Abstract
Pancreatic islet transplantation is a physiologically advantageous and minimally invasive procedure for the treatment of type 1 diabetes mellitus. Here, we describe the first reported case of successful allogeneic islet transplantation alone, using single-donor, marginal-dose islets in a Korean patient. A 59-yr-old patient with type 1 diabetes mellitus, who suffered from recurrent severe hypoglycemia, received 4,163 islet equivalents/kg from a single brain-death donor. Isolated islets were infused intraportally without any complications. The immunosuppressive regimen was based on the Edmonton protocol, but the maintenance dosage was reduced because of mucositis and leukopenia. Although insulin independence was not achieved, the patient showed stabilized blood glucose concentration, reduced insulin dosage and reversal of hypoglycemic unawareness, even with marginal dose of islets and reduced immunosuppressant. Islet transplantation may successfully improve endogenous insulin production and glycemic stability in subjects with type 1 diabetes mellitus.
Graphical Abstract
Keywords: Hypoglycemia, Islet Transplantation, Korea, Diabetes Mellitus Type 1
INTRODUCTION
Pancreatic islet transplantation is a physiologically advantageous and minimally invasive procedure for the treatment of type 1 diabetes mellitus (1). The success rate of islet transplantation has been improved by transplanting a higher mean islet mass prepared from two to four donor pancreases and using glucocorticoid-free immunosuppressants (1, 2). However, a shortage of donors and the use of potentially islet-toxic immunosuppressants are the main hurdles to successful islet transplantation. Few data are available on islet isolation from the pancreas of Asian people, and most of the guidelines on islet isolation techniques are based on the procedures used on Western subjects.
Reports on allogeneic islet transplantation alone in the Korean population are lacking. According to data from the Korean Network for Organ Sharing, 10 cases of allogeneic islet transplantation have been performed, and most were islet-after-kidney transplantations (3). Several autologous islet transplantations have been reported (4), but islet isolation was performed using a living-donor pancreas and an inadequate quantity of isolated islets was not a limitation to performing the transplantation. Here, we report on a case of allogeneic islet transplantation alone, using a marginal dose from a single-donor pancreas in a Korean patient with type 1 diabetes mellitus.
CASE DESCRIPTION
Islet transplantation recipient
A 59-yr-old woman who had been diagnosed with type 1 diabetes mellitus for 25 yr, suffered from recurrent severe hypoglycemia and hypoglycemic unawareness since October 2011. Her body weight was 52 kg and her body mass index (BMI) was 20.31 kg/m2. Fasting and postprandial serum C-peptide concentrations were 0.07 ng/mL and 0.23 ng/mL, respectively. She had visited the emergency room twice complaining of decreased consciousness related to severe hypoglycemia (<50 mg/dL) in the previous year. She did not experience hypoglycemic symptoms until her blood glucose level decreased to 50 mg/dL. Her total insulin requirement was 34 U/day, and her glycated hemoglobin (HbA1c) level ranged from 7.8% to 9.6% with a mean value of 8.54% in the preceding 2 yr. The estimated glomerular filtration rate (eGFR) was 91.67 mL/min/1.73 m2 and albumin to creatinine ratio (ACR) was 16.45 mg/g. An ophthalmological evaluation showed mild nonproliferative retinopathy of both eyes. There was no evidence of macrovascular complication.
