Abstract
Context:
Resting heart rate variability (HRV) is a measure of the modulation of autonomic nervous system (ANS) at rest. Increased HRV achieved by the exercise is good for the cardiovascular health. However, prospective studies with comparison of the effects of yogic exercises and those of other endurance exercises like walking, running, and swimming on resting HRV are conspicuous by their absence.
Aims:
Study was designed to assess and compare the effects of yogic training and swimming on resting HRV in normal healthy young volunteers.
Settings and Design:
Study was conducted in Department of Physiology in a medical college. Study design was prospective randomized comparative trial.
Subjects and Methods:
One hundred sedentary volunteers were randomly ascribed to either yoga or swimming group. Baseline recordings of digital electrocardiogram were done for all the subjects in cohorts of 10. After yoga training and swimming for 12 weeks, evaluation for resting HRV was done again.
Statistical Analysis Used:
Percentage change for each parameter with yoga and swimming was compared using unpaired t-test for data with normal distribution and using Mann-Whitney U test for data without normal distribution.
Results:
Most of the HRV parameters improved statistically significantly by both modalities of exercise. However, some of the HRV parameters showed statistically better improvement with yoga as compared to swimming.
Conclusion:
Practicing yoga seems to be the mode of exercise with better improvement in autonomic functions as suggested by resting HRV.
Keywords: Autonomic functions, resting heart rate variability, swimming, yoga
INTRODUCTION
Emphasis is being placed worldwide on adopting physical activity into one's lifestyle. The modality of exercise that is most beneficial and economic for masses has now become a topic of research.[1] The conventional exercises (endurance exercises like walking, jogging, running, swimming, cycling, etc.), which give stress on cardiovascular and respiratory systems and test the responses of these systems are very popular. On the other hand, ancient yogic exercises which have been claimed to benefit human body on multiple fronts are also getting more popularity all over the world.[2]
Lot of studies are published in India demonstrating positive effects of yogic exercises on multiple body functions.[3] However, most of these studies are cross-sectional. Furthermore, the positive effects of yogasanas and pranayama may be the outcome of any exercise per se and may be same as that of other exercises. Comparison of the effects of yogic exercises and those of other endurance exercises like walking, running, and swimming on major body functions has not been done to reveal the efficacy of yogic exercises over other exercises, if any. Efficacy of different modules must be compared as practicing any one exercise module may not be feasible by different people. With this background, this study was designed to assess the effects of yogic exercises on resting heart rate variability (HRV), a measure of the modulation of autonomic nervous system (ANS) at rest,[4] in normal healthy young sedentary volunteers, and to compare these effects with those obtained with swimming in sedentary volunteers.
SUBJECTS AND METHODS
Participants
Healthy males and females with normal physical examination and with sedentary occupations between 18–40 years of age were included in the study. The volunteers from the socio-cultural gathering of general populations were motivated to participate in the study by explaining plan of the study to them. The recruitment was purely on the voluntary basis. After screening and fulfilments of inclusion and exclusion criteria, volunteers were recruited in the study. Initially, 100 volunteers were recruited but at the end of the study, Yoga group consisted of 41 subjects (n = 41), out of which 16 were males and 25 females. Swimmer group comprised 40 subjects with 18 males and 22 females. Volunteers had not been engaged in yoga practice or swimming in the past nor were they doing any physical exercise at least during 3 years preceding the study as assessed by enquiring in detail. Smokers, subjects consuming alcohol even occasionally, subjects who were in non-sedentary occupations, post-operative patients, and subjects suffering from any hernia, pregnant females, subjects with history of any cardiovascular disorder, subjects with a history of respiratory tract infection symptoms during previous 6 weeks, and subjects suggestive of any active respiratory disorders were excluded by thorough history and clinical examination.
Study protocol
The screening of subjects was done and clearance of Institutional Ethics Committee was obtained. After selection of the subjects, they were explained about the detailed plan of work and aim of present research project. The volunteers were briefed about the study protocol, they were motivated for the training and for compliance needed till the end of the study and written informed consent was obtained from them. The appointments for recording of study parameters were given to each subject.
