were carried out to determine the exact rate of the enhancement of these five genes expression. The real-time PCR results were converted to mRNA, and fold changes were calculated. Our data show that CCL4 expression on the leukocytes in RA PB was higher than in the control group PB (1.65-fold, p<0.05) (Fig. 1). However, CCL4 expression was significantly up-regulated in the ST of RA patients as compared with their expression in normal ST (8.65-fold, p<0.05) (Fig. 2). We also detected that CCR5 expression was higher on leukocytes in RA PB compared with its expression in normal PB (1.36-fold, p<0.05) (Fig. 1), and significantly higher in RA ST than in the control group ST (6.84-fold, p<0.05) (Fig. 2).
Fig. 1. Real-time polymerase chain reaction analysis of CCL2, CCL4, CCR5, c-Fos, and c-Jun mRNA levels on peripheral blood leukocytes. The relative mRNA expression levels were normalized to the levels of GAPDH. The significance of differences was determined using Student's t-test. *p<0.05, n=10 (open bars healthy controls, coarse stippling bars rheumatoid arthritis patients). mRNA, messenger RNA.
Fig. 2. Real-time polymerase chain reaction analysis of CCL2, CCL4, CCR5, c-Fos, and c-Jun mRNA levels in synovial tissue. The relative mRNA expression levels were normalized to the levels of GAPDH. The significance of differences was determined using Student's t-test. *p<0.05, n=6 (open bars healthy controls, coarse stippling bars rheumatoid arthritis patients). mRNA, messenger RNA.
Our results have also shown that both c-Jun and c-Fos were highly expressed in the RA synovium (2.88-fold and 20.4-fold increase, respectively, compared with controls, p<0.05) (Fig. 2). It is worth noting that RA ST exhibits significantly higher c-Jun expression (20.4-fold) compared with controls. We showed that the high expression of c-Jun and c-Fos on PB leukocytes in RA was higher than those in healthy controls (3.9-fold and 3.4-fold, p<0.05) (Fig. 1). The constitutive expression of CCL2 was 7.18-fold (p<0.05) (Fig. 2) higher in RA ST than control ST, the CCL2 expression was 1.91-fold (p<0.05) (Fig. 1) higher on RA PB leukocytes than control leukocytes, as determined by quantitative RT-PCR. The relative mRNA levels of these five genes and fold changes on RA PB leukocytes and in synovium (normalized to GAPDH), determined by the real-time PCR are summarized in Table 2.
Table 2. Summary of CCL2, CCL4, CCR5, c-Jun, c-Fos Gene Expression in RT-PCR.
| Gene | Relative mRNA expression in RA ST (RA/control) | Fold | Relative mRNA expression on RA PB leukocytes (RA/control) | Fold |
|---|---|---|---|---|
| CCL2 | (10.91±1.11)/(1.52±0.61) | 7.18* | (5.85±14.23)/(3.07±3.79) | 1.91* |
| CC4 | (9.43±0.96)/(1.09±0.51) | 8.65* | (1.42±0.50)/(0.86±0.42) | 1.65* |
| CC5 | (7.46±1.21)/(1.09±0.40) | 6.84* | (1.53±0.82)/(1.12±0.53) | 1.36* |
| c-Jun | (20.80±0.87)/(1.02±0.24) | 20.40* | (5.60±9.30)/(1.65±181) | 3.39* |
| c-Fos | (5.38±1.69)/(1.87±0.05) | 2.88* | (2.81±1.82)/(0.72±0.41) | 3.90* |
RT-PCR, real-time polymerase chain reaction; mRNA, messenger RNA; RA, rheumatoid arthritis; ST, synovial tissues; PB, peripheral blood.
*p<0.05, n=6 (in ST) and n=10 (on leukocytes) for RA patients versus healthy control.
