Abstract
We evaluated the association of the APOE polymorphism with serum C-reactive protein levels and white blood cell count in two large population-based studies in Korean. The datasets included the Dong-gu study (n = 8,893) and the Namwon Study (n = 10,032). APOE genotypes were identified by polymerase chain reaction-restriction fragment length polymorphism. Multivariable linear regression analysis was performed to evaluate the relationship of APOE genotypes with C-reactive protein levels and white blood cell count with adjustments for age, sex, body mass index, smoking, diabetes, hypertension, and serum lipids. In the multivariate model, carriers of E3E4 or E4E4 genotype had significantly lower C-reactive protein levels compared with carriers of E3E3 genotype group (0.50 mg/L vs. 0.67 mg/L; 0.37 mg/L vs. 0.67 mg/L, respectively, for the Dong-gu Study and 0.47 mg/L vs. 0.66 mg/L; 0.45 mg/L vs. 0.66 mg/L, respectively, for the Namwon Study). However, there was no difference in white blood cell count among APOE genotypes. We found that the APOE E4 allele is associated with lower C-reactive protein levels, but not white blood cell count. Our results suggest that APOE genotype may influence C-reactive protein levels through non-inflammatory pathway.
Graphical Abstract
Keywords: C-reactive Protein; Apolipoprotein E; Polymorphism, Genetic; Inflammation
INTRODUCTION
C-reactive protein (CRP) is a sensitive and nonspecific systemic marker of inflammation (1). Increased CRP levels are associated with mortality, coronary heart disease, and stroke (2, 3). Serum CRP levels are substantially influenced by genetic factors, with a heritability of 35% to 40% (4). White blood cell count (WBC) is also a marker of systemic inflammation and higher WBC count has been associated with cardiovascular disease incidence and mortality (5, 6). WBC count is also moderately influenced by heritable factors, with heritability estimates ranging from 14% to 40% across the WBC subtypes (7).
Apolipoprotein E (apoE = protein, APOE = gene) has a central role in the metabolism of cholesterol and triglycerides (8, 9, 10). APOE gene has three common alleles (E2, E3, and E4) arising from two single nucleotide polymorphisms (rs429358 and rs7412) in exon 4 that give six possible genotypes (E2E2, E2E3, E2E4, E3E3, E3E4, and E4E4) (11). APOE genotypes have been associated with ischemic cerebrovascular disease (12) and coronary heart disease (13). Differences among APOE genotypes in the risk of cardiovascular disease have traditionally been explained by lipid metabolism. In previous our study of the same data set, APOE genotypes was associated with carotid atherosclerosis and this association was partly mediated through blood lipid (14). However, APOE genotype may also affect the risk of cardiovascular disease through anti-inflammatory and antioxidant properties of apoE protein (15, 16).
Since Manttari and colleagues first described the association of APOE E4 allele with low CRP levels in 2001, many studies have investigated relationships between APOE genotype and CRP levels. Most studies have reported that E4 allele is associated with low levels of CRP (17, 18, 19, 20, 21, 22, 23, 24, 25, 26). However, there is little research on the relationship between other markers of inflammation and APOE genotype. In addition, most studies were carried out in Caucasians, and few studies have been carried out in Asians. Therefore, we evaluated the association of the APOE genotype with serum CRP levels and WBC in two large population-based studies in South Korea.
MATERIALS AND METHODS
Subjects
The Dong-gu Study and Namwon Study are ongoing prospective studies designed to investigate the prevalence, incidence, and risk factors for chronic disease in urban and rural populations, respectively. Details of the study subjects and measurements have been published previously (27).
In the Dong-gu Study, 9,260 subjects aged 50 yr and older were recruited in the baseline survey between April 2007 and June 2010 in the Dong-gu district of Gwangju Metropolitan City in South Korea. Of these, 101 subjects were excluded because of missing data on APOE genotype, CRP, WBC, blood lipids and medical history and smoking. Subjects with WBC counts of less than 2,000 cells/μL or more than 12,000 cells/μL and/or CRP ≥ 10 mg/L were excluded because of a high probability of acute inflammation and other medical disorders, which left 8,893 (3,525 men and 5,368 women) for analysis.
