Abstract
The phenomenon of coral fluorescence in mesophotic reefs, although well described for shallow waters, remains largely unstudied. We found that representatives of many scleractinian species are brightly fluorescent at depths of 50–60 m at the Interuniversity Institute for Marine Sciences (IUI) reef in Eilat, Israel. Some of these fluorescent species have distribution maxima at mesophotic depths (40–100 m). Several individuals from these depths displayed yellow or orange-red fluorescence, the latter being essentially absent in corals from the shallowest parts of this reef. We demonstrate experimentally that in some cases the production of fluorescent pigments is independent of the exposure to light; while in others, the fluorescence signature is altered or lost when the animals are kept in darkness. Furthermore, we show that green-to-red photoconversion of fluorescent pigments mediated by short-wavelength light can occur also at depths where ultraviolet wavelengths are absent from the underwater light field. Intraspecific colour polymorphisms regarding the colour of the tissue fluorescence, common among shallow water corals, were also observed for mesophotic species. Our results suggest that fluorescent pigments in mesophotic reefs fulfil a distinct biological function and offer promising application potential for coral-reef monitoring and biomedical imaging.
Introduction
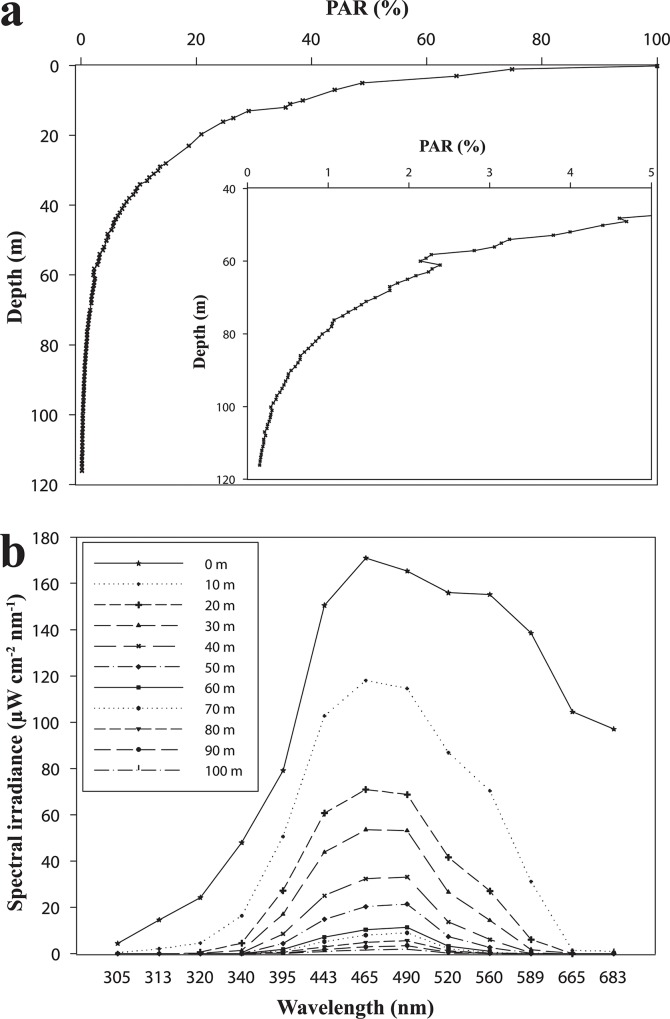
The obligatory symbiotic association with photosynthetic algae of the genus Symbiodinium (zooxanthellae) renders the major builders of shallow, warm-water reef scleractinian corals, dependent on sufficient amounts of photosynthetically-active radiation (PAR) [1–4]. Both the intensity and spectral composition of the irradiating sunlight change dramatically with increasing water depth [5–7]. Ultraviolet and red wavelengths are gradually removed from the spectrum resulting in a blue-green underwater light field at greater depths (Fig 1).
Fig 1. Changes in downwelling photosynthetic active radiation (PAR) and spectral composition of the underwater light in different depths in the Gulf of Aqaba off the Interuniversity Institute in Eilat on the 29th October 2015.
(a) Percentage of PAR at various depths expressed as % of surface PAR (1400 μmol*m-2*s-1). The inset shows the values for mesophotic depths. (b) Depth-dependent changes in spectral irradiance.
In the shallowest parts of a reef, corals and their zooxanthellae often experience stress due to excessive light exposure. With increasing water depth, light becomes limiting rather quickly for the coral-zooxanthellae symbiosis [5,8,9]. Zooxanthellate corals are often most abundant and diverse at depths down to 30 m [10,11]. However, numerous species also occur at mesophotic depths (30–150 m), among them Leptoseris fragilis, with a distribution that extends to a maximal depth of ~145 m [11]. Light can thus be considered one of the most important environmental factors structuring coral-reef communities, confronting both the coral host and the algal symbiont with a range of physiological challenges. For instance, mesophotic reef corals live at depths at which high light stress is not relevant, but in which their algal symbionts need to succeed under a limited supply of PAR. Fluorescence has been suggested to enhance the performance of zooxanthellae in low-light environments at depths down to 40 m [12]. Interestingly, “light-harvesting” by fluorescent chromatophores has been found to extend the distribution of the zooxantellate coral L. fragilis to depths beyond 100 m in the Red Sea [6]. In this coral, as well as in other deep-water representatives of the family Agariciidae from the Red Sea, autofluorescent chromatophores were found in the oral gastrodermis emitting brilliant blue/turquoise fluorescence (between 430 and 500 nm wavelength) under excitation with light at 365–410 nm [13,14]. The chromatophores are organised in dense layers underneath the zooxanthellae and are thought to transform the irradiating light from shorter into longer wavelengths, thereby enhancing algal photosynthesis. Some of the pigments could be extracted in chloroform, but the biochemical nature of the chromophores is as yet unknown [6]. The fluorescence of colour morphs of Leptoseris spp. from mesophotic depths in Hawaii could be assigned to chromophores with spectral properties indicative of the presence of cyan and green fluorescent representatives from green fluorescent protein (GFP) family [15].
In shallow-water corals, GFP-like fluorescent proteins are among the most prevalent pigments [16]. Together with the biochemically closely-related non-fluorescent chromoproteins (CPs), these fluorescent proteins (FPs) are responsible for most of the green, orange-red and purple-blue colours of reef corals [16]. The colour diversity arises from variations in the chromophore-forming amino acid triplet and the interactions of the chromophore with the surrounding protein scaffold [17,18]. In many shallow-water corals, the production of the pigments is regulated at the transcriptional level by light, in particular from the blue spectral range [19]. A light-driven upregulation of FPs and CPs is also involved in growth-related processes such as repair of mechanical damage or defence against epibionts [20]. Other environmental factors including temperature stress can also affect the expression of FPs and CPs [21–23]. The genes coding for these proteins occur in multiple copies in the coral genomes and the striking colour polymorphisms of reef corals are associated with large intraspecific differences in expression levels resulting from variation in the number of actively expressed genes [24].
Both FPs and CPs have been suggested to function as sunscreens for the corals and their symbionts [25–28], and recent experimental studies have provided evidence of their ability to reduce photo damage in zooxanthellae under acute light stress [24,29]. FPs have also been found in corals such as Montastraea cavernosa, Lobophyllia hemprichii and Galaxea fascicularis [30–33] which typically grow in lower-light habitats. In these species the expression of the pigments is less dependent on the intensity of irradiating light compared to that of acroporids from shallow-water habitats [24,31,32]. The differences in gene regulation support the notion that GFP-like proteins fulfil multiple functions in different light environments [19,32].
