Abstract
Total knee arthroplasty has witnessed a significant increase in recent years. Despite the advantages of this surgical procedure, it has some complications, the most serious of which is prosthetic infection. The discrimination of bacterial infections from sterile inflammatory processes is of great importance in the management of periprosthetic infection (PPI). Ubiquicidin (UBI) is a synthetic antimicrobial peptide fragment reported to be highly infection-specific. Tc99m-UBI has recently been reported to be a promising radiotracer for infection imaging. We report a case of left knee PPI diagnosed using 99mTc-UBI scintigraphy and compared with F-18 fluorodeoxy-glucose positron emission tomography.
Keywords: Knee joint, prosthetic infection, Tc99m-ubiquicidin, fluorodeoxy-glucose-positron emission tomography
INTRODUCTION
As the number of orthopedic procedures continues to rise, there is a substantial increase in surgical implantation of internal devices and prostheses. Accordingly, the diagnosis of orthopedic implant infections, as one of the most important complications of the procedure, is becoming an increasingly common challenge. The noninvasive diagnostic differentiation between periprosthetic infection (PPI) and sterile inflammation is problematic, while clinical management decisions need to be made promptly in order to avoid subsequent serious complications.[1] The current available imaging approaches, despite having high sensitivity, lack specificity for infections.[2] Therefore, advances in the noninvasive differentiation between PPI and sterile inflammation are needed. Ubiquicidin (UBI) is a synthetic cationic antimicrobial peptide that preferentially binds to bacterial cell membrane at the site of infection. Considering its affinity for bacterial components, UBI has been labeled with Tc99m and tested as a potential scintigraphic agent for diagnosis of suspected PPI.[3] We report diagnostic value of Tc99m-UBI scintigraphy in differentiation of bacterial infection from sterile inflammation in suspected orthopedic knee implants.
CASE REPORT
A 55-year-old woman underwent bilateral knee replacement before 1-year. She developed left knee pain and swelling. In view of suspected PPI, she was referred for three phase bone scintigraphy. Blood pool phase showed increased tracer pooling in the left knee region [Figure 1]. Delayed whole body images show increased uptake in the left knee prosthetic bone interface [Figure 2]. Whole body Tc99m-UBI scintigraphy showed pooling of tracer in the same area of left knee region as in bone scintigraphy [Figure 3]. Static images of knee joint at 2 h showed interval increase in pooling of tracer [Figure 4]. 18F fluorodeoxy-glucose positron emission tomography (F-18 FDG PET/CT) images of the knee joint also show increased uptake in the left knee prosthesis-bone interface [Figure 5]. In view of increased uptake in all three scans, scan findings suggestive of infection were raised. Patient underwent debridement of the prosthesis, and bacterial culture was positive. She was started on antibiotics.
Figure 1.
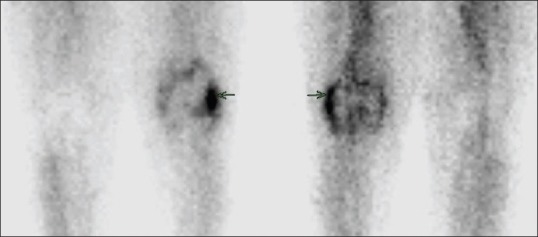
Tc99m-methylene diphosphonate blood pool phase showed increased tracer pooling in the left knee region (arrow)
Figure 2.
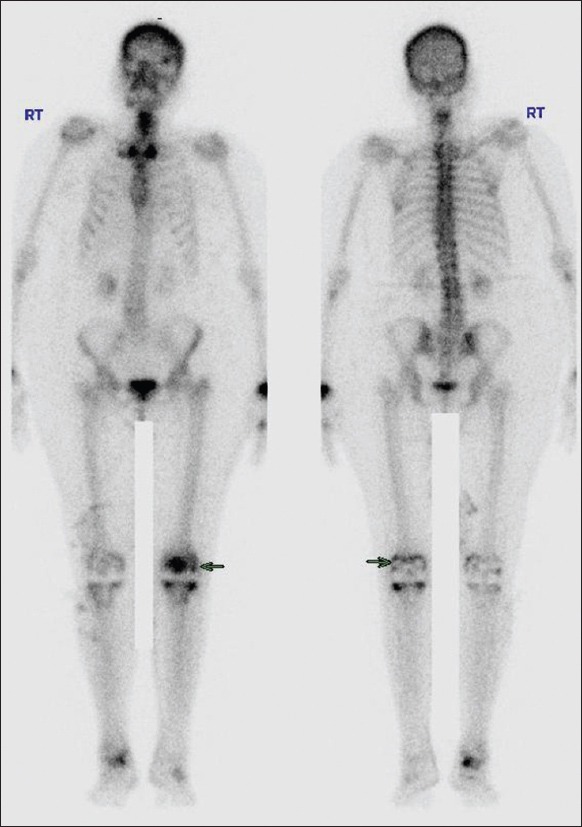
Tc99m-methylene diphosphonate whole body bone scan increased uptake in the left knee prosthetic bone interface (arrow)
Figure 3.
