Abstract
Cholecystokinin (CCK) receptors are G-protein coupled receptors (GPCR) which are present on lung cancer cells. CCK-8 stimulates the proliferation of lung cancer cells, whereas the CCK2R receptor antagonist CI-988 inhibits proliferation. GPCR for some gastrointestinal hormones/neurotransmitters mediate lung cancer growth by causing epidermal growth factor (EGF)R transactivation. Here, the role of CCK/gastrin and CI-988 on EGFR transactivation and lung cancer proliferation were investigated. Addition of CCK-8 or gastrin-17 (100 nM) to NCI-H727 human lung cancer cells increased EGFR Tyr1068 phosphorylation after 2 min. The ability of CCK-8 to cause EGFR tyrosine phosphorylation was blocked by CI-988, gefitinib (EGFR tyrosine kinase inhibitor), PP2 (Src inhibitor), GM6001 (matrix metalloprotease inhibitor) and tiron (superoxide scavenger). CCK-8 nonsulfated and gastrin-17 caused EGFR transactivation and bound with high affinity to NCI-H727 cells, suggesting that the CCK2R is present. CI-988 inhibited the ability of CCK-8 to cause ERK phosphorylation and elevate cytosolic Ca2+. CI-988 or gefitinib inhibited the basal growth of NCI-H727 cells or that stimulated by CCK-8. The results indicate that CCK/gastrin may increase lung cancer proliferation in an EGFR-dependent manner.
Keywords: CCK, gastrin, CI-988, EGFR transactivation, lung cancer proliferation
Introduction
Cholecystokinin (CCK), an 8 amino acid peptide, is biologically active in the central nervous system and periphery (Mutt and Jorpes, 1971). In the CNS, CCK alters rodent behavior, neuronal activity and causes satiety (Gibbs et al., 1973, Crawley, 1988; Wang 1986). In the periphery, CCK is localized to the myenteric plexus and endocrine cells. Upon secretion, CCK causes release of enzymes from the pancreas and contraction of the gallbladder (Jensen et al., 1980). In the stomach, gastrin-17 causes release of gastric acid which facilitates digestion of fats and proteins. The actions of CCK-8/gastrin are mediated by two receptors (R), the CCK1R and CCK2R (Dufresne et al., 2006). The CCK1R binds CCK-8 sulfated but not nonsulfated (NS) CCK-8 with high affinity and is antagonized by L-364,718 (Jensen et al., 1989). The CCK2R binds gastrin-17, CCK-8 and CCK-8NS with high affinity and is antagonized by L-365,260 (Innes and Snyder, 1980). In the normal CNS, pancreas and stomach, both the CCK1R and CCK2R are present in many species (Dufresne el al., 2006).
The signal transduction mechanisms of the CCK2R have been extensively investigated. The CCK2R is a 447 amino acid G-protein coupled receptor (GPCR) which causes phosphatidyl inositol (PI) turnover (Wank et al., 1998). The diacyglycerol released activates protein kinase (PK) C, whereas the inositol-1,4,5-trisphosphate released causes increased cytosolic Ca2+ (Staley et al., 1989; Sethi and Rozengurt, 1991). Other downstream targets include ERK, FAK, Jun kinase, p38 MAP kinase and PI-3-K (Dockray et al., 2012). CCK-8 stimulates whereas L-365,260 and CI-988 inhibit SCLC basal growth or that stimulated by CCK-8 (Moody and Jensen, 2001). Several cancers make gastrin which functions as an autocrine growth factor (Rehfeld, 2006). The CCK2R is present in many cancers, including small cell lung cancer (SCLC), medullary thyroid carcinomas, astrocytomas and stromal ovarian cancers (Reubi et al., 1997).
SCLC is a neuroendocrine tumor with p53 mutations which kills approximately 30,000 patients is the United States (USA) annually. SCLC patients are usually treated with chemotherapy. In contrast, non-SCLC (NSCLC) is an epithelial tumor with epidermal growth factor receptor (EGFR) amplifications and mutations, which kills approximately 130,000 USA patients annually (Kaufman et al., 2011). In the world, lung cancer kills approximately 1,500,000 patients annually. NSCLC patients who fail chemotherapy can be treated with the tyrosine kinase inhibitors (TKI) erlotinib and gefitinib (Lynch et al., 2004; Paez et al., 2004). The EGFR is activated by endogenous ligands such as transforming growth factor (TGF) α resulting in tyrosine phosphorylation of the EGFR, ERK and PI-3-K (Lemmon and Schlessinger, 2010). Also, GPCR can cause EGFR tyrosine phosphorylation through transactivation (George et al. 2013). Previously, we found that GPCR for bombesin, neurotensin or pituitary adenylate cyclase activating polypeptide caused EGFR transactivation in NSCLC cells (Moody et al., 2010, Moody et al., 2012; Moody et al., 2014). In this communication, CCK-8 causes lung cancer EGFR transactivation and proliferation. Further CI-988 antagonized the increase in EGFR transactivation and proliferation caused by CCK-8 addition to lung cancer cells.
Materials and Methods
Cell culture and receptor binding
NCI-H727 cells were cultured in RPMI-1640 containing 10% fetal bovine serum. The cells were split weekly 1/20 with trypsin-ethylenediaminotetraacetic acid (EDTA). The cells were mycoplasma free and were used when they were in exponential growth phase after incubation at 37°C in 5% CO2/95% air. For the radioreceptor assay NCI-H727 cells were placed in 24 well plates. When confluent the cells were washed 3 times in SIT medium (RPMI-1640 containing 3 × 10−8 M sodium selenite, 5 µg/ml bovine insulin and 10 µg/ml transferrin (Sigma-Aldrich, St. Louis, MO)). The cells were incubated in SIT buffer containing 0.25% bovine serum albumin and 250 µg/ml bacitracin (Sigma-Aldrich, St. Louis, MO) and 125I-Bolton Hunter(BH)-CCK-8 (50,000 cpm) added as well as various concentrations of CCK-8, nonsulfated (NS)CCK-8, CCK-4, gastrin-17 or CI-988. After incubation at 37°C for 30 min, free 125I-BH-CCK8 was removed by washing 3 times in buffer and the cells which contained bound 125I-BH-CCK8 dissolved in 0.2 N NaOH and counted in a gamma counter. The Ki was calculated for each unlabeled competitor.