Up to now, the role of fluorescence in corals in low-light habitats at depths >40m has remained largely unstudied due to the difficulties in accessing these habitats using standard SCUBA diving. Recent advantages in technical diving approaches [34,35] and fluorescence detection techniques [36–38] facilitate the exploration of coral fluorescence in mesophotic corals. Here, we provide first insights in the prevalence, spectral properties and regulation of coral fluorescence in a mesophotic coral community in the Red Sea in Eilat, Israel.
Materials and Methods
Coral collection
Coral samples were collected under the permits No. 40613, No. 40621, No. 40230, No. 38726 and No. 38249 issued by the Israel Nature and Parks Authority. Sixteen mesophotic stony coral species, comprising Acropora squarrosa, Alveopora allingi, Alveopora ocellata, Blastomussa merleti, Echinophyllia aspera, Euphyllia paradivisa, Favites cf.bestae, Goniopora minor, Goniopora somaliensis, Leptoseris glabra, Leptoseris sp., Oxypora egyptensis, Pleuractis granulosa, Stylocoeniella guentheri, Stylophora pistillata, Turbinaria reniformis, and two corallimorpharians: Discosoma unguja and Rhodactis rhodostoma, were collected from 50–60 m depth off Dekel Beach (29°32'20.02"N 34°56'44.80"E) and the IUI (29°30'6.19"N 34°55'3.64"E) for examination of their fluorescence spectra. In addition, four dominant shallow coral species: Acropora sp., Hydnophora exesa, Pocillopora damicornis and Stylophora pistillata, were collected from 1–25 m depth off the IUI. One to three fragments (~10 cm) collected from different colonies were photographed in situ with a standard underwater camera and with a fluorescence imaging system which was composed of a standard Cannon 5DII SLR camera with a Tiffen #12 yellow filter and Xenon strobes fitted with blue filters for illumination [37]. Fluorescent images of G. somaliensis were taken in situ using the excitation produced by the natural blue light prevalent at this depth in order to prevent polyp retraction, which is promoted by changes in light level in Goniopora species.
Representative specimens were then collected and transferred to the IUI laboratory in separate Ziploc bags and stored in black plastic containers for further analyses. Samples of S. pistillata were collected from a depth range of 1–65 m. We sampled horizontal branches with equal exposure to down-welling light on the upper part and bottom reflectance on the lower part of the branches. The fragments were kept in the IUI flow-through aquarium system inside a dark room until they were photographed and fluorescence spectra were recorded.
Characterisation of the underwater light field
Downwelling spectral irradiance measurements were conducted using a profiling reflectance radiometer PRR800 (Biospherical Instruments Inc., San Diego, USA) with 19 channel detection (300–900nm) and an integrated Photosynthetically Active Radiation (PAR) sensor. The instrument was deployed at midday (11:00–13:00) on the 29th October 2014 from a boat, using the free fall technique in order to avoid shading or reflectance by the vessel and to keep the light sensor in a vertical position. The instrument was lowered from the boat while its distance from sea bed was kept constant (~1 m) comparing the boat sonar data to the depth sensor data of the instrument. Data were analysed using PROFILER software (Biospherical Instruments Inc., San Diego, USA).
Depth distribution and fluorescence of prevalent coral species
Fluorescence in shallow water corals (depths between 0.5 and 3 m) was surveyed at night using a blue-light torch and yellow-barrier filter goggles (Nightsea). The reefs in front of the IUI were assessed over a 500 m transect.
Additionally, coral fluorescence was explored during technical SCUBA diving along one belt transect of 50 m2 at depths of 2, 5, 10, 15, 40, 50 and 65 m using the underwater fluorescence imaging system for excitation and fluorescence detection. Four localities were explored (Dekel Beach, Oil Jetty, Nature Reserve and IUI) in the Gulf of Eilat/Aqaba during 2010 to 2015. All localities had a fringing reef structure and mesophotic submerged bunks down to a maximal depth of 72 m. Coral identification was achieved by (1) using keys provided by Veron [39,40], Scheer & Pillai [41], Wallace [42] and Hoeksema [43], (2) comparison with the coral collection at the Tel-Aviv University Steinhardt Museum of Natural History and National Research Center, and (3) evaluation by expert coral taxonomists.
Coral culture
Five to ten fragments from each colony of the studied E. paradivisa colonies were kept in “light” and “dark” conditions in the flow-through aquarium system at the IUI for one year. Light levels experienced by the “light”-treated fragments were determined with a Li-Cor LI-193SA 4 pi probe attached to a Li-1400 data logger (Li-Cor) at noon on several days over the different seasons. The upper parts of the corals were exposed to maximum fluxes of 10–20 μmol photons m-2 s-1. The “dark”-treated fragments were kept in complete darkness. At the end of the experiment, the fluorescence of the corals from the different treatments was documented by spectroscopic measurements and photography (see below).
Replicate fragments of a red colour morph of Echinophyllia sp. were cultured in the experimental mesocosm of the Coral Reef Laboratory at the University of Southampton [44]. To test the effect of short wavelengths on the coral fluorescence, the five replicate corals were exposed to light with different spectral compositions but with identical photon fluxes of ~20 μmol photons m-2 s-1 for six months. One group was kept under full spectrum white light (containing violet wavelengths) provided by a metal halide lamp fitted with an Aqualine 10000 burner (13000 K; AquaMedic). The other group was incubated under cyan light (~480nm, lacking violet wavelengths <430 nm) emitted from Aqua Ray Marine Blue LEDs (Tropical Marine Centre).
Fluorescence photography in the laboratory
Coral fluorescence was photographed through a yellow long-pass filter (Nightsea) upon excitation of the tissue fluorescence with a ~450-nm light source (Nightsea). Standardised conditions (excitation light intensity and exposure times) were established to produce images that allow a comparison of tissue fluorescence intensity.
Fluorescence spectroscopy
Fluorescence was excited with blue light torches (440–460 nm) and a yellow long-pass filter (Nightsea) to block out the excitation light.
Fluorescence emission spectra were measured with a USB2000 spectrometer and analysed with the SpectraSuite—Spectrometer Operating Software (Ocean Optics Inc.). The background was subtracted and the spectra were smoothed by a running median smoother in SigmaPlot 12 (Systat Software Inc.).
Fluorescence emission spectra of the aquarium-cultured specimens of Echinophyllia sp. were recorded with a fibre optic probe coupled to a Cary Eclipse fluorescence spectrometer (Varian). A 0.5 cm spacer attached to the probe was used to measure the fluorescence of comparable areas and enable a quantitative comparison of tissue fluorescence between the experimental samples from the different light treatments.
Photoconversion under the fluorescence microscope
Following a six-month exposure to cyan light lacking violet wavelengths, the tissue fluorescence of Echinophyllia sp. was imaged with an MZ10 Olympus fluorescence microscope equipped with a long-pass GFP filter set (AHF). Green-to-red photoconversion of the fluorescent pigments was induced by local exposure to ~380 nm light for 10 minutes using a UV filter set (AHF). The photoconversion was subsequently imaged using the above mentioned GFP filter set.
Results
Green fluorescence in shallow water corals was found in most of the colonies surveyed in the reefs in front of the IUI. Notably, essentially no orange-red coral fluorescence was observed with the exception of two orange-red fluorescent individuals, one Dipsastraea sp. coral and one corallimorpharian (Discosoma sp.), both of which were growing in crevices underneath massive corals.