In the Namwon Study, 10,667 participants (4,201 men and 6,466 women) were recruited in the baseline survey between January 2004 and February 2007 in Namwon city of Jeollabuk-do province in South Korea. Of these, 225 subjects were excluded because of missing data on APOE genotype, CRP, WBC, blood lipids and medical history and smoking. Subjects with WBC counts of less than 2,000 cells/μL or more than 12,000 cells/μL and/or CRP ≥ 10 mg/L were excluded, which left 10,032 (3,909 men and 6,123 women) for analysis.
APOE genotyping
Genomic DNA was extracted from peripheral blood with an AccuPrep Genomic DNA Extraction Kit (Bioneer, Seoul, Korea) or a QIAamp DNA Mini Kit (Qiagen Inc., Chatsworth, CA, USA) according to the manufacturer's protocol. APOE genotypes were determined as described by Hixson and Vernier, with slight modifications (28). Our APOE genotyping method has been reported previously (29). The genotyping method was validated by direct sequencing (ABI PRISM 3100 Genetic Analyzer, Applied Biosystems) of 96 subjects with 100% concordance.
Other clinical variables
Demographic characteristics, lifestyle and medical history were obtained by standardized questionnaires. Smoking status was classified into non-smoker and current smoker. Height was measured to the nearest 0.1 cm, and weight to the nearest 0.1 kg. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
Diabetes was defined by a fasting plasma glucose ≥ 126 mg/dL or use of antidiabetic medication. Blood pressure was measured in the right upper arm using a mercury sphygmomanometer (Baumanometer; WA Baum Co, Inc, Copiague, NY, USA) with an appropriately sized cuff after subjects rested at least 5 min while seated. Three consecutive measurements of systolic and diastolic blood pressures were performed at 1-min intervals, and the average was used in the analysis. Hypertension was defined by systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mmHg, or use of antihypertensive medication.
Blood samples were drawn from an antecubital vein in the morning after a 12-hr overnight fast. Serum was separated within 30 min and stored at -70℃ until analyzed. Serum total cholesterol, high-density lipoprotein cholesterol (HDL), triglycerides, and fasting blood glucose levels were measured using enzymatic methods. All samples were analyzed using an automatic analyzer (model 7600 chemical analyzer; Hitachi Ltd, Tokyo, Japan). CRP level was determined by means of particle-enhanced immunonephelometry using BN II nephelometer (Dade Behring, Marburg, Germany). The lower detection limit for CRP was 0.2 mg/L. White blood cell (WBC) count was determined using a cell counter (Micro 60, ABX, Montpellier, France).
Statistical analysis
Data are presented as mean ± standard deviation (SD) or percentage for categorical variables. Because the distribution of triglycerides and CRP levels was skewed, triglycerides and CRP values were logarithmically transformed, and geometric means with 95% confidence intervals are presented. The APOE genotypes were categorized into E2E2, E2E3, E2E4, E3E3, E3E4, and E4E4. The APOE genotype was also categorized into three groups for analytical purposes: APOE E2 (E2E2 and E2E3), APOE E3 (E3E3), and APOE E4 (E3E4 and E4E4). Subjects with the E2E4 genotype were excluded because of the opposing biological effects of the E2 and E4 alleles. Multivariable linear regression analysis was performed to evaluate the association between APOE genotypes and CRP levels and white blood cell count after adjusting for age, sex, BMI, smoking, diabetes and hypertension, total cholesterol, HDL cholesterol, and log-transformed triglycerides. The Bonferroni method was used to correct multiple comparisons. Hardy-Weinberg equilibrium was tested by use of a chi-square goodness of fit test. Statistical analyses were performed using the SPSS version 21.0 (SPSS, Inc., an IBM Company, Chicago, IL, USA).
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki guidelines. The study protocol was approved by the institutional review board of Chonnam National University Hospital (Dong-gu Study, IRB No. I-2008-05-056; Namwon Study, IRB No. I-2007-07-062), and informed consent was obtained from each subject.