Western Blot
The ability of CCK-8, CCK-8NS, CCK-4 or gastrin-17 (Bachem Inc., Torrence, CA) to stimulate tyrosine phosphorylation of the EGFR or ERK (p42/p44 MAP kinase) was investigated by Western blot. Lung cancer cells were cultured in 10 cm dishes. When a monolayer of cells formed they were placed in SIT media for 3 hr. Routinely, lung cancer cells were treated with CI-988 (Provided by Dr. J. Hughes and dissolved in DMSO at a concentration of 30 mM), gefitinib (Tocris Bioscience, Bristol, UK, and dissolved in DMSO at a concentration of 30 mM), PP2 (Sigma-Aldrich, St. Louis, MO and dissolved in DMSO at a concentration of 10 mM), GM6001 (Sigma-Aldrich, St. Louis, MO and dissolved in DMSO at a concentration of 1 mM), N-acetylcysteine (NAC, Sigma-Aldrich, St. Louis, MO and dissolved in DMSO at a concentration of 5 M), Tiron (Sigma-Aldrich, St. Louis, MO and dissolved in DMSO at a concentration of 5 M) or no additions for 30 minutes. Then cells were incubated with 0.1 µM CCK-8 for 2 min, washed twice with PBS and lysed in buffer containing 50 mM Tris.HCl (pH 7.5), 150 mM sodium chloride, 1% Triton X-100, 1% deoxycholate, 1% sodium azide, 1 mM ethyleneglycoltetraacetic acid, 0.4 M EDTA, 1.5 µg/ml aprotinin, 1.5 µg/ml leupeptin, 1 mM phenylmethylsulfonylfluoride and 0.2 mM sodium vanadate (Sigma-Aldrich, St. Louis, MO). The lysate was sonicated for 5 s at 4°C and centrifuged at 10,000 × g for 15 min. Protein concentration was measured using the BCA reagent (Pierce Chemical Co., Rockford, IL), and 400 µg of protein was incubated with 4 µg of anti-phosphotyrosine (PY) monoclonal antibody, 4 µg of goat anti-mouse immunoglobulin IgG and 15 µl of immobilized protein G overnight at 4°C. The immunoprecipitates were washed 3 times with phosphate buffered saline and analyzed by sodium dodecyl sulfate/polyacrylamide gel electrophoresis and Western blotting. Immunoprecipitates were fractionated using 4–20% polyacrylamide gels (Novex, San Diego, CA). Proteins were transferred to nitrocellulose membranes and the membranes were blocked overnight at 4°C using blotto (5% non-fat dried milk in solution containing 50 mM Tris/HCl (pH 8.0), 2 mM CaCl2, 80 mM sodium chloride, 0.05% Tween 20 and 0.02% sodium azide) and incubated for 16 h at 4°C with 1 µg/ml anti-EGFR antibody (Cell Signaling Technologies, Danvers, MA) followed by anti-rabbit immunoglobulin G-horseradish peroxidase conjugate (Upstate Biotechnologies, Lake Placid, NY). The membrane was washed for 10 min with blotto and twice for 10 min with washing solution (50 mM Tris/HCl (pH 8.0), 2 mM CaCl2, 80 mM sodium chloride, 0.05% Tween 20 and 0.02% sodium azide). The blot was incubated with enhanced chemiluminescence detection reagent for 5 min and exposed to Kodak XAR film. The band intensity was determined using a densitometer.
Alternatively, 20 µg of cellular extract was loaded onto a 15 well 4–20% polyacrylamide gels. After transfer to nitrocellulose, the blot was probed with anti PY1068-EGFR, anti-EGFR, anti-PY204ERK, anti-ERK or anti-tubulin (Cell Signaling Technologies, Danvers, MA).
Cytosolic Ca2+
NCI-H727 cells were treated with trypsin-EDTA and harvested. After centrifugation the cells were resuspended in SIT medium (2.5 × 106 cells/ml) containing Fura-2AM (Calbiochem, La Jolla, CA) at 37°C for 30 min (Staley et al., 1990). The cells were centrifuged at 1000 × g for 5 min and resuspended at a concentration of 2.5 × 106/ml and 2 ml placed in a Quartz cuvette containing a stirbar. The excitation ratio was determined at 340 and 380 nm and the emission at 510 nm using a spectrofluorometer equipped with a magnetic stirring mechanism and temperature (37°C) regulated cuvette holder before and after addition of drug.
Reactive oxygen species
NCI-H727 cells were placed in black 96 well plates (30,000 cells/well) and cultured overnight. The cells were treated with 10 µM dichlorofluoresceindiacetate for 1 h and washed 3 times with serum- free SIT medium. Some of the cells were treated with 10 µM CI-988 and then stimuli such as 10 nM CCK-8 or 10 µM H2O2 added. Fluorescence measurements were taken at the various times using an excitation wavelength of 485 nm and emission wavelength of 585 nm.
Proliferation
Growth studies in vitro were conducted using the 3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyl-2H-tetrazolium bromide (MTT, Sigma-Aldrich, St. Louis, MO) and clonogenic assays. In the MTT assay, NCI-H727 cells were placed in SIT medium and various concentrations of CI-988 or gefitinib added. After 2 days, 15 µl of 0.1 % MTT solution added. After 4 h, 150 µl of dimethylsulfoxide was added. After 16 h, the optical density at 570 nm was determined. In the clonogenic assay, the effects of CI-988 and gefitinib were investigated on NCI-H727 cells. The bottom layer contained 0.5% agarose in SIT medium containing 5% FBS in 6 well plates. The top layer consisted of 3 ml of SIT medium in 0.3% agarose, CCK-8, CI-988 and/or gefitinib using 5 × 104 lung cancer cells. Triplicate wells were plated and after 2 weeks, 1 ml of 0.1% p-iodonitrotetrazolium violet (Sigma-Aldrich, St. Louis, MO) was added and after 16 hours at 37°C, the plates were screened for colony formation; the number of colonies larger than 50 µm in diameter were counted using an Omnicon image analysis system.
Results
CCK2R agonists cause EGFR transactivation and bind with high affinity
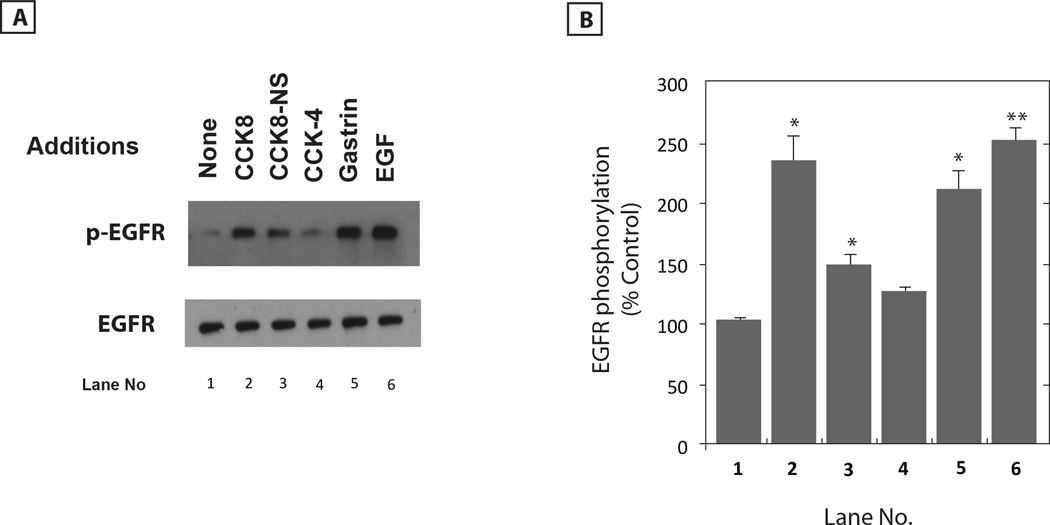
The ability of CCK/gastrin analogs to cause EGFR transactivation was investigated. Figure 1A shows that addition of 0.1 µM CCK-8, CCK-8NS, gastrin-17 or EGF strongly, whereas CCK-4 moderately increased EGFR tyrosine phosphorylation 2 min after addition to NCI-H727 cells. Fig. 1B shows that CCK-8, CCK-8NS, gastrin-17 and EGF increased significantly EGFR tyrosine phosphorylation by 233, 149, 208 and 250%, respectively. CCK-4 increase EGFR tyrosine phosphorylation by 125% but it was not significant. In contrast, the ligands had no effect on total EGFR (Fig. 1A). These results indicate that CCK causes EGFR transactivation in lung cancer cells.