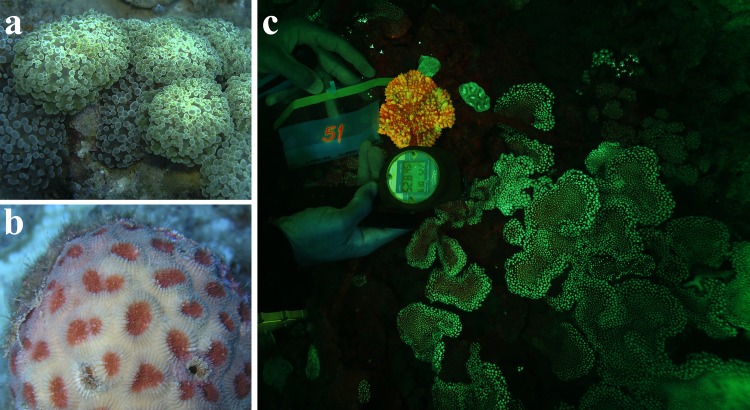
Visual inspection of corals within the belt transects in depths between 40–65 m revealed that green and orange-red fluorescence excited by the natural, blue-dominated environmental light was widespread and often bright enough to be detected by the naked eye or by a standard underwater camera. The fluorescence imaging system proved helpful in improving the contrast between the fluorescence signal and the background (Fig 2).
Fig 2. In-situ survey of fluorescence in mesophotic corals.
(a) Green fluorescence of Euphyllia paradivisia and (b) Orange-red fluorescence of Dipsastraea favus photographed under excitation by the blue-dominated natural light at depths of ~60 m. (c) Fluorescence imaging system photography showing the orange-red fluorescence emission of Echinophyllia aspera and the green fluorescence of Alveopora ocellata at ~53 m prior to collection for laboratory analyses.
Spectral properties
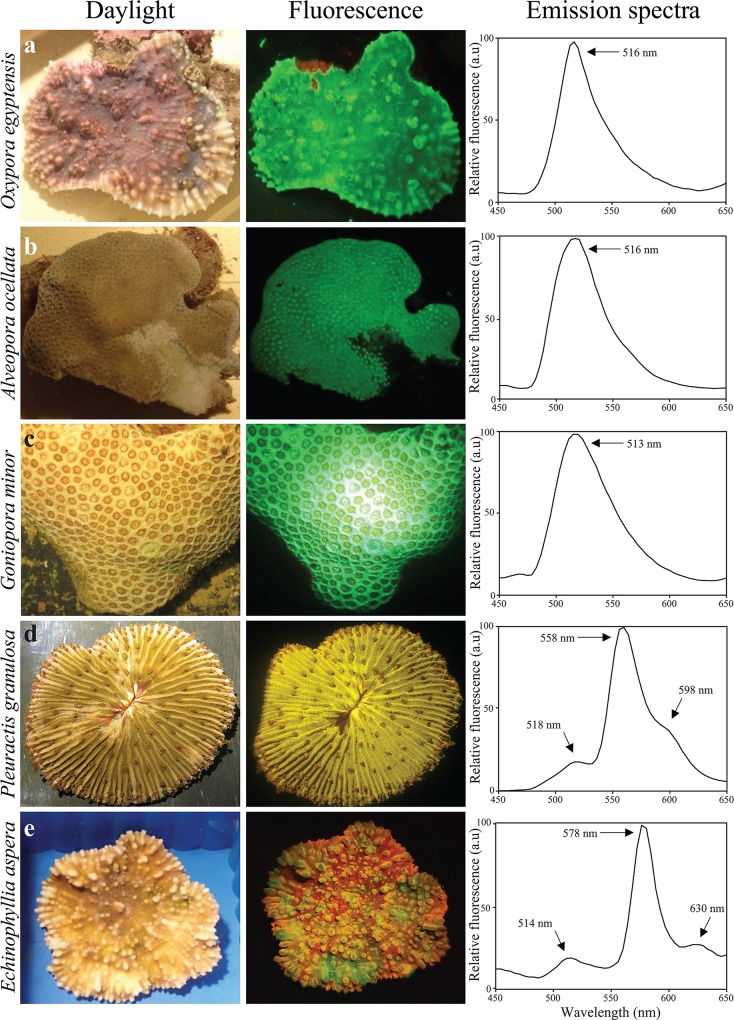
We collected corals from mesophotic depths with representative and visually significant fluorescence in order to gain an overview of the range of the fluorescent pigments present at these depths. After transfer to the laboratory, the fluorescence emission of the living corals was immediately recorded (Figs 3–5, Table 1). Green fluorescence with maxima in the range of 512–522 nm could be found in essentially all examined species. Yellow fluorescence with emission maxima between 522 and 558 nm was detected in more than a third of the species. Emission maxima in the orange-red range from 578 to 584 were recorded in six species. Most of the fluorescence spectra were dominated by a single, narrow peak characteristic of GFP-like proteins [18]. The spectroscopic data of some individuals of E. paradivisa and G. somaliensis showed variable contributions of green and yellow fluorescent pigments with overlapping emission spectra.
Fig 3. Fluorescence signature of corals collected from mesophotic depths in the Gulf of Eilat/Aqaba.
Photographs show the appearance of representative specimens under daylight (left column) or imaged under blue light through a yellow long-pass filter (Nightsea) (middle column). Fluorescence spectra (right column) were recorded after excitation with 450 nm light. Peak positions are indicated by arrows. (a) Oxypora egyptensis, (b) Alveopora ocellata, (c) Goniopora minor, (d) Pleuractis granulosa and (e) Echinophyllia aspera.
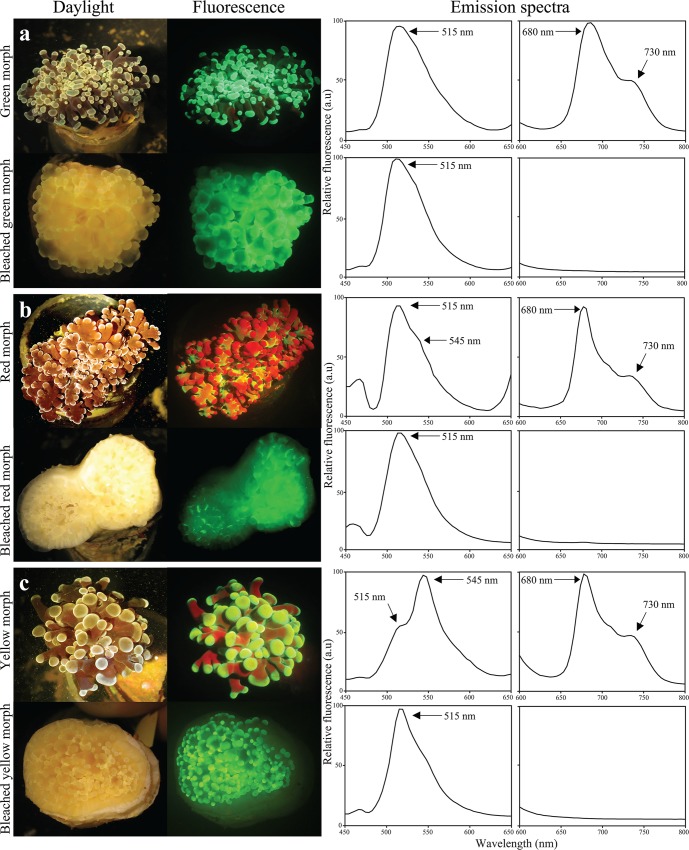
Fig 5. Changes in fluorescence of colour morphs of Euphyllia paradivisa from mesophotic depths in response to altered light environments.
The daylight appearance and blue-light- induced fluorescence of the green (a), red (b) and yellow (c) colour morphs are imaged after one year of culture under low intensity daylight (upper row) and in complete darkness (lower row). Fluorescence emission spectra in the range of GFP-like proteins (450–650 nm) and chlorophyll (650–800 nm), measured by excitation with ~450 nm, are shown for the displayed individuals. Peak positions are indicated by arrows.
