Abstract
Purpose:
The aim of the present study is to evaluate the possible role of fluorine-18 fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET-CT) in the management of mycosis fungoides (MF).
Materials and Methods:
Nineteen patients (mean age, 40.6 years; median age 41 years; 16 males and 3 females) with risk of secondary lymph node (LN) involvement (those with large cell transformation, tumors, erythroderma, or enlarged LNs on physical examination) were included in the study. All patients underwent PET-CT scan by injecting 0.06 mCi/kg of F18 FDG. The maximum standard uptake value (SUVmax) was recorded for each patient.
Results:
The 18F-FDG PET-CT was positive in 15 patients. PET-CT detected local cutaneous disease in 13 cases. The range of SUVmax is 2.8–14.1. Out of 19 patients, hypermetabolic adenopathy is found in 9 patients and visceral involvement in one.
Conclusions:
Although the study population is small our findings suggests that 18F-FDG PET-CT can detect cutaneous and extracutaneous lesions in MF and may guide biopsies especially in patients with risk of secondary LN involvement.
Keywords: Cutaneous T-cell lymphoma, fluorine-18 fluorodeoxyglucose positron emission tomography-computed tomography, mycosis fungoides
INTRODUCTION
Mycosis fungoides (MF) is a nonHodgkin lymphoma (NHL) of T-cell origin that primarily involves the skin. The disease is relatively rare, with an annual incidence of 0.36/100,000.[1] Accurate staging is important clinically for prognostic purposes and to help determine appropriate treatment options. Prognosis for MF is related to the staging, mainly concerning the extension and skin involvement type and the presence or absence of extra-cutaneous disease.[2]
The traditional approach to staging involves general physical examination, including palpation of superficial lymph nodes (LNs). Staging based on a computed tomography (CT) scan is based on size criteria and provides anatomic information only. LNs in patients with MF can be enlarged from nonspecific inflammatory changes, such as dermatopathic lymphadenopathy, rather than malignant infiltration.[3] Fluorine-18 fluorodeoxyglucose positron emission tomography-CT (18F-FDG PET-CT) has been used in the staging and assessment of tumor response in nodal-based lymphomas and solid tumors.[4] Only few study and case reports evaluate the potential role of PET-CT in diagnosing primary cutaneous lymphomas and specifically MF.[5] Our study assessed the role of F18 FDG PETCT in patients with MF. We also evaluate the clinical usefulness and suitability of 18F-FDG PET-CT in MF patients that have risk of secondary LN involvement.
MATERIALS AND METHODS
Patients with histological proven MF were referred for FDG PET-CT if they are considered at risk of secondary LN involvement (those with large cell transformation, tumors, erythroderma, or enlarged LNs on physical examination) were retrospectively included in the study from a period of September 2010 to December 2013. FDG PET-CT was performed at the time of initial diagnosis or suspected relapse. We excluded patients who had done scans at mid-treatment. Clinical examination and local staging are done by the primary physician. Only four high-risk patients are referred for follow-up 18F-FDG PET-CT scan by primary physician to assess response of multimodality therapy (radiotherapy, chemotherapy, psoralen ultra violet A (PUVA) and interferon). No follow-up scan was referred in other patients either because of loss of clinical follow-up or they are clinically in remission. The retrospective analysis was approved by hospital research and ethical committee. The following data were recorded: Age, sex, histologic type, site of disease and maximum standardized uptake value (SUVmax). PET positivity was defined as a site of abnormal FDG uptake in tissue histologically proven or clinically or radiographically suspected to represent lymphomatous involvement. The sites of disease were characterized as cutaneous and extracutaneous sites. LN biopsies were performed on the regions with highest SUV values. The lymph node biopsy samples were from axillary, inguinal or cervical regions. Our total population comprised of 19 patients (16 men and 3 women), with a mean age of 40.6 years (range: 15–72 years).