Islet isolation using prepurification gradient tests
The pancreas was procured from a 49-yr-old brain-death donor with a BMI of 23.13 kg/m2. The organ was transported in chilled histidine-tryptophan-ketoglutarate solution and transferred to the Good Manufacturing Practice facility. The pancreas was distended by controlled ductal perfusion with Liberase human islet enzyme (Roche Diagnostics, Indianapolis, USA) and digested mechanically in a Ricordi chamber. Pancreatic tissue density was tested before purification using a COBE 2991 cell processor (Gambro BCT Inc., Lakewood, CO, USA) to predict the pancreatic tissue density. Prepurification gradient tests were performed using a Ficoll (Biochrom, Berlin, Germany)-based standard gradient (1.100-1.077 g/cm3), OptiPrep (Sigma-Aldrich, St. Louis, MO, USA)-based gradient-1 (1.100-1.085-1.060 g/cm3) and OptiPrep-based gradient-2 (1.090-1.075-1.050 g/cm3). The tests showed little differences in the density and produced a single layer of islet-acinar cell mixture. By contrast, a gradient test using OptiPrep-based gradient-3 (1.080-1.065-1.040 g/cm3) produced two distinct layers of the different cell types. Islet purification using the COBE was performed using the gradient-3 combination with OptiPrep solution. The total amount of purified islets was 216,500 islet equivalents (IEQ) (4,163 IEQ/recipient body weight in kg), which is the minimally adequate quantity for transplantation (5). The purity of the isolated islets was 79.6%, and the islet viability was >90%. Glucose stimulated insulin secretion of isolated islet was tested in vitro and showed an insulin concentration of 10.1 µU/mL at low (2.8 mM) glucose media and 17.1 µU/mL at high (16.8 mM) glucose media during 1 hr each. Gram staining of the media was negative, and the endotoxin concentration was <1EU/kg.
Islet transplantation and immunosuppressant
The islet transplantation was performed in November 2013. The patient was sedated and a percutaneous transhepatic approach was used to access to the portal vein under fluoroscopic guidance. The portal venous pressure was measured at baseline and after islet infusion. Doppler ultrasonography of the portal vein was performed within 24 hr after transplantation, and no definite evidence of flow disturbance was observed. The immunosuppressive regimen was based on that previously described in the Edmonton protocol (5). Instead of daclizumab, basiliximab was given intravenously