Abstract
Purpose of the Study:
The present study was undertaken to evaluate the diagnostic utility of fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (F-18 FDG PET/CT) in patients presenting as pyrexia of unknown origin (PUO).
Materials and Methods:
Forty-seven patients (31 males and 16 females; mean age of 42.7 ± 19.96 years) presenting as PUO to the Department of Medicine at the All India Institute of Medical Sciences, New Delhi over a period of 2 years underwent F-18 FDG PET/CT. PET ⁄ CT was considered supportive when its results correlated with the final definitive diagnosis. Final diagnosis was made on the basis of combined evaluation of history, clinical findings, investigations, and response to treatment.
Results:
Thirty-five PET/CT studies (74.5%) were positive. However, only 18 (38.3%) were supportive of the final diagnosis. In three patients (6.4%), PET/CT was considered diagnostic as none of the other investigations including contrast-enhanced computed tomography of chest and abdomen, and directed tissue sampling could lead to the final diagnosis. All these three patients were diagnosed as aortoarteritis.
Conclusion:
Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography is an important emerging modality in the workup of PUO. It supported the final diagnosis in 38% of our patients and was diagnostic in 6.4% of patients. Thus, PET/CT should only be considered as second-line investigation for the diagnostic evaluation of PUO; especially in suspected noninfectious inflammatory disorders.
Keywords: Fever, fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography, pyrexia of unknown origin, pyrexia
INTRODUCTION
Fever continues to be one of the most challenging situations faced by the physician since ages. Since the classic study by Petersdorf and Beeson in 1961,[1] the profile of patients with unexplained fever has evolved considerably. The routine hematological investigations including various serological tests, along with the conventional imaging modalities, that is, ultrasound (USG) abdomen and contrast-enhanced computed tomography (CECT) of chest and abdomen contribute to the diagnosis in a large number of patients with pyrexia of unknown origin (PUO). In others, blind or guided biopsies based on imaging findings help to reach the specific diagnosis. However, despite extensive workup, the proportion of undiagnosed cases of PUO has ranged from 7% to 51% in various studies.[1,2,3,4,5,6,7,8,9,10,11,12] Often, these undiagnosed cases turn out to be rather uncommon presentation of common diseases.[13] Thus, there has always been a search for modalities that could be helpful in unmasking the cause of fever in these patients. Positron emission tomography (PET) using radiolabelled fluorodeoxyglucose (FDG) and combined with computed tomography (CT), fluorine-18 (F-18) FDG PET/CT is one such emerging modality. Be it infection, non-infectious inflammatory disorders (NIIDs) or malignancy; PET/CT can detect a site with abnormal metabolism much before the appearance of abnormal anatomical features thereby helping in early diagnosis and thus, early initiation of treatment. Various studies on the role of FDG PET and PET/CT in PUO have reported it to be contributory to the final diagnosis in 16–69% cases.[14,15,16,17,18,19] Studies have also shown that negative F-18 FDG PET/CT excluded any significant disease and pyrexia in such patients often resolved spontaneously.[14]
We performed a prospective study at a tertiary care institute to evaluate the utility of F-18 FDG PET/CT in the diagnosis of patients with PUO.
MATERIALS AND METHODS
A total of 100 patients of ≥12 years of age presenting as PUO at both out-patient and in-patient in the Department of Medicine at our institute over a period of 2 years were screened and evaluated. Axillary temperature was recorded using a digital thermometer at regular intervals (every 6 h), and patients were included if several reading above 101°F were observed. Initial investigations included routine hematological, biochemical, and serological investigations, blood and urine culture, body fluid analysis, chest X-ray (CXR), USG abdomen, and CECT chest and abdomen followed by histo/cytopathological evaluation of an abnormal site in CT if the previous investigations did not reveal a specific diagnosis. Apart from these, bone marrow examination, thyroid function tests, tumor markers, and two-dimensional echocardiography were also done depending upon patient's clinical presentation. If a definitive diagnosis was achieved after these investigations, patients were excluded from the current study. Neutropenic PUO, nosocomial PUO, patient with known malignancy on chemotherapy, human immunodeficiency virus-positive patients and pregnant and lactating females were also excluded from the study.
Forty-seven out of 100 patients, who fulfilled the revised Petersdorf's criteria[20] for PUO, that is, illness characterized by temperature exceeding 38.3°C (101°F) evolving during at least 3 weeks and no diagnosis reached after 1-week of relevant and intensive investigations were included in the study and subsequently underwent PET/CT. The study was approved by the Institutional Ethics Committee.
Positron emission tomography/computed tomography scans were done with a dedicated PET/CT scanner (SIEMENS, BIOGRAPH 2). After fasting for 4 h to ensure blood glucose less than 150 mg/dL, 370 MBq (10 mci) of FDG was injected intravenously and scan taken after 45–60 min. Data obtained from CT acquisition were used for low noise attenuation correction of PET emission data and for fusion of attenuation corrected PET images with corresponding CT images. The reconstructed attenuation corrected PET images, CT images, and fused PET/CT images of matching pairs were available for review in axial, coronal, and sagittal planes and in maximum intensity projections, three-dimensional cine mode. A region of interest (ROI) was carefully drawn around the site of the abnormal FDG uptake in consecutive 4–6 PET/CT scan slices. The slice with a maximal FDG uptake in the ROI was chosen for quantitative measurement of metabolic activity of the tracer, that is, standardized uptake values (SUV). The SUV was calculated according to the formula below:
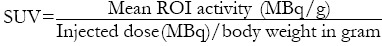
Where, MBq = Mega Becquerel and g = gram.