Serum levels of CCL2 in RA patients
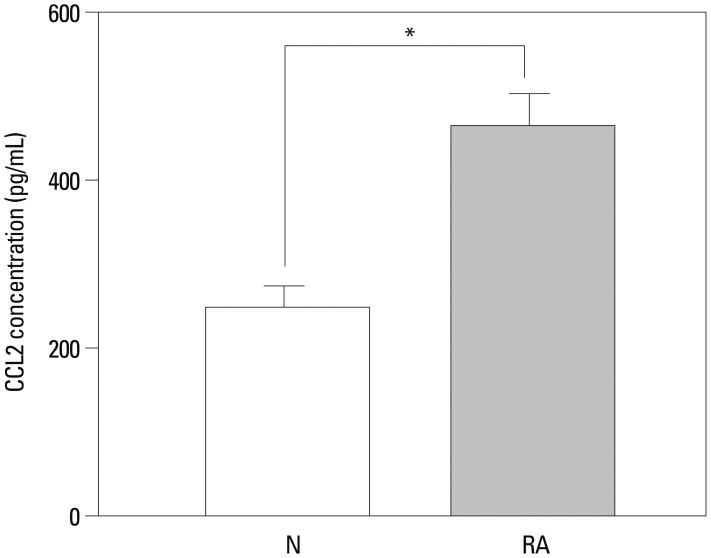
To determine whether PB from patients with RA also contained significant quantities of CCL2 compared to normal volunteers, serum CCL2 levels were determined by ELISA using 11 RA samples. Serum levels of RA CCL2 ranged from 251.26 pg/mL to 748.54 pg/mL (mean 464±180 pg/mL). In contrast, normal sera in 11 volunteers contained significantly less CCL2 (179.89 pg/mL to 357.34 pg/mL) (mean 270±73.5 pg/mL, p<0.05) (Fig. 3).
Fig. 3. Concentrations of CCL2 protein in serum, assessed by enzyme-linked immunosorbent assays technique. The significance of differences was determined using Student's t-test. *p<0.05, n=11 (open bars healthy controls, coarse stippling bars rheumatoid arthritis patients).
Expression of CCL2 in inflamed synovium
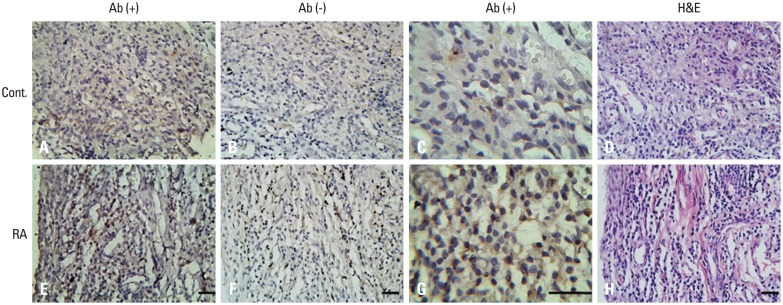
Tissue samples from all six patients with RA showed clear CCL2 signals in mononuclear round cells and lymphocytes cells. In contrast, specimens from six normal donors contained only a few positive cells that were scattered throughout the tissue. Fig. 4 shows adjacent synovial tissue sections from a patient with RA. Positive cells are predominantly located in the ST (Fig. 4E and G). Fig. 4C and G show a representative area at higher magnification. Slides were negative when the primary antibody was omitted. Histologic examination of RA ST demonstrated a significant increase in chronic synovial inflammation (Fig. 4H). The number of immunoreactive CCL2 cells increased with increasing severity of inflammation. Of note is the intense staining in the synovial membrane, characterized histologically in RA by the infiltration of leukocytes and marked hyperplasia.
Fig. 4. Immunohistochemical study of CCL2 expression in synovial tissue of knee joints. Photomicrographs were taken from healthy controls (A-D), rheumatoid arthritis patients (E-H). (A, C, E, and G) CCL2 immuoreactivity (brown staining). (B and F) Background staining with CCL2 antibody omitted (blue staining). (H) Maximal inflammatory cell infiltration (hematoxylin and eosin staining in D and H; avidin-biotin-peroxidase staining in A-G. Original magnification ×400 in A, B, D, E, F, and H; ×1000 in C and G). Bar=40 µm.
