Abstract
Treatment of infectious diseases caused by the carbapenem-resistant Pseudomonas aeruginosa (CRPA) is becoming more challenging with each passing year. We conducted a meta-analysis to assess the impact of carbapenem resistance on mortality of patients with P. aeruginosa infection. We searched PUBMED, Web of science, EMBASE, Google Scholar and the Cochrane Library up to December 25, 2014, to identify published cohort or case-control studies. 17 studies, including 6660 patients carrying P. aeruginosa, were identified. The pooling analysis indicated that patients infected with CRPA had significantly higher mortality than those infected with carbapenem-susceptible P. aeruginosa (CSPA) (crude OR = 1.64; 95%CI = 1.40, 1.93; adjusted OR = 2.38; 95%CI = 1.53, 3.69). The elevated risk of mortality in patients with CRPA infection was not lessened when stratified by study design, sites of infection, or type of carbapenem, except that the estimate effect vanished in CRPA high-incidence region, South America (crude OR = 1.12; 95%CI = 0.64, 1.99). Begg’s (z = 0.95, p = 0.34) and Egger’s test (t = 1.23, p = 0.24) showed no evidence of publication bias. Our results suggest that carbapenem resistance may increase the mortality of patients with P. aeruginosa infection, whether under univariate or multivariate analysis.
Being a glucose non-fermentative Gram-negative bacillus, with minimal requirements for survival and a considerable ability to adapt to various environmental challenges, Pseudomonas aeruginosa has become an increasingly notorious pathogen of nosocomial infection in recent years. And this pathogen is regarded as an unfortunate by-product of progress in the modern medical treatment1. Although there are successive advances in antimicrobial therapy, P. aeruginosa associated infection remains to be alarming, with mortality ranging from 18% to 61%2,3,4.
Owing to intrinsic resistance and rapid acquisition of additional resistance to commonly used antibiotics, the therapeutic options for P. aeruginosa infection are limited5. For P. aeruginosa infection, carbapenem may be only one of choice of antibiotic. As a result, the emerging of carbapenem resistance is of great concern. According to epidemiologic studies, the prevalence of carbapenem-resistant P. aeruginosa (CRPA) varied by geographic regions. Based on the data of the Carbapenem Antimicrobials Pseudomonas Isolate Testing At regional Locations (CAPITAL) surveillance program in 2010, the overall rates of CRPA isolates among all P. aeruginosa isolates ranged from 7.4% to 35.4%6. However, the proportion of carbapenem resistance in P. aeruginosa has reached even more than 60% in several other regions of the world, especially in South America7. As carbapenem resistance, partly through altering outer membrane proteins or via over-expression of efflux pumps, may enhance excretion of carbapenem and other agents, multi-drug resistance in CRPA is not surprising, which poses even more serious problem to the selection of anti-pseudomonal agents from limited arsenal8.
It is noted that the prevalence of CRPA has been increasing steadily in recent years, which exerts deleterious effects on clinical outcomes, such as length of hospital stay or health-care costs9,10. Complicated by the severity of the underlying disease and the virulence of the pathogens, the detrimental impact of carbapenem resistance on mortality of patients with P. aeruginosa infection, however, remains obscure11,12. Several researches showed higher mortality rates among patients infected with CRPA isolates13, while others didn’t repeated the same tendency14. In order to effectively guide antibiotic policy during empirical therapy and to prioritize future interventions to these types of drug-resistant infections, it is paramount to identify the effect of resistance on mortality. Although Nathwani D, et al. reported increased all-cause mortality among hospitalized patients with resistant and MDR P. aeruginosa infections in a recent meta-analysis, their reseach was limited by heterogeneity of definition of resistance and multi-drug resistance15. Up till now, there is no meta-analysis reported focusing on the clincal impact of CRPA associated infection. Considering this scenario, we performed a meta-analysis to determine the influence of carbapenem resistance on mortality of patients with P. aeruginosa infection.