RESULTS
The baseline characteristics of the study subjects are presented in Table 1. The mean age was 65.1±8.2 yr in the Dong-gu Study, and 61.6±8.0 yr in the Namwon Study. The APOE genotype frequencies were consistent with Hardy-Weinberg equilibrium (P=0.86 for the Dong-gu Study, P=0.91 for the Namwon Study) and not significantly different. The frequency of APOE genotypes for E2E2, E2E3, E2E4, E3E3, E3E4, and E4E4 was 0.4, 10.7, 1.3, 71.0, 15.6, and 1.0%, respectively in the Dong-gu Study and 0.4, 10.2, 1.1, 72.6, 14.9, and 0.9%, respectively in the Namwon Study.
Table 1. Baseline characteristics of the study subjects.
| Parameters | Dong-gu study (n = 8,893) | Namwon study (n = 10,032) | P value |
|---|---|---|---|
| Age (yr) | 65.1 ± 8.2 | 61.6 ± 8.0 | < 0.001 |
| Men (%) | 3,525 (39.6) | 3,909 (39.0) | 0.344 |
| Body mass index (kg/m2) | 24.4 ± 2.9 | 24.4 ± 3.1 | 0.617 |
| Current smoking (%) | 962 (10.8) | 1,512 (15.1) | < 0.001 |
| Hypertension (%) | 3,960 (44.5) | 3,945 (39.3) | < 0.001 |
| Diabetes mellitus (%) | 1,662 (18.7) | 1,205 (12.0) | < 0.001 |
| Total cholesterol (mg/dL) | 201.3 ± 39.9 | 189.3 ± 37.0 | < 0.001 |
| HDL cholesterol (mg/dL) | 51.6 ± 11.9 | 47.6 ± 12.0 | < 0.001 |
| Triglycerides (mg/dL)* | 118.0 (84.0-172.0) | 130.0 (89.0-193.0) | < 0.001 |
| C-reactive protein (mg/L)* | 0.60 (0.30-1.20) | 0.60 (0.30-1.30) | 0.104 |
| White cell blood count ( × 103/µL) | 5.81 ± 1.53 | 6.20 ± 1.60 | < 0.001 |
| Myocardial infarction (%) | 114 (1.3) | 37 (0.4) | < 0.001 |
| Stroke (%) | 369 (4.1) | 352 (3.5) | 0.022 |
Data are means±SD, medians (interquartile range)* or n (%). HDL, high density lipoprotein.
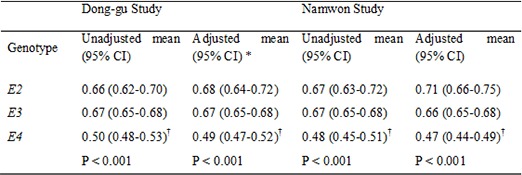
The associations of APOE genotypes and allele with CRP are shown in Table 2. There was significant difference in CRP across the APOE genotypes and alleles in both studies in all Models. Carriers of E3E4 or E4E4 genotype had significantly lower CRP levels compared with carriers of APOE E3E3 genotype group in both studies (0.51 mg/L vs. 0.67 mg/L; 0.37 mg/L vs. 0.67 mg/L, respectively, for the Dong-gu Study and 0.48 mg/L vs. 0.67 mg/L; 0.47 mg/L vs. 0.67 mg/L, respectively, for the Namwon Study). This association was not attenuated after adjustment for age, sex, BMI, smoking, diabetes and hypertension, total cholesterol, HDL cholesterol, and log-transformed triglycerides (0.50 mg/L vs. 0.67 mg/L; 0.37 mg/L vs. 0.67 mg/L, respectively, for the Dong-gu Study and 0.47 mg/L vs. 0.66 mg/L; 0.45 mg/L vs. 0.66 mg/L, respectively, for the Namwon Study).