One hundred volunteers were divided into cohorts of ten subjects each and were randomly assigned by block randomization method to undergo either yogic training or swimming for a duration of 12 weeks. Before the actual training period, resting blood pressure, resting heart rate, and resting digital electrocardiogram were recorded in a week's time for one cohort. In the same week, the subjects of that cohort were motivated for the exercise regimen they had to follow during the entire 12-weeks period. After 12-weeks exercise by all ten subjects in that cohort, all the parameters were again studied (2 or 3 subjects daily) in about a week's time. After baseline parameters were recorded for one cohort and the training started for that cohort, the next cohort was subjected to same treatment. Their cardiovascular parameters and digital electrocardiogram (ECG) were recorded both before training and after training. For women, both pre- and post-training recordings were postponed in case they were menstruating till their menstruation stopped. Because of overlapping of training duration for cohorts, the baseline and post-exercise evaluation became easy.
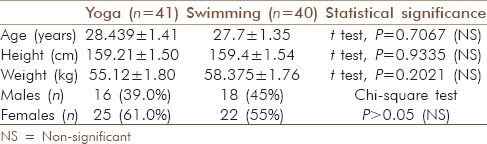
Out of the 100 subjects, nine from the yoga group and ten from the swimmer group dropped out in due course of the study. As subjects who dropped out were non-compliant, per protocol analysis method was used to analyze the data. Thus, at the end of the study, data of 41 subjects from yoga group and 40 subjects from swimming group were analyzed [characteristics of the population shown in Table 1].
Table 1.
Composition of both groups of exercise modality namely yoga and swimming
The yoga group subjects were instructed not to practice any yogic technique other than the prescribed ones and swimmer group was advised to refrain from other physical exercises during the study. We supervised the subjects early in the morning (5.00–6.00 a.m.) during yoga classes and swimmers from 6.00–7.00 a. m. everyday during the training period. Participants of both the groups were allowed to do their routine activities during the study period.
The subjects were taught yogasanas and pranayamas and then they practised the same, 6 days/week for 60 minutes daily, for a total duration of 12 weeks. Iyengar yoga techniques were followed by the yoga trainers.[5] Different yogasanas (yogic postures) viz. tādāsana, konāsana, utkatāsana, sarvāngāsana, halāsana, chakrāsana, padmāsana, dhanurāsana, makarāsana, pashchimottānāsana, vajrāsana, virāsana, and shavāsana were practiced for 40 minutes and pranayamic breathing exercises with purak, rechak and kumbhak, anulom-vilom, bhastrikā, bhramari prānāyām, and kapalbhāti were practised for 20 minutes. Swimming was practiced 6 days/week for 60 minutes daily. Swimming comprised freestyle in first 6 weeks (including training in first 2–3 weeks) and freestyle and breast stroke in last 6 weeks including 10 minutes of floating on the water. For novice swimmers, continuous swimming for 60 minutes is difficult; therefore, intermittent floating with deep slow breathing (total 10 minutes) was introduced. It also helped to keep similarity with yoga group who practiced shavāsana for 10 minutes (lying still and relaxed with slow deep breathing).
An important limitation of the methodology was inability to assess and compare the intensities of two modalities of exercise during 12-weeks duration. The reason for this inability is that tools for intensity assessment of endurance exercise like exercise and post-exercise heart rates or O2 consumption during exercise are not valid for measuring intensity of yogic exercises. Immediate heart rates after some yogasanas have shown variable response with decrease in heart rate seen more often than increase in heart rate with yogasanas.[6] Therefore, the whole module lasting for same duration for swimming and yoga was compared.
Measurement of autonomic activity
On the day of recordings, the subjects were familiarized with the laboratory environment and their anthropometric measurements were taken. Pre- and post-training measurements were recorded in the morning 2 hours after a light breakfast. All parameters like heart rate and right brachial blood pressure as well as ECG were recorded after 20 minutes of rest in supine position.
For autonomic activity, ECG was recorded continuously on a computerized data acquisition and analysis system (Polyrite, Recorders, and Medicare Systems, Chandigarh, India).