Results
Flow of included studies
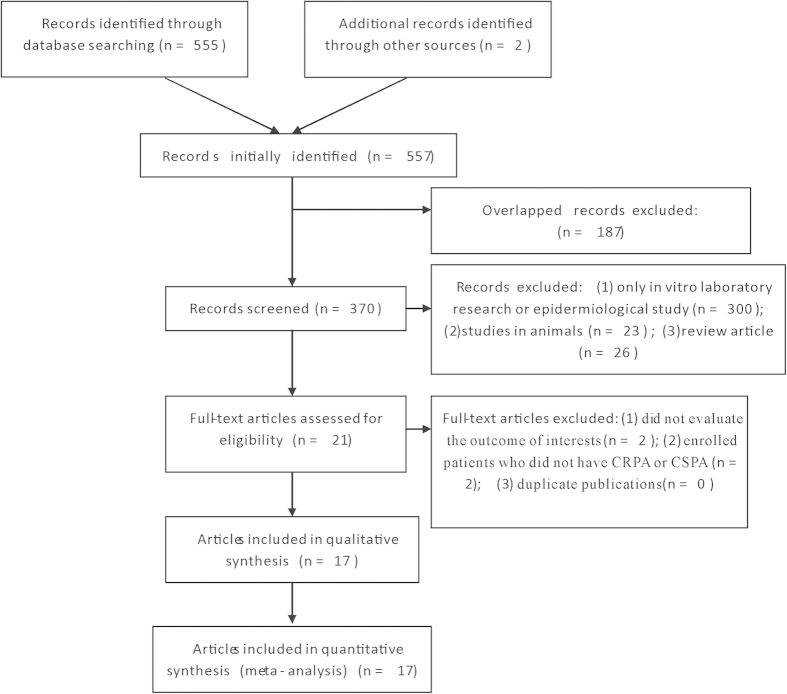
Initially, 557 relevant articles were identified. After excluding those overlapped records between the databases, the remaining 370 abstracts were retrieved for further evaluation. Subsequently, 21 articles with full texts that met the inclusion criteria were assessed. Then four articles were excluded for being not relevant to the influence of carbapenem resistance on mortality of patients with P. aeruginosa infection. Hence, a total of 17 studies were included in this meta-analysis (Fig. 1).
Figure 1. Flow diagram of included studies.
Study characteristics
The basic characteristics of each included study are listed in Table 18,13,14,16,17,18,19,20,21,22,23,24,25,26,27,28,29. These 17 studies were published from 1996 to 2014, including 6660 patients carrying P. aeruginosa, from hospitals distributed in Asia (three in Korea, one in India, one in China), Europe (one in Slovakia, two in Spain), North America (Seven in USA), and South America (two in Brazil). Carbapenem resistance rates ranged from 8.7% to 50.4%, showing the overall trend to be on the rise over the time and obviously different distribution among various regions. Among those researches, three were designed as matched case-control studies, while 14 belonged to cohort studies. 10 studies focused on bacteraemia as the source of infections, while the other seven studies aimed at any infection or colonization with P. aeruginosa.
Table 1. Characteristics of studies included in meta-analysis.