Fig. 1.
CCK peptides increase EGFR tyrosine phosphorylation. (A) The ability of CCK-8, CCK-8NS, CCK-4, gastrin-17 or EGF to increase EGFR tyrosine phosphorylation was investigated using NCI-H727 cells. Total EGFR is indicated. (B) The mean value ± S.E. of 3 experiments is indicated; p < 0.05, *; p < 0.01, ** relative to control using the Student’s t-test.
Table I shows that CCK-8, CCK-8NS, gastrin-17 and CCK-4 bound with high affinity to NCI-H727 cells with Ki values of 0.8, 1.2, 5.9 and 22 nM, respectively. In contrast, gefitinib, an EGFR tyrosine kinase inhibitor, has no effect. The CCK2R antagonist, CI-988, inhibited specific 125I-BH-CCK-8 binding to NCI-H727 cells with high affinity (Ki = 4.5 nM). These results suggest that the CCK2R is present on NCI-H727 cells. RT-PCR results verified that CCK2R but not CCK1R mRNA was present in NCI-H727 cells (data not shown).
Table I.
Binding of CCK analogs
| Peptide | Ki, nM |
|---|---|
| CCK-8 | 0.8 ± 0.2 |
| CCK-8NS | 1.2 ± 0.3 |
| Gastrin-17 | 5.9 ± 0.91 |
| CCK-4 | 22 ± 4 |
| CI-988 | 4.5 ± 0.9 |
| Gefitinib | >1800 |
| Gastrin-17 | Met-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu- Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2 |
| CCK-8NS | Asp-Tyr-Met-Gly-Trp-Met-Asp-Phe-NH2 |
CI-988 is a CCK2R antagonist
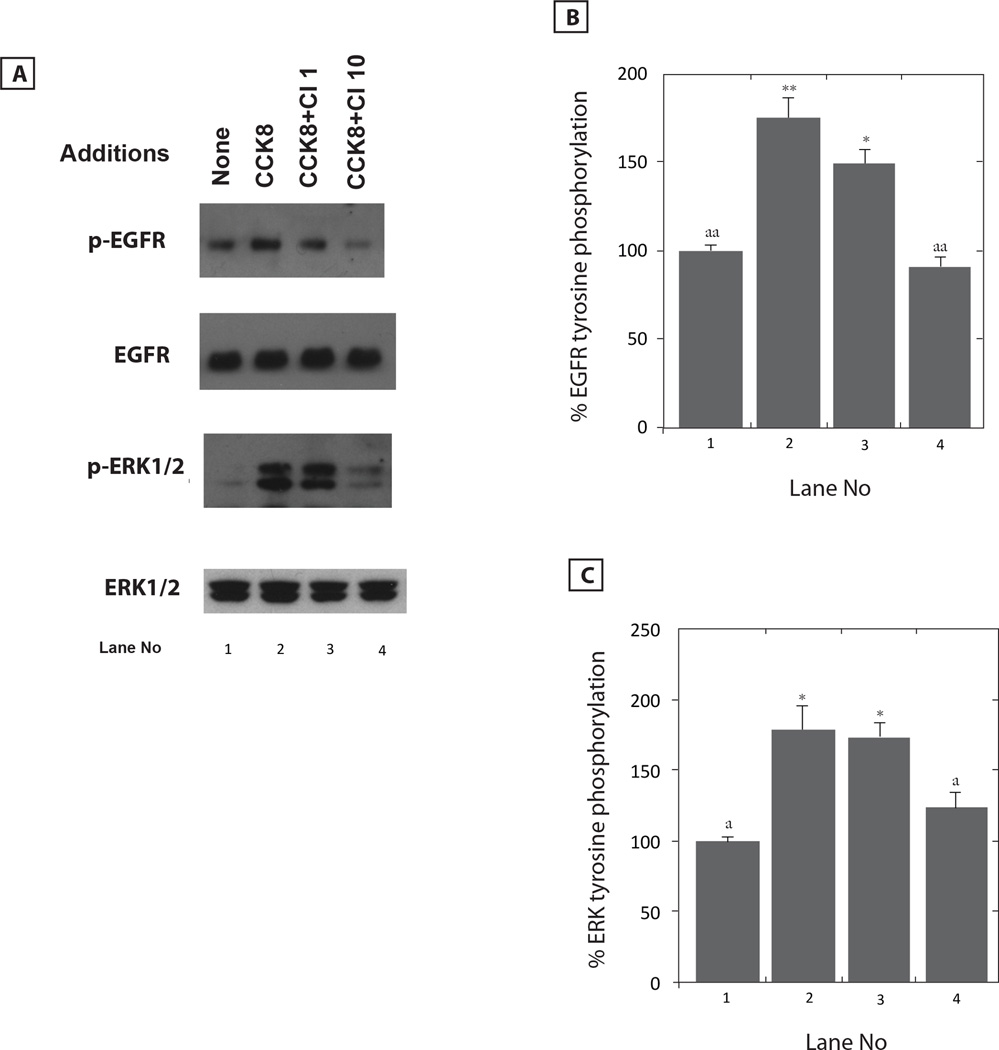
The ability of CI-988 to antagonize the effects of CCK/gastrin was investigated. Figure 2A shows that CI-988 inhibited in a dose-dependent manner the ability of CCK-8 to cause EGFR transactivation in NCI-H727 cells. CI-988 at doses of 1 and 10 µM weakly and strongly, respectively, inhibited the ability of 0.1µM CCK-8 to increase EGFR tyrosine phosphorylation. Figure 2B shows that CCK-8 addition to NCI-H727 cells increased EGFR tyrosine phosphorylation by 175% and the increase caused by CCK-8 was significantly inhibited by 10 but not 1 µM CI-988. Similarly, 10 µM but not 1 µM CI-988 antagonized the ability of CCK-8 to cause ERK tyrosine phosphorylation (Fig. 2A). Figure 2C shows that CCK-8 addition to NCI-H727 cells significantly increased ERK tyrosine phosphorylation by 178% and the increase caused by CCK-8 was significantly inhibited by 10 but not 1 µM CI-988. In contrast, CI-988 had no effect on total EGFR or ERK. The results indicate that CI-988 antagonizes the ability of CCK-8 to cause lung cancer EGFR or ERK tyrosine phosphorylation. Similarly, CI-988 antagonized the ability of gastrin-17 to cause EGFR or ERK tyrosine phosphorylation (data not shown).
Fig. 2.