at a dose of 20 mg 2 hr before islet transplantation and 4 days after transplantation. Sirolimus was administered administered once daily to achieve a target trough therapeutic range of 12-15 ng/mL for the first 3 months, after which the target trough range was planned to be lowered to 7-12 ng/mL. Tacrolimus was administered twice daily and adjusted to achieve a target trough level of 3-6 ng/mL. One month after transplantation, the dosage of sirolimus was reduced because of systemic mucositis and leukopenia, and this dosage was maintained at the trough level of 5-9 ng/mL. The islet transplantation protocol was approved by the institutional review board of The Catholic University of Korea (No. KC10CISI0438) and written informed consent was obtained from the patient. This study was conducted according to the principles expressed in the Declaration of Helsinki.
Assessment of glycemic control after islet transplantation
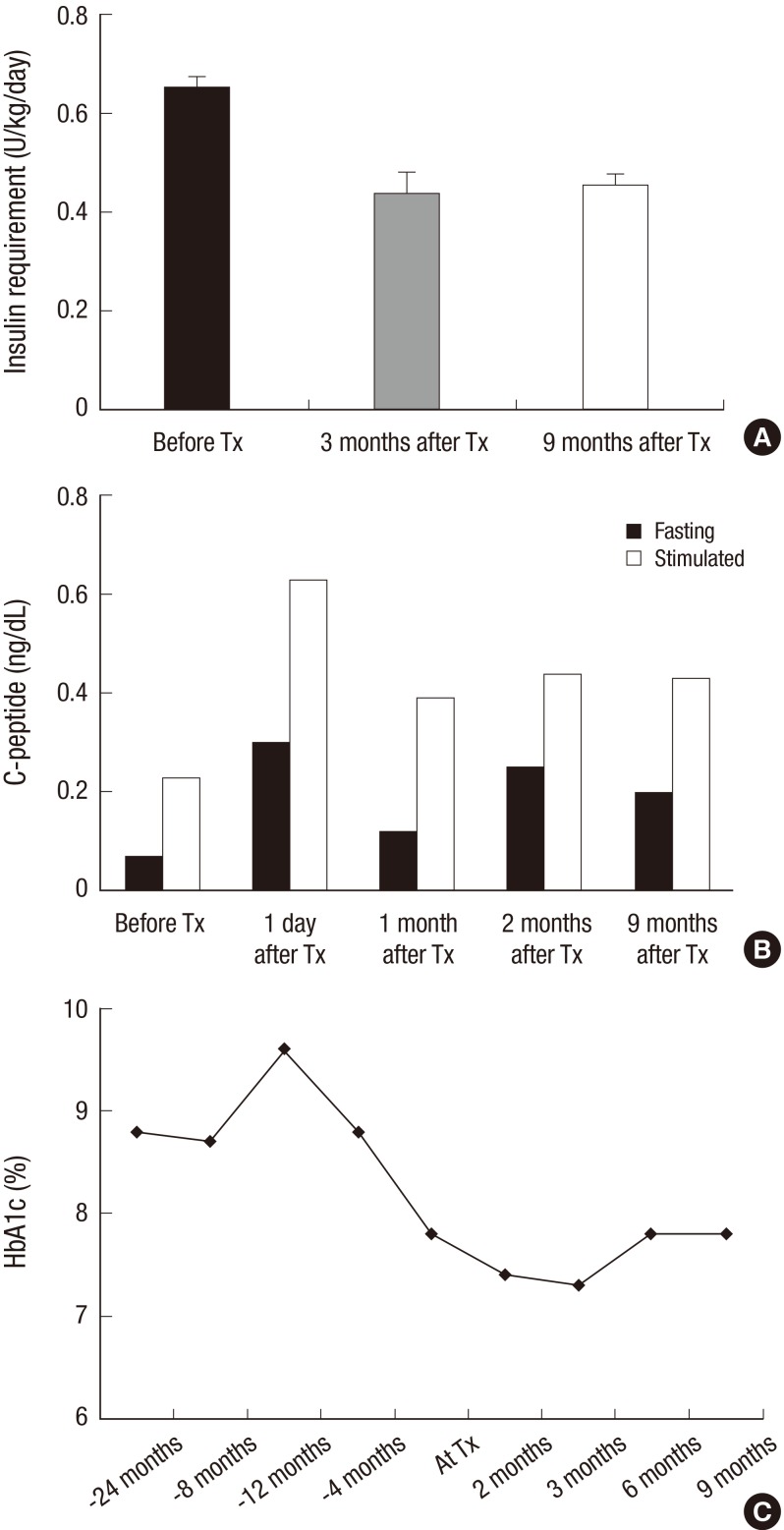
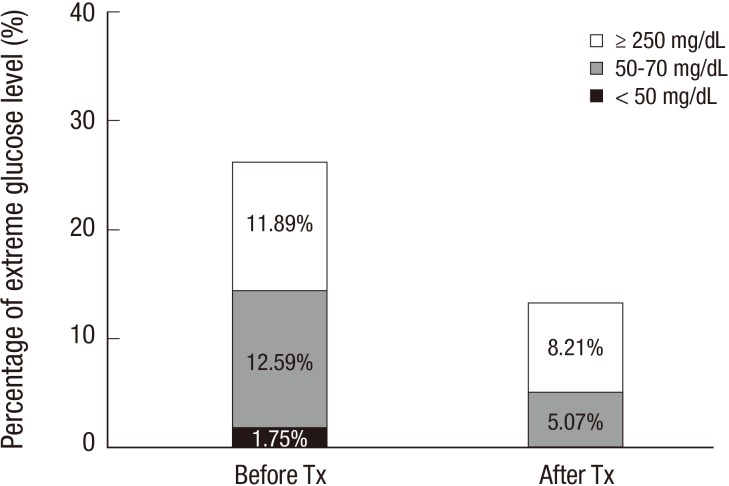
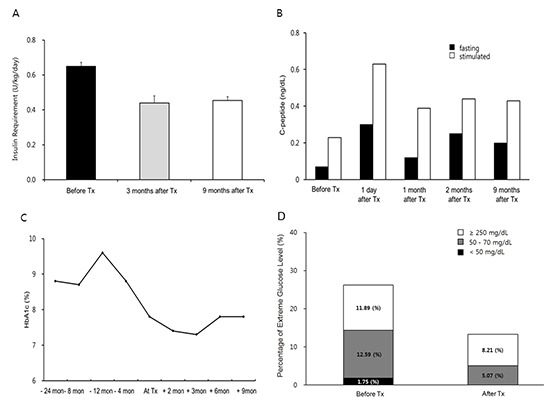
After islet transplantation, the patient's insulin requirement decreased to 22 U/day, which was about 65% of the previous requirement (Fig. 1). Fasting and postprandial C-peptide concentrations improved to 0.25 ng/mL and 0.44 ng/mL, respectively, at 2 months after transplantation. The HbA1c level improved to 7.3% at 3 months after transplantation. Self-monitored blood glucose concentration was measured four times a day (before each meal and at bedtime), and the results showed marked improvement in the frequencies of both hypoglycemia and extreme hyperglycemia after transplantation (Fig. 2). Before transplantation, she experienced severe hypoglycemia (<50 mg/dL) on average twice a month. However, she did not experience further severe hypoglycemia and became aware of hypoglycemia at a glucose concentration of 70 mg/dL. We calculated the Glycemic Risk Assessment Diabetes Equation (GRADE) score to assess the glycemic variability and the percentage contribution of hypoglycemia events, before and at 3 and 9 months after the transplantation (6). Also the Average Daily Risk Range (ADRR) index was calculated; the values are stratified into three categories: low risk <20; moderate risk, 20-≤40; and high risk >40 (7, 8). Before the transplantation, the GRADE score was 10.31 with 28.5% of hypoglycemia (<70 mg/dL) events, and improved to GRADE score of 7.94 with 2.4% of hypoglycemia events