| Author (year) | Country | Type of study | Sample size, n: CRPA vs. CSPA | Sites of infection | Definition of resistance | Type of carbapenem | Carbapenem Resistance | NOS score |
|---|---|---|---|---|---|---|---|---|
| Krcmery (1996)15 | Slovakia | Retrospective cohort | 10 vs. 91 | Bacteremia | NA | Imipenem | 9.9% | 5 |
| Cofsky (2002)16 | USA | Retrospective case-control | 10 vs. 10 | Any infection | NA | Carbapenem | NA | 9 |
| Kang (2005)17 | Korea | Retrospective cohort | 28 vs. 162 | Bacteremia | NCCLS | Imipenem | 14.7% | 7 |
| Marra (2005)18 | USA | Retrospective cohort | 16 vs. 72 | Bacteremia | NA | Imipenem | 18.2% | 7 |
| Gasink (2006)8 | USA | Retrospective cohort | 872 | Any infection or colonization | NCCLS | Imipenem | NA | 7 |
| Lautenbach (2006)19 | USA | Retrospective cohort | 135 vs. 719 | Any infection or colonization | CLSI | Imipenem | 15.8% | 7 |
| Eagye (2009)20 | USA | Retrospective case-control | 58 vs. 125 | Any infection or colonization | NA | Meropenem | NA | 9 |
| Lautenbach (2010)21 | USA | Retrospective cohort | 253 vs. 2289 | Any infection or colonization | CLSI | Imipenem | 10.0% | 9 |
| Suárez (2010)22 | Spain | Retrospective cohort | 33 vs. 88 | Bacteremia | CLSI (2005) | Carbapenem | 27.3% | 9 |
| Joo (2011)13 | Korea | Retrospective cohort | 46 vs. 156 | Bacteremia | NA | Imipenem | 22.8% | 9 |
| Babu (2011)23 | India | Prospective cohort | 24 vs. 86 | Any infection | CLSI (2007) | Imipenem | 21.8% | 5 |
| Pena (2012)24 | Spain | Prospective cohort | 145 vs. 487 | Bacteremia | CLSI | Carbapenem | 22.9% | 9 |
| Tuon (2012)25 | Brazil | Retrospective cohort | 29 vs.48 | Bacteremia | CLSI (2010) | Carbapenem | 37.7% | 9 |
| Hattemer (2013)14 | USA | Retrospective cohort | 13 vs. 137 | Bacteremia | CLSI (2012) | Carbapenem | 8.7% | 9 |
| Lin (2014)26 | China | Retrospective case-control | 82 vs. 82 | Any infection or colonization | CLSI (2011) | Carbapenem | NA | 7 |
| Dantas (2014)27 | Brazil | Retrospective cohort | 55 vs. 65 | Bacteremia | CLSI | Carbapenem | 45.8% | 7 |
| Kim (2014)28 | Korea | Retrospective cohort | 118 vs. 116 | Bacteremia | CLSI (2008) | Carbapenem | 50.4% | 9 |
Abbreviations: CRPA, carbapenem-resistant Pseudomonas aeruginosa; CSPA, carbapenem-susceptible Pseudomonas aeruginosa; NOS, Newcastle-Ottawa Scale; NA, not available; NCCLS, National Committee for Clinical Laboratory Standards; CLSI, Clinical and Laboratory Standard Institute.
The results of all included studies are shown in Supplementary Table S1. Totally, 16 studies reported crude odds ratios (OR) or risk ratio (RR) concerning the influence of carbapenem resistance on mortality of P. aeruginosa infection, while only four studies provided the adjusted OR or RR. As inappropriate antimicrobial treatment and severity of underlying diseases were generally regarded as the leading confounding factors affecting both the risk of antibiotic resistance and mortality, we also attempted to assess these variables. When comparing patients infected with CRPA and carbapenems susceptible P. aeruginosa (CSPA), three studies didn’t detect any difference in severity of underlying diseases, while two studies reported significant differences in inappropriate antimicrobial treatment. Although six studies didn’t find any increased risk for mortality regarding inappropriate antimicrobial treatment in P. aeruginosa infection, another study showed higher risk [OR = 5.54; 95% confidence interval (95%CI) = 2.15, 14.56] after adjusting the potential influencing factors in multivariate analysis. Additionally, seven studies reported elevated risk for more severe underlying diseases in P. aeruginosa infection, despite that statistical significance was not obvious in two studies.
Methodological quality
The methodological quality scores of all included studies range from 5 to 9, with 88.24% of the studies (15 of 17) identified to be high quality (Supplementary Table S2 and S3). Two studies scored only five mainly because they didn’t establish a consistent follow-up time for mortality during observation period. What’s more, they didn’t adjust for potential confounders in analyses.