The analogue to digital conversion of the ECG signal was done using A/D converter with the sampling frequency 512 Hz. Both frequency and time domain analysis of HRV was done using HRV analysis software of Recorders and Medicare System proided with the data acquisition and analysis system. For frequency domain analysis, Fast Fourier Transformation was done using Welch's periodogram method with a Hann window. Entire spectrum of frequencies was divided into three major bands, very low frequency (VLF, 0–0.04 Hz), low frequency (LF, 0.04–0.15 Hz), and high frequency (HF, 0.15– 0.4 Hz). For computing HRV indices during supine rest, recommendations of the Task Force on HRV were followed.[4]
Statistical analysis
All the data obtained was analyzed group-wise by descriptive statistics using mean, standard deviation, and standard error of mean. For differences in sex-wise composition of two study groups, Chi-squared test was used. For each parameter in both yoga and swimming groups before and after training period of 12 weeks, data distribution was tested for normality of distribution by Kolmogorov-Smirnov test.
The paired data before and after 12 weeks of training for both yoga and swimming groups was tested by Student's paired t-test for parametric data with normal distribution and by Wilcoxon signed rank test for parametric data without normal distribution as well as for non-parametric data.
The change in different parameters with 12 weeks of training was studied by calculating delta i. e., difference in value before and after 12-weeks exercise training of both modalities. The percent change was also calculated for each parameter as percentage of change with respect to pre-exercise training level of that parameter. Increase or decrease in value of a parameter (delta) with yoga and swimming was also compared using unpaired t-test for parametric data with normal distribution and using Mann-Whitney U test for parametric data without normal distribution as well as for non-parametric data.
The statistical significance was considered at probability value less than 0.05.
The statistical calculations were done using Data Analysis tool of Microsoft Excel and Systat 12 (Systat Software, Inc. Chicago).
RESULTS
Comparison between compositions of study groups
Randomly assigned yoga and swimming groups showed statistical similarities for basic parameters like age, sex, height, and weight [Table 1]. Even after similarities in these two groups, percentage improvements in HRV parameters after 12 weeks of training with respect to baseline level were considered for comparisons of efficacy of exercise modality. This reduced the effect of differences in the baseline HRV parameters of two groups obtained because of sampling error.
Effect of yoga and swimming on cardiovascular parameters
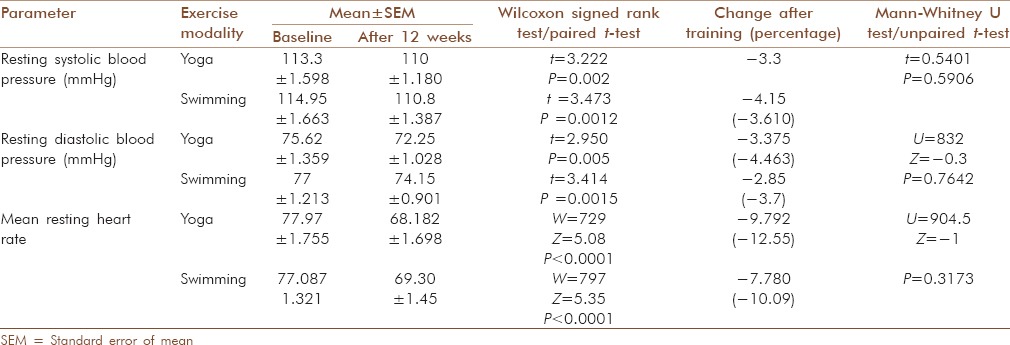
Two basic parameters viz. resting arterial blood pressure (BP) and resting heart rate (RHR) were recorded both before and after training period for both yoga and swimming group.
Average resting systolic blood pressure (RSBP) as well as resting diastolic blood pressure (RDBP) in both yoga and swimming groups decreased significantly [P < 0.01, Table 2]. Similarly, mean RHR decreased significantly in both the groups [P < 0.0001, Table 2]. The difference in percent change among both groups for all the above three parameters was statistically non-significant [Unpaired t-test and Mann-Whitney U test, P > 0.05, Table 2].