Any focal areas of increased FDG uptake other than physiological uptake were considered as a positive result for PET/CT. The extent of the disease in terms of the organs involved and the degree of uptake in the involved organs in terms of SUV were assessed. If a patient had uptake at multiple sites, then the highest SUVmax was used for analysis. The PET/CT fusion images were interpreted by an experienced nuclear medicine expert. In addition to this, independent interpretation of CT was done by an expert in radiodiagnosis.
Abnormal PET/CT studies were categorized as “contributory to diagnosis” when the abnormal uptake pointed to the organ or tissue where the cause of the fever was eventually found by additional techniques; “noncontributory to diagnosis” when the detected abnormality was considered to be unrelated to the illness causing the PUO and “diagnostic” if PET/CT was the only modality that helped to achieve the final diagnosis. The contributory and diagnostic scans were collectively considered as “supportive.” Final diagnoses were made on the basis of combined evaluation of history, clinical findings, investigations, and response to treatment and were classified into four groups: Infections, malignancies, NIIDs, and miscellaneous causes.
Data collection and statistical analysis
Epidemiologic data; number, type, and results of all diagnostic tests performed; final diagnosis and mode of diagnosis data were recorded for all patients in a structured database. The data were analyzed using SPSS version 20 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed as number and percentage. All potential diagnostic clues including sex (male or female), duration of fever (<6 or >6 months), presence or absence of anorexia, weight loss, lymphadenopathy, hepatomegaly, splenomegaly, anemia, leukopenia/leukocytosis, thrombocytopenia/thrombocytosis, elevated erythrocyte sedimentation rate (ESR), abnormal liver function test (LFT), and abnormal imaging studies (CXR, USG abdomen, CECT chest and abdomen, and PET/CT) were dichotomized as categorical variables. Patients who achieved a final diagnosis were compared with those remaining undiagnosed using Fischer exact test with respect to the above parameters. Patients with a confirmed final diagnosis were grouped into three diagnostic categories of infection, malignancy, and NIID, and these groups were compared to each other using Fisher exact test to ascertain difference in the above parameters between each diagnostic group. SUVmax values were compared between supportive and non-contributory PET/CT groups using Mann–Whitney U-test and between infection, malignancy, and NIID groups using Kruskal–Wallis test. Log transformation was used for normalizing the skewed data. A P < 0.05 was considered statistically significant.
RESULTS
Of the 100 patients screened, 47 satisfied the inclusion criteria [Figure 1]. Baseline characteristics of the patients in terms of demographic and clinical characteristics are shown in Table 1.
Figure 1.
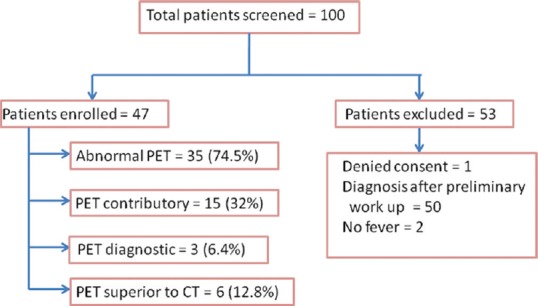
Screening of study participants and summary of positron emission tomography/computed tomography findings
Table 1.
Demographic and clinical characteristics of patients
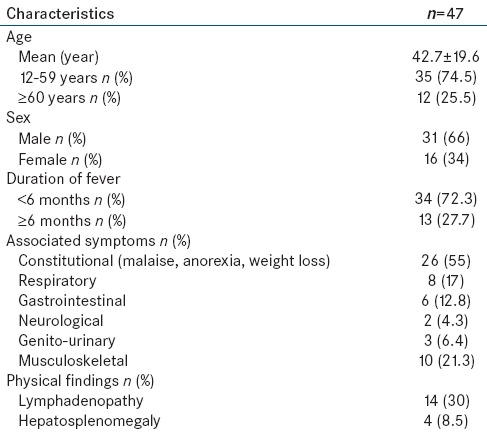
Figure 2.
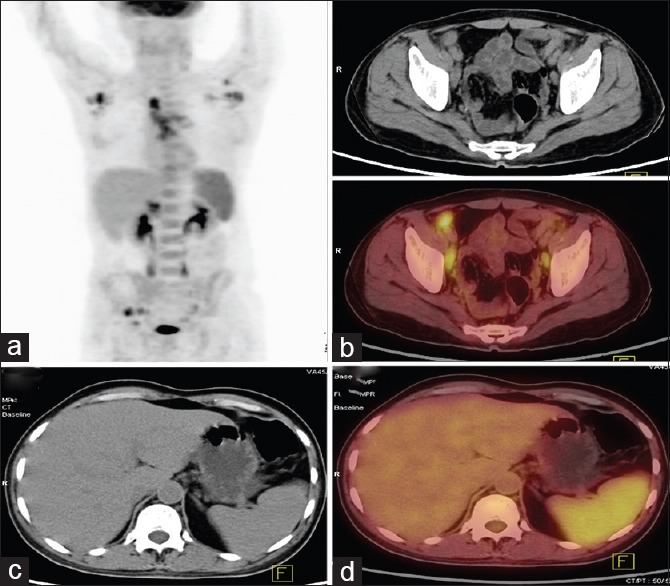
52-year-old man presented with fever of 1-year duration and large joints arthritis. (a) Maximum intensity projection whole body fluorine-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) image with abnormal FDG uptake in bilateral axillae, mediastinum, pelvis and enlarged spleen. (b and c) Trans-axial computed tomography (CT) and PET/CT images of the pelvis reveal enlarged FDG avid bilateral iliac lymph nodes (maximum standardized uptake value [SUVmax] = 3.3). (d and e) Transaxial CT and PET/CT images at the level of spleen show splenomegaly with increased FDG uptake (SUVmax = 1.6). FNAC from axillary lymph node showed reactive changes. Based on PET and clinical findings, a diagnosis of Still's disease was made
Figure 3.