Table 1. Fluorescence emission maxima of corals and corallimorpharians from Eilat reefs.
| Species | Collection depth (m) | Green (nm) | Yellow (nm) | Red (nm) | Red sea depth range (m) |
|---|---|---|---|---|---|
| Scleractinian corals: | |||||
| Acropora sp. | 6 | - | - | - | 1–60 a |
| Acropora squarrosa | 5,60 | 522 | - | - | 5–60 a , b |
| Alveopora allingi | 50,60 | 522 | - | - | 20–65 a |
| Alveopora ocellata | 50,60 | 516 | - | - | 30–65 a |
| Blastomussa merleti | 50 | 520 | - | - | 5–60 a , b |
| Echinophyllia aspera | 53,58 | 514 | 535 | 578 (630) | 30–60 a |
| Euphyllia paradivisa | 50 | 515 | 545 | - | 36–72 c |
| Favites cf. bestae | 60 | 512 | - | - | 5–60 a |
| Goniopora minor | 50 | 513 | - | - | 40–65 a |
| Goniopora somaliensis | 55,60 | 512 | 530 | 583 | 40–65 a |
| Hydnophora exesa | 6 | 512 | - | - | 1–25 a , b |
| Leptoseris glabra | 60 | 516 | - | - | 20–72 a |
| Leptoseris sp. | 60 | 513 | - | - | 20–60 a |
| Oxypora egyptensis | 60 | 516 | - | 580 | 30–60 a |
| Pleuractis granulosa | 50 | 518 | 558 | 598 | 40–50 a |
| Pocillopora damicornis | 6 | 507 | 523 | - | 1–40 a , b |
| Stylocoeniella guentheri | 62 | 513 | 524 | - | 5–65 a , b |
| Stylophora pistillata | 1,3,5,10,25,60,65 | 516 | - | - | 0–65 b , d |
| Turbinaria reniformis | 60 | 512 | - | - | 3–60 a |
| Corallimorpharians: | |||||
| Rhodactis rhodostoma | 55 | - | - | 584 | 0.5–65 a , e |
| Discosoma unguja | 55 | 513 | - | - | 0.5–60 a , f |
The dominant emission peaks are indicated in bold numbers.
a This study
b WoRMS Editorial Board (2015). World Register of Marine Species. Available from http://www.marinespecies.org at VLIZ
c Eyal et al, unpublished
d [45]
e [46]
f [47]
In E. aspera, the typical signature of green-to-red photoconvertible proteins was detected [17,32,48]. The emission spectrum shows a local maximum at ~514 nm which can be attributed to the green, unconverted chromophore, and a second peak at ~578 nm with the characteristically pronounced vibronic sideband at ~630 nm, emitted by the red, photoconverted chromophore (Fig 3).
All species showed detectable chlorophyll fluorescence with an emission maximum around ~680 nm. Representative chlorophyll emission spectra in the corals G. somaliensis and E. paradivisa are shown in Figs 4 and 5, respectively.
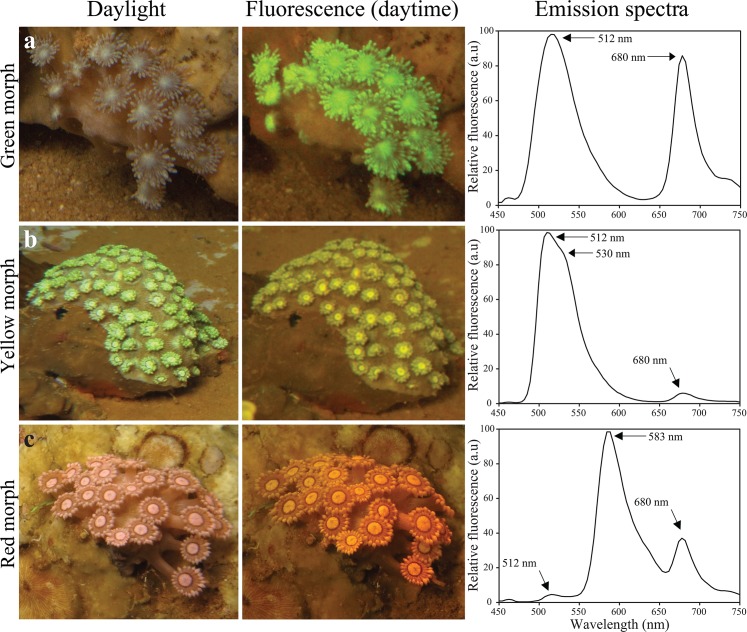
Fig 4. Colour polymorphism of Goniopora somaliensis.
Representative colonies of the (a) green, (b) yellow and (c) red colour morphs were imaged in situ at 50–60 m depth by flashlight photography (left column). Their fluorescence, excited by the natural, down-welling blue-dominated light during daytime was bright enough to be photographed using a yellow long-pass barrier filter (Nightsea) (middle column). Fluorescence emission spectra were recorded under blue light excitation (450 nm) after transfer of the corals to the laboratory (right column). Peak positions are indicated by arrows.
Fluorescence patterns and colour polymorphisms
Many species collected from depths >50 m were characterised by a uniform distribution of fluorescence over the upper colony surface: e.g. O. egyptensis, A. ocellata, G. minor, P. granulosa and E. aspera (Fig 3), while G. somaliensis was represented by three distinct colour morphs which could be easily distinguished by their green, yellow and orange-red fluorescence (Fig 4). The colour morphs also showed differences in the tissue-specific accumulation of the pigments. Whereas in the green and yellow morphs the fluorescence was concentrated in the polyps, the fluorescent pigments in the red morph were distributed over the whole colony. In E. paradivisa too, three colour morphs (green, yellow and red) could be distinguished based on the spectral signature and the localisation of tissue fluorescence (Fig 5). In the green and yellow morphs, fluorescence was concentrated in the tips of the tentacles. In the red morph, in contrast, green light emission was evenly distributed over the length of the tentacles and absent from the tentacle tips, which showed instead a dark red fluorescence emitted by the chlorophyll of the zooxanthellae.
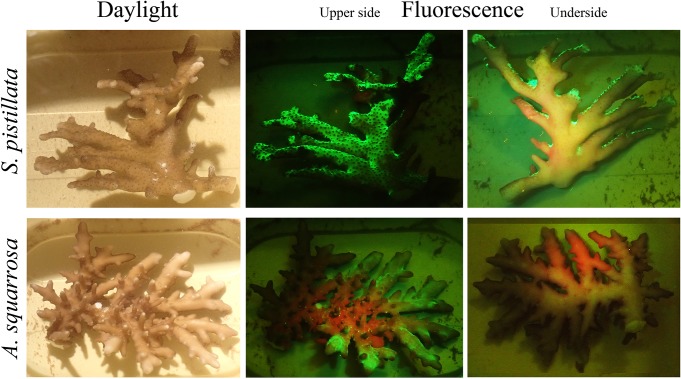
Deep-water individuals of the branching species S. pistillata and A. squarrosa showed a flattened branch morphology. In both cases, the green fluorescence was restricted to the upper part of the branches (Fig 6).
Fig 6. Fluorescence of S. pistillata and A. squarrosa from depths ≥60 m.
Images, taken after the specimens were collected, show the daylight appearance (left column) and the blue-light-induced fluorescence of the upper and underside of the branches (middle/right column).