Table 2.
Effects of exercise on cardiovascular parameters (resting heart rate and blood pressure) in both groups and comparison of effects obtained between groups
Effect of yoga and swimming on resting supine heart rate variability
The average variances/total powers increased significantly in both training groups (P < 0.0001) after training. This increase in variance was significantly more in yoga group compared to swimmer group (P < 0.01) [Table 3].
Table 3.
Effects of exercise on different time domain and frequency domain parameters of resting heart rate variability in both groups and comparison of effects obtained between groups
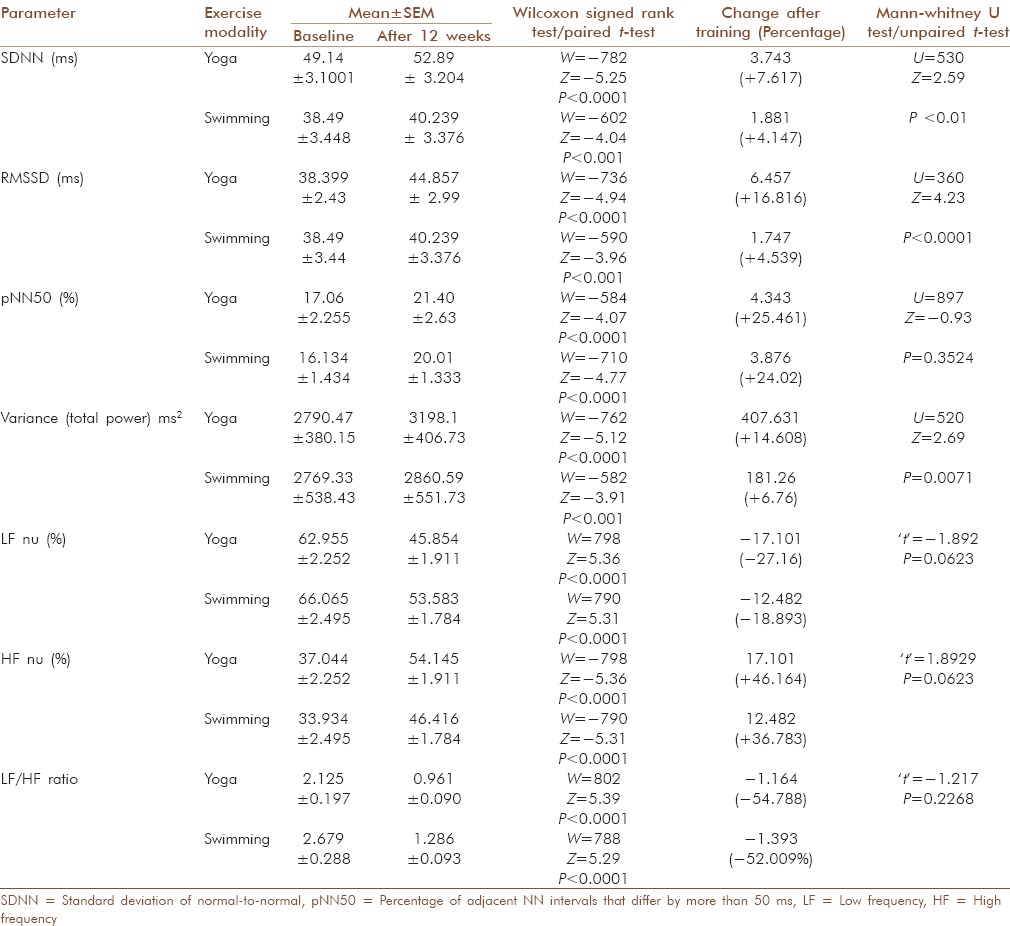
The average standard deviation of normal-to-normal (NN) (SDNN) RR interval increased in both groups (yoga group: P <0.0001 and swimmer group: P < 0.001) with better improvement in yoga group than swimmer group (P < 0.01) [Table 3].
The average square root of the mean squared differences of successive NN intervals (RMSSD) increased in both groups (yoga group: P < 0.0001 and swimmer group: P < 0.001) with better improvement in yoga group than swimmer group (P < 0.001) [Table 3].
