Abstract
The prognostic value of the left ventricle ejection fraction (LVEF) after acute myocardial infarction (AMI) has been questioned even though it is an accurate marker of left ventricle (LV) systolic dysfunction. This study aimed to examine the prognostic impact of LVEF in patients with AMI with or without high-grade mitral regurgitation (MR). A total of 15,097 patients with AMI who received echocardiography were registered in the Korean Acute Myocardial Infarction Registry (KAMIR) between January 2005 and July 2011. Patients with low-grade MR (grades 0-2) and high-grade MR (grades 3-4) were divided into the following two sub-groups according to LVEF: LVEF ≤ 40% (n = 2,422 and 197, respectively) and LVEF > 40% (n = 12,252 and 226, respectively). The primary endpoints were major adverse cardiac events (MACE), cardiac death, and all-cause death during the first year after registration. Independent predictors of mortality in the multivariate analysis in AMI patients with low-grade MR were age ≥ 75 yr, Killip class ≥ III, N-terminal pro-B-type natriuretic peptide > 4,000 pg/mL, high-sensitivity C-reactive protein ≥ 2.59 mg/L, LVEF ≤ 40%, estimated glomerular filtration rate (eGFR), and percutaneous coronary intervention (PCI). However, PCI was an independent predictor in AMI patients with high-grade MR. No differences in primary endpoints between AMI patients with high-grade MR (grades 3-4) and EF ≤ 40% or EF > 40% were noted. MR is a predictor of a poor outcome regardless of ejection fraction. LVEF is an inadequate method to evaluate contractile function of the ischemic heart in the face of significant MR.
Keywords: Mitral Regurgitation, Acute Myocardial Infarction, Left Ventricular Ejection Fraction
INTRODUCTION
Mitral regurgitation (MR) is a frequent complication and a powerful predictor of long-term cardiovascular mortality after acute myocardial infarction (AMI) (1, 2, 3, 4, 5). Although left ventricular (LV) ejection fraction (EF) is a determinant of poor prognosis and an accurate marker of LV systolic dysfunction, the prognostic value of LVEF after MI has been questioned (6). In patients with chronic MR, LVEF underestimates the degree of LV systolic dysfunction because of volume overload. LVEF is also highly influenced not only by LV contractility but also by LV geometry, loading condition, and MR severity (7, 8). In patients with AMI, low LVEF can be the result of reduced contractile function due to extensive myocardial damage, LV dilatation, or myocardial stunning (9).
The role of LVEF as a prognostic factor in AMI patients with significant MR has been poorly addressed (10, 11). Furthermore, the prognostic value of LVEF in AMI patients with severe MR has not been previously reported. This study aimed to examine the prognostic significance of LVEF in the long-term outcome of AMI patients with or without severe MR in the clinical setting.
MATERIALS AND METHODS
Study population
The Korean Acute Myocardial Infarction Registry (KAMIR) is a Korean, prospective, open, observational, multicenter, on-line registry of AMI data with support from the Korean Society of Cardiology that was initiated in November 2005. The 50 participating hospitals are capable of performing primary percutaneous coronary intervention (PCI). Details of the KAMIR have been published previously (2, 12, 13). A total of 15,097 patients with AMI who received echocardiography were registered in the KAMIR between January 2005 and July 2011. Patients with low-grade MR (grade 0-2) were divided into two groups according to LVEF, namely, LVEF≤40% (n=2,426) or EF>40% (n=12,252). Patients with high-grade MR (grade 3-4) were similarly divided into two groups (EF≤40% [n=197] or EF>40% [n=226]).
The endpoints of the study were major adverse cardiac events (MACE), cardiac death, and all-cause death during the year following registration. MACEs were defined as the composite of all-cause death, MI, and repeated PCI or coronary artery bypass grafting (CABG) during 12 months of clinical follow-up.
The follow-ups in the outpatient clinic occurred immediately after hospital discharge, one month post-discharge, and at intervals of less than 6 months thereafter. Information on events and mortality was obtained from hospital records and phone calls.
The diagnosis of non-ST elevation myocardial infarction (NSTEMI) and ST elevation myocardial infarction (STEMI) was based on the definitions from the American College of Cardiology/American Heart Association (ACC/AHA guidelines) (14, 15).
Coronary angiogram
Coronary angiograms were performed using standard techniques. Significant coronary artery disease (CAD) was defined as ≥70% stenosis of an epicardial coronary artery. The extent of CAD was characterized by one-, two-, or three-vessel disease or left main disease (14, 15). Percutaneous coronary intervention (PCI) was performed according to the physician's discretion. Coronary blood flow in the infarct-related artery before and after stent implantation was graded according to the classification used in the Thrombolysis in Myocardial Infarction trials.
Echocardiography
All index transthoracic echocardiographs were recorded during routine clinical practice according to the current guidelines (16). Two-dimensional M-mode echocardiography and Doppler ultrasound examinations were performed within 3 days of the PCI.