Light-dependent accumulation of fluorescent pigments
We incubated replicate nubbins of the different colour morphs of E. paradivisa for 12 months under low-intensity full spectrum daylight and in complete darkness. The photosynthetic pigments of the zooxanthellae were lost completely from the specimens in the dark treatment as evident from the bleached appearance of the corals and lack of the chlorophyll emission peak at ~680 nm (Fig 5). The yellow fluorescence too (emission maximum at ~545 nm) vanished completely. However, under these conditions, the intensity of the green tentacle fluorescence remained essentially unaltered.
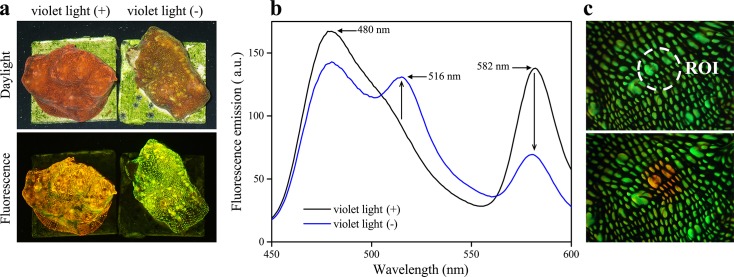
Accumulation of fluorescent pigments was restricted to the upper side of outer branch parts in S. pistillata and A. squarrosa collected from ≥60 m depth (Fig 6). Long term experimental exposure of Echinophyllia sp. to low intensity of ~480 nm-light, lacking wavelengths <430 nm resulted in a greening of the otherwise orange-red fluorescent corals (Fig 7). Short-term local exposure with light around ~380 nm resulted in a green-to-red photoconversion of tissue fluorescence.
Fig 7. Changes in the tissue fluorescence of Echinophyllia sp. in response to experimentally-altered light environments.
(a) The daylight appearance (upper panel) and blue-light-induced fluorescence (lower panel) were imaged after 6 months of culture under low intensity, full spectrum white light (violet light [+]) or cyan light (~480 nm, violet light [-]). (b) Fluorescence emission per area upon excitation with 420 nm light was quantified for violet light [+] and violet light [–] treated corals. Spectra represent the means of 10 individual measurements taken from 3 different replicate colonies exposed to the corresponding light environments. Peak positions are indicated by arrows. (c) A violet light [–] treated specimen was photographed with a Leica digital video camera attached to an Olympus fluorescence stereomicroscope using a GFP filter set before (upper image) and after (lower image) exposure of the region of interest (ROI) to focused 380 nm light.
Discussion
Spectral properties
Our assessment of coral fluorescence in Eilat reefs in shallow water (depths between 0.5 and 3 m) encountered essentially no yellow (rarely found also in other reef regions [16]) or red fluorescent specimens. In contrast, green, yellow and red fluorescent pigments were found frequently in corals from mesophotic depths ≥50m. Fluorescence at depths >40m is readily visible by eye and can be documented photographically even without optical filter systems owing to the fact that at these depths longer wavelengths are depleted relative to the fluorescence-exciting shorter wavelengths (Fig 1). Thereby, the contrast of the emitted light in the yellow-green and orange-red spectral region is enhanced.
The specific signature of the emission spectrum of E. aspera with emission maxima at 514 nm and 578 nm identified its dominant pigment as a photoconvertible protein of the class of green-to-red photoconvertible proteins [32,48]. The chromophore of this specific type of GFP-like protein requires exposure to light in the spectral range between 360 to 430 nm (maximal efficiency at 390 nm) in order to undergo the irreversible photochemical reaction that changes the chromophore from a green(514 nm) to a red (578 nm) emitting form [48]. A GFP-like protein with an amino acid sequence with high similarity to the green-to-red photoconvertible EosFP has been isolated from Echinophyllia echinata [16].
Several previously studied fluorescent proteins show a reversible increase of their fluorescence intensity and small shifts in the position of their emission maxima with increasing pressure in the range of 0.1–500 MPa [49,50]. The fluorescence intensity of the red fluorescence protein dsRed, for example, was maximally increased by ~8% at a pressure of 200 MPa as compared to normal atmospheric levels [49]. Since the coral pigments in mesophotic depths are exposed to increased pressure of only ~0.5–1.1 MPa, it is unlikely that their spectral properties in situ deviate substantially from those determined in our measurements at atmospheric pressure
Our analysis of 16 species of corals (Table 1) from mesophotic depths did not detect a significant contribution of cyan fluorescence with emission maxima in the range of 470–500 nm to the overall fluorescence of these animals. In contrast, cyan fluorescent proteins are frequently encountered in shallow water corals and can dominate the quality of light emission of several species [16,19]. A pronounced cyan fluorescent maximum was detected in the strain of Indo-Pacific Echinophyllia sp. that was cultured for the long-term laboratory experiments and in colour morphs of M. cavernosa [30,32], suggesting that the absence of CFP might not be a uniform feature of corals with a preference for less light-exposed habitats.
Fluorescence patterns and colour polymorphism
Shallow-water corals display a broad range of fluorescence patterns that result from the tissue-specific expression of GFP-like proteins [51]. Many of the corals from depths >50 m in this work showed a rather homogenous distribution of light emission over the whole colony. Exceptions were found among individuals of E. paradivisa, which showed a variable localisation of the fluorescent pigments in the tentacles; and among representatives of G. somaliensis, in which the fluorescence was either distributed evenly over the colony or restricted to the polyps. The latter two species also showed a pronounced colour polymorphism related to variations in the expression of the dominant fluorescent pigment, a phenomenon that is common among anthozoans [30,32,52–54]. As demonstrated for the coral Acropora millepora, these colour polymorphisms can be the result of the pigment-encoding genes being present in multiple copies [55]. Depending on how many genes are active, the corals will become more or less colourful. In shallow waters, where the GFP-like proteins can fulfil a photoprotective function, this genetic framework can enable corals to invest either in expensive high-level pigmentation, offering benefits under light stress, or to rely on low tissue pigment concentrations and use the conserved resources for other purposes, a preferable strategy in low light-exposed environments.
Regulation of pigment production
Previous studies showed that the expression of GFP-like proteins in shallow water corals is often upregulated at the transcriptional level by the light intensity, specifically by blue light [19]. At low-light intensities, the tissue fluorescence decreases, usually to almost undetectable levels [19,24].
In contrast, corals such as L. hemprichii or M. cavernosa, which frequently occupy less light-exposed habitats showed a constitutive expression of their fluorescent pigments [31,32].
We incubated Echinophyllia sp. and E. paradivisa under reduced light intensity to examine whether the constitutive expression of fluorescent pigments was a common phenomenon in corals from low-light environments.
Long-term experimental exposure of Echinophyllia sp. to low intensity of cyan light (~480 nm), which lacked wavelengths <430 nm resulted in a colour change from orange-red to green. This greening could be reversed by a brief, local exposure to short wavelength light (~380 nm). A comparable response was previously observed for the red fluorescent protein mcavRFP in the tissue of M. cavernosa, suggesting that the exposure to short-wavelength light is essential for these corals to develop their dominant red tissue fluorescence [31]. Since the production of the pigment in its native green state is sustained at low light levels [31], the relevant genes are most likely constitutively expressed and light is only required for the post-translational modification of the pigments. The E. aspera specimens analysed in the present study showed bright orange-red fluorescence in situ at a depth of >50 m, indicating that even at this depth, the underwater light field contains sufficient amounts of photons in the short—wavelength range to induce efficient photoconversion.
Variable responses were observed after prolonged incubation of the different colour morphs of E. paradivisa in darkness. All the morphs completely lost the chlorophyll fluorescence of the zooxanthellae. Accordingly, the prevailing red emission of the red morph was lost. In contrast, in all the morphs, the green fluorescence with an emission maximum at ~515 nm persisted essentially unaltered in corals cultured in the dark. This indicates that the relevant genes are constitutively expressed.