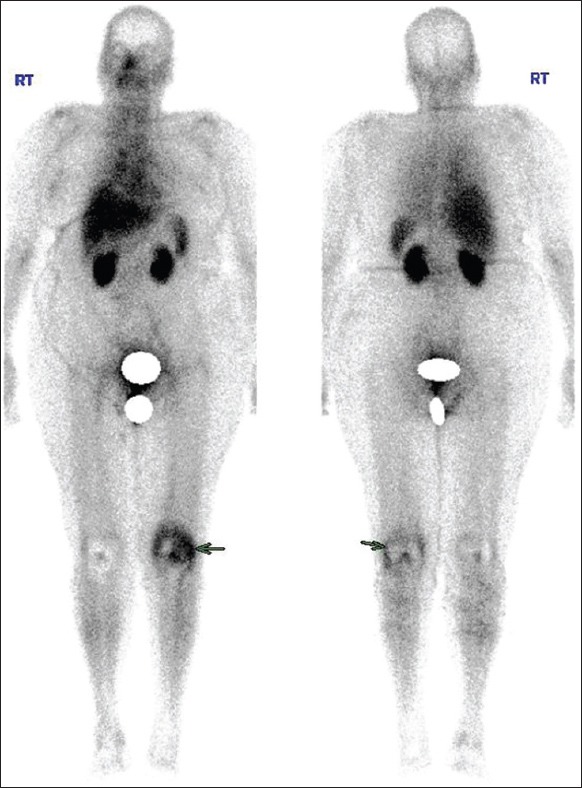
Tc99m-ubiquicidin whole body bone scan increased uptake in the left knee region (arrow). Also physiological uptake noted in liver and spleen
Figure 4.
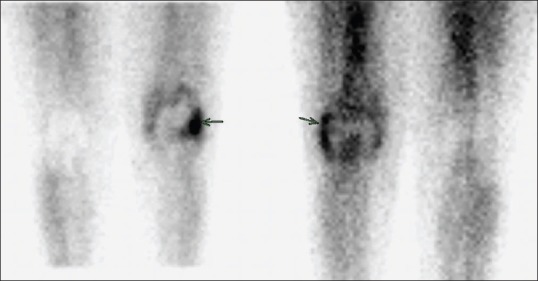
Static images of 99mTc-ubiquicidin showing increased uptake in left knee localized exactly to blood pool uptake in methylene diphosphonate (arrow)
Figure 5.