In each patient, LVEF was measured using bidimensional echocardiography from two- and four-chamber apical views by the modified Simpson's method (17). The wall motion score index was derived according to a 17 segment model (18). For each segment, wall motion was scored from 1 (normal) to 4 (dyskinesia). The presence and degree of MR were measured using the proximal isovelocity surface area (PISA) method and a validated nomogram for semi-quantitative estimation (19). MR was classified into four degrees of severity (I: mild, II: mild to moderate, III: moderate, IV: severe).
Statistical analysis
Data are expressed as the means±SD for continuous variables and absolute numbers (proportions) for categorical variables. All comparisons between baseline variables were performed by the Pearson chi-square test for categorical variables and the t-test for continuous variables.
Cox proportional hazards regression was used to estimate the relative mortality risk at 1 yr. We controlled for all available variables considered potentially relevant in all regression analysis of low-grade MR (grade 0-2) and high-grade MR (grade 3-4): age, gender, Killip class, Q wave on electrocardiography (ECG), Ischemic heart disease history (IHD), diabetes mellitus (DM), N-terminal pro-B type natriuretic peptide (NT-proBNP), glucose level, high-sensitivity C-reactive protein (Hs-CRP), LVEF, estimated glomerular filtration rate (eGFR), and PCI. Best cut-off values of continuous variables were assessed by the receiver operating curve. All statistical tests were 2-sided, and a P value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS 18.0 for Windows (SPSS, Inc., Chicago, IL, USA). Survival analysis was performed using the Kaplan-Meier method with log-rank tests to compare survival between groups.
Ethics statement
The institutional review board of all participating institutions approved the study protocol. The approval number of Chonnam National University Hospital was 05-49. Written informed consent was obtained from all participating patients.
RESULTS
Baseline clinical characteristics and laboratory findings
The baseline clinical characteristics are presented in Table 1. Among the patients with low-grade MR (grade 0-2), the patients in the LVEF≤40% group were older, were more likely to be women, had decreased body mass indexes (BMIs) and blood pressures, had higher heart rates and Killip classes and were more likely to have histories of hypertension, DM, and IHD compared with the patients in the LVEF>40% group. Patients with reduced LVEF were more likely to present STEMI, Q waves, and atrial fibrillation/flutter on electrocardiography. However, among the patients with high-grade MR (grade 3-4), the patients in the LVEF≤40% group were more likely to be men, have higher heart rates, have histories of DM and IHD, and present Q waves on ECG compared with the patients in the LVEF>40% group. Regardless of MR grade, the LVEF≤40% group had higher glucose, Hs-CRP, and NT-proBNP levels compared with the LVEF >40% group (Table 2).
Table 1. Clinical characteristics of patients.
| Clinical characteristics | MR Grades 0-2 | MR Grades 3-4 | ||||
|---|---|---|---|---|---|---|
| EF ≤ 40% (n = 2,422) |
EF > 40% (n = 12,252) |
P value | EF ≤ 40% (n = 197) |
EF > 40% (n = 226) |
P value | |
| Age (mean ± SD) (yr) | 67.0 ± 12.2 | 63.4 ± 12.5 | < 0.001 | 71.1 ± 11.7 | 72.6 ± 10.6 | 0.164 |
| Men (%) | 1,695 (70.1) | 8,906 (72.6) | 0.006 | 106 (54.1) | 97 (42.9) | 0.022 |
| Body mass index, median (IQR) | 23 (21-25.4) | 24 (22-26) | < 0.001 | 23 (21-24) | 23 (21-25) | 0.791 |
| Heart rate (beats/min) | 83 (72-99.5) | 74 (64-84) | < 0.001 | 90 (72-107) | 78 (64-91) | < 0.001 |
| Blood pressure (mmHg) | ||||||
| Systolic | 121 (110-140) | 130 (110-150) | < 0.001 | 120 (100-146) | 128 (105-140) | 0.518 |
| Diastolic | 80 (69-90) | 80 (70-90) | < 0.001 | 71 (60-87) | 77 (63.5-89.5) | 0.626 |
| Killip class ≥ III | 673 (28.7) | 1,003 (8.5) | < 0.001 | 91 (46.4) | 87 (38.7) | 0.094 |
| Risk factor (%) | ||||||
| Hypertension | 1,209 (50.2) | 5,818 (47.8) | 0.018 | 103 (53.1) | 130 (61.3) | 0.693 |
| Diabetes mellitus | 829 (34.2) | 3,078 (25.3) | < 0.001 | 93 (47.2) | 70 (31.4) | < 0.001 |
| Currently smoking | 1,169 (48.9) | 6,552 (54.0) | < 0.001 | 74 (38.1) | 67 (30.0) | 0.081 |
| Dyslipidemia* | 224 (9.3) | 1,407 (11.6) | 0.001 | 27 (13.9) | 37 (16.4) | 0.459 |
| Ischemic heart disease history | 500 (20.8) | 1,688 (13.9) | < 0.001 | 62 (31.5) | 45 (20.4) | 0.009 |
| STEMI | 1,535 (63.4) | 6,927 (56.5) | < 0.001 | 77 (39.1) | 81 (36.0) | 0.546 |
| NSTEMI | 887 (36.6) | 5,325 (43.5) | < 0.001 | 120 (60.9) | 144 (64.0) | 0.540 |
| Q wave | 473 (19.6) | 1,547 (12.6) | < 0.001 | 43 (22.5) | 19 (8.7) | < 0.001 |
| Atrial fibrillation/ flutter | 130 (5.4) | 389 (3.2) | < 0.001 | 23 (11.8) | 22 (10.0) | 0.546 |
Data are expressed as the mean±SD or number (%), or median (IQR) as appropriate. *Defined as patients who were previously diagnosed by a physician and/or patients receiving lipid-lowering drugs. NSTEMI, non-ST elevation myocardial infarction; STEMI, ST elevation myocardial infarction; MR, mitral regurgitation; EF, ejection fraction.