DISCUSSION
The recruitment and retention of inflammatory cells in the synovial tissue is a cardinal feature in RA. The chemokines and counter-receptors play an important role for initial recruitment of circulating leukocytes to the site of inflammation. CCL4 may be major chemoattractants for CCR5+ leukocytes from the PB into the RA joint. The role of CCL4 in leukocyte recruitment to sites of inflammation is also underlined by the high level of expression of the chemokine receptor CCR5 (receptor for CCL4) in ST and on leukocytes observed in the PB of RA patients.
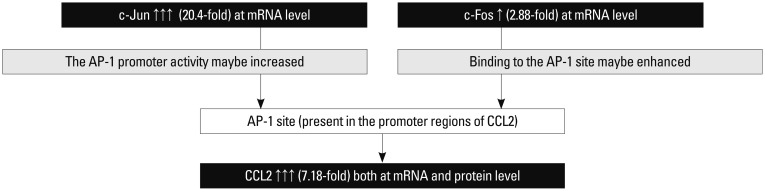
High expressions of the c-Jun and c-Fos can increase DNA-binding activity of activator protein (AP-1).12 It is worth noting that RA ST exhibited significantly higher c-Jun expression (20.4-fold) compared to controls. It has been reported that c-Jun is a major transcription factor in AP-1 activation.13 The AP-1 site is present in the promoter regions of CCL2 and likely to be one of the more important sites for CCL2 production.14 Asahara, et al.15 demonstrated significantly higher AP-1 DNA binding activity in RA ST, detected by electrophoretic mobility shift assay. Chromatin immunoprecipitation assay revealed that AP-1 was recruited to CCL2 promoter as a result of stimulation with osteopontin in PB mono-nuclear cells.16 AP-1 is upstream of CCL2. The expression of AP-1 and CCL2 were up-regulated in this study. Therefore, when c-Jun binds to the AP-1 element on the CCL2 promoter, the binding of c-Jun to the AP-1 site may be enhanced. The AP-1 promoter activity may be increased as well. The c-Fos is also highly expressed in the present study (2.88-fold). Coexpression of c-Jun and c-Fos may dramatically amplified AP-1 binding activity.17 A high level of AP-1 expression may play an important role in certain aspects of the pathogenesis of RA, such as synovial hyperplasia, overproduction of tissue-degrading molecules, and local immune disorders.18,19 The AP-1 proteins c-Jun and c-Fos may directly cause phenotypic activation and induction of CCL2.17 Therefore, increased CCL2 expression is most likely caused by the constitutive activation of AP-1 in RA synovial tissue (Fig. 5). AP-1 can also bind to NF-κB and NF-AT. Thus, AP-1 may stimulate the proliferation of leukocytes.20 CCL2 produced by synovial fibroblasts is considered to be a prototypical chemokine for the recruitment of monocytes.21
Fig. 5. The gene expressions that we have confirmed are shown in black, and confirmed pathways are shown with a solid line. The deductive results that may be involved are shown in coarse stippling. mRNA, messenger RNA; AP-1, activator protein.
Leukocytes are believed to be recruited from the circulation into the synovial tissue and then migrate into the joint space. Within the tissue, leukocyte migration is thought to follow chemokine gradients, which direct the cells toward the site of inflammation. In the present study, the expressions of CCL4, CCR5, c-Jun, c-Fos, and CCL2 were shown to significantly changed in the RA ST, and that such a large change was diminished on PB leukocytes from RA patients, suggesting that these five genes included in the chemokine signaling pathway (CCL4/CCR5/c-Jun and c-Fos/CCL2) play a role in chemoattractants for PB leukocytes into joint and in mediating the amplification and the perpetuation of the chronic synovial inflammation persent in RA patients.