Table 2. Adjusted geometric means and 95% confidence intervals for C-reactive protein (mg/L) in different APOE genotypes or alleles.
| Genotypes | Dong-gu study | Namwon study | ||||
|---|---|---|---|---|---|---|
| No. (%) | Unadjusted mean (95% CI) | Adjusted mean (95% CI)* | No. (%) | Unadjusted mean (95% CI) | Adjusted mean (95% CI) | |
| E2E2 | 40 (0.4) | 0.57 (0.43-0.77) | 0.61 (0.46-0.81) | 40 (0.4) | 0.62 (0.44-0.87) | 0.67 (0.48-0.93) |
| E2E3 | 954 (10.7) | 0.66 (0.62-0.70) | 0.68 (0.64-0.72) | 1,026 (10.2) | 0.67 (0.63-0.72) | 0.71 (0.66-0.76) |
| E2E4 | 115 (1.3) | 0.52 (0.44-0.62) | 0.53 (0.45-0.63) | 106 (1.1) | 0.62 (0.51-0.77) | 0.65 (0.53-0.79) |
| E3E3 | 6,312 (71.0) | 0.67 (0.65-0.68) | 0.67 (0.65-0.68) | 7,280 (72.6) | 0.67 (0.65-0.68) | 0.66 (0.65-0.68) |
| E3E4 | 1,386 (15.6) | 0.51 (0.49-0.54)† | 0.50 (0.48-0.53)† | 1,493 (14.9) | 0.48 (0.45-0.51)† | 0.47 (0.44-0.49)† |
| E4E4 | 86 (1.0) | 0.37 (0.31-0.46)† | 0.37 (0.30-0.45)† | 87 (0.9) | 0.47 (0.37-0.59)† | 0.45 (0.36-0.57)† |
| P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | |||
| Partial R2‡ = 0.014 | Partial R2 = 0.016 | Partial R2 = 0.012 | Partial R2 = 0.016 | |||
| E2 | 994 (11.2) | 0.66 (0.62-0.70) | 0.68 (0.64-0.72) | 1,066 (10.6) | 0.67 (0.63-0.72) | 0.71 (0.66-0.75) |
| E3 | 6,312 (71.0) | 0.67 (0.65-0.68) | 0.67 (0.65-0.68) | 7,280 (72.6) | 0.67 (0.65-0.68) | 0.66 (0.65-0.68) |
| E4 | 1,472 (16.6) | 0.50 (0.48-0.53)† | 0.49 (0.47-0.52)† | 1,580 (15.7) | 0.48 (0.45-0.51)† | 0.47 (0.44-0.49)† |
| P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | |||
| Partial R2 = 0.012 | Partial R2 = 0.015 | Partial R2 = 0.012 | Partial R2 = 0.016 | |||
Values are geometric mean (95% confidence interval). *Adjusted for age, sex, BMI, smoking, hypertension, diabetes mellitus, total cholesterol, HDL cholesterol, and log triglycerides. †P<0.05 compared with E3/E3 or E3 with a Bonferroni multiple comparisons test; ‡Variance explained by APOE genotype after adjustment for covariates.
Carriers of E4 allele had significantly lower CRP levels compared with carriers of APOE E3 allele in both studies (0.50 mg/L vs. 0.67 mg/L for the Dong-gu Study and 0.48 mg/L vs. 0.67 mg/L for the Namwon Study). This association was not attenuated after adjustment for potential confounders (0.49 mg/L vs. 0.67 mg/L for the Dong-gu Study and 0.47 mg/L vs. 0.66 mg/L for the Namwon Study). There was no difference in CRP levels between carriers of E2 allele and carriers of E3 alleles.
The variance of CRP explained by the APOE genotype after adjustment for covariates was 1.6% in both studies. However, there was no difference in WBC among APOE genotypes in both studies (Table 3).
Table 3. Adjusted means and 95% confidence intervals for white blood cell count in different APOE genotypes or alleles.