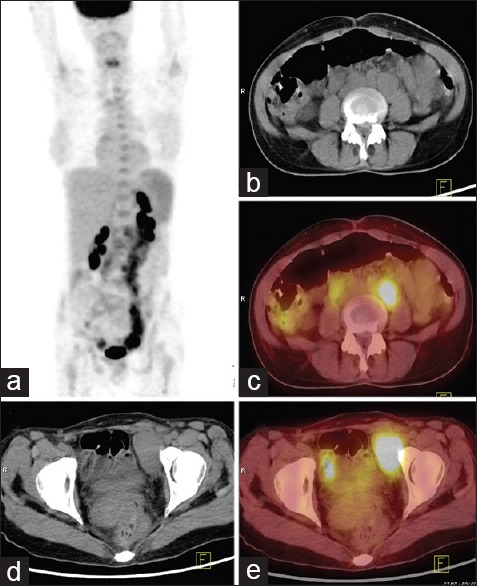
43-year-old lady presented with fever of five months duration associated with anorexia and weight loss. (a) Maximum intensity projection fluorine-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) whole body image reveals irregular areas of uptake in abdomen and pelvis. Trans-axial computed tomography (CT) and PET/CT images at the level of abdomen (b and c) and pelvis (d and e) reveal enlarged retroperitoneal nodes and pelvic nodes with increased FDG uptake (maximum standardized uptake value = 21). Diagnosis of lymphoma/TB made on PET/CT. Mesenteric and mesocolic lymph nodes of reportedly normal size were biopsied by mini-laparotomy and confirmed the diagnosis of Hodgkin's disease
Figure 4.
38-year-old lady presented with fever of four months duration associated with anorexia, weight loss and myalgia. Maximum intensity projection fluorine-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) whole body image reveals multiple areas of increased FDG uptake throughout the body. Coronal PET/computed tomography (CT) image (b) localizes the patchy uptake to multiple skeletal muscles (arrow) (maximum standardized uptake value [SUVmax] = 2.4). (d) Diffusely increased FDG uptake in the enlarged spleen (SUVmax = 3.5). Diagnosis of inflammatory pathology involving muscles was made on PET/CT. Although creatine phosphokinase levels were normal but electromyography showed myopathic pattern and muscle biopsy revealed dermatomyositis
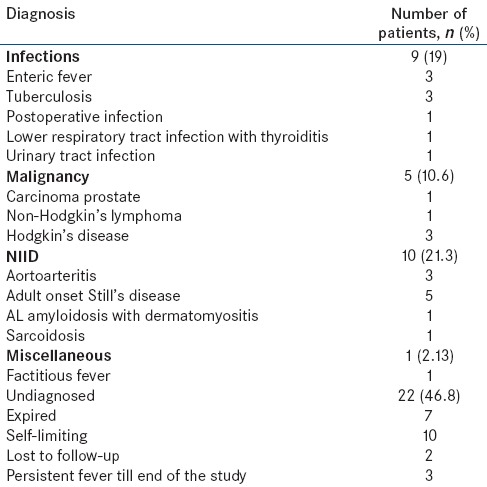
Of the 47 patients evaluated, a definitive diagnosis could be arrived at in 25 patients (53.2%; 95% confidence interval: 38.1–67.9%) while 22 (46.8%) patients remained undiagnosed. Distribution of patients according to the final confirmed diagnosis is shown in Table 2.
Table 2.
Distribution of patients according to the final diagnosis
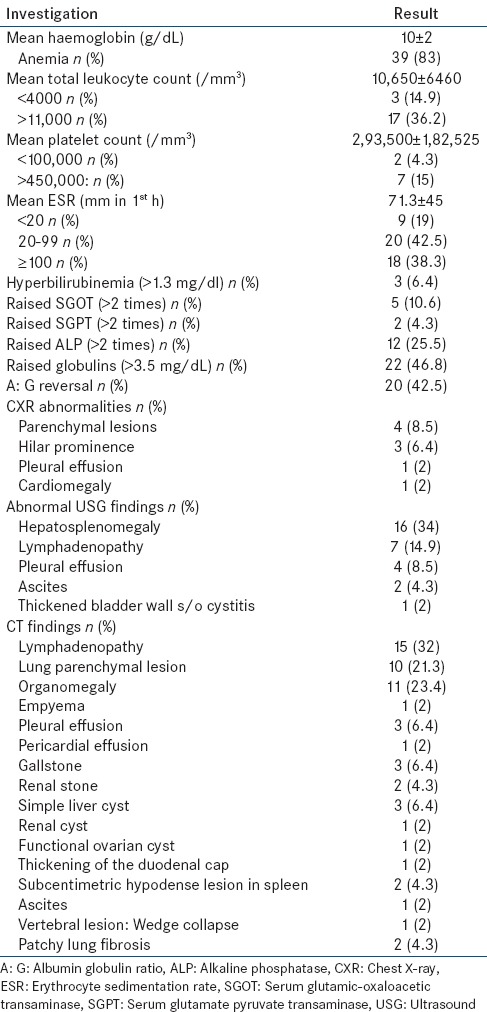
All patients underwent screening investigations including routine hematology, biochemistry, imaging (CXR, USG abdomen, and CECT chest and abdomen) and other relevant investigations based on history and examination findings. Key abnormalities on baseline investigations are shown in Table 3. Baseline investigations were followed by PET/CT in all the 47 patients. A summary of PET/CT findings is shown in Figure 1.
Table 3.
Investigative profile of patients
Thirty-five PET/CT studies (74.5%) demonstrated foci of increased F-18 FDG uptake, suggesting the presence of a disease process that could represent the cause of PUO. In 15 of these 35 positive studies (32% of total), PET/CT findings correlated with the final diagnosis and thus, PET/CT was considered “contributory” in those patients. These included four patients with infections, six patients with NIIDs and five patients with malignancy.