CI-988 dose-response. (A) Varying doses of CI-988 (CI) were used to antagonize the ability of CCK-8 to increase tyrosine phosphorylation of EGFR and ERK. Total EGFR and ERK are shown. (B) The % EGFR tyrosine phosphorylation mean value ± S.E. of 3 experiments is indicated; p < 0.05, *; p < 0.01, ** relative to control; p < 0.01, aa: relative to CCK-8 using the Student’s t-test. (C) The % ERK tyrosine phosphorylation mean value ± S.E. of 3 experiments is indicated; p < 0.05, * relative to control; p < 0.05,a; relative to CCK-8 using the Student’s t-test.
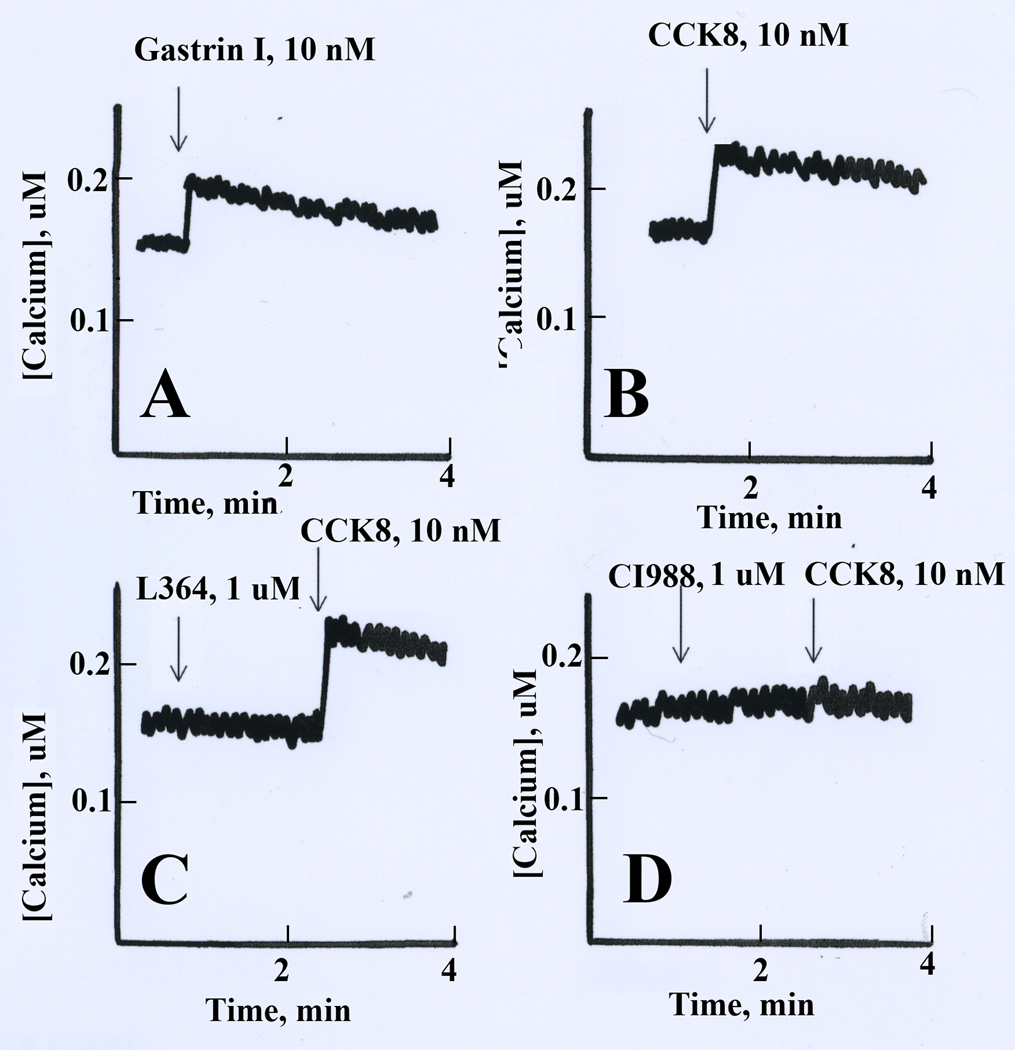
The effects of CCK/gastrin on cytosolic Ca2+ was investigated. Fig. 3 shows that 10 nM CCK-8 or gastrin-17 addition to Fura-2AM loaded NCI-H727 cells increased the cytosolic Ca2+ transiently. The cytosolic Ca2+ increased rapidly in NCI-H727 cells from 160 to 210 nM within seconds after addition of CCK-8, peaked after about 15 sec and then slowly declined over a 2 min period. L-364,718 had no effect on basal cytosolic Ca2+ or the increase caused by CCK-8 (Fig. 3C). CI-988 (1 µM) had no effect on basal cytosolic Ca2+ but blocked the increase in cytosolic Ca2+ caused by CCK-8 addition to NCI-H727 cells (Fig. 3D). The results demonstrate that CI-988 antagonizes the ability of CCK-8 to cause second messenger production in NCI-H727 cells.
Fig. 3.
Cytosolic Ca2+. The ability of gastrin-17 (A) and CCK-8 (B) to increase cytosolic in Fura-2AM loaded NCI-H727 cells is shown. (C) L364,718 (L364) had no effect on basal cytosolic Ca2+ or the ability of CCK-8 to increase cytosolic Ca2+. (D) CI-988 had no effect on basal cytosolic Ca2+ but antagonized the ability of CCK-8 to increase cytosolic Ca2+. This experiment is representative of 3 others.