Table 2. Laboratory findings and echocardiographic parameters.
| Variables | MR Grades 0-2 | MR Grades 3-4 | ||||
|---|---|---|---|---|---|---|
| EF ≤ 40% (n = 2,422) |
EF > 40% (n = 12,252) |
P value | EF ≤ 40% (n = 197) |
EF > 40% (n = 226) |
P value | |
| Peak CK-MB (U/L) | 162.7 ± 332.3 | 118.1 ± 223.1 | < 0.001 | 121.8 ± 310.8 | 98.4 ± 146.8 | 0.314 |
| Glucose (mg/dL) | 188.2 ± 92.3 | 164.1 ± 75.6 | < 0.001 | 205.6 ± 100.1 | 179.7 ± 90.2 | 0.006 |
| Glomerular filtration rate (mL/min/1.73 m2) | 59.9 ± 37.3 | 73.6 ± 40.3 | < 0.001 | 49.9 ± 26.2 | 53.6 ± 28.1 | 0.183 |
| Total cholesterol (mg/dL) | 178.1 ± 45.7 | 183.8 ± 43.4 | < 0.001 | 169.6 ± 50.1 | 174.0 ± 49.8 | 0.370 |
| Triglycerides (mg/dL) | 113.4 ± 73.8 | 132.9 ± 111.2 | < 0.001 | 101.4 ± 56.9 | 107.7 ± 57.3 | 0.273 |
| High-density lipoprotein cholesterol (mg/dL) | 44.3 ± 15.3 | 44.9 ± 21.4 | 0.221 | 43.4 ± 14.5 | 43.7 ± 13.6 | 0.845 |
| Low-density lipoprotein cholesterol (mg/dL) | 114.2 ± 42.8 | 117.1 ± 39.6 | 0.003 | 109.6 ± 46.3 | 110.2 ± 40.2 | 0.904 |
| High-sensitivity C-reactive protein (mg/dL) | 12.8 ± 41.4 | 7.28 ± 35.8 | < 0.001 | 19.3 ± 86.4 | 12.7 ± 45.1 | 0.035 |
| NT-pro BNP (pg/mL) | 5,472.8 ± 9,110.1 | 1,626.0 ± 443.6 | < 0.001 | 8,984.9 ± 10,105.8 | 6,116.4 ± 9,101.3 | 0.009 |
| Echocardiographic parameters | ||||||
| Left ventricular ejection fraction (%) | 33.4 ± 6.25 | 56.0 ± 8.8 | < 0.001 | 31.4 ± 6.7 | 51.9 ± 7.7 | < 0.001 |
| Regional wall motion score | 25.3 ± 12.6 | 18.5 ± 9.3 | < 0.001 | 28.4 ± 13.8 | 21.3 ± 10.3 | < 0.001 |
| Left ventricular end-diastolic dimension (mm) | 52.9 ± 9.30 | 48.4 ± 8.0 | < 0.001 | 56.1 ± 9.8 | 50.1 ± 8.3 | < 0.001 |
| Left end-systolic dimension (mm) | 42.1 ± 9.2 | 33.3 ± 7.2 | < 0.001 | 43.3 ± 12.3 | 36.2 ± 8.2 | < 0.001 |
Data are expressed as the mean±SD. MR, mitral regurgitation; EF, ejection fraction; CK-MB, creatine kinase myocardial band; NT-pro BNP, N-terminal pro-B-type natriuretic peptide.
Coronary angiogram
In AMI patients with low-grade MR (grade 0-2), the LVEF≤40% group had more severe coronary angiogram findings than the LVEF>40% group (Table 3). However, in patients with high-grade MR (grade 3-4), the LVEF≤40% group had no significantly different coronary angiographic findings, except for left anterior coronary artery disease, compared with the LVEF>40% group. PCI was performed in a significantly lower number of patients in the high grade MR group as compared to the low grade MR group. However, based on LVEF, there was no significant difference between the number of PCI performed on patients in the high grade MR group (Table 3).