The average values of percentage of adjacent NN intervals that differ by more than 50 ms (pNN50) after training increased significantly in both groups (P < 0.0001). The values of pNN50 after training showed similar increments in both training groups (P > 0.05) [Table 3].
The average values of LF normalized units (nu-relative value of each power component in proportion to the total power minus very low frequency component) after training decreased significantly in both the groups (P < 0.0001) and the changes were similar in both the training groups (P > 0.05) [Table 3]. The average values of HF nu after training increased significantly in both the groups (P < 0.0001) and the improvement was similar in both the training groups (P < 0.05) [Table 3]. Average LF/HF ratio in both groups decreased after training (P < 0.0001). The changes in both groups were similar (P > 0.05) [Table 3].
DISCUSSION
On extensive literature search, it was found that there are no randomized prospective comparative studies comparing HRV and other cardiovascular changes after 12-weeks training of yogic exercises and swimming.
Effect of yoga and swimming on cardiovascular parameters
With both modalities of exercise, there was significant decrease in mean RHR, mean RSBP, and mean RDBP. However, the extent of such reduction in these parameters was similar with yoga and swimming.
Similar findings of significant decrease in basal heart rate after yoga training have been reported by many workers.[7,8,9] Yogic exercises particularly Pranayama improves cardio-respiratory functions and alters autonomic status[10,11] and results in a significant decrease in basal heart rate and blood pressure.[12,13,14]
Significant reduction in RHR after yoga training is supposed to be due to algebraic summation of sympathetic and parasympathetic activation modulated by hypocapnia and lung stretch receptor responses following pulmonary inflation which accounts for bradycardia responses of deep breathing during yogasanas as well as during pranayama anulom-vilom, an alternate nostril breathing ANB.[15,16,17]
Normally exercise produces a marked and significant increase in heart rate (HR), systolic blood pressure (SBP), mean pressure (MP), and pulse pressure (PP), whereas diastolic blood pressure (DP) shows a significant decrease in response to exercise stress.[18] After yoga training, these exercise induced changes (i. e., decrease in DP and increase in other parameters) are significantly reduced.[12,13]
Yogic asanas like Sarvāngāsana, Pavanamuktāsana, Bhujangāsana, Dhanurāsana, Chakrāsana, and Shavāsana could reactivate the sluggish baroreflex mechanism in patients of essential hypertension and relieve the stress-induced sympathetic hyperactivity, thereby restoring the blood pressure to normal even in elderly patients with long history of essential hypertension.[18] Reduction in sympathetic activity and plasma renin activity to optimal levels after yoga training has been reported.[19]
Decrease in the RHR as well as blood pressure after short-term training of endurance exercises has also been well documented.[18,20,21] There are number of studies showing a decreased heart rate in individuals undergoing endurance exercise training.[22,23]
Endurance training lowers BP and that the BP reduction is based on a decrease in systemic vascular resistance (SVR), in which the sympathetic nervous system and the renin-angiotensin system appear to be involved.[20] The reduction in the activity of the sympathetic nervous system also affects the kidney, which is the most potent factor in long-term BP regulation.[20] The reduction of insulin resistance may also contribute to the favorable effect on BP.[20] Improvement of endothelial function is another potentially important mechanism.[20]
Effect of yoga and swimming on resting supine heart rate variability
With both modalities of exercise, there was significant increase in resting heart rate variance (total power) and high-frequency power, though low-frequency power did not increase significantly. HF and total power responded better with yoga than with swimming. SDNN, RMSSD, and pNN50 increased significantly with both modalities though SDNN and RMSSD improvements were statistically better with yoga than with swimming.