Interestingly, the 545 nm-peak of the yellow fluorescent pigment (most pronounced in the yellow morph) was not detected in the spectra measured after dark incubation. This may indicate that this pigment is either a yellow fluorescent protein with a light-dependent expression or that it represents a derivative of the green fluorescent emitter that is formed in a light-dependent reaction. Further studies will elucidate the putative mechanism responsible for this observation.
The restricted accumulation of green fluorescent pigments in the upper-side of S. pistillata and A. squarrosa branches collected from ≥60 m depth suggests an influence of the irradiating light on the regulation of pigment production. This localised accumulation in the most light-exposed branch parts has been frequently observed for light-regulated GFP-like proteins in shallow water corals, in particular among acroporids [24,29].
Biological function of fluorescence at mesophotic depths
Recent studies have demonstrated the ability of both FPs and CPs to fulfil a photoprotective function for the zooxanthellae in some shallow-water corals by screening photosynthetically-active radiation (PAR) [24,29]. In line with their function, these photoprotective pigments are tightly controlled by the light environment. The need for photoprotection can be ruled out for corals from mesophotic reefs and may explain the absence of dominant cyan fluorescence at greater depths, since the absorption properties of these pigments appear to be well suited for screening of algal chlorophyll [19]. Nevertheless, species such as S. pistillata with a broad depth distribution ranging from very shallow water to mesophotic depths (Table 1), may still reveal the light-mediated regulation mechanisms associated with the photoprotective function that fluorescence fulfils in the shallow water population.
We have found that a number of brightly fluorescent species, including E. aspera and E. paradivisa, have their maximal abundance at depths deeper than 30 m. The production of individual pigment molecules might be considered comparably cheap [31], since only a single gene is required to produce the functional FP and their turnover is slow, presumably due to the stable beta-can fold. However, the high tissue concentrations of up to 7–14% of the total soluble protein [31,32] can only be reached by the simultaneous expression of multiple copies of the same gene [24]. Hence, maintaining high tissue fluorescence is energetically costly, and it is therefore very likely that the fluorescent pigments in mesophotic corals have a particular biological function that differs from photoprotection. This assumption is supported by the light-independent, constitutive expression of their FP genes, which is atypical for the photoprotective pigments from shallow water corals [31,32]. Furthermore, in contrast to shallow water corals, yellow and orange-red fluorescence were more abundant in the species with a preference for greater depths (Table 1). Also Leptoseris spp. from Hawaii showed a shift of the dominant FPs from shorter (cyan) in shallow waters to longer (green) wavelengths in mesophotic depths [15]. The trend that fluorescence in mesophotic corals tends to be red-shifted compared to the shallow water representatives further points to a distinct biological function in corals from deeper habitats. Alternative functions of FPs, which have been discussed, include modulation of the activity of regulatory photosensors analogous to phytochromes and cryptochromes of higher plants [56], links to visual ecology of the reef fishes [57,58] and PAR enhancement [12]. Future experimental studies are required to confirm the function of fluorescence in mesophotic corals.
Coral reef monitoring
Coral fluorescence can be detected with a high signal-to-background ratio and has been suggested as an indicator of coral health and live coral cover in reef-monitoring approaches [19,20,59–61]. Indeed, the ability to detect coral recruits in situ is increased by screening for their fluorescence [62,63]. The use of advanced wide field-of-view fluorescence imaging shows the potential of coral fluorescence to be used in semi-automated image analysis for habitat mapping [37]. Since coral fluorescence in mesophotic reefs appears to be widespread, bright and spectrally diverse, fluorescence imaging represents a promising approach for the monitoring of mesophotic reefs.
Bio-prospecting
Fluorescent proteins of the GFP-family have revolutionised biomedical research, as in vivo labels of proteins or markers of gene expression [64,65]. The range of differently coloured FPs isolated from marine invertebrates has facilitated multicolour imaging of biological processes in live cells, while photoconvertible proteins have enabled advanced tracking of dynamic processes in living cells and organisms, such as protein movement [66,67]. Furthermore, they are also essential tools for super-resolution microscopy [68]. Red fluorescent proteins are particularly desirable for imaging application, since their detection is facilitated by the better penetration of cells and tissues by long-wavelength light and reduced cellular autofluorescence in the red emission range [69]. The high number of long-wave emitting FPs that we have encountered in the present study suggests that mesophotic reefs may hold novel marker proteins useful for biomedical research applications.
Acknowledgments
We would like to thank the staff of Interuniversity Institute for Marine Sciences of Eilat (IUI) for their invaluable support and for hosting the Second International Mesophotic Workshop (MCEIsrael). We are grateful to M. Pichon and Z. Dinesen for their help with the coral taxonomy, to N. Paz for proofreading the manuscript and to all members of the Loya-lab for their support.
Data Availability
All relevant data are within the paper.
Funding Statement
GE was supported by the Israel Taxonomy Initiative (ITI) and Sciences-based Management (SBM) doctoral Fellowships (http://taxonomy.tau.ac.il/en/). YL acknowledges funding by the Israel Science Foundation (ISF) (http://www.isf.org.il) grant No. 341/12, USAID/MERC grant No. M32-037, The Israel Ministry of Environmental Protection (MoEP) (http://www.sviva.gov.il/english/pages/homepage.aspx) and the Israel Nature and Parks Authority (INPA) (http://www.parks.org.il/parks/Pages/WhoWeAre.aspx). JW/CDA acknowledge funding from Natural Environment Research Council (http://www.nerc.ac.uk/) (NE/I01683X/1, NE/I01683X/1 & NE/I012648/1), Deutsche Forschungsgemeinschaft (DFG) (http://www.dfg.de/en/) (Wi1990/2-1), ASSEMBLE and the European Research Council (http://erc.europa.eu/) under the European Union’s Seventh Framework Programme (ERC Grant Agreement no. 311179) and support from Tropical Marine Centre (TMC) London (http://www.tropicalmarinecentre.co.uk/en/index.aspx). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Falkowski PG, Dubinsky Z (1981) Light-shade adaptation of Stylophora pistillata, a hermatypic coral from the Gulf of Eilat. Nature 28: 172–174. [Google Scholar]