Fluorine-18 fluorodeoxyglucose positron emission tomography-computed tomography
All patients underwent 18F-FDG PET-CT systems (discovery 690 and 710, from GE Healthcare). Images were acquired after intravenous injection of 0.06 mCi/kg FDG, and a 60- to 90-min uptake period. Blood glucose level was <200 mg/dL before injection. PET emission images were obtained at 2–3 min/bed position according to patient BMI. Patients with a diagnosis of MF were imaged from the vertex to toes. PET, CT, and fusion images were reviewed on a workstation integrated with a PACS on Hermes Hybrid viewer version 2.2, Hermes Medical Solutions, Stockholm, Sweden.
RESULTS
The 18F-FDG PET-CT was positive in 15 patients. General characteristics of the patients are described in Table 1. The range of SUVmax is 2.8–14.1 [Table 2].
Table 1.
Characteristics of mycosis fungoides patients at the time of 18F-FDG PET-CT

Table 2.
Summary of 18F-FDG PET-CT findings in mycosis fungoides patients
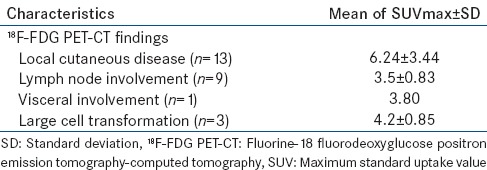
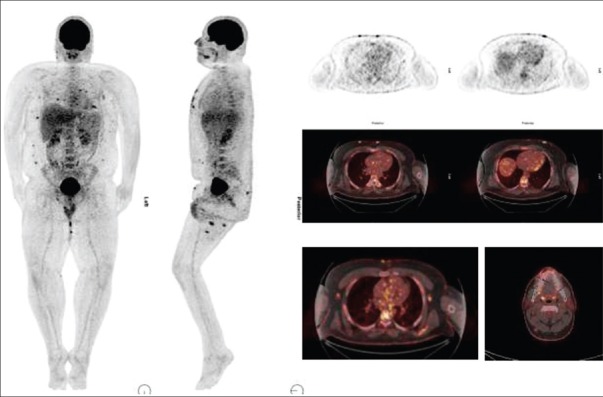
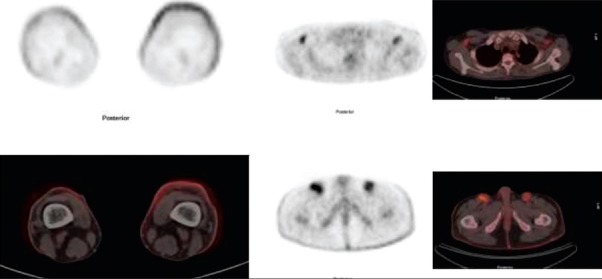
Fluorine-18 fluorodeoxyglucose positron emission tomography-computed tomography detected cutaneous disease in 13 cases with SUVmax 6.24 ± 3.44 [Figure 1]. We found hypermetabolic adenopathy in nine out of 19 patients with plaque or tumor stage disease with SUVmax 3.5 ± 0.83 [Figure 2]. One patient showed visceral involvement. Four high-risk patients underwent follow-up 18F-FDG PET-CT scan to assess response of multimodality therapy (radiotherapy, chemotherapy, PUVA and interferon) and they shows complete metabolic response [Figure 3].
Figure 1.
24-year-old male presented with multiple cutaneous lesions. Fluorine-18 fluorodeoxyglucose positron emission tomography-computed tomography scan shows multiple hypermetabolic cutaneous lesions at face, anterior chest, anterior abdomen and thighs. Hypermetabolic lymph nodes are also noted at right nuchal and bilateral axillary region
Figure 2.
A 28-year-old male with mycosis fungoides. Fluorine-18 fluorodeoxyglucose (FDG) positron emission tomography-computed tomography shows diffuse increase FDG uptake in the cutaneous tissue on both lower legs. Hypermetabolic lymph nodes are also noted at bilateral axillary and inguinal region
Figure 3.