Similar increase in the HRV measured by variance of RR intervals (also called as Total Power) has been reported by many workers. Increase in parasympathetic activity as indicated by increased high frequency power (absolute power, HF% or HF n. u.) has been demonstrated after endurance exercises as well as yogic exercises. Similar change in LF/HF ratio which changes the sympathovagal balance in favor of parasympathetic component has also been reported by many workers.[19,22,24,25,26,27,28]
However, Martinelli et al.,[26] have described that endurance-trained athletes present indications of increased HRV in the time domain but not in the frequency domain. Some cross-sectional and more prolonged longitudinal studies show no difference in HRV between endurance athletes and untrained subjects or with prolonged endurance training.[18]
Measures of HRV are more sensitive to subtle changes than traditional tests of autonomic function.[29] SDNN, which encompasses all components responsible for RR variability, is a simple time domain measure of overall HRV.[4] High-frequency spectral power reflects parasympathetic modulation of RR intervals at respiratory frequency.[4] LF power in absolute units of power quantifies baroreflex-mediated modulation of RR intervals in the 0.04–0.15 Hz range. Changes in sympathetic as well as vagal nerve traffic to the heart are thought to contribute to LF power.[4] LF power is more closely related to stretch-induced changes in firing of baroreceptor afferents rather than the tonic discharge in baroreceptor afferents in response to the prevailing mean blood pressure.[12] Physical training improves baroreflex sensitivity and can be associated with a reduced risk for cardiac mortality after myocardial infarction.[18] Increase in HF power after both yoga and swimming training depicts a balance in favor of parasympathetic component of ANS. Even in diseased conditions, exercise training can restore a more normal cardiac autonomic balance by increasing cardiac parasympathetic regulation and also reduce the incidence of malignant arrhythmias.[23] Heart rate power (LF and HF) decreases with increasing age; however, the effects of age are not significant between all deciles of age.[30]
Deep psycho-physiological relaxation obtained in shavasana, a relaxing yogic posture, which is practised intermittently and at the end of training sessions, causes the reversal of the “flight or fight” response which results in maximum reduction of heart rate and blood pressure below normal resting level. Due to such conditioning, the parasympathetic predominance may be established.[17,31]
The ANS response to athletic training and rehabilitative exercise programs is thought to be a conditioning phenomenon.[4] HRV is a product of the dynamic interplay of many of the body's systems and is considered a measure of neurocardiac function that reflects heart–brain interactions and ANS dynamics. Yoga, pranayam, or endurance exercise may be modifying this complex interplay of heart–brain interactions.[32] Whatever are the mechanisms in yoga or endurance exercises, a swift increase in HRV is seen, particularly favoring the parasympathetic dominance.
To conclude, the present study has shown significant improvements in resting HRV after both the modalities viz. yoga and swimming. However, results are more in favor of yoga, though the results of the present study needs further confirmation on a larger sample size as adequacy of the sample size was not tested in the present study.
Apart from the preventive value of yoga there is also an emerging realization of its benefit as a complementary therapy in therapeutic and rehabilitative medicine.[33,34] In developing and poor countries, the facilities for recreational exercise and sports are not easily available and many low socio-economic group people cannot afford to utilize the available resources. Some individuals with physical constraints and for other reasons like lack of infrastructure and training may not be able to undertake exercises like swimming, though they can easily undergo yogic training. In light of these facts, yogic exercise can become the most important way of lifestyle intervention and physical activity for prevention of many diseases as prescribed by World Health Organization.[35]