| Genotypes | Dong-gu study | Namwon study | ||||
|---|---|---|---|---|---|---|
| No. (%) | Unadjusted mean (95% CI) | Adjusted mean (95% CI)* | No. (%) | Unadjusted mean (95% CI) | Adjusted mean (95% CI) | |
| E2E2 | 40 (0.4) | 5.60 (5.13-6.07) | 5.56 (5.11-6.01) | 40 (0.4) | 5.70 (5.21-6.20) | 5.82 (5.35-6.28) |
| E2E3 | 954 (10.7) | 5.82 (5.72-5.92) | 5.83 (5.73-5.92) | 1,026 (10.2) | 6.21 (6.11-6.31) | 6.22 (6.12-6.31) |
| E2E4 | 115 (1.3) | 5.72 (5.44-6.00) | 5.72 (5.46-5.99) | 106 (1.1) | 6.04 (5.73-6.34) | 6.07 (5.79-6.36) |
| E3E3 | 6,312 (71.0) | 5.80 (5.76-5.84) | 5.80 (5.77-5.84) | 7,280 (72.6) | 6.20 (6.17-6.24) | 6.20 (6.17-6.24) |
| E3E4 | 1,386 (15.6) | 5.88 (5.80-5.96) | 5.87 (5.79-5.94) | 1,493 (14.9) | 6.16 (6.08-6.25) | 6.16 (6.08-6.23) |
| E4E4 | 86 (1.0) | 5.75 (5.43-6.07) | 5.73 (5.42-6.03) | 87 (0.9) | 6.18 (5.84-6.51) | 6.11 (5.8-6.43) |
| P = 0.412 | P = 0.512 | P = 0.328 | P = 0.414 | |||
| E2 | 994 (11.2) | 5.81 (5.72-5.91) | 5.82 (5.73-5.91) | 1,066 (10.6) | 6.19 (6.1-6.29) | 6.20 (6.11-6.29) |
| E3 | 6,312 (71.0) | 5.80 (5.76-5.84) | 5.80 (5.77-5.84) | 7,280 (72.6) | 6.20 (6.17-6.24) | 6.21 (6.17-6.24) |
| E4 | 1,472 (16.6) | 5.88 (5.80-5.95) | 5.86 (5.78-5.93) | 1,580 (15.7) | 6.16 (6.09-6.24) | 6.16 (6.08-6.23) |
| P = 0.204 | P = 0.415 | P = 0.667 | P = 0.493 | |||
Values are arithmetic mean (95% confidence interval) for white blood cell count (×103/µL). *Adjusted for age, sex, BMI, smoking, hypertension, diabetes mellitus, total cholesterol, HDL cholesterol, and log triglycerides.
DISCUSSION
In this population based study, we observed that the E3E4 and E4E4 genotypes were associated with lower C-reactive protein compared with the E3E3 genotype group, while WBC count was not associated with APOE genotype. To our knowledge, this is the largest population-based study on the association of the APOE genotype and CRP levels and WBC count.
Our results that E3E4 and E4E4 genotype are associated with lower CRP levels are in agreement with previous studies (17, 18, 19, 20, 21, 22, 23, 24, 25, 26). In a few studies, there was no association between APOE E4 allele and low CRP levels (30, 31). The small sample size of these studies might have contributed to the negative findings. Recently, Hubacek et al. (25) observed the lowest CRP levels in carriers of the APOE E3E4 and E4E4 genotypes in a large general population sample of 6,230 aged 45-69 yr. In a meta-analysis in Asians including a Korean cohort and two Filipino cohorts, APOE E4 haplotype was significantly associated with decreased CRP levels compared to the APOE E3 haplotype (32).
There is still limited research on the association of APOE genotype with pro- and anti-inflammatory markers except for CRP in humans. In the present study, APOE genotype was not associated with WBC count. There was only one study that reported an association between APOE genotype and WBC count (18), which is in accordance with our study. This study showed that the APOE genotype is associated with CRP levels, but not white blood cells. However, a limitation of this study is the small sample number of participants who had undergone coronary angiography. Tziakas et al. (33) reported that E3E4 carriers were not only associated with lower levels of IL-10, an anti-inflammatory cytokine, but also with lower CRP levels, an inflammatory marker in patients with acute coronary syndrome and chronic stable angina. Tziakas et al. (33) speculated that in E4 carriers, the deleterious effects of low IL-10 levels may outweigh the protective effects of low CRP levels found in these patients, and that an imbalance between anti- and pro-inflammatory forces may be responsible for the increased CAD risk associated with the E4 allele. Drabe et al. (34) reported that the presence of the E4 allele is associated with increased release of IL-8 and TNF-alpha in 22 patients receiving standard coronary artery bypass grafting. Further studies are needed to clarify the association of APOE genotype and inflammation markers.