Inhibitors of EGFR transactivation
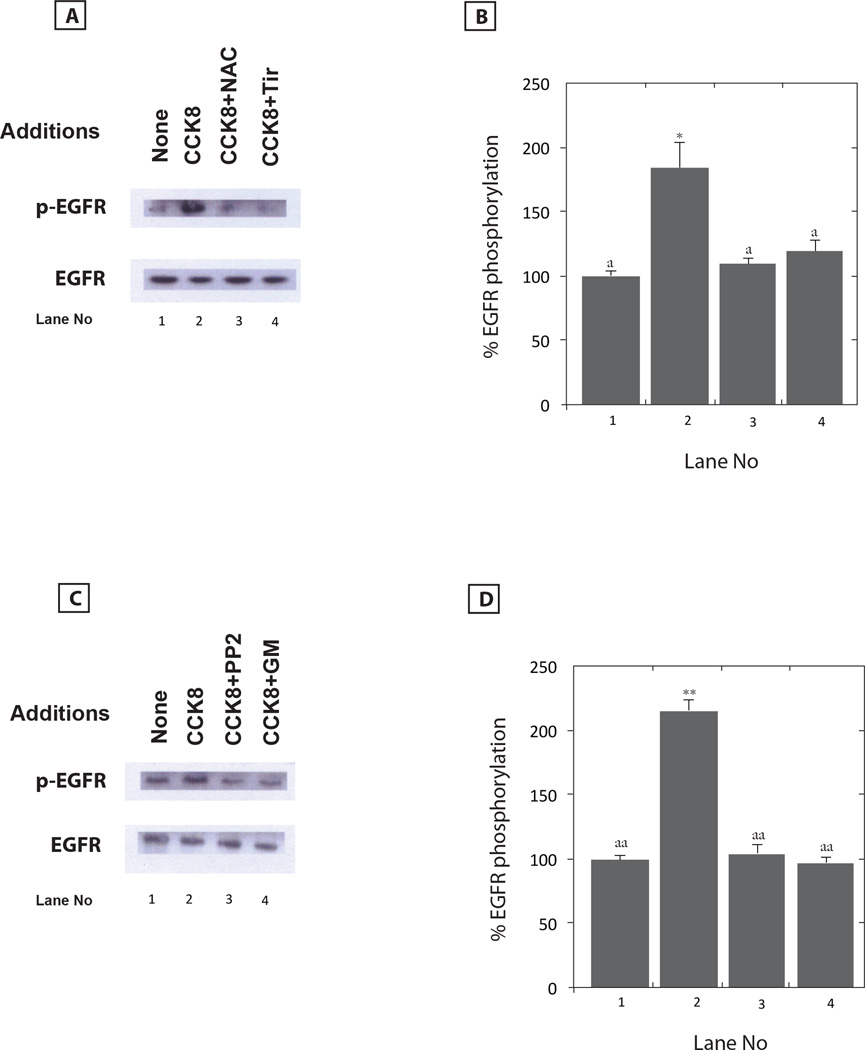
The cellular mechanism by which CCK-8 causes EGFR transactivation was investigated. Five mM N-acetyl cysteine (NAC) or 5 mM Tiron (Tir) block the ability of CCK-8 to cause EGFR transactivation (Fig. 4A). Figure 4B shows that CCK-8 significantly increased EGFR transactivation by 184% and that the increased in EGFR tyrosine phosphorylation was inhibited significantly by NAC or Tiron. The results indicate that CCK-8 requires reactive oxygen species (ROS) to cause EGFR transactivation. Table II shows that CCK-8 addition to NCI-H727 cells increases significantly reactive oxygen species (ROS) by 128%. The increase in ROS caused by CCK-8 addition to NCI-727 cells was blocked significantly by CI-988. Fig. 4C shows that CCK-8 addition to NCI-H727 cells increased EGFR tyrosine phosphorylation which was blocked by 10 µM PP2 (Src inhibitor) or 1 µM GM60001 (matrix metalloprotease (MMP) inhibitor). Figure 4D shows that CCK-8 increased the EGFR tyrosine phosphorylation significantly by 214% and the increase was significantly inhibited by PP2 or GM6001. Src may activate MMP causing the metabolism of EGFR precursor ligands to biologically active TGFα (Moody et al., 2010).
Fig. 4.
EGFR transactivation inhibitors. (A) Five mM NAC or Tiron (Tir) had no effect on basal EGFR tyrosine phosphorylation but inhibited the increase in EGFR transactivation caused by CCK8 addition to NCI-H727 cells. (B) The mean value ± S.E. of 3 experiments is indicated; p < 0.05, *; relative to control; ; p < 0.05,a; relative to CCK-8 using the Student’s t-test. (C) Ten µM PP2 or GM6001 (GM) had no effect on basal EGFR tyrosine phosphorylation but inhibited the increase in EGFR transactivation caused by CCK-8 addition to NCI-H727 cells. (D) The mean value ± S.E. of 3 experiments is indicated; p < 0.01, ** relative to control; p < 0.01, aa: relative to CCK-8 using the Student’s t-test.
Table II.
Reactive oxygen species
NCI-H727 cells were treated with 100 nM CCK-8 for 60 min and the relative fluorescence determined. The mean value ± S.D. of 8 determinations is indicated; p < 0.05,
relative to control; p < 0.05,
relative to CCK-8 using the Student’s t-test.
CCK-8 stimulates whereas CI-988 or gefitinb inhibit proliferation
The effects of CCK8, CI-988 and/or gefitinib were investigated on NCI-H727 proliferation. Table III shows that addition of 10 nM CCK-8 increases NCI-H727 colony number by 76%. The increase in colony number caused by CCK-8 was reversed by addition of 3 µM CI-988 or 1 µM gefitinib. Surprisingly, CI-988 and gefitinib together reduced NCI-H727 colony number more than either agent alone. In the absence of CCK-8, CI-988 or gefitinib significantly reduced NCI-H727 colony number by 50% and 37% respectively. CI-988 and gefitinib strongly reduced the number of NCI-H727 colonies by 79%. The results indicate that CCK-8 stimulates NCI-H727 proliferation whereas CI-988 or gefitinib inhibit proliferation.
Table III.
Clonal growth of NCI-H727 cells.
| Addition | Colony number | Colony number + 10 nM CCK-8 |
|---|---|---|
| None | 42± 6 | 74 ± 9* |
| CI-988, 3 uM | 21 ± 3* | 44 ± 5 |
| Gef, 1 uM | 26± 4* * | 49 ± 7 |
| CI-988 + Gef | 9 ± 2** | 28 ± 5* |
The mean value ± S.D. of 3 determinations is indicated;
, p < 0.05;
, p < 0.01 relative to control using Student’s t-test.
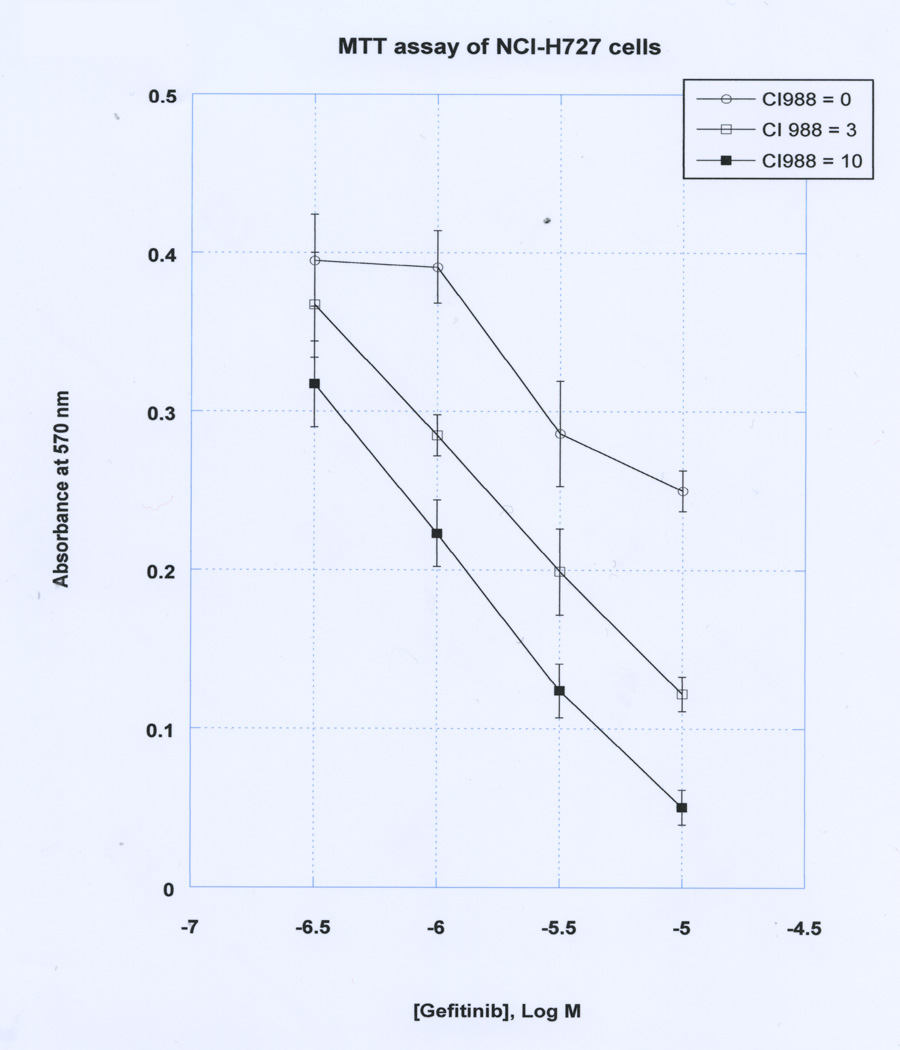
The effects of CI-988 and gefitinib on NCI-H727 proliferation were further investigated. Figure 5 shows that in the absence of CI-988, gefitinib inhibits weakly lung cancer proliferation with an IC50 of approximately 30 µM. In the presence of 3 µM CI-988, the gefitinib dose-response curve shifted to the left and the IC50 was approximately 5 µM. In the presence of 10 µM CI-988, the IC50 for gefitinib was approximately 2 µM. The results indicate that in the presence of CI-988, the cytotoxicity of gefitinib to kill NCI-H727 cells increased by approximately an order of magnitude. The results demonstrate that CI-988 and gefitinib can act synergistically inhibit the proliferation of lung cancer cells.