ACKNOWLEDGEMENT
We acknowledge help of yoga instructors of Anekant Swadhyaya Mandir, a yoga and naturopathy centre at Wardha and swimming coaches of Municipal Swimming Pool managed by Police Welfare Fund at Wardha.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Shimamoto H, Adachi Y, Takahashi M, Tanaka K. Low impact aerobic dance as a useful exercise mode for reducing body mass in mildly obese middle-aged women. Appl Human Sci. 1998;17:109–14. doi: 10.2114/jpa.17.109. [DOI] [PubMed] [Google Scholar]
- 2.Saper RB, Eisenberg DM, Davis RB, Culpepper L, Phillips RS. Prevalence and Patterns of adult yoga use in the United States: Results of a national survey. Altern Ther Health Med. 2004;10:44–9. [PubMed] [Google Scholar]
- 3.Khalsa SB. Yoga as a therapeutic intervention: A bibliometric analysis of published research studies. Indian J Physiol Pharmacol. 2004;48:269–85. [PubMed] [Google Scholar]
- 4.Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–81. [PubMed] [Google Scholar]
- 5.Iyengar BK. Schocken Books; 1995. Light on Yoga: Yoga Dipika; p. 544. [Google Scholar]
- 6.Bhavanani AB, Ramanathan M, Balaji R, Pushpa D. Comparative immediate effect of different yoga asanas on heart rate and blood pressure in healthy young volunteers. Int J Yoga. 2014;7:89–95. doi: 10.4103/0973-6131.133870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph S, Sridharan K, Patil SK, Kumaria ML, Selvamurthy W, Joseph NT, et al. Study of some physiological and biochemical parameters in subjects undergoing yogic training. Indian J Med Res. 1981;74:120–4. [PubMed] [Google Scholar]
- 8.Nayar HS, Mathur RM, Kumar RS. Effect of yogic exercises on human physical efficiency. Indian J Med Res. 1975;63:1369–76. [PubMed] [Google Scholar]
- 9.Pal GK, Velkumary S, Madanmohan Effect of short-term practice of breathing exercises on autonomic functions in normal human volunteers. Indian J Med Res. 2004;120:115–21. [PubMed] [Google Scholar]
- 10.Raghuraj P, Ramakrishnan AG, Nagendra HR, Telles S. Effect of two selected yogic breathing techniques on Heart rate variability. Indian J Physiol Pharmacol. 1998;42:467–72. [PubMed] [Google Scholar]
- 11.Telles S, Nagarathna R, Nagendra HR. Breathing through a particular nostril can alter metabolism and autonomic activities. Indian J Physiol Pharmacol. 1994;38:133–7. [PubMed] [Google Scholar]
- 12.Madanmohan, Udupa K, Bhavanani AB, Shatapathy CC, Sahai A. Modulation of cardiovascular response to exercise by yoga training. Indian J Physiol Pharmacol. 2004;48:461–5. [PubMed] [Google Scholar]
- 13.Ray US, Sinha B, Tomer OS, Pathak A, Dasgupta T, Selvamurthy W. Aerobic capacity and perceived exertion after practice of Hatha yogic exercises. Indian J Med Res. 2001;114:215–21. [PubMed] [Google Scholar]
- 14.Schell FJ, Allolio B, Schonecke OW. Physiological and psychological effects of Hatha-yoga exercise in healthy women. Int J Psychosom. 1994;41:46–52. [PubMed] [Google Scholar]
- 15.Khanam AA, Sachdeva U, Guleria R, Deepak KK. Study of pulmonary and autonomic functions of asthma patients after yoga training. Indian J Physiol Pharmacol. 1996;40:318–24. [PubMed] [Google Scholar]
- 16.Srivastava RD, Jain N, Singhal A. Influence of alternate nostril breathing on cardiorespiratory and autonomic functions in young healthy adults. Indian J Physiol Pharmacol. 2005;49:475–83. [PubMed] [Google Scholar]
- 17.Telles S, Joshi M, Dash M, Raghuraj P, Naveen KV, Nagendra HR. An evaluation of the ability to voluntarily reduce the heart rate after a month of yoga practice. Integr Physiol Behav Sci. 2004;39:119–25. doi: 10.1007/BF02734277. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki K, Zhang R, Zuckerman JH, Levine BD. Dose-response relationship of the cardiovascular adaptation to endurance training in healthy adults: How much training for what benefit? J Appl Physiol (1985) 2003;95:1575–83. doi: 10.1152/japplphysiol.00482.2003. [DOI] [PubMed] [Google Scholar]