The underlying mechanisms by which APOE genotype affects CRP levels are poorly understood. This mechanism may not be related to inflammation for following reasons. First, the negative association between E4 allele and CRP levels is in contradiction to the observation that E4 allele is a risk factor for CVD and Alzheimer's disease (13, 35) and has been associated with increased brain and macrophage inflammation (36). Second, in our study, there was no association between APOE genotype and WBC count, an important cellular marker of systemic inflammation. März et al. (18) postulated that the metabolism of CRP could be related to the activity of the mevalonate pathway in the liver, since in E4 carriers, the mevalonate pathway that produces cholesterol in vivo is downregulated, which could also lead to low production of CRP. This postulation can be supported by the findings that statins, the inhibitor of the mevalonate pathway, cannot only reduce the cholesterol biosynthesis but also CRP levels (18, 37). However, this hypothesis has not been verified and the possible biological mechanisms are not entirely clear. It may be necessary to see whether the APOE genotype would modify the association between CRP and inflammation-related disease. Because of the two opposing effects of APOE E4 on inflammation, APOE E4 allele may influence atherosclerosis in a positive or negative way and using CRP as the biomarker in assessing CVD risk could underestimate the CVD risk in the carriers of E4 allele (36).
The strengths of this study are its very large sample size and adequate statistical power to assess whether APOE genotype is associated with CRP levels. Our study has some limitations. First, we measured the CRP levels and WBC count only once in this study, which may have affected the results. However, the within-person biologic variation of CRP is low (38) and so there may be little bias. Second, we did not measure other inflammatory markers such as interleukin-6, tumor necrosis factor-α, and fibrinogen which might have helped us to better clarify the relationship between APOE genotype and inflammation.
In conclusion, our data demonstrated that the APOE E4 allele is associated with lower CRP levels, but not WBC count. Our results suggest that APOE genotype may influence CRP levels through a non-inflammatory pathway.
Footnotes
This study was supported by a grant (CRI11020-1) Chonnam National University Hospital Biomedical Research Institute.
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception and design: Shin MH, Kweon SS. Acquisition of data: Yun YW, Kweon SS, Choi JS, Rhee JA, Lee YH, Nam HS, Jeong SK, Park KS, Ryu SY, Choi SW, Kim HN, Shin MH. Analysis and interpretation of data: Yun YW, Shin MH. Preparation, critical revision and final approval of manuscript approval: all authors.
References
- 1.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 4.Pankow JS, Folsom AR, Cushman M, Borecki IB, Hopkins PN, Eckfeldt JH, Tracy RP. Familial and genetic determinants of systemic markers of inflammation: the NHLBI family heart study. Atherosclerosis. 2001;154:681–689. doi: 10.1016/s0021-9150(00)00586-4. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Anderson K, Wilson PW. White blood cell count and cardiovascular disease. Insights from the Framingham Study. JAMA. 1992;267:1253–1256. [PubMed] [Google Scholar]
- 6.Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: atherosclerosis risk in communities study. Am J Epidemiol. 2001;154:758–764. doi: 10.1093/aje/154.8.758. [DOI] [PubMed] [Google Scholar]