Table 3. Coronary angiography findings.
| Variables | MR Grades 0-2 | MR Grades 3-4 | ||||
|---|---|---|---|---|---|---|
| EF ≤ 40% (n=2,422) |
EF > 40% (n = 12,252) |
P value | EF ≤ 40% (n = 197) |
EF > 40% (n = 226) |
P value | |
| Coronary angiographic findings, n (%) | 2,236/2,422 (92.3) | 11,942/12,252 (97.4) | < 0.001 | 168/197 (85.2) | 199/ 226 (88.0) | 0.563 |
| Number of vessels with significant stenotic lesions | < 0.001 | 0.958 | ||||
| 0 | 44/2,236 (2.0) | 489/11,942 (4.1) | 6/168 (3.6) | 6/199 (2.6) | ||
| 1 | 1,370/2,236 (61.3) | 7,439/11,942 (62.3) | 91/168 (54.0) | 114/199 (57.4) | ||
| 2 | 446/2,236 (19.9) | 2,531/11,942 (21.2) | 37/168 (22.1) | 41/199 (21.0) | ||
| 3 | 259/2,236 (11.6) | 1,158/11,942 (9.7) | 24/168 (14.2) | 29/199 (14.6) | ||
| LMA involvement | 117/ 2,236 (5.2) | 331/ 11,942 (2.8) | 10/ 168 (6.1) | 9/ 199 (4.4) | ||
| Infarct-related artery | ||||||
| Left anterior descending coronary artery | 1,737/2,236 (77.6) | 6,448/11,942 (54.0) | < 0.001 | 94/168 (56.4) | 80/199 (40.4) | 0.003 |
| Left circumflex coronary artery | 499/ 2,236 (22.3) | 3,295/11,942 (27.6) | < 0.001 | 43/168 (26.1) | 77/199 (38.9) | 0.013 |
| Right coronary artery | 606/ 2,236 (27.1) | 5,087/11,942 (42.6) | < 0.001 | 74/168 (44.2) | 112/199 (56.5) | 0.026 |
| LMA | 117/ 2,236 (5.2) | 331/11,942 (2.8) | < 0.001 | 10/168 (6.1) | 9/199 (4.4) | 0.083 |
| ACC/AHA lesion type | < 0.001 | 0.616 | ||||
| A | 84/2,236 (3.8) | 394/11,942 (3.3) | 6/168 (3.4) | 5/199 (2.3) | ||
| B1 | 330/2,236 (14.8) | 2,161/11,942 (18.1) | 31/168 (18.5) | 27/199 (13.8) | ||
| B2 | 595/2,236 (26.6) | 4,119/11,942 (34.5) | 41/168 (24.5) | 49/199 (24.9) | ||
| C | 1,225/2,236 (54.8) | 5,268/11,942 (44.1) | 90/168 (53.6) | 118/199 (59.0) | ||
| Initial TIMI flow grade 0 | 1,113/2,236 (49.8) | 5,230/11,942 (43.8) | < 0.001 | 79/168 (47.4) | 93/199 (46.9) | 1.000 |
| Final TIMI flow grade 3 | 1,994/2,236 (89.2) | 11,321/11,942 (94.8) | < 0.001 | 147/168 (87.5) | 187/199 (94.0) | 0.072 |
| PCI | 2,117/2,422 (87.4) | 11,291/12,252 (92.1) | < 0.001 | 136/197 (69.0) | 170/226 (75.2) | 0.229 |
| Number of stents | 1.56 ± 0.87 | 1.53 ± 0.83 | 0.180 | 1.65 ± 0.98 | 1.61 ± 0.94 | 0.730 |
| Total stent length (mm) | 24.86 ± 7.23 | 24.01 ± 7.0 | < 0.001 | 24.4 ± 6.8 | 24.8 ± 6.3 | 0.657 |
| Thrombolytic therapy | 88/2,422 (3.6) | 656/12,252 (5.3) | 0.007 | 3/197 (1.5) | 5/226 (2.2) | 0.347 |
| CABG | 17/2,422 (0.7) | 34/12,252 (0.2) | 0.001 | 2/197 (1.0) | 4/226 (1.8) | 0.690 |
Data are expressed as the number of patients (%). MR, mitral regurgitation; EF, ejection fraction; ACC/AHA, American College of Cardiology/American Heart Association; TIMI, Thrombolysis In Myocardial Infarction; CABG, coronary artery bypass graft; LMA, left main coronary artery; PCI, percutaneous coronary intervention.