There were only three patients (6.4%) who had normal CECT chest and abdomen but uptake in PET/CT and thus, PET/CT was considered “diagnostic” in them. All these three patients had normal looking vessels in CECT but showed abnormal FDG uptake in aorta and large vessels and were diagnosed as aortoarteritis [Figure 5]. Combining the results of contributory and diagnostic groups, there were 18 (38.3%) patients in whom PET/CT was “supportive.” A summary of patients where PET/CT was supportive toward achieving a definitive diagnosis is shown in Table 4.
Figure 5.
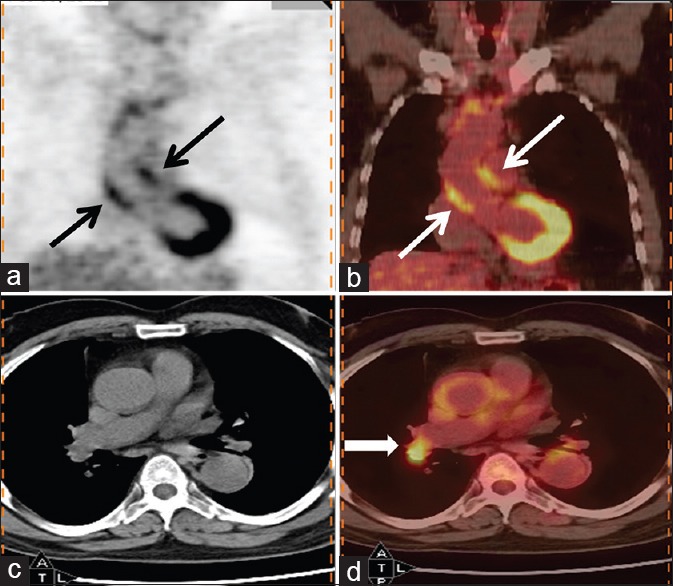
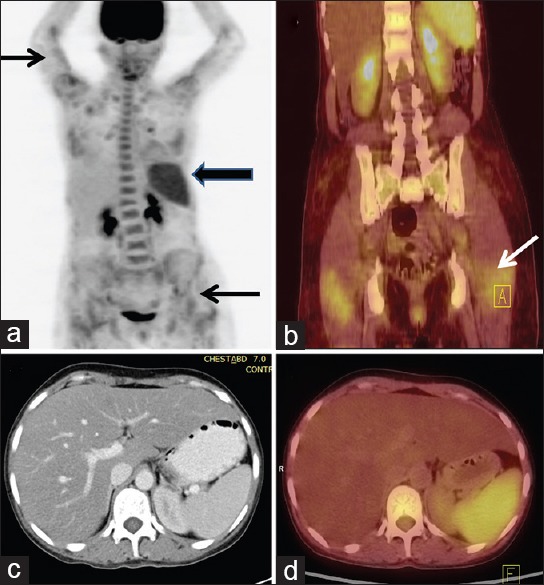
69-year-old man presented with fever of 5 months duration. His erythrocyte sedimentation rate was >100 mm and contrast-enhanced computed tomography chest, and abdomen was reported as normal. Coronal (b) and transaxial (d) PET/computed tomography images of thorax reveal increased fluorodeoxyglucose (FDG) uptake in walls of ascending thoracic aorta (thin arrow) (maximum standardized uptake value = 4). Also noted was increased FDG uptake in right hilar node (bold arrow) which was considered to be reactive. A diagnosis of large vessel vasculitis (aortoarteritis) was made, and patient responded to corticosteroids
Table 4.
Clinical and imaging findings in patients with contributory PET/CT

Non-contributory positron emission tomography/computed tomography
In 17 of the 35 positive PET/CT studies (36.2% of total), PET/CT was noncontributory to the final diagnosis and further patient management. Some of these patients had uptake in more than one site, and if accessible, all these sites were sampled. Three patients had uptake in large bowel on PET/CT, but colonoscopy with or without biopsy did not show any abnormality; one was diagnosed as enteric fever, one had factitious fever, and one of these patients remained undiagnosed. One patient had uptake in tongue and floor of the mouth, but ENT evaluation did not reveal any abnormality, and he later expired undiagnosed. Similarly, another patient had uptake in left infraspinatus muscle but ultrasonography did not reveal any localized lesion amenable to sampling and later, he succumbed to his illness undiagnosed. Ten patients had uptake in multiple lymph nodes and three of these patients also had a bone marrow uptake, however, neither lymph node sampling nor bone marrow examination was diagnostic. Four such patients had spontaneous resolution of fever, one was diagnosed as enteric fever (same patient discussed above), two patients expired undiagnosed, one patient was lost to follow-up, one continued to have fever till the last follow-up visit and one was finally diagnosed as urinary tract infection (UTI). One patient had patchy uptake in bilateral lungs and showed no response to a trial of antibiotics. As the patient was not willing for bronchoscopy, he was discharged on empirical ATT. However, after discharge, his condition worsened and he expired. Another patient had uptake in adenoids, but his fever resolved spontaneously. One patient had uptake in cervical lymph nodes which were subcentimetric and not amenable to sampling. This patient also had spontaneous resolution of fever. Thus, in these 17 patients, findings of PET/CT were noncontributory to the final outcome and definite diagnosis was achieved in only 2 (12%) patients. The patient given the diagnosis of enteric fever had rising Widal titers initially and received appropriate antibiotics, but fever persisted and even Widal titers normalized. Thus, she was evaluated for other causes of fever but no other cause could be elucidated and fever subsided with prolonged antibiotic treatment. Thus, a diagnosis of enteric was kept on the basis of initial Widal test and response to treatment. Similarly, in the patient diagnosed with UTI, initial urine examination revealed pus cells but repeated urine cultures were sterile. Urine examination for acid-fast bacilli was negative, and USG abdomen did not reveal any abnormality of the urinary tract. Patient also had the neutrophilic leukocytosis and continued to have fever despite 2 weeks of oral and 10 days of intravenous antibiotics and thus, was evaluated for other causes of fever. However, fever subsided with continued intravenous antibiotic administration with resolution of pus cells in urine. Thus, a diagnosis of UTI was given on the basis of the presence of pus cells in urine, raised TLC, and response to antibiotics. Thus, in both these cases, diagnosis was made on the basis of response to treatment.