Discussion
CCK and gastrin as well as the CCK1R and CCK2R are present in certain cancer cells (Clerc et al., 1997; Coulson et al., 2003; Ocejo-Garcia et al., 2001; Sethi et al, 1993). In SCLC cell lines NCI-H209 and NCI-H345, high affinity CCK binding sites were identified (Yoder and Moody, 1987). Both CCK-8 and CCK-8NS bind with high affinity to SCLC cells and increase cytosolic Ca2+ (Staley et al., 1989). The effects of CCK-8 on SCLC cells were blocked by L-365–260, the CCK2R antagonist, but not L-364,718, the CCK1R antagonist (Staley et al., 1990). Subsequently CI-988 was developed as a CCK2R antagonist (Hughes et al., 1990). CI-988 inhibits the ability of CCK-8 to increase cytosolic Ca2+, ERK and FAK tyrosine phosphorylation, and c-fos as well as VEGF expression after addition to various SCLC cells (Moody and Jensen 2001). SCLC cells have few EGFR, whereas the EGFR is overexpressed in NSCLC cells which lack the CCK2R (Reubi et al., 1997). Here NCI-H727 cells, a lung carcinoid, were identified as having both EGFR and CCK2R.
The CCK2R activates phospholipase C leading to PI metabolism (Paulson et al., 2000; Jensen et al., 1989). The PI metabolites activate PKC and increase cytosolic Ca2+ (Piiper et al., 2003; Staley et al., 1990). High densities of CCK2R mRNA are present on SCLC cell lines NCI-H510, H345 and H69 (Sethi et al., 1993) and addition of 0.1 µM gastrin or CCK-8 to NCI-H510 cells increased cytosolic Ca2+ and the increase in cytosolic Ca2+ caused by CCK-8 was inhibited by CAM-2200 (CCK2R antagonist). In contrast, addition of gastrin to SCLC GLC19 cells had no effect whereas addition of CCK-8 increased cytosolic Ca2+. The increase in cytosolic Ca2+ caused by CCK-8 was inhibited by CAM-1481 (CCK1R antagonist) but not CAM-2200. The results indicate that NCI-H510 has a functional CCK2R whereas GLC19 has a functional CCK1R. Using lung carcinoid cell line NCI-H727, the addition of 10 nM Gastrin or CCK-8 increased the cytosolic Ca2+. The increase in cytosolic Ca2+ caused by CCK-8 was inhibited by CI-988 (CCK2R antagonist) but not L364,718 (CCK1R antagonist). Thus the CCK2R but not CCK1R is present on NCI-H727 cells.
Previous studies show that neuropeptides can stimulate cancer cellular proliferation as a result of EGFR transactivation (Moody et al., 2010, 2012, 2014). Addition of CCK-8 to pancreatic cancer cell line AR42J causes tyrosine phosphorylation of numerous proteins. P-Src and P-ERK are significantly increased after CCK-8 addition to AR42J cells (Ferrand et al., 2006). The CCK-8 stimulated increase in P-Src and P-ERK is inhibited by PP2 or GP2A, a Gq inhibitor. Further the increase in EGFR and ERK tyrosine phosphorylation caused by CCK-8 addition to AR42J cells is inhibited by AG1478, an EGFR TKI (Piiper et al., 2003). Gastrin increases heparin binding (HB) EGF, an EGFR ligand, in a PKC-dependent manner (Sinclair et al., 2004). Also, gastrin increases the expression of HB-EGF and amphiregulin which was blocked by the CCK-BR antagonist L-740,093 (Tsutsui et al., 1997). In the present study addition of CCK-8, CCK-8NS or gastrin-17 to NCI-H727 cells significantly increased EGFR tyrosine phosphorylation. The increase in EGFR and ERK tyrosine phosphorylation caused by addition of CCK-8 to NCI-H727 cells was significantly inhibited by CI-988, PP2, gefitinib and GM6001, a MMP inhibitor. In gastric epithelial cells, gastrin increases MMP9 expression (Wroblewski et al., 2002). Preliminary data (T. Moody unpublished) indicate that GM6001 inhibits CCK-8 stimulated secretion of TGF α, an EGFR ligand, from NCI-H727 cells supporting the important role of CCK-8 in MMP activation. Addition of CCK-8 to NCI-H727 cells significantly increased ROS in the present study. The increase in ROS caused by CCK-8 was inhibited by CI-988 and Tiron (T. Moody, unpublished). Also, Tiron inhibited the ability of the CCK2R to transactivate the EGFR. It remains to be determined if CCK increases NAD(P)H oxidase activity resulting in increased ROS production. The results emphasize the importance of MMP-activation, release of EGFR ligands and ROS in the CCK2R regulation of the EGFR.
EGFR transactivation caused by CCK or gastrin addition cells causes numerous downstream events. CCK-8 addition to AR42J cells increases formation of the Shc-Grb2 adaptor protein complex (Piiper et al., 2003). The Src kinase, Yes, is activated by CCK and mediates EGFR Shc-Grb2 interactions (Clerc et al., 2002). Gastrin increases tyrosine phosphorylation of the EGFR as well as ERK and degradation of PPARγ (Chang et al., 2006). Ras, a GTP binding protein, Raf-1, a serine-threonine kinase, and MEK1/2, which phosphorylates ERK, were activated by CCK-8 in a PKC-dependent manner using AR42J cells (Piiper et al., 2003). Transfection of pancreatic cancer cells with a dominant-negative Ras reduces the ability of CCK to cause EGFR transactivation and ERK tyrosine phosphorylation in AR42J cells (Piiper et al., 2003). MEK1/2, which phosphorylates ERK, is phosphorylated after CCK addition to AR42J cells (Piiper et al., 2003). In the present study, the phosphorylation of ERK caused by CCK-8 is blocked by PD98059, a MEK inhibitor, CI-988 or gefitinib using NCI-H727 cells (T. Moody, unpublished). The results indicate that CCK-8 addition to NCI-H727 cells activates the Ras, Raf, MEK, ERK pathway and that EGFR transactivation is important for this activation.