- 7.Keller MF, Reiner AP, Okada Y, van Rooij FJ, Johnson AD, Chen MH, Smith AV, Morris AP, Tanaka T, Ferrucci L, et al. CHARGE Hematology; COGENT; BioBank Japan Project (RIKEN) Working Groups. Trans-ethnic meta-analysis of white blood cell phenotypes. Hum Mol Genet. 2014;23:6944–6960. doi: 10.1093/hmg/ddu401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC. Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am J Epidemiol. 2002;155:487–495. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- 9.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 10.Song Y, Stampfer MJ, Liu S. Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Ann Intern Med. 2004;141:137–147. doi: 10.7326/0003-4819-141-2-200407200-00013. [DOI] [PubMed] [Google Scholar]
- 11.Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8:1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- 12.McCarron MO, Delong D, Alberts MJ. APOE genotype as a risk factor for ischemic cerebrovascular disease: a meta-analysis. Neurology. 1999;53:1308–1311. doi: 10.1212/wnl.53.6.1308. [DOI] [PubMed] [Google Scholar]
- 13.Bennet AM, Di Angelantonio E, Ye Z, Wensley F, Dahlin A, Ahlbom A, Keavney B, Collins R, Wiman B, de Faire U, et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007;298:1300–1311. doi: 10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- 14.Shin MH, Choi JS, Rhee JA, Lee YH, Nam HS, Jeong SK, Park KS, Kim HY, Ryu SY, Choi SW, et al. APOE polymorphism and carotid atherosclerosis in Korean population: the Dong-gu Study and the Namwon Study. Atherosclerosis. 2014;232:180–185. doi: 10.1016/j.atherosclerosis.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Bellosta S, Mahley RW, Sanan DA, Murata J, Newland DL, Taylor JM, Pitas RE. Macrophage-specific expression of human apolipoprotein E reduces atherosclerosis in hypercholesterolemic apolipoprotein E-null mice. J Clin Invest. 1995;96:2170–2179. doi: 10.1172/JCI118271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorngate FE, Rudel LL, Walzem RL, Williams DL. Low levels of extrahepatic nonmacrophage ApoE inhibit atherosclerosis without correcting hypercholesterolemia in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:1939–1945. doi: 10.1161/01.atv.20.8.1939. [DOI] [PubMed] [Google Scholar]
- 17.Mänttäri M, Manninen V, Palosuo T, Ehnholm C. Apolipoprotein E polymorphism and C-reactive protein in dyslipidemic middle-aged men. Atherosclerosis. 2001;156:237–238. doi: 10.1016/s0021-9150(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 18.Marz W, Scharnagl H, Hoffmann MM, Boehm BO, Winkelmann BR. The apolipoprotein E polymorphism is associated with circulating C-reactive protein (the Ludwigshafen risk and cardiovascular health study) Eur Heart J. 2004;25:2109–2119. doi: 10.1016/j.ehj.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Judson R, Brain C, Dain B, Windemuth A, Ruaño G, Reed C. New and confirmatory evidence of an association between APOE genotype and baseline C-reactive protein in dyslipidemic individuals. Atherosclerosis. 2004;177:345–351. doi: 10.1016/j.atherosclerosis.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Eiriksdottir G, Aspelund T, Bjarnadottir K, Olafsdottir E, Gudnason V, Launer LJ, Harris TB. Apolipoprotein E genotype and statins affect CRP levels through independent and different mechanisms: AGES-Reykjavik Study. Atherosclerosis. 2006;186:222–224. doi: 10.1016/j.atherosclerosis.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Chasman DI, Kozlowski P, Zee RY, Kwiatkowski DJ, Ridker PM. Qualitative and quantitative effects of APOE genetic variation on plasma C-reactive protein, LDL-cholesterol, and apoE protein. Genes Immun. 2006;7:211–219. doi: 10.1038/sj.gene.6364289. [DOI] [PubMed] [Google Scholar]
- 22.Berrahmoune H, Herbeth B, Siest G, Visvikis-Siest S. Heritability of serum hs-CRP concentration and 5-year changes in the Stanislas family study: association with apolipoprotein E alleles. Genes Immun. 2007;8:352–359. doi: 10.1038/sj.gene.6364395. [DOI] [PubMed] [Google Scholar]
- 23.Grönroos P, Raitakari OT, Kähönen M, Hutri-Kähönen N, Marniemi J, Viikari J, Lehtimäki T. Association of high sensitive C-reactive protein with apolipoprotein E polymorphism in children and young adults: the Cardiovascular Risk in Young Finns Study. Clin Chem Lab Med. 2008;46:179–186. doi: 10.1515/CCLM.2008.033. [DOI] [PubMed] [Google Scholar]