Positron emission tomography/CT showed no abnormality in 12 (25.5%) patients. Of these, 4 (33%) patients were finally diagnosed as partially treated enteric fever (n = 2), postoperative infection (n = 1), and adult-onset Still's disease (AOSD) (n = 1). In four of them, there was spontaneous resolution of fever and one was lost to follow-up. One patient had persistent fever till end of the study with no definite cause identified. Two patients expired in the hospital, and postmortem liver biopsy was done in one of them which was inconclusive.
Thus, among the patients with non-contributory or negative PET/CT (n = 29), a final diagnosis could be established in only 6 (21%) patients. In five of these patients (enteric [3], postoperative infection [1], UTI 1]), final diagnosis was given on the basis of response to treatment while one patient with AOSD was diagnosed on the basis of Yamaguchi criteria, with the exclusion of other causes.
When compared to CECT chest and abdomen, PET/CT was superior to CT in six patients (12.8%). Three of them were diagnosed as aortoarteritis, solely on the basis of PET/CT findings. One patient had uptake in normal size lymph nodes which on biopsy revealed Hodgkin's disease. Similarly, one patient who had shown increased uptake in otherwise insignificantly enlarged mediastinal nodes, negative Mantoux test and elevated angiotensin-converting enzyme levels, was diagnosed as sarcoidosis and responded to corticosteroids. Another patient who had an inconclusive CT showed increased FDG uptake in pelvic and thigh muscles in PET/CT and was diagnosed as dermatomyositis after confirmation by electromyography and muscle biopsy findings.
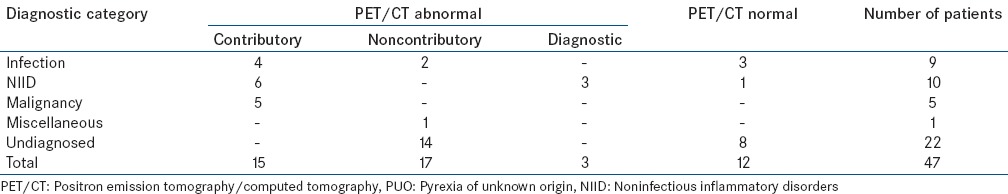
Thus, in the present study, out of 47 patients of PUO who underwent PET/CT, PET/CT was contributory to final diagnosis in 15 (32%) patients, noncontributory in 17 (36.2%) patients, negative in 12 (25.5%), diagnostic in 3 (6.4%) patients and superior to CT in 6 (12.8%) patients. Result of FDG PET/CT in different diagnostic categories of PUO is summarized in Table 5.
Table 5.
Result of FDG PET/CT in different diagnostic categories of PUO
The mean SUVmax for supportive PET/CT was 9.3 ± 6.5 (range: 3.1–23.6) while the mean SUVmax for noncontributory PET/CT was 5.6 ± 3.1 (range: 0.9–11.7). There was no significant difference between the two groups (P = 0.092). Since there were just 35 abnormal PET/CT and the data for SUVmax had a wide range, log transformation was used for normalizing the data. After log transformation, the means for supportive PET/CT and non-contributory PET/CT were 7.4 ± 2.01 and 4.5 ± 2.07, respectively, with a borderline significant P = 0.05.
Among the patients in whom a diagnosis was achieved, mean SUVmax was highest for infectious etiology (11.98 ± 6.6) followed by NIIDs (8.8 ± 7.6) and malignancy (8.2 ± 4.4). There was no significant difference in the SUVmax of the three groups (P = 0.44).
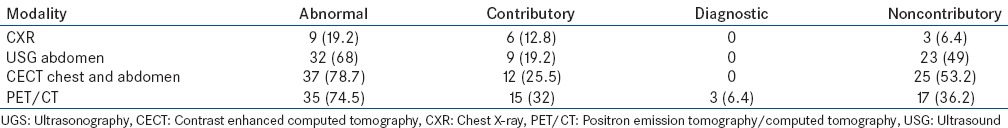
Various diagnostic modalities that helped to reach a final diagnosis are shown in Table 6, and the contribution of various imaging modalities in the evaluation of PUO is shown in Table 7.
Table 6.
Mode of final diagnosis in various categories of PUO

Table 7.
Comparison of various imaging modalities (n=47)
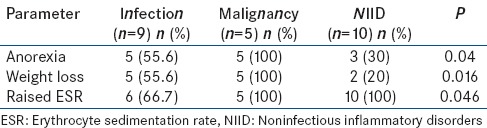
All potentially diagnostic clues including sex, duration of fever, anorexia, weight loss, lymphadenopathy, hepatomegaly, splenomegaly, anemia, leukopenia/leukocytosis, thrombocytopenia/thrombocytosis, elevated ESR, abnormal LFT, and abnormal imaging studies (CXR, USG abdomen, CECT chest and abdomen, and PET/CT) were statistically analyzed to assess if they had any association with the likelihood of establishing a diagnosis. None of these parameters were statistically different between patients who were diagnosed and remained undiagnosed (data not shown) at the end of the study. However, among the patients in three diagnostic categories, that is, infections, malignancies, and NIIDs, it was observed that the proportion of patients with anorexia, weight loss, and elevated ESR was significantly different between the three diagnostic categories [Table 8].