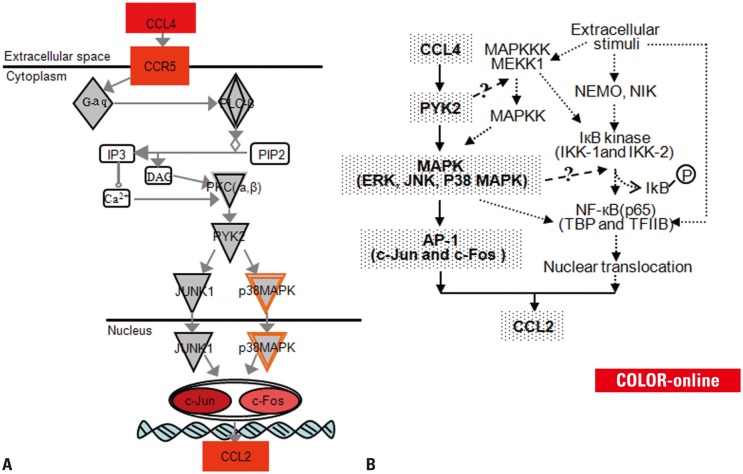
By the comprehensive microarray-based pathway analysis (ingenuity pathway analysis), we have earlier demonstrated that CCL4 stimulated CCL2 expression through the chemokine signaling pathway in CIA rat joint tissue.22 A key finding of the present study is the significant up-regulation of CCL4, CCR5, c-Jun, c-Fos, and CCL2 production in ST and on PB leukocytes of clinically RA patients possibly through the chemokine signaling pathway (CCL4/CCR5/c-Jun and c-Fos/CCL2). CCL4 exerts its effects by binding to CCR5 on the surface of the targeted leukocytes. CCR5, once activated, triggers a set of cellular signaling processes that result in activation of phospholipase C (PLC) together with inositol triphosphate (IP3) and diacyl glycerol formation, intracellular calcium release, and protein kinase C (PKC) activation. PKC activation and/or cytosolic Ca2+ are both necessary and sufficient to activate the prolinerich tyrosine kinase 2 (PYK2). PYK2 regulates the activity of Jun N-terminal kinase (JNK) and p38 in the MAP kinase (MAPK) pathway, leading to increased DNA binding of an AP-1 to the CCL2 promoter, selectively through c-Jun and c-Fos. The c-Jun and c-Fos components of AP-1 are phosphorylated by JNK and p38 MAPK, respectively. One of the key transcription factors activated by JNK and p38 MAPK is AP-1. Fig. 6A shows a schematic of this signaling pathway.
Fig. 6. (A) Schematic representation of CCL4/CCR5/c-Jun and c-Fos/CCL2 signaling pathway in RA. Red color indicates gene increased expression, the darker the higher expression. (B) Signal transduction cascades in rheumatoid arthritis and factors that stimulate the CCL2 transcription. Signaling molecules that we have confirmed are shown in coarse stippling, and confirmed pathways are shown with bold arrows. Suspected factors that may be involved are shown in white, and potential pathways are shown with dashed arrows. DAG, diacyl glycerol; PIP2, phosphatidylinositol 4,5-trisphosphate; PKC, protein kinase C; PYK2, prolinerich tyrosine kinase 2; JUNK1, Jun N-terminal kinase 1; MAPK, MAP kinase; NEMO, NF-κB essential modulator; NIK, NF-κB-inducing kinase; IKB, inhibitors of NF-κB; IKK, IκB kinases; ERK, extracellular signal-regulated kinase; JNK, Jun N-terminal kinase; AP-1, activator protein; TBP, TATA-binding protein.