The activation of ERK by gastrin or CCK leads to a number of important cellular signal cascades. When ERK is phosphorylated it can enter the nucleus and alter gene expression (Whitmarsh and Davies, 1996). Gastrin increases c-fos expression (Stepan et al., 1999) and the c-fos may form a heterodimer with c-jun, activating AP-1 sites. Gastrin addition to intestinal epithelial cells causes increased ELK-1 and COX-2 expression (Guo et al., 2002). Gastrin addition to colon cancer cells increases COX-2 and prostaglandin E2 expression (Colucci et al., 2005). CCK2R activation increases expression of Reg proteins during the early steps of carcinogenesis in the Elas CCK2R mouse pancreas (Gigoux et al, 2008). CCK causes activation of ERK-1 and p85/p110-PI-3-K in a Src- dependent manner (Daulhac et al., 1999). Tyr438 of the CCK2R is essential for interacting with SHP-2, a protein tyrosine phosphatase, and Akt (Vantinel et al., 2006). Downstream, p70S6-kinase is phosphorylated after addition of CCK-8 (Seva et al., 1997). Activation of the Akt pathway by CCK or gastrin may increase survival of cancer cells.
The EGFR TKI gefitinib or erlotinib are used effectively in NSCLC patients with EGFR mutations who fail chemotherapy. Previously, we showed that non-peptide GRCR antagonists for bombesin, neurotensin and pituitary adenylate cyclase activating polypeptide, increased the potency of gefitinib in NSCLC cells, which have wild type EGFR (Moody et al., 2010, 2012, 2014). Unfortunately, the CCK2R is not abundant in NSCLC cells. In this communication a lung carcinoid cell line NCI-H727, which has wild type EGFR and CCK2R, was identified. We found that CI-988 potentiated the growth inhibitory effects of gefitinib in NCI-H727 cells. Preliminary data indicate that in SCLC cell line NCI-H345, which has wild type EGFR and CCK2R, CI-988 potentiated the growth inhibitory effects of gefitinib (T. Moody, unpublished). Our results suggest that CI-988 can potentiate the effects of gefitinib in some lung carcinoid and SCLC cells suggesting a novel therapeutic approach.
In summary, the CCK2R regulated EGFR transactivation in lung carcinoid cells. CCK-8 or gastrin addition to NCI-H727 cells causes increased tyrosine phosphorylation of the EGFR which is inhibited by CI-988 or gefitinib. CI-988 and gefitinib inhibited synergistically the proliferation of NCI-H727 cells in vitro. The results indicate that some of the growth effects of CCK or gastrin in lung carcinoid cells occur in an EGFR-dependent manner and this could lead to new treatment strategies.
Fig. 5.
CI-988 and gefitinib growth inhibition. Varying doses of gefitinib were added with (○) no additions, (▫) 3 µM CI-988 and (▪) 10 µM-CI988 to NCI-H727 cells using the MTT assay. The mean value ± S.E. of 8 determinations is indicated. This experiment is representative of 3 others.
Acknowledgments
The authors thank Dr. J. Hughes for the CI-988 and Dr. D. Chan for helpful discussions. This research is supported by the NIH intramural programs of NIDDK and NCI.
Footnotes
Disclosure Statement: The authors state that there are no conflicts of interest to disclose.
References
- Chang AJ, Song DH, Wolfe MM. Attenuation of peroxisome proliferator-activated receptor gamma (PPARgamma) mediatres gastrin-stimulated colorectal cancer cell proliferation. J Biol Chem. 2006;281:14700–14710. doi: 10.1074/jbc.M602623200. [DOI] [PubMed] [Google Scholar]
- Clerc P, Dufresne M, Saillan C, et al. Differential expression of the CCK-A and CCK-B/gastrin receptor genes in human cancers of the esophagus, stomach and colon. Int J Cancer. 1997;72:931–936. doi: 10.1002/(sici)1097-0215(19970917)72:6<931::aid-ijc2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Clerc P, Leung-Theung-Long S, Wang TC, et al. Expression of CCK2 receptors in the murine pancreas: Proliferation, transdifferentiation of acinar cells and neoplasia. Gastroenterology. 2002;122:428–437. doi: 10.1053/gast.2002.30984. [DOI] [PubMed] [Google Scholar]
- Colucci R, Blandizzi C, Tanini M, et al. Gastrin promotes human colon cancer cell growth via CCK-2 receptor-mediated cyclooxygenase-2 induction and prostaglandin E2 production. Br J Pharmacol. 2005;144:338–348. doi: 10.1038/sj.bjp.0706053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson JM, Ocejo-Garcia M, Woll PJ. Neuroendocrine phenotype of small cell lung cancer. Methods Mol Med. 2003;74:61–73. doi: 10.1385/1-59259-323-2:61. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Modulation of mesolimbic dopaminergic behaviors by cholecystokinin. Ann N Y Acad Sci. 1988;547:380–396. doi: 10.1111/j.1749-6632.1988.tb42121.x. [DOI] [PubMed] [Google Scholar]
- Daulhac L, Kowalski-Chauvel A, Pradayrol L, et al. Src-family kinases in activation of ERK-1 and p85/p110-phosphatidylinositol 3-kinase by G/CCKB receptors. J Biol Chem. 1999;274:20657–20663. doi: 10.1074/jbc.274.29.20657. [DOI] [PubMed] [Google Scholar]
- Dockray GJ, Moore A, Varro A, Pritchard DM. Gastrin receptor pharmacology. Curr Gastroenterol Rep. 2012;14:453–459. doi: 10.1007/s11894-012-0293-1. [DOI] [PubMed] [Google Scholar]
- Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev. 2006;86:805–847. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- Ferrand A, Vatinel S, Kowalski-Chauvel A, et al. Mechanism for Src activation by the CCK2 receptor: Patho-physiological functions of this receptor in pancreas. World J Gastroenterol. 2006;12:4498–4503. doi: 10.3748/wjg.v12.i28.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George AJ, Hannan RD, Thomas WG. Unravelling the molecular complexity of GPCR-mediated EGFR transactivation using functional genomics approaches. FEBS Journal. 2013;280:5258–5268. doi: 10.1111/febs.12509. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Young RC, Smith GP. Cholecystokinin elicits satiety in rats with open gastric fistulas. Nature. 1973;245:323–325. doi: 10.1038/245323a0. [DOI] [PubMed] [Google Scholar]
- Gigoux V, Clerc P, Sanchez D, et al. Reg genes are CCK2 receptor targets in Elas CCK2 mice pancreas. Reg Peptides. 2008;146:88–98. doi: 10.1016/j.regpep.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Guo YS, Cheng JZ, Jin GF, et al. Gastrin stimulates cyclooxygenase-2 expression in intestinal epithelial cells through multiple signaling pathways. J Biol Chem. 2002;277:48755–48763. doi: 10.1074/jbc.M209016200. [DOI] [PubMed] [Google Scholar]