Table 8.
Significant differences between patients with infection, malignancy, and NIID (n=24)
DISCUSSION
The definition of PUO was first given by Petersdorf and Beeson[1] followed by a modification by Durack and Street.[21] In 1992, in an editorial, Petersdorf proposed another change in the definition of PUO that in view of the expense of hospitalization, 1-week in-hospital investigations be changed to 1-week of intelligent and intensive investigation which, in most patients, could be conducted on an outpatient basis.[20]
The present study evaluated the role of 18-F FDG PET/CT in 47 patients presenting as PUO. Of the 47 patients, a definitive diagnosis could be arrived at in 25 (53.2%) patients. NIIDs (21.3%) accounted for the majority of the diagnoses followed by infections (19%). Malignancy (10.6%) and miscellaneous causes (2.13%) contributed to a small number of patients. However, in a large proportion of patients (46.8%), the exact cause of PUO could not be ascertained, and they remained undiagnosed.
The observation that NIIDs accounted for a larger proportion of cases of PUO in our study is in concordance with the results of recent Western studies where proportion of patients with NIIDs was as high as 37%.[10,11,12,17,22] However, this observation is different from many of the previous Indian studies in which infections particularly extra-pulmonary tuberculosis was found to be the predominant cause of PUO.[23,24,25,26,27,28,29,30] This difference may be accounted for by referral bias as the present study was done at a tertiary care institute and in the present scenario, majority of the infections are diagnosed at an earlier stage because of improved imaging modalities as well as guided invasive procedures. Increase in the proportion of NIID cases could also be due to better awareness of these disorders; both among the clinicians and the patients, well-defined diagnostic criteria and most importantly, highly sensitive serological tests for these diseases. With the use of PET/CT, aortoarteritis is being recognized with increasing frequency, which otherwise would have remained undiagnosed or diagnosed at an advanced stage with complications.
In the present study, a large proportion of patients presenting with PUO remained undiagnosed. Similar observations have been made in Western studies with undiagnosed cases accounting for 51% and 40%, respectively, in studies by Bleeker-Rovers et al. and Pedersen et al.[11,12] An increase in the proportion of undiagnosed cases may reflect the increasing difficulty in diagnosing patients that make up the PUO cohort currently in tertiary care centers as these patients have already been extensively evaluated prior to referral. Patients who fail to receive a definitive diagnosis despite CECT chest and abdomen are less likely to be diagnosed even with PET/CT as was seen in our study. Many of these undiagnosed patients improved spontaneously suggesting a minor benign pathology which is not picked up by current investigative modalities. However, a substantial proportion of these undiagnosed patients died or continued to have fever, highlighting the limitations of the present diagnostic modalities and knowledge of causes of PUO.
Positron emission tomography/CT was contributory to diagnosis in 15 out of 47 (32%) cases and diagnostic in three patients (6.4%). These results are in concordance with the previous studies in which FDG PET or FDG PET/CT has been reported to be helpful in diagnosing 16–69% cases of PUO.[11,12,14,15,16,18,19,31,32] In the present study, PET/CT was contributory in all patients with malignancy and many of the patients with infectious etiology (including disseminated tuberculosis and lower respiratory tract infection with thyroiditis). These results are comparable with previous studies in which FDG PET has been found helpful in disseminated tuberculosis, focal infections, lymphomas, and solid organ malignancy with or without metastasis.[14,16,33,34]
The biggest advantage of PET/CT in this study was observed for the diagnosis of NIIDs. The overall superiority of PET/CT over CECT was seen in only six (12.8%) patients out of whom five had NIIDs. Three were diagnosed as aortoarteritis on the basis of uptake in aorta with no abnormality seen in CECT chest and abdomen. The importance of PET/CT in the diagnosing vasculitis has been emphasized in many previous studies.[15,16,31,34,35] In a study by Bleeker Rovers et al., temporal artery biopsy was done in 14 patients with PUO (10 with potentially diagnostic clues for vasculitis, two as part of second level test and two in patients with PET suggestive of vasculitis). However, it was useful in the diagnosis only in one patient in whom PET had already pointed to large vessel vasculitis.[15] Studies employing FDG PET and magnetic resonance imaging (MRI) for early diagnosis and follow-up of vasculitis revealed comparable sensitivities for both the methods.[36,37] In fact, FDG PET may have the advantage that it identifies affected vascular regions at an early stage, reflecting the fact that metabolic changes normally precede morphologic changes in inflammation.
Rosenbaum et al.[38] and Federici et al.[17] have also compared the utility of CT and FDG PET/CT in patients presenting with PUO and found that PET was superior to CT in 11/18 (61%) patients and 3/13 (23%) patients (two had large vessel vasculitis), respectively. Similarly, in our study, PET/CT was superior to CT in 6/47 patients (12.8%). These observations emphasize the role of PET/CT in picking up disease at an early stage before anatomical changes are seen on CT.
In the present study, PET/CT was found to be noncontributory in 17 patients (36.2%). Because a definitive diagnosis could not be established in majority of these patients (n = 15), and five of these patients expired undiagnosed, all these abnormal results cannot be said to be false positives instead should be considered clinically noncontributory. Indeed, if the involved body area is not completely anatomically or histopathologically investigated, the abnormal tracer uptake cannot be considered to be a false-positive finding. Four out of these five patients expired in the hospital, and postmortem liver biopsy was performed in three patients. It was inconclusive in all the three cases. This observation highlights the fact that complete/partial autopsy should be performed in all the patients of PUO, who expire undiagnosed so that an appropriate tissue could be sampled that might help to reach a definitive diagnosis.