and 5.99 with 2.1% of hypoglycemia events at 3 and 9 months after the transplantation, respectively. Similarly, the ADRR index was 26.88 before the transplantation and improved to 17.56 and 14.60 at 3 and 9 months after transplantation, respectively. Currently (about 9 months after the transplantation), her HbA1c is 7.8%, and fasting and postprandial C-peptide concentrations are 0.20 and 0.43 ng/mL, respectively. There is no evidence of leukopenia and her renal function is in normal range with an eGFR of 106.04 mL/min/1.73 m2.
Fig. 1. Assessment of glycemic control after islet transplantation. (A) Average dosage of insulin requirement for 2 weeks before and 3 and 9 months after islet transplantation. The data are presented by mean±standard deviation. (B) Fasting and stimulated C-peptide level before and after islet transplantation. (C) Changes in HbA1c level before and after islet transplantation. Tx, transplantation.
Fig. 2. Assessment of glucose variability after islet transplantation. Percentage of extreme blood glucose measurements classified as hypoglycemia (< 50 or < 70 mg/dL) or extreme hyperglycemia (≥ 250 mg/dL) measured by patient's self-monitoring of blood glucose four times a day (before each meal and at bedtime) before and after islet transplantation. Tx, transplantation.
DISCUSSION
To our knowledge, this is the first reported case on allogeneic islet transplantation alone in Korea. Although insulin independence was not achieved, the patient showed stabilized blood glucose concentration, reduced insulin dosage and reversal of hypoglycemic unawareness, even with a marginal dose of islets and reduced immunosuppressant. There are only a few reports on islet transplantation in Asian subjects. According to the Collaborative Islet Transplantation Registry, centers from North America, Europe and Australia are registered, but none from Asian countries (9).
When islets are purified with a COBE, the purification gradient used is often fixed. However, this method does not appropriately adjust for interpancreatic variations in exocrine and islet tissue density, which is influenced by donor characteristics, the secretory status of exocrine cells, and the islet isolation procedure (10). It is not known whether the density of pancreatic tissue differs between ethnic groups. Predicting pancreatic tissue density using prepurification density tests may improve the islet isolation yield, as we performed in this case.