Independent predictors of mortality
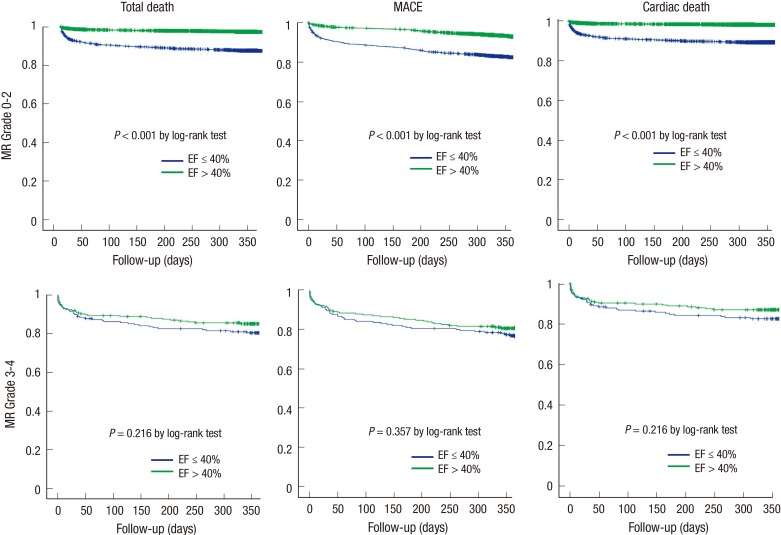
The independent predictors of cardiac death in the multivariate analysis in AMI patients with low-grade MR were age≥75 yr, Killip class≥III, IHD history, NT-proBNP>4,000 pg/mL, Hs-CRP ≥2.59 mg/L, eGFR, PCI, and LVEF≤40% (Table 4). However, PCI was an independent predictor in patients with AMI with high-grade MR (Table 5). Primary endpoints in the MR grade 0-2 group based on Kaplan-Meier analysis were significantly different according to EF (EF≤40% vs. EF>40%, total death, 330/2,422 vs. 373/12,252, P<0.001; MACEs, 489/2,422 vs. 1,091/12,252, P<0.001; cardiac death, 281/2,422 vs. 254/12,252, P<0.001 by the log-rank test). However, primary endpoints among AMI patients with MR grade 3-4 was not different according to EF (EF≤40% vs. EF>40%, total death, 42/197 vs. 37/226, P=0.216; MACEs, 55/197 vs. 49/226, P=0.357; cardiac death, 37/197 vs. 31/226, P=0.216 by the log-rank test) (Fig. 1). Fig. 1 reveals that patients with grades 3-4 MR and preserved EF exhibit a poor prognosis given the advanced age of this patient population compared with groups with low-grade MR (Table 1).
Table 4. Cox regression analysis of cardiac death by 1 yr in the mitral regurgitation grades 0-2 groups of acute myocardial infarction patients.
| Variables | Univariate HR | 95% CI | P value | Multivariate HR | 95% CI | P value | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||
| Age ≥ 75 | 3.214 | 2.743 | 3.765 | < 0.001 | 1.527 | 1.191 | 1.957 | 0.001 |
| Gender (Male) | 0.532 | 0.453 | 0.625 | < 0.001 | 1.148 | 0.895 | 1.473 | 0.276 |
| Killip ≥ III | 5.645 | 4.797 | 6.643 | < 0.001 | 1.833 | 1.405 | 2.39 | < 0.001 |
| Qwave | 1.307 | 1.06 | 1.611 | 0.012 | 1.021 | 0.76 | 1.372 | 0.891 |
| Ischemic heart disease | 1.876 | 1.56 | 2.256 | < 0.001 | 1.308 | 0.997 | 1.716 | 0.052 |
| Diabetes mellitus | 1.877 | 1.597 | 2.206 | < 0.001 | 0.954 | 0.733 | 1.241 | 0.725 |
| NT-proBNP > 4,000 (pg/mL) | 8.134 | 6.628 | 9.982 | < 0.001 | 1.807 | 1.349 | 2.42 | < 0.001 |
| Glucose ≥ 160 (mg/dL) | 2.008 | 1.71 | 2.358 | < 0.001 | 1.173 | 0.915 | 1.505 | 0.208 |
| Hs-CRP ≥ 2.59 (mg/dL) | 3.063 | 2.553 | 3.675 | < 0.001 | 1.668 | 1.31 | 2.125 | < 0.001 |
| LVEF ≤ 40% | 8.223 | 5.81 | 11.637 | < 0.001 | 3.802 | 2.174 | 6.65 | < 0.001 |
| LVEF ≤ 40%*log (time) | 1.177 | 1.07 | 1.295 | 0.001 | 1.204 | 1.046 | 1.386 | 0.010 |
| eGFR (mL/min/1.73 m2) | 0.969 | 0.967 | 0.972 | < 0.001 | 0.98 | 0.975 | 0.985 | < 0.001 |
| PCI | 0.216 | 0.182 | 0.257 | < 0.001 | 0.368 | 0.283 | 0.479 | < 0.001 |
CK-MB was excluded because it was not primary variable and had interaction with time. *LVEF≤40% had time dependent hazard ratio (HR=9.452 on 30 day, 10.504 on 180 day, 10.951 on 365 day). LVEF, Left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; PCI, percutaneous coronary intervention;CK-MB, creatine kinase myocardial band; Hs-CRP, high-sensitivity C-reactive protein; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Table 5. Cox regression analysis of cardiac death by 1 yr in the mitral regurgitation grades 3-4 groups of acute myocardial infarction patients.