If PET/CT is negative, the definite cause of fever was found in only 33% cases. A recent retrospective study from North India also found that only 17.5% PET/CT negative cases achieved a definite diagnosis.[33]
Although it has been emphasized that SUV values are generally higher for malignant etiology as compared with benign lesions but observation in the present study was contrary to this. Malignant lesions were found to have lower SUV values while infectious causes had the highest SUV values. Furthermore, there was no significant difference in the mean SUVmax values of supportive and noncontributory PET/CT.
The strength of the present study is that as per the definition of PUO, the patients included for evaluation had been intensively investigated with multiple relevant investigations including invasive procedures like guided biopsies in patients with lymph node enlargement on CECT chest and abdomen. Thus, the cohort of PUO in our study was a real diagnostic challenge.
An important limitation of the study was that the autopsy was not performed in any of the patients who expired undiagnosed. Performing an autopsy in such cases would be of great value to not only establish a definitive diagnosis but could help in future management of difficult cases.
Based on present data, it can be concluded that PET/CT can be helpful to diagnose more patients as compared to CECT chest and abdomen. However, given the high cost and limited availability, PET/CT should only be considered a second-line investigation in diagnostic evaluation of PUO; especially in patients with suspected NIIDs.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Petersdorf RG, Beeson PB. Fever of unexplained origin: Report on 100 cases. Medicine (Baltimore) 1961;40:1–30. doi: 10.1097/00005792-196102000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Larson EB, Featherstone HJ, Petersdorf RG. Fever of undetermined origin: Diagnosis and follow-up of 105 cases, 1970.1980. Medicine (Baltimore) 1982;61:269–92. [PubMed] [Google Scholar]
- 3.Gleckman R, Crowley M, Esposito A. Fever of unknown origin: A view from the community hospital. Am J Med Sci. 1977;274:21–5. doi: 10.1097/00000441-197707000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Barbado FJ, Vazquez JJ, Peña JM, Seoane JG, Arnalich F, Gil A, et al. Fever of unknown origin: A survey on 133 patients. J Med. 1984;15:185–92. [PubMed] [Google Scholar]
- 5.Barbado FJ, Vázquez JJ, Peña JM, Arnalich F, Ortiz-Vázquez J. Pyrexia of unknown origin: Changing spectrum of diseases in two consecutive series. Postgrad Med J. 1992;68:884–7. doi: 10.1136/pgmj.68.805.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knockaert DC, Vanneste LJ, Vanneste SB, Bobbaers HJ. Fever of unknown origin in the 1980s. An update of the diagnostic spectrum. Arch Intern Med. 1992;152:51–5. [PubMed] [Google Scholar]
- 7.Kazanjian PH. Fever of unknown origin: Review of 86 patients treated in community hospitals. Clin Infect Dis. 1992;15:968–73. doi: 10.1093/clind/15.6.968. [DOI] [PubMed] [Google Scholar]
- 8.de Kleijn EM, van der Meer JW. Fever of unknown origin (FUO): Report on 53 patients in a Dutch university hospital. Neth J Med. 1995;47:54–60. doi: 10.1016/0300-2977(95)00037-n. [DOI] [PubMed] [Google Scholar]
- 9.de Kleijn EM, Vandenbroucke JP, van der Meer JW. Fever of unknown origin (FUO). I A. prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO Study Group. Medicine (Baltimore) 1997;76:392–400. doi: 10.1097/00005792-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Vanderschueren S, Knockaert D, Adriaenssens T, Demey W, Durnez A, Blockmans D, et al. From prolonged febrile illness to fever of unknown origin: The challenge continues. Arch Intern Med. 2003;163:1033–41. doi: 10.1001/archinte.163.9.1033. [DOI] [PubMed] [Google Scholar]
- 11.Bleeker-Rovers CP, Vos FJ, de Kleijn EM, Mudde AH, Dofferhoff TS, Richter C, et al. A prospective multicenter study on fever of unknown origin: The yield of a structured diagnostic protocol. Medicine (Baltimore) 2007;86:26–38. doi: 10.1097/MD.0b013e31802fe858. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen TI, Roed C, Knudsen LS, Loft A, Skinhoj P, Nielsen SD. Fever of unknown origin: A retrospective study of 52 cases with evaluation of the diagnostic utility of FDG-PET/CT. Scand J Infect Dis. 2012;44:18–23. doi: 10.3109/00365548.2011.603741. [DOI] [PubMed] [Google Scholar]
- 13.Wolff SM, Fauci AS, Dale DC. Unusual etiologies of fever and their evaluation. Annu Rev Med. 1975;26:277–81. doi: 10.1146/annurev.me.26.020175.001425. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzen J, Buchert R, Bohuslavizki KH. Value of FDG PET in patients with fever of unknown origin. Nucl Med Commun. 2001;22:779–83. doi: 10.1097/00006231-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Bleeker-Rovers CP, de Kleijn EM, Corstens FH, van der Meer JW, Oyen WJ. Clinical value of FDG PET in patients with fever of unknown origin and patients suspected of focal infection or inflammation. Eur J Nucl Med Mol Imaging. 2004;31:29–37. doi: 10.1007/s00259-003-1338-3. [DOI] [PubMed] [Google Scholar]