Statistical results
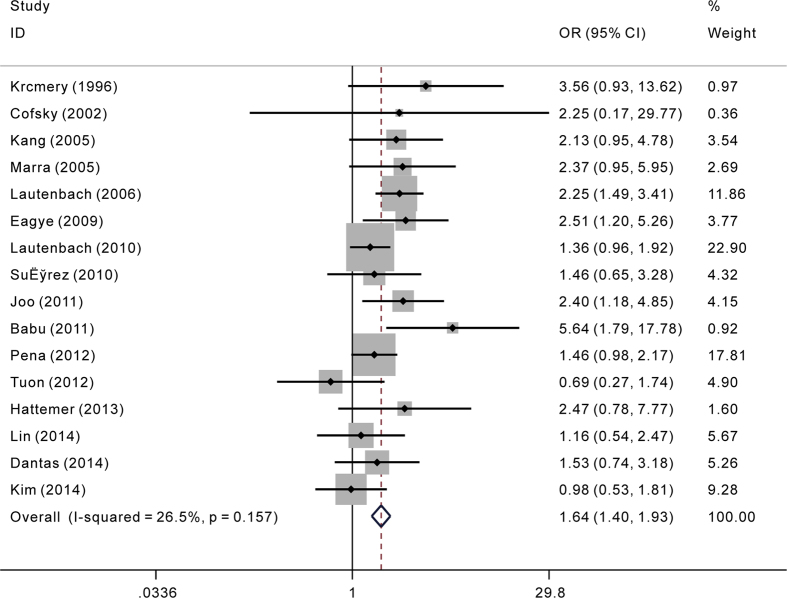
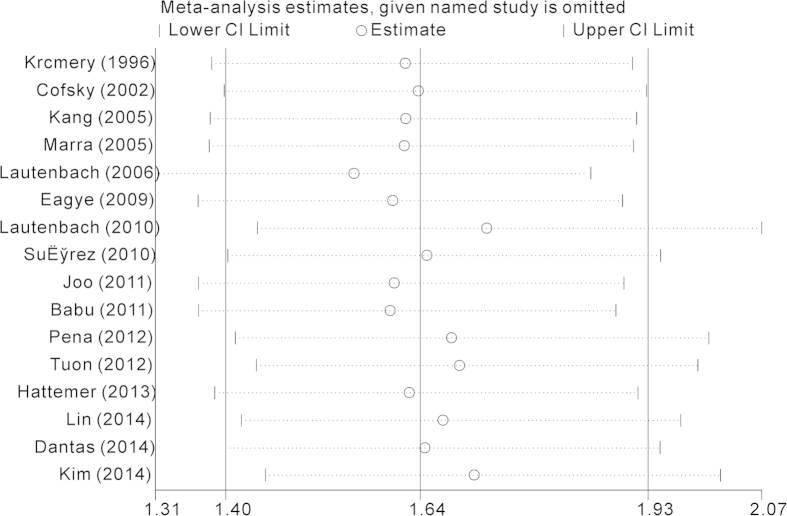
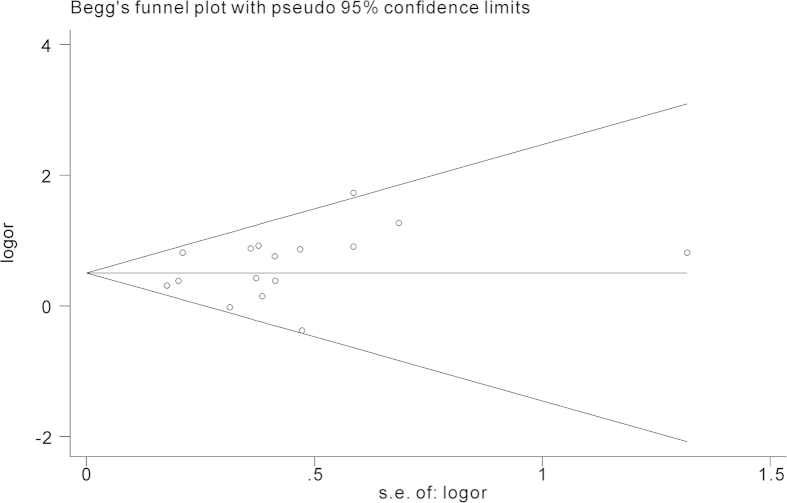
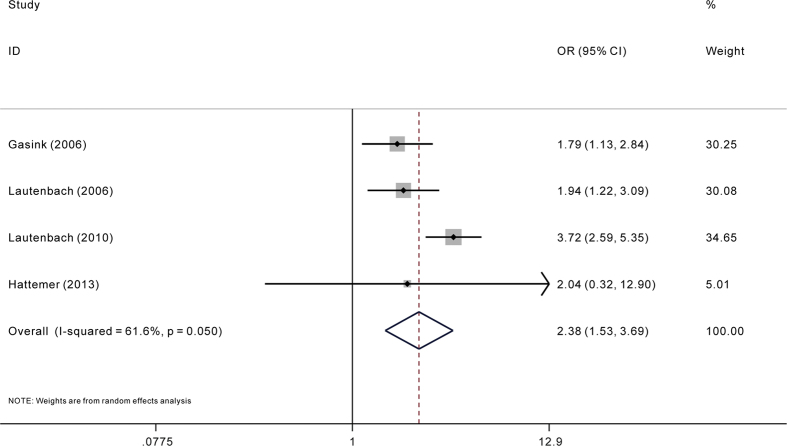
The overall results of the relationship between carbapenem resistance and risk for crude mortality in P. aeruginosa infections are presented in Fig. 2. 16 studies involving 5788 patients reported effect estimates in the univariate analysis. No statistical heterogeneity was observed among studies (p = 0.157, I2 = 26.5%). The pooling analysis indicated that patients with CRPA had significantly higher mortality than patients with CSPA (crude OR = 1.64; 95%CI = 1.40, 1.93). We further performed subgroup analyses by study design, region, sites of infection, and type of carbapenem respectively (Table 2). The elevated risk of mortality in patients with CRPA infection was not lessened when stratified by the above potential confounding factors, except that the estimate effect conducted in South America proved to be insignificant. During sensitivity analysis, after excluding each study one by one, we got almost the same results (Fig. 3). Begg’s (z = 0.95, p = 0.34) and Egger’s test (t = 1.23, p = 0.24) showed no evidence of publication bias concerning crude ORs (Fig. 4).