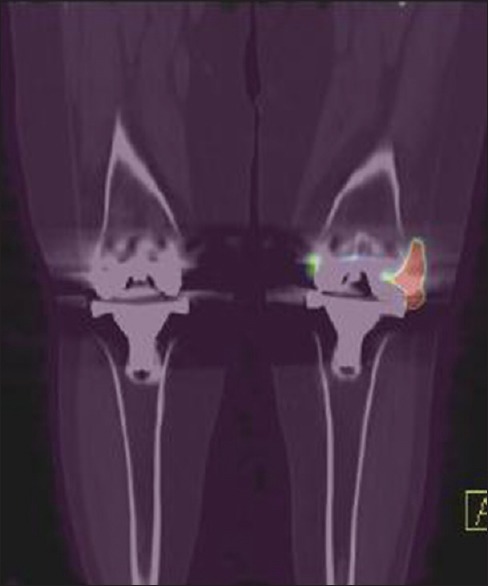
Coronal fused positron emission tomography-computed tomography also showing increased uptake in left knee prosthesis region correlating with methylene diphosphonate and ubiquicidin
DISCUSSION
Total knee arthroplasty (TKA) is a common surgery for end-stage knee arthritis that is associated with significant improvements in pain, function, and quality-of-life. Outcomes following TKA are excellent in the majority of the patients. Perhaps the most challenging complication following TKA is PPI. PPI occurs in 1–2% of primary TKAs and 3–5% of revision TKAs.[4] The differentiation of infection from aseptic loosening is of great importance because loosening with PPI is a catastrophic complication. Although the differential diagnosis of the two conditions is essential to the success of further revision surgery, the detection of PPI remains difficult. Although one stage revision surgery is indicated for patients with aseptic loosening, two-stage revision surgery in which implantation after eradication of infection by several surgical steps including removal of the implants, debridement, and antibiotics-loaded cement beads, is usually required for the patients with PPI.[5]
Several imaging modalities are available to assist surgeons in diagnosing PPI in TKA. Radiographs showing periosteal new bone formation, scattered foci of osteolysis and subchondral bone resorption are highly suggestive of infection but are typically late findings. Periprosthetic radiolucency may be unrelated to a septic process, and serial radiographs help rule out other conditions like wear, osteolysis or fracture.[6] Radionuclide scintigraphy may be helpful in the diagnosis of PPI in TKA, because their results are not impacted by the presence of metallic implants. Triple-phase Tc99m-methylene diphosphonate bone scintigraphy (TPBS) is a simple, widely available test which is quite sensitive in detecting bone remodeling changes around TKA components; however, it cannot distinguish between aseptic loosening and TKA infection TPBS does have a high negative predictive value, however, making it a useful initial screening test. Labeled antigranulocyte antibody or labeled leukocyte study has Sensitivity, specificity, and accuracy of 100%, 97%, and 98%, respectively.[7] Boubaker et al. reported that Tc99m-besilesomab was 67% sensitive and 75% specific for diagnosing prosthetic hip infection.[8]
F-18 fluorodeoxy-glucose PET/CT has been recently evaluated for diagnosis of PPI in TKA. Inflammatory cells express more glucose transporters, resulting in intracellular accumulation of F-18 FDG-6 phosphate which cannot be metabolized by the cell and can be identified by PET imaging. The advantages of a PET scan are that only one injection is required and the results are available within 4 h. However, it is not widely available, is expensive and can produce false positives secondary to uptake of FDG in aseptic inflammation around implants. In their meta-analysis, Reinartz reported that the accuracy of the TPBS, white blood cell imaging, and FDG-PET scan of PPI for TKA was 81%, 84%, and 83% respectively.[9] Aksoy et al. showed sensitivity, specificity, and positive and negative predictive values of FDG-labeled leukocyte PET/CT were 93.3%), 97.4%, 93.3%, and 97.4%, respectively.[10]
Antimicrobial peptides bind to the bacterial cell membrane. Their expression may be constant or induced on contact with microbes. They also can be transported via leukocytes.[11] Among all the human-derived antimicrobial peptides tested, the UBI has demonstrated the greatest promise. Tc99m-UBI is a small synthetic peptide, which originated from human UBI and which attaches preferentially to bacteria in vitro and not to activated leukocytes.[12] The Tc99m-UBI scans have depicted the most promising results for delineation between infection and inflammation in animal models and human investigations.[13,14] Meta-analysis by Ostovar showed that Tc99m-UBI scans had a reasonably high differentiation ability in recognizing an infection among patients who had possible infection in the soft tissue, musculoskeletal system, and prosthesis, and overall accuracy was 93.7%.[15]