MAPK pathways regulate the activity of AP-1 and NF-κB. The extracellular signal-regulated kinase, JNK, and p38 families of MAPKs have been implicated in the pathogenesis of RA.23 They contribute to up-regulation of c-Fos gene transcription through phosphorylation, however, transcription of the c-Jun gene is primarily controlled by c-Jun itself.24 MAP kinase signaling pathways influence AP-1 activity by both increasing the abundance of AP-1 components and by directly stimulating their activity.25 AP-1 includes members of the Jun and Fos families of transcription factors. AP-1 proteins bind to DNA and activate transcription as Jun homodimers, Jun-Jun heterodimers, or Jun-Fos heterodimers.26
The same cellular signals that activate the JNK and p38 MAPK pathways can also activate NF-κB. NF-κB is engaged after extracellular stimuli activate IκB kinases (IKK1 and IKK2) that phosphorylate IκB, resulting in its degradation. Subsequently, NF-κB translocates to the nucleus and results in transcription of various cytokines and inflammatory mediators. The activated MAPK/MEKK may activate IKK.27 CCL2 regulation by the cytokine-inducible MKK6/p38 pathway is also mediated by NF-κB sites.28 Carter, et al.29 found that the p38 MAP kinase regulates NF-κB-dependent transcription in part by modulating the activation of basal transcription factors, such as TFIIB, and TATA-binding protein. It has been reported that c-Jun and c-Fos are capable of physically interacting with NF-κB p65 through the Rel homology domain. The complex of NF-κB p65 and Jun or Fos can enhanced DNA binding and biological function via both the κB and AP-1 response elements.30 The MAPK cascade may act as the upstream signaling for the activation of AP-1 and NF-κB, which induce chemokine expression. NF-κB and AP-1 may be cross-coupled for the transcription of the chemokines CCL2. Overexpression of NF-κB-inducing kinase (NIK) in cells leads to activation of both IKK-1 and IKK-2. Several studies have shown that MEKK1, an upstream activator of the p38 MAP kinase, regulates NF-κB-dependent transcription by activating IKK. In fact, ME-KK1 interacts directly with both IKK-1 and IKK-2 and phosphorylates them.31 The regulatory role of the p38 MAP kinase is downstream of IKK.
The binding sites for NF-κB and AP-1 are in the 5'region of the CCL2 gene.13 Therefore, CCL2 gene transcription may require the activation of NF-κB and AP-1, or the combination of NF-κB and AP-1. This may depend on the types of cell or stimuli since there are binding sites for NF-κB, AP-1 in the promoter regions of CCL2 gene. Therefore, it is a possible that the PYK2/MAPK/AP-1 pathway (in the present study), the MAPK/MEKK1/NF-κB pathway, and the NIK or NF-κB essential modifier/IKK/NF-κB pathway32 lead to chemokine expression and may be activated upon CCL2 expression in PB and ST of RA patients (Fig. 6B). This hypothesis is consistent with the observations that the significant up-regulation observed in CCL4, CCR5, c-Jun, c-Fos, and CCL2 production may be due to the chemokine signaling pathway [CCL4/CCR5/PLC2/PKC/PYK2/MA-PK/AP-1 (c-Jun and c-Fos)/CCL2] in this study. The other pathways may need to be proved in future.
CCL2 is produced at the site of joint inflammation, whereas CCL4-expressing cells are found in both locally and systemically. CCR5 polymorphisms showed a significant association with radiographic severity, and they were found to be an independent predictor of radiographic severity.33 The c-Jun and c-Fos high expression in RA may cause proliferation of the synovium, up-regulation of tissue degrading molecules, and an abnormal immune network. Thus, we suggest that these five genes measured in ST and on PB leukocytes of RA patients reflect the local inflammatory activity of several components of the arthritic joint and systematic inflammation in RA. Taken together, these data strengthen the notion that these five genes may be a prognostic marker for progression of RA, as well as a marker of pharmacological response.
In conclusion, this study shows that the chemokine signaling pathway (CCL4/CCR5/c-Jun and c-Fos/CCL2) is involved in CCL2 expression in RA patient tissues, which may contribute to chronic inflammation associated with RA. This mechanism may be more general on other inflammatory conditions. We showed previously that low-level laser irradiation treatment significantly reduces CCL2, CCL4, CCR5, c-Jun, and c-Fos expression in CIA rat rheumatoid synovia via the CCL4/CCR5/c-Jun and c-Fos/CCL2 signaling pathway,22 thus targeting this pathway may provide a novel therapeutic avenue in RA.