Figure 2. Crude odds ratio (OR) for the association between carbapenem resistance and mortality of patients with Pseudomonas aeruginosa infection.
Table 2. Subgroup analyses of studies included in meta-analysis.
| Factors | Subgroups | Number of included studies | Crude OR(95%CI) | Statistical model | I2 | Pheterogeneity |
|---|---|---|---|---|---|---|
| Study design | Case-control study | 3 | 1.72(1.03–2.89) | Fixed | 4.1% | 0.352 |
| Cohort study | 13 | 1.63(1.38–1.93) | Fixed | 34.3% | 0.108 | |
| Sources of infection | Bacteraemia | 10 | 1.54(1.23–1.93) | Fixed | 11.6% | 0.336 |
| Any infection or colonization | 6 | 1.92(1.31-2.81) | Random | 47.7% | 0.089 | |
| Region | Europe | 3 | 1.55(1.10–2.18) | Fixed | 0.0% | 0.454 |
| Asia | 5 | 1.82(1.06–3.11) | Random | 57.8% | 0.05 | |
| North America | 6 | 1.82(1.44–2.29) | Fixed | 1.8% | 0.405 | |
| South America | 2 | 1.12(0.64–1.99) | Fixed | 43.5% | 0.183 | |
| Type of carbapenem | Carbapenem | 8 | 1.30(1.02–1.67) | Fixed | 0.0% | 0.698 |
| Meropenem | 1 | 2.51(1.20–5.26) | Fixed | NA | NA | |
| Imipenem | 7 | 1.92(1.54–2.39) | Fixed | 35.0% | 0.161 |
Abbreviations: NA, not available.