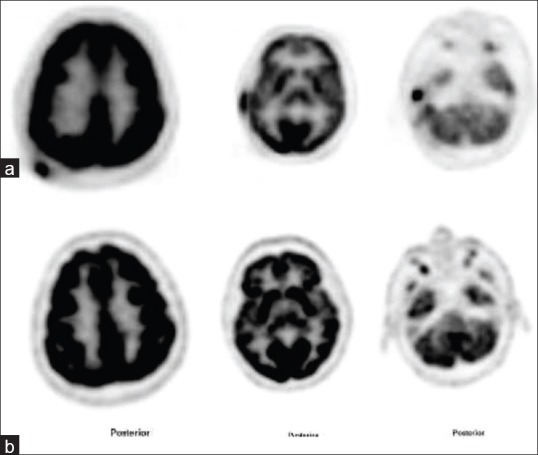
A 50-year-old male with mycosis fungoides. (a) Fluorine-18 fluorodeoxyglucose positron emission tomography-computed tomography (F18 FDG PET-CT) shows multiple hypermetabolic cutaneous lesions of maximum standard uptake value 14.0 at right scalp and right external ear region. Patient was treated with chemotherapy. (b) Follow-up F18 FDG PET-CT shows complete metabolic response at right scalp and right external ear lesions
DISCUSSION
Mycosis fungoides is a cutaneous T-cell lymphoma (CTCL) attributed to the clonal expansion of epidermotropic CD4+ helper T-cells that exists in three clinical phases: Patches, plaques, and tumors.[6] Accurate staging of MF is important for providing the patient with appropriate prognostic information and for guiding the clinician in the selection of treatment options.[7] Although physical examination with palpation of superficial LNs is routinely performed, it often incorrectly estimates the sizes of LNs. F18 FDG PET-CT is standard of care for the staging, monitoring of response to therapy, and detection of disease recurrence for Hodgkin's disease and NHL[8,9] But only few study and isolated case reports are seen in literature for the role of FDG PET-CT in CTCL and its common subtype MF.[10] FDG PET-CT has better sensitivity and specificity as compare to CT alone, and it provides a definitive localization of metabolic activity within specific lesions. In a series of 19 patients with CTCL (5 with MF), the highest accuracy of PET-CT was for the detection of metastatic disease, especially at restaging with sensitivity and specificity of 100%.[11] Same study also concluded that 18F-FDG PET-CT is more accurate than CT in detecting both cutaneous and extracutaneous disease.
Usually securing a diagnosis of MF can be difficult. Obtaining adequate tissue can often be a rate-limiting step. Fine needle aspirates are rarely if ever sufficient to allow a conclusive diagnosis and even core biopsies often do not contain enough material to render a diagnosis. Feeney et al.[12] describe that PET-CT identifies avid sites of disease in CTCL including MF and is particularly useful at identifying cutaneous involvement, nodal, extranodal sites of disease not always detected on CT. Similarly, our study shows FDG avidity in cutaneous lesions in 13 out of 19 patients [Figure 4].
Figure 4.