- 2. Edmunds PJ, Davies PS (1986) An energy budget for Porites porites (Scleractinia). Marine Biology 92: 339–347. [Google Scholar]
- 3. Falkowski PG, Jokiel PL, Kinzie RA 3rd (1990) Irradiance and corals In: Dubinsky Z, editor. Ecosystems of the world: Coral Reefs: Elsevier; pp. 89–107. [Google Scholar]
- 4. Muscatine L (1990) The role of symbiotic algae in carbon and energy flux in reef corals In: Dubinsky Z, editor. Ecosystems of the world: Coral Reefs: Elsevier; pp. 5–87. [Google Scholar]
- 5. Dustan P (1982) Depth-dependent photoadaption by zooxanthellae of the reef coral Montastrea annularis. Marine Biology 68: 253–264. [Google Scholar]
- 6. Schlichter D, Fricke HW, Weber W (1986) Light harvesting by wavelength transformation in a symbiotic coral of the Red Sea twilight zone. Marine Biology 91: 403–407. [Google Scholar]
- 7. Mass T, Kline DI, Roopin M, Veal CJ, Cohen S, Iluz D, et al. (2010) The spectral quality of light is a key driver of photosynthesis and photoadaptation in Stylophora pistillata colonies from different depths in the Red Sea. Journal of Experimental Biology 213: 4084–4091. 10.1242/jeb.039891 [DOI] [PubMed] [Google Scholar]
- 8. Lesser MP, Slattery M, Stat M, Ojimi M, Gates RD, Grottoli A (2010) Photoacclimatization by the coral Montastraea cavernosa in the mesophotic zone: light, food, and genetics. Ecology 91: 990–1003. [DOI] [PubMed] [Google Scholar]
- 9. Mass T, Einbinder S, Brokovich E, Shashar N, Vago R, Erez J, et al. (2007) Photoacclimation of Stylophora pistillata to light extremes: metabolism and calcification. Marine Ecology Progress Series 334: 93–102. [Google Scholar]
- 10. Loya Y (1972) Community structure and species diversity of hermatypic corals at Eilat, Red Sea. Marine Biology 13: 100–123. [Google Scholar]
- 11. Fricke HW, Schuhmacher H (1983) The Depth Limits of Red Sea Stony Corals: An Ecophysiological Problem (A Deep Diving Survey by Submersible). Marine Ecology 4: 163–194. [Google Scholar]
- 12. Salih A, Cox G, Szymczak R, Coles SL, Baird AH, Dunstan A, et al. The role of host-based color and fluorescent pigments in photoprotection and in reducing bleaching stress in corals In: Suzuki Y, Nakamori T, Hidaka M, Kayanne H, Casareto BE et al. , editors; 2006 May 20, 2006; Okinawa, Japan:. Japanese Coral Reef Society, Tokyo, Japan; pp. 746–756. [Google Scholar]
- 13. Schlichter D, Fricke HW (1991) Mechanisms of amplification of photosynthetically active radiation in the symbiotic deep-water coral Leptoseris fragilis . Hydrobiologia 216–217: 389–394. 11538047 [Google Scholar]
- 14. Schlichter D, Meier U, Fricke HW (1994) Improvement of photosynthesis in zooxanthellate corals by autofluorescent chromatophores. Oecologia 99: 124–131. [DOI] [PubMed] [Google Scholar]
- 15. Roth M, Padilla-Gaminio J, Pochon X, Bidigare R, Gates R, Smith C, et al. (2015) Fluorescent proteins in dominant mesophotic reef-building corals. Marine Ecology Progress Series 521: 63–79. [Google Scholar]
- 16. Alieva NO, Konzen KA, Field SF, Meleshkevitch EA, Hunt ME, Beltran-Ramirez V, et al. (2008) Diversity and evolution of coral fluorescent proteins. PLoS ONE 3: e2680 10.1371/journal.pone.0002680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nienhaus K, Nienhaus GU, Wiedenmann J, Nar H (2005) Structural basis for photo-induced protein cleavage and green-to-red conversion of fluorescent protein EosFP. Proceedings of the National Academy of Science USA 102: 9156–9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nienhaus GU, Wiedenmann J (2009) Structure, dynamics and optical properties of fluorescent proteins: Perspectives for marker development. Chemphyschem 10: 1369–1379. 10.1002/cphc.200800839 [DOI] [PubMed] [Google Scholar]
- 19. D’Angelo C, Denzel A, Vogt A, Matz MV, Oswald F, Salih A, et al. (2008) Blue light regulation of host pigment in reef-building corals. Marine Ecology Progress Series 364: 97–106. [Google Scholar]
- 20. D’Angelo C, Smith EG, Oswald F, Burt J, Tchernov D, Wiedenmann J (2012) Locally accelerated growth is part of the innate immune response and repair mechanisms in reef-building corals as detected by green fluorescent protein (GFP)-like pigments. Coral Reefs 31: 1045–1056. [Google Scholar]
- 21. Zawada DG, Jaffe JS (2003) Changes in the fluorescence of the Caribbean coral Montastraea faveolata during heat-induced bleaching. Limnology and Oceanography 48: 412–425. [Google Scholar]
- 22.Smith-Keune C, Dove S (2007) Gene expression of a green fluorescent protein homolog as a host-specific biomarker of heat stress within a reef-building coral. Marine Biotechnology: 1–15. [DOI] [PubMed]
- 23. Roth MS, Deheyn DD (2013) Effects of cold stress and heat stress on coral fluorescence in reef-building corals. Scientific Reports 3: 1421 10.1038/srep01421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gittins JR, D'Angelo C, Oswald F, Edwards RJ, Wiedenmann J (2015) Fluorescent protein-mediated colour polymorphism in reef corals: multicopy genes extend the adaptation/acclimatization potential to variable light environments. Molecular Ecology 24: 453–465. 10.1111/mec.13041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kawaguti S (1944) On the physiology of reef corals VI. Study on the pigments. Palao Trop Biol Stn Stud 2. [Google Scholar]
- 26. Kawaguti S (1969) Effect of the green fluorescent pigment on the productivity of the reef corals. Micronesica 5: 313. [Google Scholar]
- 27. Wiedenmann J, Röcker C, Funke W (1999) The morphs of Anemonia aff. sulcata (Cnidaria, Anthozoa) in particular consideration of the ectodermal pigments. Verhandlungen der Gesellschaft fuer Oekologie 29: 497–503. [Google Scholar]
- 28. Salih A, Larkum A, Cox G, Kuhl M, Hoegh-Guldberg O (2000) Fluorescent pigments in corals are photoprotective. Nature 408: 850–853. [DOI] [PubMed] [Google Scholar]
- 29. Smith EG, D’Angelo C, Salih A, Wiedenmann J (2013) Screening by coral green fluorescent protein (GFP)-like chromoproteins supports a role in photoprotection of zooxanthellae. Coral Reefs 32: 463–474. [Google Scholar]
- 30. Kelmanson IV, Matz MV (2003) Molecular basis and evolutionary origins of color diversity in great star coral Montastraea cavernosa (Scleractinia: Faviida). Molecular Biology and Evolution 20: 1125–1133. [DOI] [PubMed] [Google Scholar]
- 31. Leutenegger A, D'Angelo C, Matz MV, Denzel A, Oswald F, Salih A, et al. (2007) It's cheap to be colorful. Anthozoans show a slow turnover of GFP-like proteins. FEBS Journal 274: 2496–2505. [DOI] [PubMed] [Google Scholar]
- 32. Oswald F, Schmitt F, Leutenegger A, Ivanchenko S, D'Angelo C, Salih A, et al. (2007) Contributions of host and symbiont pigments to the coloration of reef corals. FEBS Journal 274: 1102–1109. [DOI] [PubMed] [Google Scholar]
- 33.Ben-Zvi O, Eyal G, Loya Y (2014) Light-dependent fluorescence in the coral Galaxea fascicularis. Hydrobiologia: 1–12.