Figure 3. Sensitivity analysis of Crude odds ratio (OR) for the association between carbapenem resistance and mortality of patients with Pseudomonas aeruginosa infection.
Figure 4. The funnel plot for selected studies on Crude odds ratio (OR) for the association between carbapenem resistance and mortality of patients with Pseudomonas aeruginosa infection.
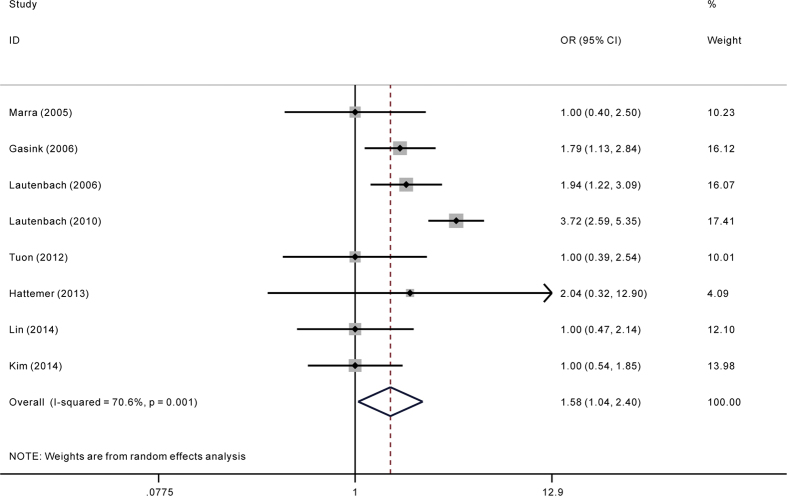
Among all included studies, only five reported adjusted ORs. The pooling analysis of adjusted ORs indicated significant association between carbapenem resistance and mortality risk in P. aeruginosa infection (adjusted OR = 2.38; 95%CI = 1.53, 3.69) (Fig. 5). When including studies with assumed adjusted OR in the sensitivity analysis, the trend of increased risk still didn’t changed (adjusted OR = 1.58; 95%CI = 1.04, 2.40) (Fig. 6). For less than ten studies were included in the pooling analysis of adjusted ORs, assessing publication bias were then not carried out in case of inadequacy of power of test.
Figure 5. Adjusted odds ratio (OR) for the association between carbapenem resistance and mortality of patients with Pseudomonas aeruginosa infection.
Figure 6. Sensitivity analysis of adjusted odds ratio (OR) for the association between carbapenem resistance and mortality of patients with Pseudomonas aeruginosa infection.
Discussion
Treatment of infectious diseases caused by the opportunistic pathogen P. aeruginosa is becoming more challenging with each passing year, especially with the advent of carbapenem resistance. CRPA infection was already proved to be independently associated with increased hospital costs and length of hospital stay, even after controlling for important predictors of these outcomes22. However, it is still in debate regarding the influence of carbapenem resistance on mortality of P. aeruginosa infection. It is considered that many studies had small numbers of patients with P. aeruginosa infection, thereby decreasing the statistical power. In the present meta-analysis, patients with CRPA exhibited significantly higher risk of mortality than those with CSPA, when pooling results under univariate analysis or multivariate analysis. Our findings were similar to a recently conducted meta-analysis which aimed to evaluate the influence of carbapenem resistance on mortality in patients infected with another pathogen, Acinetobacter baumannii and achieved almost the same tendency of increased mortality in patients with carbapenem-resistant pathogen30.