| Variables | Univariate HR | 95% CI | P value | Multivariate HR | 95% CI | P value | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||
| Age ≥ 75 | 1.880 | 1.168 | 3.026 | 0.009 | 1.867 | 0.886 | 3.934 | 0.101 |
| Gender (Male) | 0.601 | 0.370 | 0.976 | 0.040 | 1.149 | 0.574 | 2.302 | 0.694 |
| Killip ≥ III | 1.697 | 1.053 | 2.735 | 0.030 | 1.036 | 0.498 | 2.153 | 0.925 |
| Q wave | 0.820 | 0.407 | 1.653 | 0.580 | 1.093 | 0.449 | 2.660 | 0.845 |
| Ischemic heart disease | 1.732 | 1.062 | 2.824 | 0.028 | 1.087 | 0.514 | 2.297 | 0.827 |
| Diabetes mellitus | 0.913 | 0.562 | 1.483 | 0.713 | 1.026 | 0.465 | 2.262 | 0.949 |
| NT-proBNP > 4,000 (pg/mL) | 2.234 | 1.232 | 4.050 | 0.008 | 1.165 | 0.558 | 2.432 | 0.684 |
| Glucose ≥ 160 (mg/dL) | 1.893 | 1.161 | 3.087 | 0.011 | 0.936 | 0.429 | 2.042 | 0.869 |
| Hs-CRP ≥ 2.59 (mg/dL) | 2.285 | 1.250 | 4.175 | 0.007 | 1.616 | 0.768 | 3.400 | 0.206 |
| LVEF ≤ 40% | 1.341 | 0.841 | 2.138 | 0.217 | 0.836 | 0.400 | 1.747 | 0.633 |
| CK-MB (U/L) | 0.999 | 0.997 | 1.001 | 0.296 | 0.997 | 0.992 | 1.001 | 0.170 |
| PCI | 0.310 | 0.195 | 0.494 | < 0.001 | 0.381 | 0.192 | 0.755 | 0.006 |
eGFR was excluded because it was not primary variable and had interaction with time. LVEF, Left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; PCI, percutaneous coronary intervention; CK-MB, creatine kinase myocardial band; NT-pro BNP, N-terminal pro-B type natriuretic; Hs-CRP, high-sensitivity C-reactive protein.
Fig. 1. Kaplan-Meier analysis of all-cause mortality, major adverse cardiac event (MACE), and cardiac death in the mitral regurgitation grades 0-2 (upper panel) and grades 3-4 (lower panel) groups of acute myocardial infarction patients. Primary endpoints were significant in the groups with mild mitral regurgitation (MR) according to ejection fraction (EF) (≤ 40% and > 40%). However, the groups with severe MR did not exhibit significant differences in all-cause mortality based on EF (≤ 40% and > 40%).
DISCUSSION
The present study showed that in the presence of high-grade MR, LVEF is not an independent predictor of mortality. LVEF is the most widely used variable to represent LV systolic function in patients with AMI. Furthermore, low LVEF was associated with high one-year mortality after AMI in patients without high-grade MR in the present study. Because the majority (97%) of the patients with AMI did not have high-grade MR. However, the prognostic value of LVEF after AMI has been questioned in several studies that could not confirm LVEF as a prognostic factor in AMI patients. Left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV) were described as more meaningful prognostic factors than LVEF because a low EF might be attributed to extensive myocardial damage, LV dilatation, or a hibernating or stunned myocardium (6, 9). The present study suggests that ischemic high grade MR could be one reason for this result because LVEDV and LVESV could be increased by volume overload disease, such as ischemic MR. A previous study reported that the presence of MR was associated with an increased likelihood of adverse outcomes but not LV EF in patients with AMI with MR (11).
Ischemic MR could be caused by global LV remodeling with spherical LV enlargement or local inferior wall remodeling with predominantly posterior leaflet restriction. In case of non-ischemic MR, an increase in preload and/or a decrease in afterload will result in a falsely higher EF because the EF is load-sensitive (20). However, some have argued that the 'low impedance leak' effect might exclusively apply to acute severe MR and that the afterload is likely increased when the LV is dilated. Furthermore, the decline in EF following MVR could result from chordal transection. Whitlow et al. (21) have shown that the EF is essentially unchanged at 12 months among patients experiencing significant reductions in MR and 'reverse' remodeling using edge-to-edge clip devices without the confounding effects of CABG, sternotomy, or chordal transection.
Alternatively, a portion of the LV dysfunction in AMI patient results from afterload excess; this finding could explain why reduced LVEF has no impact on survival in patients with severe MR. Accordingly, contractile function might be better than the EF suggests because the LV is managing afterload excess.