ACKNOWLEDGEMENTS
This study is supported by the grant of the National Natural Science Foundation of China (No. 81273056), Science and Technology Development Project for Universities in Tianjin of China funded by Tianjin Education Commission (No. 20100103).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Szekanecz Z, Kim J, Koch AE. Chemokines and chemokine receptors in rheumatoid arthritis. Semin Immunol. 2003;15:15–21. doi: 10.1016/s1044-5323(02)00124-0. [DOI] [PubMed] [Google Scholar]
- 3.Shahrara S, Amin MA, Woods JM, Haines GK, Koch AE. Chemokine receptor expression and in vivo signaling pathways in the joints of rats with adjuvant-induced arthritis. Arthritis Rheum. 2003;48:3568–3583. doi: 10.1002/art.11344. [DOI] [PubMed] [Google Scholar]
- 4.Dayer JM. The saga of the discovery of IL-1 and TNF and their specific inhibitors in the pathogenesis and treatment of rheumatoid arthritis. Joint Bone Spine. 2002;69:123–132. doi: 10.1016/s1297-319x(02)00363-9. [DOI] [PubMed] [Google Scholar]
- 5.Akahoshi T, Wada C, Endo H, Hirota K, Hosaka S, Takagishi K, et al. Expression of monocyte chemotactic and activating factor in rheumatoid arthritis. Regulation of its production in synovial cells by interleukin-1 and tumor necrosis factor. Arthritis Rheum. 1993;36:762–771. doi: 10.1002/art.1780360605. [DOI] [PubMed] [Google Scholar]
- 6.Shahrara S, Proudfoot AE, Park CC, Volin MV, Haines GK, Woods JM, et al. Inhibition of monocyte chemoattractant protein-1 ameliorates rat adjuvant-induced arthritis. J Immunol. 2008;180:3447–3456. doi: 10.4049/jimmunol.180.5.3447. [DOI] [PubMed] [Google Scholar]
- 7.Schall TJ, Bacon K, Camp RD, Kaspari JW, Goeddel DV. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821–1826. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundy SK, Sarkar S, Tesmer LA, Fox DA. Cells of the synovium in rheumatoid arthritis. T lymphocytes. Arthritis Res Ther. 2007;9:202. doi: 10.1186/ar2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreira AP, Cavassani KA, Massafera Tristão, FS, Campanelli AP, Martinez R, Rossi MA, et al. CCR5-dependent regulatory T cell migration mediates fungal survival and severe immunosuppression. J Immunol. 2008;180:3049–3056. doi: 10.4049/jimmunol.180.5.3049. [DOI] [PubMed] [Google Scholar]
- 10.Katschke KJ, Jr, Rottman JB, Ruth JH, Qin S, Wu L, LaRosa G, et al. Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis Rheum. 2001;44:1022–1032. doi: 10.1002/1529-0131(200105)44:5<1022::AID-ANR181>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 11.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto H, Cujec TP, Yamanaka H, Kamatani N. Molecular aspects of rheumatoid arthritis: role of transcription factors. FEBS J. 2008;275:4463–4470. doi: 10.1111/j.1742-4658.2008.06582.x. [DOI] [PubMed] [Google Scholar]
- 13.Schonthaler HB, Guinea-Viniegra J, Wagner EF. Targeting inflammation by modulating the Jun/AP-1 pathway. Ann Rheum Dis. 2011;70(Suppl 1):i109–i112. doi: 10.1136/ard.2010.140533. [DOI] [PubMed] [Google Scholar]
- 14.Kok SH, Hong CY, Kuo MY, Wang CC, Hou KL, Lin YT, et al. Oncostatin M-induced CCL2 transcription in osteoblastic cells is mediated by multiple levels of STAT-1 and STAT-3 signaling: an implication for the pathogenesis of arthritis. Arthritis Rheum. 2009;60:1451–1462. doi: 10.1002/art.24452. [DOI] [PubMed] [Google Scholar]
- 15.Asahara H, Fujisawa K, Kobata T, Hasunuma T, Maeda T, Asanuma M, et al. Direct evidence of high DNA binding activity of transcription factor AP-1 in rheumatoid arthritis synovium. Arthritis Rheum. 1997;40:912–918. doi: 10.1002/art.1780400520. [DOI] [PubMed] [Google Scholar]
- 16.Zheng W, Li R, Pan H, He D, Xu R, Guo TB, et al. Role of osteopontin in induction of monocyte chemoattractant protein 1 and macrophage inflammatory protein 1beta through the NF-kappaB and MAPK pathways in rheumatoid arthritis. Arthritis Rheum. 2009;60:1957–1965. doi: 10.1002/art.24625. [DOI] [PubMed] [Google Scholar]