The main reasons for association between carbapenem resistance and elevated mortality are speculated to as follows. Firstly, it is often recognized that infections with CRPA may lead to raised probability of receiving initially inappropriate antimicrobial therapy. The latter were demonstrated to be associated with higher risk for mortality31. Secondly, a number of studies reported elevated mortality risk for patients with P. aeruginosa infection under more severe underlying diseases. Thus, it is suspected that there may be interaction between carbapenems resistance and the severity of co morbid diseases31. However, as is shown in our research, when comparing patients infected with CRPA and CSPA, the majority of included studies didn’t detect any difference in severity of underlying diseases. Beyond that increased virulence in resistant pathogens was also proposed to explain their adverse impact on clinical outcomes31. Yet, Bjorkman et al. reported that CRPA group had a slower death than the CSPA group in the first 48 h after the bacteraemia, which might suggest a lower virulence of CRPA strains32. What’ more, in vitro evidence also showed that resistance genes or mutations can alter the fitness of microorganisms, reducing their capacity to generate deleterious host inflammatory responses, which further make it hard to the justify the hypothesis of increased virulence in resistant pathogens33. On the whole, it is now still difficult to clearly elucidate the specific reason regarding the elevated mortality in CRPA infection.
We obtained consistent results of increased mortality among CRPA after further stratified analyses by study design, sites of infection, and type of carbapenems. However, in the subgroup of patients from South America, no statistically difference was demonstrated in our research. Regarding this point, it is estimated that differences in virulence of P. aeruginosa, antibiotics prescription, medical treatment strategy and environmental factors in different regions may contribute to this negative result26.
Carbapenem resistance in P. aeruginosa is reported to result from the complex interaction of several mechanisms including the production of carbapenemase, the over-expression of efflux systems, and the loss of outer membrane porins34. Among these resistance mechanisms, production of carbapenemase is most important because it occupies a large majority and is associated with higher mortality rate35. Along with the increasingly clearer understanding of drug-resistant mechanisms, investigating the clinical outcome of CRPA infection classified by different resistance mechanisms may further promote future study.
The strength of our meta-analysis include exhaustively search strategy as well as large sample size, which may add more statistical power to detect differences and allow for subgroup analyses. However, some limitations should also be addressed. Firstly, some detailed information, such as the inappropriate antimicrobial treatment, the severity of underlying diseases between CRPA and CSPA, and resistance mechanisms in clinical isolates of P. aeruginosa was not all available, which limited our further assessment by performing stratified analysis. Secondly, some included studies identified subjects based on the database of clinical culture results, thus not all elements were available to apply CDC criteria fully to determine which of these culture results represented true infection or colonization36. Under this situation, blood cultures that yield P. aeruginosa would be more reliable to determine true pathogens or colonizers. However, in our subsequent analyses by sources of infection, no substantive differences were discovered. Thirdly, not all included studies in our meta-analysis reported definitions of definite cut off value used to judge the susceptibility of P. aeruginosa to imipenem and/or meropenem. Accordingly, there may exist potential heterogeneity. Fourthly, none of our included studies are randomised controlled trial, thus some confounded factors cannot be adjusted, which is a compromise. Lastly, we only included studies published in English, which may lead to the miss of some relevant reports published in other languages.
In conclusion, the pooling analysis indicated that patients infected with CRPA had significantly higher mortality than those infected with CSPA, whether under unvariate or multivariate analysis. However, the impact of CRPA on the mortality in an index particular patient was more or less subjective and should be assessed in the background of other risk factors prevailing in a patient.
Methods
Search strategy
We searched the following databases from their inception until December 25, 2014: PUBMED, Web of science, EMBASE, Google Scholar, the Cochrane Library. The search strategies were based on the following terms: ‘aeruginosa’ in combination with ‘mortality’ or ‘death’, and in combination with ‘carbapenem’ or ‘imipenem’ or ‘meropenem’. Furthermore, the reference lists of reports identified by this search strategy were also searched for relevant articles.