A study by Akhtar et al. showed Tc99m-UBI is a highly sensitive and specific agent for localizing infective foci in bone and soft tissues of humans. The optimum imaging time for delineation between infectious and inflammatory process is 30 min after intravenous administration of radiotracer.[16] In a study by Aryana et al. evaluated the feasibility of Tc99m-UBI scintigraphy in the detection of infectious foci in painful hip prosthesis. The sensitivity, specificity, negative and positive predictive values and accuracy of the study were all 100%.[3] Our case demonstrates uptake in bone scintigraphy, 99mTc-UBI and FDG PET/CT confirming PPI in the knee joint.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Hanssen AD, Rand JA. Evaluation and treatment of infection at the site of a total hip or knee arthroplasty. Instr Course Lect. 1999;48:111–22. [PubMed] [Google Scholar]
- 2.Love C, Marwin SE, Palestro CJ. Nuclear medicine and the infected joint replacement. Semin Nucl Med. 2009;39:66–78. doi: 10.1053/j.semnuclmed.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Aryana K, Hootkani A, Sadeghi R, Davoudi Y, Naderinasab M, Erfani M, et al. (99m) Tc-labeled ubiquicidin scintigraphy: A promising method in hip prosthesis infection diagnosis. Nuklearmedizin. 2012;51:133–9. doi: 10.3413/Nukmed-0444-11-11. [DOI] [PubMed] [Google Scholar]
- 4.Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688–91. doi: 10.1302/0301-620x.86b5.14887. [DOI] [PubMed] [Google Scholar]
- 5.Rand JA. Evaluation and management of infected total knee arthroplasty. Semin Arthroplasty. 1994;5:178–82. [PubMed] [Google Scholar]
- 6.Bauer TW, Parvizi J, Kobayashi N, Krebs V. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006;88:869–82. doi: 10.2106/JBJS.E.01149. [DOI] [PubMed] [Google Scholar]
- 7.Palestro CJ. Nuclear medicine and the failed joint replacement: Past, present, and future. World J Radiol. 2014;6:446–58. doi: 10.4329/wjr.v6.i7.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boubaker A, Delaloye AB, Blanc CH, Dutoit M, Leyvraz PF, Delaloye B. Immunoscintigraphy with antigranulocyte monoclonal antibodies for the diagnosis of septic loosening of hip prostheses. Eur J Nucl Med. 1995;22:139–47. doi: 10.1007/BF00838944. [DOI] [PubMed] [Google Scholar]
- 9.Reinartz P. FDG-PET in patients with painful hip and knee arthroplasty: Technical breakthrough or just more of the same. Q J Nucl Med Mol Imaging. 2009;53:41–50. [PubMed] [Google Scholar]
- 10.Aksoy SY, Asa S, Ozhan M, Ocak M, Sager MS, Erkan ME, et al. FDG and FDG-labelled leucocyte PET/CT in the imaging of prosthetic joint infection. Eur J Nucl Med Mol Imaging. 2014;41:556–64. doi: 10.1007/s00259-013-2597-2. [DOI] [PubMed] [Google Scholar]
- 11.Lupetti A, Pauwels EK, Nibbering PH, Welling MM. 99mTc-antimicrobial peptides: Promising candidates for infection imaging. Q J Nucl Med. 2003;47:238–45. [PubMed] [Google Scholar]
- 12.Ferro-Flores G, Arteaga de Murphy C, Pedraza-López M, Meléndez-Alafort L, Zhang YM, Rusckowski M, et al. In vitro and in vivo assessment of 99mTc-UBI specificity for bacteria. Nucl Med Biol. 2003;30:597–603. doi: 10.1016/s0969-8051(03)00054-4. [DOI] [PubMed] [Google Scholar]
- 13.Sarda-Mantel L, Saleh-Mghir A, Welling MM, Meulemans A, Vrigneaud JM, Raguin O, et al. Evaluation of 99mTc-UBI 29-41 scintigraphy for specific detection of experimental Staphylococcus aureus prosthetic joint infections. Eur J Nucl Med Mol Imaging. 2007;34:1302–9. doi: 10.1007/s00259-007-0368-7. [DOI] [PubMed] [Google Scholar]
- 14.Nazari B, Azizmohammadi Z, Rajaei M, Karami M, Javadi H, Assadi M, et al. Role of 99mTc-ubiquicidin 29-41 scintigraphy to monitor antibiotic therapy in patients with orthopedic infection: A preliminary study. Nucl Med Commun. 2011;32:745–51. doi: 10.1097/MNM.0b013e3283483964. [DOI] [PubMed] [Google Scholar]
- 15.Ostovar A, Assadi M, Vahdat K, Nabipour I, Javadi H, Eftekhari M, et al. A pooled analysis of diagnostic value of (99m) Tc-ubiquicidin (UBI) scintigraphy in detection of an infectious process. Clin Nucl Med. 2013;38:413–6. doi: 10.1097/RLU.0b013e3182867d56. [DOI] [PubMed] [Google Scholar]
- 16.Akhtar MS, Qaisar A, Irfanullah J, Iqbal J, Khan B, Jehangir M, et al. Antimicrobial peptide 99mTc-ubiquicidin 29-41 as human infection-imaging agent: Clinical trial. J Nucl Med. 2005;46:567–73. [PubMed] [Google Scholar]