- Hughes J, Boden P, Costall B, et al. Development of a class of selective cholecystokinin type B antagonists having potent anxiolytic activity. Proc Natl Acad Sci USA. 1990;87:6728–6732. doi: 10.1073/pnas.87.17.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes RB, Snyder SH. Distinct cholecystokinin receptor in brain and pancreas. Proc Natl Acad Sci USA. 1980;77:6917–6921. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RT, Lemp GF, Gardner JD. Interaction of cholecystokinin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci USA. 1980;77:2079–2083. doi: 10.1073/pnas.77.4.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RT, Wank SA, Rowley WH, Sato S, Gardner JD. Interaction of CCK with pancreatic acinar cells. Trends Pharmacol Sci. 1989;10:418–423. doi: 10.1016/0165-6147(89)90192-2. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Horn L, Carbone D. Molecular biology of lung cancer. In: DeVita V Jr, Lawrence TS, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Lippincott: Williams & Wilkins Philadelphia; 2011. pp. 789–798. [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Moody TW, Jensen RT. CI-988 inhibits the growth of small cell lung cancer cells. J Pharmacol Expt Ther. 2001;299:1154–1160. [PubMed] [Google Scholar]
- Moody TW, Berna MJ, Mantey S, et al. Neuromedin B receptors regulate EGF receptor tyrosine phosphorylation in lung cancer cells. Eur J Pharm. 2010;637:38–45. doi: 10.1016/j.ejphar.2010.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody TW, Osefo N, Nuche-Berenguer B, Ridnour L, Wink D, Jensen RT. Pituitary adenylate cyclase activating polypeptide causes tyrosine phosphorylation of the EGF receptor in lung cancer cells. J Pharm Exp Ther. 2012;341:873–881. doi: 10.1124/jpet.111.190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody T, Chan D, Mantey S, Moreno P, Jensen RT. SR48692 inhibits non-small-cell lung cancer growth in an EGFR-dependent manner. Life Sci. 2014;10:25–34. doi: 10.1016/j.lfs.2014.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutt V, Jorpes E. Hormonal polypeptides of the upper intestine. Biochem J. 1971;125:57P–58P. doi: 10.1042/bj1250057p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocejo-Garcia M, Ahmed SI, Coulson JM, Woll PJ. Use of RT-PCR to detect co-expression of neuropeptides and their receptors in lung cancer. Lung Cancer. 2001;33:1–9. doi: 10.1016/s0169-5002(00)00248-8. [DOI] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Paulssen RH, Fraeyman N, Florholmen J. Activation of phospholipase C by cholecystokinin receptor subtypes with different G-protein –coupling specificities in hormone-secreting pancreatic cell lines. Biochem Pharmacol. 2000;60:865–875. doi: 10.1016/s0006-2952(00)00383-x. [DOI] [PubMed] [Google Scholar]
- Piiper A, Elez R, You SJ, et al. Cholecystokinin stimulates extracellular signal-regulated kinase through activation of the epidermal growth factor receptor, yes and protein kinase C. Signal amplification at the level of Raf by activation of protein kinase C epsilon. J Biol Chem. 2003;278:7065–7072. doi: 10.1074/jbc.M211234200. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF. Gastrin and Cancer. In: Kastin A, editor. Handbook of Biologically Active Peptides. Philadelphia: Academic Press; 2006. pp. 467–471. [Google Scholar]
- Reubi JC, Schaer JC, Waser B. Cholecystokinin (CCK)-A and CCK-B/gastrin receptors in human tumors. Cancer Res. 1997;57:1377–1386. [PubMed] [Google Scholar]
- Sethi T, Rozengurt E. Multiple neuropeptides stimulate clonal growth of small cell lung cancer: Effects of bradykinin, vasopressin, cholecystokinin, galanin and neurotensin. Cancer Res. 1991;51:3621–3623. [PubMed] [Google Scholar]
- Sethi T, Herget T, Wu SV, Walsh JH, Rozengurt E. CCKA and CCKB receptors are expressed in small cell lung cancer lines and mediate Ca2+ mobilization and clonal growth. Cancer Res. 1993;53:5208–5213. [PubMed] [Google Scholar]
- Seva C, Kowalski-Chauvel A, Daulhac L, et al. Wortmannin-sensitive activation of p70S6-kinase and MAP-kinase by the G protein-coupled receptor, G/CCKB. Biochem Biophys Res Commun. 1997;238:202–206. doi: 10.1006/bbrc.1997.7163. [DOI] [PubMed] [Google Scholar]
- Sinclair NF, Ai W, Raychowdhury R, et al. Gastrin regulates the heparin-binding epidermal-like growth factor promoter via a PKC/EGFR dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2004;286:G992–G999. doi: 10.1152/ajpgi.00206.2002. [DOI] [PubMed] [Google Scholar]
- Staley J, Fiskum G, Moody TW. Cholecystokinin elevates cytosolic calcium in small cell lung cancer cells. Biochem Biophys Res Commun. 1989;163:605–610. doi: 10.1016/0006-291x(89)92180-3. [DOI] [PubMed] [Google Scholar]
- Staley J, Jensen RT, Moody TW. CCK antagonists interact with CCK-B receptors on human small cell lung cancer cells. Peptides. 1990;11:1033–1036. doi: 10.1016/0196-9781(90)90029-5. [DOI] [PubMed] [Google Scholar]
- Stepan VM, Tatewaki M, Matsushima M, et al. Gastrin induces c-fos gene transcription via multiple signaling pathways. Am J Physiol Gastrointest Liver Physiol. 1999;276:G415–G424. doi: 10.1152/ajpgi.1999.276.2.G415. [DOI] [PubMed] [Google Scholar]
- Tsutsui S, Shinomura Y, Higashiyama S, et al. Induction of heparin binding epidermal growth factor-like growth factor and amphiregulin mRNAs by gastrin in rat stomach. Biochem Biophys Res Commun. 1997;235:520–523. doi: 10.1006/bbrc.1997.6824. [DOI] [PubMed] [Google Scholar]
- Vatinel S, Ferrand A, Lopez F, et al. An ITIM-like motif within the CCK2 receptor sequence required for interaction with SHP-2 and the activation of the AKT pathway. Biochim Biophys Acta. 2006;1763:1098–1107. doi: 10.1016/j.bbamcr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Wang RY, Hu XT. Does cholecystokinin potentiate dopamine action in the nucleus accumbens? Brain Res. 1986;380:363–367. doi: 10.1016/0006-8993(86)90236-2. [DOI] [PubMed] [Google Scholar]
- Wank SA. G protein-coupled receptors in gastrointestinal physiology. I. CCK receptors: an exemplary family. Am J Physiol. 1998;274:G607–G613. doi: 10.1152/ajpgi.1998.274.4.g607. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davies RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- Wroblewski LE, Pritchard DM, Carter S, Varro A. Gastrin-stimulated gastric epithelial cells invasion: the role and mechanism of increased matrix metalloproteinase 9 expression. Biochem J. 2002;365:873–879. doi: 10.1042/BJ20020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder D, Moody TW. Cholecystokinin binds with high affinity to small cell lung cancer cells. Peptides. 1987;8:103–107. doi: 10.1016/0196-9781(87)90171-9. [DOI] [PubMed] [Google Scholar]