- 34. Pyle RL (2000) Assessing undiscovered fish biodiversity on deep coral reefs using advanced self-contained diving technology. Marine Technology Society Journal 34: 82–91. [Google Scholar]
- 35. Kahng SE, Copus JM, Wagner D (2014) Recent advances in the ecology of mesophotic coral ecosystems (MCEs). Current Opinion in Environmental Sustainability 7: 72–81. [Google Scholar]
- 36. Mazel CH (2005) Underwater fluorescence photography in the presence of ambient light. Limnology and Oceanography: Methods 3: 499–510. [Google Scholar]
- 37. Treibitz T, Neal BP, Kline DI, Beijbom O, Roberts PLD, Mitchell BG, et al. (2015) Wide field-of-view fluorescence imaging of coral reefs. Scientific Reports 5: 7694 10.1038/srep07694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vogt A, D’Angelo C, Oswald F, Denzel A, Mazel CH, Matz M, et al. (2008) A Green Fluorescent Protein with Photoswitchable Emission from the Deep Sea. PLoS ONE 3: e3766 10.1371/journal.pone.0003766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Veron JEN (1990) New scleractinia from Japan and other indo-west pacific countries. Galaxea 9: 95–173. [Google Scholar]
- 40. Veron JEN (2000) Corals of the world; Stafford-Smith M, editor. Townsville: Australian Institute of Marine Science; [Google Scholar]
- 41. Scheer G, Pillai CSG (1983) Report on the stony corals from the Red Sea. Zoologica 133: 1–198. [Google Scholar]
- 42. Wallace CC (1999) Staghorn corals of the world: a revision of the coral genus Acropora (Scleractinia; Astrocoeniina; Acroporidae) worldwide, with emphasis on morphology, phylogeny and biogeography / Carden C. Wallace; Csiro, editor. Collingwood, Vic: CSIRO Publishing. [Google Scholar]
- 43. Hoeksema BW (1989) Taxonomy, phylogeny and biogeography of mushroom corals (Scleractinia: Fungiidae). Zoologische Verhandelingen 254: 1–295. [Google Scholar]
- 44. D'Angelo C, Wiedenmann J (2012) An experimental mesocosm for long-term studies of reef corals. Journal of the Marine Biological Association of the United Kingdom 92: 769–775. [Google Scholar]
- 45. Einbinder S, Mass T, Brokovich E, Dubinsky Z, Erez J, Tchernov D (2009) Changes in morphology and diet of the coral Stylophora pistillata along a depth gradient. Marine Ecology Progress Series 381: 167–174. [Google Scholar]
- 46. Kuguru B, Winters G, Beer S, Santos SR, Chadwick NE (2007) Adaptation strategies of the corallimorpharian Rhodactis rhodostoma to irradiance and temperature. Marine Biology 151: 1287–1298. 17624159 [Google Scholar]
- 47. Kuguru B, Chadwick N, Achituv Y, Zandbank K, Tchernov D (2008) Mechanisms of habitat segregation between corallimorpharians: photosynthetic parameters and Symbiodinium types. Marine Ecology Progress Series 369: 115–129. [Google Scholar]
- 48. Wiedenmann J, Ivanchenko S, Oswald F, Schmitt F, Rocker C, Salih A, et al. (2004) EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion. Proceedings of the National Academy of Science USA 101: 15905–15910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Verkhusha VV, Pozhitkov AE, Smirnov SA, Borst JW, van Hoek A, Klyachko NL, et al. (2003) Effect of high pressure and reversed micelles on the fluorescent proteins. Biochimica et Biophysica Acta (BBA)—General Subjects 1622: 192–195. [DOI] [PubMed] [Google Scholar]
- 50. Pozzi EA, Schwall LR, Jimenez R, Weber JM (2012) Pressure-Induced Changes in the Fluorescence Behavior of Red Fluorescent Proteins. The Journal of Physical Chemistry B 116: 10311–10316. 10.1021/jp306093h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gruber DF, Kao H-T, Janoschka S, Tsai J, Pieribone VA (2008) Patterns of fluorescent protein expression in scleractinian corals. The Biological Bulletin 215: 143–154. [DOI] [PubMed] [Google Scholar]
- 52. Schnitzler CE, Keenan RJ, McCord R, Matysik A, Christianson LM, Haddock SH (2008) Spectral Diversity of Fluorescent Proteins from the Anthozoan Corynactis californica . Marine Biotechnology 10: 328–342. 10.1007/s10126-007-9072-7 [DOI] [PubMed] [Google Scholar]
- 53. Smith E, D’Angelo C, Oswald F, Nienhaus GU, Wiedenmann J (2012) Fluorescent proteins from the oceans: Marine macromolecules as advanced imaging tools for biomedical research In: Fattorusso E, Gerwick WH, Taglialatela-Scafati O, editors. Handbook of Marine Natural Products: Springer Netherlands; pp. 1231–1257. [Google Scholar]
- 54. Wiedenmann J, D’Angelo C, Nienhaus GU (2012) Fluorescent proteins: Nature’s colorful gifts for live cell imaging In: Jung G, editor. Fluorescent Proteins II: Springer Berlin Heidelberg; pp. 3–33. [Google Scholar]
- 55. Hume BCC, D'Angelo C, Smith EG, Stevens JR, Burt J, Wiedenmann J (2015) Symbiodinium thermophilum sp. nov., a thermotolerant symbiotic alga prevalent in corals of the world’s hottest sea, the Persian/Arabian Gulf. Scientific Reports 5: 8562 10.1038/srep08562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Field SF, Bulina MY, Kelmanson IV, Bielawski JP, Matz MV (2006) Adaptive evolution of multicolored fluorescent proteins in reef-building corals. Journal of Molecular Evolution 62: 332–U315. [DOI] [PubMed] [Google Scholar]
- 57. Matz MV, Marshall NJ, Vorobyev M (2006) Are Corals Colorful? Photochemistry and Photobiology 82: 345–350. [DOI] [PubMed] [Google Scholar]
- 58. Meadows MG, Anthes N, Dangelmayer S, Alwany MA, Gerlach T, Schulte G, et al. (2014) Red fluorescence increases with depth in reef fishes, supporting a visual function, not UV protection. Proceedings of the Royal Society of London B: Biological Sciences 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mazel CH (1997) Diver-operated instrument for in situ measurement of spectral fluorescence and reflectance of benthic marine organisms and substrates. Optical Engineering 36: 2612–2617. 9054568 [Google Scholar]
- 60. Zawada DG, Mazel CH (2014) Fluorescence-Based Classification of Caribbean Coral Reef Organisms and Substrates. PLoS ONE 9: e84570 10.1371/journal.pone.0084570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hedley JD, Mumby PJ (2002) Biological and remote sensing perspectives of pigmentation in coral reef organisms. Advances in Marine Biology 43: 277–317. [DOI] [PubMed] [Google Scholar]
- 62. Piniak G, Fogarty N, Addison C, Kenworthy WJ (2005) Fluorescence census techniques for coral recruits. Coral Reefs 24: 496–500. [Google Scholar]
- 63. Baird AH, Salih A, Trevor-Jones A (2006) Fluorescence census techniques for the early detection of coral recruits. Coral Reefs 25: 73–76. [Google Scholar]
- 64. Day RN, Davidson MW (2009) The fluorescent protein palette: tools for cellular imaging. Chemical Society Reviews 38: 2887–2921. 10.1039/b901966a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chudakov DM, Matz MV, Lukyanov S, Lukyanov KA (2010) Fluorescent proteins and their applications in imaging living cells and tissues. Physiological Reviews 90: 1103–1163. 10.1152/physrev.00038.2009 [DOI] [PubMed] [Google Scholar]
- 66. Wiedenmann J, Nienhaus GU (2006) Live-cell imaging with EosFP and other photoactivatable marker proteins of the GFP family. Expert Rev Proteomics 3: 361–374. [DOI] [PubMed] [Google Scholar]
- 67. Wacker SA, Oswald F, Wiedenmann J, Knochel W (2007) A green to red photoconvertible protein as an analyzing tool for early vertebrate development. Dev Dyn 236: 473–480. [DOI] [PubMed] [Google Scholar]
- 68. Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, et al. (2006) Imaging intracellular fluorescent proteins at nanometer resolution. Science 313: 1642–1645. [DOI] [PubMed] [Google Scholar]
- 69. Wiedenmann J, Oswald F, Nienhaus GU (2009) Fluorescent proteins for live cell imaging: Opportunities, limitations, and challenges. IUBMB Life 61: 1029–1042. 10.1002/iub.256 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.