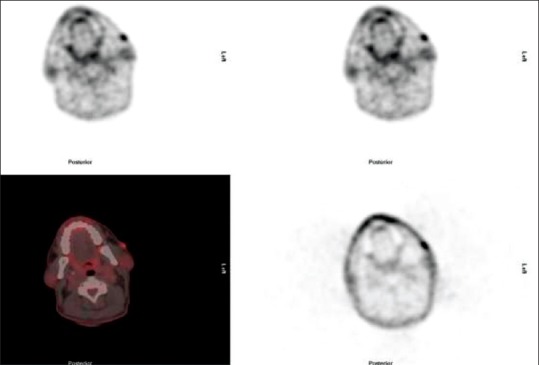
A 49-year-old male with mycosis fungoides. Fluorine-18 fluorodeoxyglucose positron emission tomography-computed tomography shows multiple hypermetabolic cutaneous lesions bilateral cheeks
In our study, nine patients have LN involvement. We found that the PET component of the 18F-FDG PET-CT was highly sensitive because it detected more diseased LNs compared with the CT component alone. A review of a series of 13 patients with MF has demonstrated the superiority of PET-CT over CT alone in detecting nodal involvement.[13] In addition, FDG PET-CT potentially increases the specificity of detecting LN involvement in MF. The higher SUVs in our series tended to correlate with pathologic findings of LNs, and even higher SUVs were associated with large cell–transformed LNs, suggesting that a node with a higher SUV would have an increased likelihood of involvement with MF and with large cell transformation. In our study, three patients showed large cell transformation with mean SUVmax 4.2 ± 0.85 [Table 2]. The International Society for Cutaneous Lymphomas and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer recommends decisional biopsy as the preferred method to evaluate abnormal LNs in MF/SS. They recommend that the largest peripheral LN draining an area of the involved skin and/or one that shows intense uptake on an FDG-PET scan should be selected for biopsy.[14]
Treatment of MF varies according to the stage of the disease. Early stage MF is highly responsive to topical treatments such as topical chemotherapy, ultraviolet irradiation (psoralen + UVA) and immunomodulatory agents such as interferon and retinoids. In contrast, systemic disease requires more aggressive and potentially more toxic therapy such as systemic chemotherapy or total skin electron beam therapy. In our study, one patient had visceral (liver) involvement on 18F FDG PET-CT and underwent systemic chemotherapy. This shows the significance of 18F-FDG PET-CT in the therapeutic management of MF and may eventually help to establish its value in staging of this rare disease. Four high-risk patients underwent follow-up 18F-FDG PET-CT scan to assess response of multimodality therapy (radiotherapy, chemotheraphy, PUVA and interferon) and they shows complete metabolic response [Figure 3]. Similarly, a study by Kuo et al.[15] in a patients with CTCL, 18F-FDG PET-CT study pre- and post-radiotherapy showed significant decreases in both SUV and lesion thickness following treatment. Another series by Shapiro et al.[10] also documented the resolution of cutaneous and visceral lesions on 18F-FDG PET following the initiation of treatment in a patient with cutaneous lymphoma.
Although all patients with active MF did not always highlight increased metabolic activity within their skin lesions on 18F-FDG PET-CT scans. However, studies have shown that the patients with tumor stage MF (T3), the PET-CT occasionally highlighted skin tumors very well.[16] In contrast to Hodgkin lymphoma and B-cell lymphoma, the imaging findings can be subtle and the disease can involve unusual sites, such as skin, muscle, or viscera, which are beyond the normal field of diagnostic CT. Therefore, at least at primary staging, patients with MF should undergo FDG PET-CT extending from the vertex to the feet to identify occult sites of disease, moreover nonattenuated corrected images should always be evaluated, which may potentially change disease stage and patient management. In our study, 18F-FDG PET-CT detected cutaneous disease in 13 out of 19 patients. 18F FDG PET-CT may be inferior to clinical examination in mapping the extent of cutaneous lesions, especially when there are macules or thin plaques, but it offer the advantage of characterizing the metabolic activity in the lesions and guide for biopsies.
Our study had some limitations related to its retrospective nature and the small numbers of patients. The primary objective of the article is to describe the spectrum of imaging findings in MF patients. Assessment of the sensitivity, specificity and impact on patient management is beyond the scope of this study. Although the study population is small, our initial experiences suggest that the 18F-FDG PET-CT is useful in nodal staging, provide prognostic information and may assess therapy response in MF patients and it can be part of routine workup of these patients. Large number of patients with longer follow-up data is required to confirm these results.