The LVEF is derived from the LV volume. Although heart rate and fiber shortening both affect LVEF, it is influenced to a far greater extent by LVEDV given that changes in stroke volume tend to be considerably smaller than changes in LVEDV (22). In the AMI setting, LVEDV is not yet fully dilated by acute severe MR. In addition, ischemic MR might have a transiently severe grade.
LVEF is a determinant of the degree of functional ischemic mitral regurgitation in patients with systolic left ventricular dysfunction (4). However, there is only a weak correlation between LVEF and MR severity (7, 8). Recently, several small population studies have examined the role of assessing LV systolic function in addition to LVEF in ischemic MR (23, 24).
PCI was performed in a significantly lower number of patients in the group of LVEF<40% with low grade MR as compared to the group of LVEF>40% with low grade MR. That could contribute the difference of survival in low grade MR not high grade MR. Furthermore, PCI was an independent predictor of lower cardiac death in high grade MR (Table 4). Reperfusion therapy for patients in AMI with severe MR could be beneficial for survival.
This study was a retrospective study. There are no detailed descriptions of MR volume or effective regurgitant orifice area (ERO) due to a lack of central readings of the echocardiograms in the core laboratory. Furthermore, the etiologies of MR and the presence of non-ischemic MR were not distinguished. Long-term follow-up echocardiography data were limited. The number of patients with severe MR was considerably lower than that in the other group. Nevertheless, this study demonstrated the prognostic impact of LVEF according to the presence of severe MR in a large, real-world population.
In conclusion, MR is a predictor of poor outcome regardless of ejection infarction. LVEF is an inadequate method to evaluate contractile function of the ischemic heart in the face of high grade MR. Other new parameters for assessing LV systolic function beyond LVEF are needed in patients with significant ischemic MR.
ACKNOWLEDGMENTS
Korean Acute Myocardial Infarction Registry (KAMIR) Investigators: Myung Ho Jeong, Young Jo Kim, Chong Jin Kim, Myeong Chan Cho, Youngkeun Ahn, Jong Hyun Kim, Shung Chull Chae, Seung Ho Hur, In Whan Seong, Taek Jong Hong, Dong Hoon Choi, Jei Keon Chae, Jae Young Rhew, Doo Il Kim, In Ho Chae, Junghan Yoon, Bon Kwon Koo, Byung Ok Kim, Myoung Yong Lee, Kee Sik Kim, Jin Yong Hwang, Seok Kyu Oh, Nae Hee Lee, Kyoung Tae Jeong, Seung Jea Tahk, Jang Ho Bae, Seung Woon Rha, Keum Soo Park, Kyoo Rok Han, Tae Hoon Ahn, Moo Hyun Kim, Joo Young Yang, Chong Yun Rhim, Hyeon Cheol Gwon, Seong Wook Park, Young Youp Koh, Seung Jae Joo, Soo Joong Kim, Dong Kyu Jin, Jin Man Cho, Wook Sung Chung, Yang Soo Jang, Jeong Gwan Cho, Ki Bae Seung, Seung Jung Park.
Footnotes
This study was supported by grants from the Korean Society of Cardiology, the Korea Centers for Disease Control and Prevention (2013-E63005-00), and the Korean Health Technology R&D Project (HI13C1527), Ministry of Health & Welfare, Republic of Korea.
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception and coordination of the study: Cho JS, Jeong MH. Acquisition of data: all authors. Data review: Cho JS, Jeong MH. Statistical analysis: Cho JS, Her SH. Manuscript preparation: Cho JS, Jeong MH. Manuscript approval: all authors.