Hering et al. (11) reported on allogeneic islet transplantation using marginal dose islets (average of 7,271 IEQ/kg) from a single donor pancreas along with potent induction immunotherapy (antithymocyte globulin, daclizumab and etanercept). However, our patient underwent islet transplantation with only a marginal quantity of islets (4,163 IEQ/kg) with reduced immunosuppressant.
In conclusion, this is the first reported case on successful islet transplantation alone in a Korean patient with type 1 diabetes. Although insulin independence was not achieved, the patient showed stabilized blood glucose concentration, reduced insulin dosage and reversal of hypoglycemic unawareness, even with a marginal dose of islets and reduced immunosuppressant.
Footnotes
This work was supported by a grant from the Korean Health Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (HI09C1555). This work was also supported by the Mystery of Life Fund of the Catholic Institute of Cell Therapy.
DISCLOSURE: The authors declare that no competing financial interests exist. Also, the funder played no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
AUTHOR CONTRIBUTION: Study design: Yang HK, Kim JW, Yoon KH. Pancreas procurement: Ham DS, Park HS, Hong TH. Islet isolation: Ham DS, Park HS, Rhee M, You YH, Kim MJ. Islet infusion: Yang HK, Ham DS, Choi BG. Writing: Yang HK. Review and revision: Yoon KH, Lee SH, Cho JH.
References
- 1.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 2.Goss JA, Schock AP, Brunicardi FC, Goodpastor SE, Garber AJ, Soltes G, Barth M, Froud T, Alejandro R, Ricordi C. Achievement of insulin independence in three consecutive type-1 diabetic patients via pancreatic islet transplantation using islets isolated at a remote islet isolation center. Transplantation. 2002;74:1761–1766. doi: 10.1097/00007890-200212270-00020. [DOI] [PubMed] [Google Scholar]
- 3.Yang TY, Oh SH, Jeong IK, Seo IA, Oh EY, Kim SJ, Chung JH, Min YK, Lee MS, Lee MK, et al. First human trial of pancreatic islet allo-transplantation in Korea--focus on re-transplantation. Diabetes Res Clin Pract. 2002;56:107–113. doi: 10.1016/s0168-8227(01)00366-7. [DOI] [PubMed] [Google Scholar]
- 4.Lee BW, Jee JH, Heo JS, Choi SH, Jang KT, Noh JH, Jeong IK, Oh SH, Ahn YR, Chae HY, et al. The favorable outcome of human islet transplantation in Korea: experiences of 10 autologous transplantations. Transplantation. 2005;79:1568–1574. doi: 10.1097/01.tp.0000158427.07084.c5. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 6.Hill NR, Hindmarsh PC, Stevens RJ, Stratton IM, Levy JC, Matthews DR. A method for assessing quality of control from glucose profiles. Diabet Med. 2007;24:753–758. doi: 10.1111/j.1464-5491.2007.02119.x. [DOI] [PubMed] [Google Scholar]
- 7.Park SA, Ko SH, Lee SH, Cho JH, Moon SD, Jang SA, Song KH, Son HS, Yoon KH, Cha BY, et al. Average daily risk range-index of glycemic variability-related factor in type 2 diabetic inpatients. Korean Diabetes J. 2009;33:31–39. [Google Scholar]
- 8.Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29:2433–2438. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
- 9.The Collaborative Islet Transplant Registry (CITR) Seventh annual report. 2011. [accessed on 23 January 2014]. Available at http://www.citregistry.org/reports/reports.htm.
- 10.Anazawa T, Matsumoto S, Yonekawa Y, Loganathan G, Wilhelm JJ, Soltani SM, Papas KK, Sutherland DE, Hering BJ, Balamurugan AN. Prediction of pancreatic tissue densities by an analytical test gradient system before purification maximizes human islet recovery for islet autotransplantation/allotransplantation. Transplantation. 2011;91:508–514. doi: 10.1097/TP.0b013e3182066ecb. [DOI] [PubMed] [Google Scholar]
- 11.Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm SH, Zhang HJ, Parkey J, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. Jama. 2005;293:830–835. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]