- 16.Jaruskova M, Belohlavek O. Role of FDG-PET and PET/CT in the diagnosis of prolonged febrile states. Eur J Nucl Med Mol Imaging. 2006;33:913–8. doi: 10.1007/s00259-006-0064-z. [DOI] [PubMed] [Google Scholar]
- 17.Federici L, Blondet C, Imperiale A, Sibilia J, Pasquali JL, Pflumio F, et al. Value of (18) F-FDG-PET/CT in patients with fever of unknown origin and unexplained prolonged inflammatory syndrome: A single centre analysis experience. Int J Clin Pract. 2010;64:55–60. doi: 10.1111/j.1742-1241.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 18.Buysschaert I, Vanderschueren S, Blockmans D, Mortelmans L, Knockaert D. Contribution of (18) fluoro-deoxyglucose positron emission tomography to the work-up of patients with fever of unknown origin. Eur J Intern Med. 2004;15:151–56. doi: 10.1016/j.ejim.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Kjaer A, Lebech AM, Eigtved A, Højgaard L. Fever of unknown origin: Prospective comparison of diagnostic value of 18F-FDG PET and 111In. granulocyte scintigraphy. Eur J Nucl Med Mol Imaging. 2004;31:622–6. doi: 10.1007/s00259-003-1425-5. [DOI] [PubMed] [Google Scholar]
- 20.Petersdorf RG. Fever of unknown origin. An old friend revisited. Arch Intern Med. 1992;152:21–2. [PubMed] [Google Scholar]
- 21.Durack DT, Street AC. Fever of unknown origin – Reexamined and redefined. Curr Clin Top Infect Dis. 1991;11:35–51. [PubMed] [Google Scholar]
- 22.Pelosi E, Skanjeti A, Penna D, Arena V. Role of integrated PET/CT with [18F]-FDG in the management of patients with fever of unknown origin: A single-centre experience. Radiol Med. 2011;116:809–20. doi: 10.1007/s11547-011-0649-x. [DOI] [PubMed] [Google Scholar]
- 23.Sood R, Agarwal V, Mukhopadhyay S. Tuberculosis mediastinal adenopathy presenting as fever of unknown origin. Lancet. 1997;350:1782. doi: 10.1016/s0140-6736(05)63618-5. [DOI] [PubMed] [Google Scholar]
- 24.Dutta AK, Sood R, Singh UB, Kapil A, Samantaray J. Diagnostic application of conventional and newer bone marrow examination in fever of unknown origin. J Indian Acad Clin Med. 2013;14:23–7. [Google Scholar]
- 25.Prasad K. New Delhi: AIIMS; 1982. Evaluation of diagnostic methods in adults with pyrexia of undetermined origin. [Google Scholar]
- 26.Sharma BK, Kumari S, Varma SC, Sagar S, Singh S. Prolonged undiagnosed fever in northern India. Trop Geogr Med. 1992;44:32–6. [PubMed] [Google Scholar]
- 27.Handa R, Singh S, Singh N, Wali JP. Fever of unknown origin: A prospective study. Trop Doct. 1996;26:169–70. doi: 10.1177/004947559602600411. [DOI] [PubMed] [Google Scholar]
- 28.Singh G. The study of prolonged fevers. J Assoc Physicians India. 2000;48:454–5. [PubMed] [Google Scholar]
- 29.Kejariwal D, Sarkar N, Chakraborti SK, Agarwal V, Roy S. Pyrexia of unknown origin: A prospective study of 100 cases. J Postgrad Med. 2001;47:104–7. [PubMed] [Google Scholar]
- 30.Bandyopadhyay D, Bandyopadhyay R, Paul R, Roy D. Etiological study of Fever of unknown origin in patients admitted to medicine ward of a teaching hospital of eastern India. J Glob Infect Dis. 2011;3:329–33. doi: 10.4103/0974-777X.91052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blockmans D, Knockaert D, Maes A, De Caestecker J, Stroobants S, Bobbaers H, et al. Clinical value of [(18) F] fluoro-deoxyglucose positron emission tomography for patients with fever of unknown origin. Clin Infect Dis. 2001;32:191–6. doi: 10.1086/318480. [DOI] [PubMed] [Google Scholar]
- 32.Manohar K, Mittal BR, Jain S, Sharma A, Kalra N, Bhattacharya A, et al. F-18 FDG-PET/CT in evaluation of patients with fever of unknown origin. Jpn J Radiol. 2013;31:320–7. doi: 10.1007/s11604-013-0190-z. [DOI] [PubMed] [Google Scholar]
- 33.Keidar Z, Gurman-Balbir A, Gaitini D, Israel O. Fever of unknown origin: The role of 18F-FDG PET/CT. J Nucl Med. 2008;49:1980–5. doi: 10.2967/jnumed.108.054692. [DOI] [PubMed] [Google Scholar]
- 34.Ferda J, Ferdová E, Záhlava J, Matejovic M, Kreuzberg B. Fever of unknown origin: A value of (18) F-FDG-PET/CT with integrated full diagnostic isotropic CT imaging. Eur J Radiol. 2010;73:518–25. doi: 10.1016/j.ejrad.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Braconier JH, Nilsson B, Oxelius VA, Karup-Pedersen F. Recurrent pneumococcal infections in a patient with lack of specific IgG and IgM pneumococcal antibodies and deficiency of serum IgA, IgG2 and IgG4. Scand J Infect Dis. 1984;16:407–10. doi: 10.3109/00365548409073969. [DOI] [PubMed] [Google Scholar]
- 36.Meller J, Strutz F, Siefker U, Scheel A, Sahlmann CO, Lehmann K, et al. Early diagnosis and follow-up of aortitis with [(18) F] FDG PET and MRI. Eur J Nucl Med Mol Imaging. 2003;30:730–6. doi: 10.1007/s00259-003-1144-y. [DOI] [PubMed] [Google Scholar]
- 37.Scheel AK, Meller J, Vosshenrich R, Kohlhoff E, Siefker U, Müller GA, et al. Diagnosis and follow up of aortitis in the elderly. Ann Rheum Dis. 2004;63:1507–10. doi: 10.1136/ard.2003.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenbaum J, Basu S, Beckerman S, Werner T, Torigian DA, Alavi A. Evaluation of diagnostic performance of 18F-FDG-PET compared to CT in detecting potential causes of fever of unknown origin in an academic centre. Hell J Nucl Med. 2011;14:255–9. [PubMed] [Google Scholar]