- 24.Haan MN, Aiello AE, West NA, Jagust WJ. C-reactive protein and rate of dementia in carriers and non carriers of Apolipoprotein APOE4 genotype. Neurobiol Aging. 2008;29:1774–1782. doi: 10.1016/j.neurobiolaging.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubacek JA, Peasey A, Pikhart H, Stavek P, Kubinova R, Marmot M, Bobak M. APOE polymorphism and its effect on plasma C-reactive protein levels in a large general population sample. Hum Immunol. 2010;71:304–308. doi: 10.1016/j.humimm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ukkola O, Kunnari A, Jokela M, Päivänsalo M, Kesäniemi YA. ApoE phenotype is associated with inflammatory markers in middle-aged subjects. Inflamm Res. 2009;58:54–59. doi: 10.1007/s00011-008-8215-2. [DOI] [PubMed] [Google Scholar]
- 27.Kweon SS, Shin MH, Jeong SK, Nam HS, Lee YH, Park KS, Ryu SY, Choi SW, Kim BH, Rhee JA, et al. Cohort Profile: The Namwon Study and the Dong-gu Study. Int J Epidemiol. 2014;43:558–567. doi: 10.1093/ije/dys244. [DOI] [PubMed] [Google Scholar]
- 28.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 29.Shin MH, Kim HN, Cui LH, Kweon SS, Park KS, Heo H, Nam HS, Jeong SK, Chung EK, Choi JS. The effect of apolipoprotein E polymorphism on lipid levels in Korean adults. J Korean Med Sci. 2005;20:361–366. doi: 10.3346/jkms.2005.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mooijaart SP, Berbée JF, van Heemst D, Havekes LM, de Craen AJ, Slagboom PE, Rensen PC, Westendorp RG. ApoE plasma levels and risk of cardiovascular mortality in old age. PLoS Med. 2006;3:e176. doi: 10.1371/journal.pmed.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin MA, Zhang C, Humphries SE, Chandler WL, Talmud PJ, Edwards KL, Leonetti DL, McNeely MJ, Fujimoto WY. Heritability of C-reactive protein and association with apolipoprotein E genotypes in Japanese Americans. Ann Hum Genet. 2004;68:179–188. doi: 10.1046/j.1529-8817.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- 32.Hong EP, Kim DH, Suh JG, Park JW. Genetic risk assessment for cardiovascular disease with seven genes associated with plasma C-reactive protein concentrations in Asian populations. Hypertens Res. 2014;37:692–698. doi: 10.1038/hr.2014.56. [DOI] [PubMed] [Google Scholar]
- 33.Tziakas DN, Chalikias GK, Antonoglou CO, Veletza S, Tentes IK, Kortsaris AX, Hatseras DI, Kaski JC. Apolipoprotein E genotype and circulating interleukin-10 levels in patients with stable and unstable coronary artery disease. J Am Coll Cardiol. 2006;48:2471–2481. doi: 10.1016/j.jacc.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 34.Drabe N, Zünd G, Grünenfelder J, Sprenger M, Hoerstrup SP, Bestmann L, Maly FE, Turina M. Genetic predisposition in patients undergoing cardiopulmonary bypass surgery is associated with an increase of inflammatory cytokines. Eur J Cardiothorac Surg. 2001;20:609–613. doi: 10.1016/s1010-7940(01)00842-9. [DOI] [PubMed] [Google Scholar]
- 35.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jofre-Monseny L, Minihane AM, Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res. 2008;52:131–145. doi: 10.1002/mnfr.200700322. [DOI] [PubMed] [Google Scholar]
- 37.Rontu R, Ojala P, Hervonen A, Goebeler S, Karhunen PJ, Nikkilä M, Kunnas T, Jylhä M, Eklund C, Hurme M, et al. Apolipoprotein E genotype is related to plasma levels of C-reactive protein and lipids and to longevity in nonagenarians. Clin Endocrinol (Oxf) 2006;64:265–270. doi: 10.1111/j.1365-2265.2006.02455.x. [DOI] [PubMed] [Google Scholar]
- 38.Carlson CS, Aldred SF, Lee PK, Tracy RP, Schwartz SM, Rieder M, Liu K, Williams OD, Iribarren C, Lewis EC, et al. Polymorphisms within the C-reactive protein (CRP) promoter region are associated with plasma CRP levels. Am J Hum Genet. 2005;77:64–77. doi: 10.1086/431366. [DOI] [PMC free article] [PubMed] [Google Scholar]