CONCLUSIONS
Our findings suggest that 18F-FDG PET-CT can detect cutaneous and extra-cutaneous lesions in MF patients. Although 18F-FDG PET-CT did not routinely identify all cutaneous lesions, this modality is more sensitive for depicting the nodal involvement. 18F-FDG PET-CT scan may help in locating sites for potential biopsy to facilitate planning of therapy.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Weinstock MA, Gardstein B. Twenty-year trends in the reported incidence of mycosis fungoides and associated mortality. Am J Public Health. 1999;89:1240–4. doi: 10.2105/ajph.89.8.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Coninck EC, Kim YH, Varghese A, Hoppe RT. Clinical characteristics and outcome of patients with extracutaneous mycosis fungoides. J Clin Oncol. 2001;19:779–84. doi: 10.1200/JCO.2001.19.3.779. [DOI] [PubMed] [Google Scholar]
- 3.Scheffer E, Meijer CJ, Van Vloten WA. Dermatopathic lymphadenopathy and lymph node involvement in mycosis fungoides. Cancer. 1980;45:137–48. doi: 10.1002/1097-0142(19800101)45:1<137::aid-cncr2820450124>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 4.Maisey MN. Overview of clinical PET. Br J Radiol. 2002;75:S1–5. doi: 10.1259/bjr.75.suppl_9.750001. [DOI] [PubMed] [Google Scholar]
- 5.Borella L, Lin M, Cains G, Watson AM, Lin P. Utility of FDG-PET for staging in a case of mycosis fungoides. Dermatology. 2007;214:185–7. doi: 10.1159/000098582. [DOI] [PubMed] [Google Scholar]
- 6.Smith BD, Wilson LD. Cutaneous lymphoma. Curr Probl Cancer. 2008;32:43–87. doi: 10.1016/j.currproblcancer.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Foss F. Mycosis fungoides and the Sézary syndrome. Curr Opin Oncol. 2004;16:421–8. doi: 10.1097/00001622-200409000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Jerusalem G, Hustinx R, Beguin Y, Fillet G. Evaluation of therapy for lymphoma. Semin Nucl Med. 2005;35:186–96. doi: 10.1053/j.semnuclmed.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Buchmann I, Moog F, Schirrmeister H, Reske SN. Positron emission tomography for detection and staging of malignant lymphoma. Recent Results Cancer Res. 2000;156:78–89. doi: 10.1007/978-3-642-57054-4_10. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro M, Yun M, Junkins-Hopkins JM, Vittorio CC, Schulman N, Saidman BH, et al. Assessment of tumor burden and treatment response by 18F-fluorodeoxyglucose injection and positron emission tomography in patients with cutaneous T- and B-cell lymphomas. J Am Acad Dermatol. 2002;47:623–8. doi: 10.1067/mjd.2002.124076. [DOI] [PubMed] [Google Scholar]
- 11.Kumar R, Xiu Y, Zhuang HM. Alavi A 18F-fluorodeoxyglucose-positron emission tomography in evaluation of primary cutaneous lymphoma. Br J Dermatol. 2006;155:357–63. doi: 10.1111/j.1365-2133.2006.07367.x. [DOI] [PubMed] [Google Scholar]
- 12.Feeney J, Horwitz S, Gönen M, Schöder H. Characterization of T-cell lymphomas by FDG PET/CT. AJR Am J Roentgenol. 2010;195:333–40. doi: 10.2214/AJR.09.3665. [DOI] [PubMed] [Google Scholar]
- 13.Tsai EY, Taur A, Espinosa L, Quon A, Johnson D, Dick S, et al. Staging accuracy in mycosis fungoides and sezary syndrome using integrated positron emission tomography and computed tomography. Arch Dermatol. 2006;142:577–84. doi: 10.1001/archderm.142.5.577. [DOI] [PubMed] [Google Scholar]
- 14.Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–22. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 15.Kuo PH, McClennan BL, Carlson K, Wilson LD, Edelson RL, Heald PW, et al. FDG-PET/CT in the evaluation of cutaneous T-cell lymphoma. Mol Imaging Biol. 2008;10:74–81. doi: 10.1007/s11307-007-0127-y. [DOI] [PubMed] [Google Scholar]
- 16.Valencak J, Becherer A, Der-Petrossian M, Trautinger F, Raderer M, Hoffmann M. Positron emission tomography with [18F] 2-fluoro-D-2-deoxyglucose in primary cutaneous T-cell lymphomas. Haematologica. 2004;89:115–6. [PubMed] [Google Scholar]