References
- 1.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 2.Cho JY, Jeong MH, Ahn Y, Jeong HC, Cho SC, Yoo JH, Song JE, Jang SY, Lee KH, Park KH, et al. Korea Acute Myocardial Infarction Registry Investigators. Different impact of mitral regurgitation on clinical outcomes according to timing of percutaneous coronary intervention in patients with non-ST segment elevation myocardial infarction. Int J Cardiol. 2013;168:4872–4874. doi: 10.1016/j.ijcard.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 3.Lancellotti P, Lebrun F, Piérard LA. Determinants of exercise-induced changes in mitral regurgitation in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 2003;42:1921–1928. doi: 10.1016/j.jacc.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Yiu SF, Enriquez-Sarano M, Tribouilloy C, Seward JB, Tajik AJ. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: A quantitative clinical study. Circulation. 2000;102:1400–1406. doi: 10.1161/01.cir.102.12.1400. [DOI] [PubMed] [Google Scholar]
- 5.Perez de Isla L, Zamorano J, Quezada M, Almería C, Rodrigo JL, Serra V, García Rubira JC, Ortiz AF, Macaya C. Prognostic significance of functional mitral regurgitation after a first non-ST-segment elevation acute coronary syndrome. Eur Heart J. 2006;27:2655–2660. doi: 10.1093/eurheartj/ehl287. [DOI] [PubMed] [Google Scholar]
- 6.Mollema SA, Nucifora G, Bax JJ. Prognostic value of echocardiography after acute myocardial infarction. Heart. 2009;95:1732–1745. doi: 10.1136/hrt.2008.161836. [DOI] [PubMed] [Google Scholar]
- 7.Magne J, Pibarot P. Left ventricular systolic function in ischemic mitral regurgitation: time to look beyond ejection fraction. J Am Soc Echocardiogr. 2013;26:1130–1134. doi: 10.1016/j.echo.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Hetzer R, Dandel M. Early detection of left ventricular dysfunction in patients with mitral regurgitation due to flail leaflet is still a challenge. Eur Heart J. 2011;32:665–667. doi: 10.1093/eurheartj/ehq399. [DOI] [PubMed] [Google Scholar]
- 9.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 10.Pecini R, Thune JJ, Torp-Pedersen C, Hassager C, Køber L. The relationship between mitral regurgitation and ejection fraction as predictors for the prognosis of patients with heart failure. Eur J Heart Fail. 2011;13:1121–1125. doi: 10.1093/eurjhf/hfr114. [DOI] [PubMed] [Google Scholar]
- 11.Amigoni M, Meris A, Thune JJ, Mangalat D, Skali H, Bourgoun M, Warnica JW, Barvik S, Arnold JM, Velazquez EJ, et al. Mitral regurgitation in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: prognostic significance and relation to ventricular size and function. Eur Heart J. 2007;28:326–333. doi: 10.1093/eurheartj/ehl464. [DOI] [PubMed] [Google Scholar]
- 12.Cho JY, Jeong MH, Ahn Y, Jeong HC, Jang SY, Kim SS, Rhew SH, Jeong YW, Lee KH, Park KH, et al. Impact of high admission blood pressure without history of hypertension on clinical outcomes of patients with acute myocardial infarction: From Korea Acute Myocardial Infarction Registry. Int J Cardiol. 2014;172:e54–e58. doi: 10.1016/j.ijcard.2013.12.063. [DOI] [PubMed] [Google Scholar]
- 13.Lee KH, Jeong MH, Ahn Y, Cho MC, Kim CJ, Kim YJ. New horizons of acute myocardial infarction: from the Korea Acute Myocardial Infarction Registry. J Korean Med Sci. 2013;28:173–180. doi: 10.3346/jkms.2013.28.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions. O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA Guideline for the management of ST-Elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, et al. 2011 writing group members; ACCF/AHA task force members. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e426–e579. doi: 10.1161/CIR.0b013e318212bb8b. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 18.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS, et al. American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 19.Enriquez-Sarano M, Miller FA, Jr, Hayes SN, Bailey KR, Tajik AJ, Seward JB. Effective mitral regurgitant orifice area: clinical use and pitfalls of the proximal isovelocity surface area method. J Am Coll Cardiol. 1995;25:703–709. doi: 10.1016/0735-1097(94)00434-R. [DOI] [PubMed] [Google Scholar]
- 20.Enriquez-Sarano M, Tajik AJ, Schaff HV, Orszulak TA, McGoon MD, Bailey KR, Frye RL. Echocardiographic prediction of left ventricular function after correction of mitral regurgitation: results and clinical implications. J Am Coll Cardiol. 1994;24:1536–1543. doi: 10.1016/0735-1097(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 21.Whitlow PL, Feldman T, Pedersen WR, Lim DS, Kipperman R, Smalling R, Bajwa T, Herrmann HC, Lasala J, Maddux JT, et al. EVEREST II Investigators. Acute and 12-month results with catheter-based mitral valve leaflet repair: the EVEREST II (Endovascular Valve Edge-to-Edge Repair) High Risk Study. J Am Coll Cardiol. 2012;59:130–139. doi: 10.1016/j.jacc.2011.08.067. [DOI] [PubMed] [Google Scholar]
- 22.Rumberger JA, Behrenbeck T, Breen JR, Reed JE, Gersh BJ. Nonparallel changes in global left ventricular chamber volume and muscle mass during the first year after transmural myocardial infarction in humans. J Am Coll Cardiol. 1993;21:673–682. doi: 10.1016/0735-1097(93)90100-f. [DOI] [PubMed] [Google Scholar]
- 23.Zito C, Cusmà-Piccione M, Oreto L, Tripepi S, Mohammed M, Di Bella G, Falanga G, Oreto G, Lentini S, Carerj S. In patients with post-infarction left ventricular dysfunction, how does impaired basal rotation affect chronic ischemic mitral regurgitation? J Am Soc Echocardiogr. 2013;26:1118–1129. doi: 10.1016/j.echo.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Gelsomino S, van Garsse L, Lucà F, Parise O, Cheriex E, Rao CM, Gensini GF, Maessen J. Left ventricular strain in chronic ischemic mitral regurgitation in relation to mitral tethering pattern. J Am Soc Echocardiogr. 2013;26:370–380.e11. doi: 10.1016/j.echo.2013.01.011. [DOI] [PubMed] [Google Scholar]