- 19.Selvamurthy W, Sridharan K, Ray US, Tiwari S, Hedge KS, Radhakrishan U, et al. A new physiological approach to control essential hypertension. Indian J Physiol Pharmacol. 1998;42:205–13. [PubMed] [Google Scholar]
- 20.Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure–regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46:667–75. doi: 10.1161/01.HYP.0000184225.05629.51. [DOI] [PubMed] [Google Scholar]
- 21.Sharma RK, Deepak KK, Bijlani RL, Rao PS. Short term physical training alters cardiovascular autonomic response amplitude and latencies. Indian J Physiol Pharmacol. 2004;48:165–73. [PubMed] [Google Scholar]
- 22.Tulppo MP, Hautala AJ, Makikallio TH, Laukkanen RT, Nissila S, Hughson RL, et al. Effects of aerobic training on heart rate dynamics in sedentary subjects. J Appl Physiol (1985) 2003;95:364–72. doi: 10.1152/japplphysiol.00751.2002. [DOI] [PubMed] [Google Scholar]
- 23.Billman GE, Kukielka M. Effects of endurance exercise training on heart rate variability and susceptibility to sudden cardiac death: Protection is not due to enhanced cardiac vagal regulation. J Appl Physiol (1985) 2006;100:896–906. doi: 10.1152/japplphysiol.01328.2005. [DOI] [PubMed] [Google Scholar]
- 24.Atlaoui D, Pichot V, Lacoste L, Barale F, Lacour JR, Chatard JC. Heart rate variability, training variation and performance in elite swimmers. Int J Sports Med. 2007;28:394–400. doi: 10.1055/s-2006-924490. [DOI] [PubMed] [Google Scholar]
- 25.Hautala AJ, Makikallio TH, Kiviniemi A, Laukkanen RT, Nissila S, Huikuri HV, et al. Cardiovascular autonomic function correlates with the response to aerobic training in healthy sedentary subjects. Am J Physiol Heart Circ Physiol. 2003;285:H1747–52. doi: 10.1152/ajpheart.00202.2003. [DOI] [PubMed] [Google Scholar]
- 26.Martinelli FS, Chacon-Mikahil MP, Martins LE, Lima-Filho EC, Golfetti R, Paschoal MA, et al. Heart rate variability in athletes and nonathletes at rest and during head-up tilt. Braz J Med Biol Res. 2005;38:639–47. doi: 10.1590/s0100-879x2005000400019. [DOI] [PubMed] [Google Scholar]
- 27.Molgaard H, Hermansen K, Bjerregaardf P. Spectral components of short-term RR interval variability in healthy subjects and effects of risk factors. Eur Heart J. 1994;15:1174–83. doi: 10.1093/oxfordjournals.eurheartj.a060650. [DOI] [PubMed] [Google Scholar]
- 28.Vinet A, Beck L, Nottin S, Obert P. Effect of intensive training on heart rate variability in prepubertal swimmers. Eur J Clin Invest. 2005;35:610–4. doi: 10.1111/j.1365-2362.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasan K, Vaz M, Sucharita S. A study of stress and autonomic nervous function in first year undergraduate medical students. Indian J Physiol Pharmacol. 2006;50:257–64. [PubMed] [Google Scholar]
- 30.Aria Y, Saul JP, Albrecht P, Hartley LH, Lilly LS, Cohen RJ, et al. Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol. 1989;256:H132–41. doi: 10.1152/ajpheart.1989.256.1.H132. [DOI] [PubMed] [Google Scholar]
- 31.Bera TK, Gore MM, Oak JP. Recovery from stress in two different postures and in shavasana–a yogic relaxation posture. Indian J Physiol Pharmacol. 1998;42:473–8. [PubMed] [Google Scholar]
- 32.McCraty R, Atkinson M, Tomasino D, Bradley RT. The coherent heart-brain interactions, phychophysiological coherence, and the emergence of System-wide order. Integral Rev. 2009;5:10–115. [Google Scholar]
- 33.Patel C, North WR. Randomised control trial of yoga and biofeedback in the management of hypertension. Lancet. 1975;2:93–5. doi: 10.1016/s0140-6736(75)90002-1. [DOI] [PubMed] [Google Scholar]
- 34.Tandon MK. Adjunct treatment with yoga in chronic severe airways obstruction. Thorax. 1978;33:514–7. doi: 10.1136/thx.33.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization; 2010. World Health Organization. Global recommendations on physical activity for health; p. 57. [PubMed] [Google Scholar]