- 17.Wang N, Verna L, Hardy S, Forsayeth J, Zhu Y, Stemerman MB. Adenovirus-mediated overexpression of c-Jun and c-Fos induces intercellular adhesion molecule-1 and monocyte chemoattractant protein-1 in human endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19:2078–2084. doi: 10.1161/01.atv.19.9.2078. [DOI] [PubMed] [Google Scholar]
- 18.Kuroki Y, Shiozawa S, Yoshihara R, Hotta H. The contribution of human c-fos DNA to cultured synovial cells: a transfection study. J Rheumatol. 1993;20:422–428. [PubMed] [Google Scholar]
- 19.Gravallese EM, Darling JM, Ladd AL, Katz JN, Glimcher LH. In situ hybridization studies of stromelysin and collagenase messenger RNA expression in rheumatoid synovium. Arthritis Rheum. 1991;34:1076–1084. doi: 10.1002/art.1780340903. [DOI] [PubMed] [Google Scholar]
- 20.Yang-Yen HF, Chambard JC, Sun YL, Smeal T, Schmidt TJ, Drouin J, et al. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990;62:1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- 21.Villiger PM, Terkeltaub R, Lotz M. Production of monocyte chemoattractant protein-1 by inflamed synovial tissue and cultured synoviocytes. J Immunol. 1992;149:722–727. [PubMed] [Google Scholar]
- 22.Zhang L, Zhao J, Kuboyama N, Abiko Y. Low-level laser irradiation treatment reduces CCL2 expression in rat rheumatoid synovia via a chemokine signaling pathway. Lasers Med Sci. 2011;26:707–717. doi: 10.1007/s10103-011-0917-y. [DOI] [PubMed] [Google Scholar]
- 23.Schett G, Tohidast-Akrad M, Smolen JS, Schmid BJ, Steiner CW, Bitzan P, et al. Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum. 2000;43:2501–2512. doi: 10.1002/1529-0131(200011)43:11<2501::AID-ANR18>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 24.Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55:875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- 25.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 26.Wagner EF. Bone development and inflammatory disease is regulated by AP-1 (Fos/Jun) Ann Rheum Dis. 2010;69(Suppl 1):i86–i88. doi: 10.1136/ard.2009.119396. [DOI] [PubMed] [Google Scholar]
- 27.Lange-Carter CA, Johnson GL. Ras-dependent growth factor regulation of MEK kinase in PC12 cells. Science. 1994;265:1458–1461. doi: 10.1126/science.8073291. [DOI] [PubMed] [Google Scholar]
- 28.Goebeler M, Kilian K, Gillitzer R, Kunz M, Yoshimura T, Bröcker EB, et al. The MKK6/p38 stress kinase cascade is critical for tumor necrosis factor-alpha-induced expression of monocyte-chemoattractant protein-1 in endothelial cells. Blood. 1999;93:857–865. [PubMed] [Google Scholar]
- 29.Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP) J Biol Chem. 1999;274:30858–30863. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]
- 30.Stein B, Baldwin AS, Jr, Ballard DW, Greene WC, Angel P, Herrlich P. Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 1993;12:3879–3891. doi: 10.1002/j.1460-2075.1993.tb06066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemoto S, DiDonato JA, Lin A. Coordinate regulation of IkappaB kinases by mitogen-activated protein kinase kinase kinase 1 and NF-kappaB-inducing kinase. Mol Cell Biol. 1998;18:7336–7343. doi: 10.1128/mcb.18.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JM, Cho SJ, Oh YK, Jung HY, Kim YJ, Kim N. Nuclear factor-kappa B activation pathway in intestinal epithelial cells is a major regulator of chemokine gene expression and neutrophil migration induced by Bacteroides fragilis enterotoxin. Clin Exp Immunol. 2002;130:59–66. doi: 10.1046/j.1365-2249.2002.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han SW, Sa KH, Kim SI, Lee SI, Park YW, Lee SS, et al. CCR5 gene polymorphism is a genetic risk factor for radiographic severity of rheumatoid arthritis. Tissue Antigens. 2012;80:416–423. doi: 10.1111/j.1399-0039.2012.01955.x. [DOI] [PubMed] [Google Scholar]