Inclusion and exclusion criteria
Studies were considered eligible for inclusion if they were cohort or case-control studies assessing the impact of carbapenem resistance on mortality of patients with P. aeruginosa infection. Carbapenem resistance in this study was defined as resistance to imipenem and/or meropenem (with no limitation on definition of definite cut off value), irrespective of susceptibility to other antibiotics. The other P. aeruginosa isolates were considered as CSPA. In-hospital all-cause mortality was defined as the primary clinical outcome in our analysis. Appropriate antimicrobial therapy was defined as at least one of the antibiotics given was later proved to be active against the P. aeruginosa isolate in vitro.
Researches focusing on animals, referring only to therapeutic effect of carbapenems on P. aeruginosa infections, concerning only the in vitro susceptibility of carbapenem in P. aeruginosa were excluded. In addition, duplicated publications were not included in our statistical analysis. When the same study was included by more than one article, we would select the study with higher sample size. We included published articles only written in English.
Data abstraction and quality assessment
Two independent investigators (Qianqian Liu and Xiaoqing Li) assumed the task of abstraction of data and discrepancies were solved by discussion and referring to the relevant literatures. The following variables were extracted from original publications: title, first author and year of publication, country of origin, region of study population, study design, sample size, baseline characteristics of the study population (age, sex, source of infection, severity of underlying disease), inappropriate antimicrobial treatment rates, crude mortality rates among patients with CRPA and CSPA, adjusted OR or RR and 95%CI of carbapenems resistance on mortality, variables adjusted in multivariate regression model. If relevant data were not displayed, the investigators would attempt to contact the corresponding author to obtain the missing data.
We assessed the methodological quality of cohort or case-control studies included in the meta-analysis according to the Newcastle-Ottawa scale (NOS) score, ranging from 0 to 9 scores37. Studies with NOS score less than three were classified as poor quality and were excluded from this meta-analysis. While studies with NOS score greater than or equal to seven were identified to be of high quality.
Statistical analysis
Between-study heterogeneity was assessed by the χ2 based Q statistics and I2 test. Fixed effect model (Mantel–Haenszel’s method) was adopted if the result of the heterogeneity test was p > 0.10, otherwise the random effect model (DerSimonian and Laird’s method) was used38,39. We transformed RR into OR when necessary using equation provided by Zhang et al.40. OR estimates and 95% CIs on a natural logarithmic scale were used to assess the strength of association between carbapenem resistance and the mortality in patients with P. aeruginosa infection in both crude and adjusted analysis. Stratified analyses were performed by region, type of carbapenem, source of infection, and study design. Sensitivity analyses of crude OR were performed to assess the stability of the results by excluding each study one by one. In addition, several studies in our research detected no significant statistical differences in mortality between patients with CRPA and CSPA infections during univariate analysis, thus they didn’t conducted the risk estimate in subsequent multivariate analysis. As excluding those studies in the pooling analysis of adjusted OR may introduce potential bias, we included them in the sensitivity analysis, assuming adjusted OR to be 1 and calculated the 95% CIs from the univariate analysis30. Begg’s and Egger’s tests were used to test the possible publication bias when more than ten studies were pooled41. All statistical analyses were done with STATA version 12.0 (StataCorp, College Station, Texas), using metan programme and two-sided p values.
Additional Information
How to cite this article: Liu, Q. et al. Influence of carbapenem resistance on mortality of patients with Pseudomonas aeruginosa infection: a meta-analysis. Sci. Rep. 5, 11715; doi: 10.1038/srep11715 (2015).
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No. 81072432, Grant No. 81170042 and Grant No. 81370121) and from science & technology department of Sichuan Province (2012SZ0126).
Footnotes
Author Contributions Q.Q.L. and X.Q.L. conceived and designed the study; Q.Q.L., X.Q.L., W.Z.L., X.M.D., J.Q.H., Y.L.F. and C.M.T analyzed the data; Q.Q.L. wrote the manuscript and all authors reviewed the manuscript.
References
- Blanc D. S., Francioli P. & Zanetti G. Molecular Epidemiology of Pseudomonas aeruginosa in the Intensive Care Units - A Review. Open Microbiol J 1, 8–11 10.2174/1874285800701010008 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti M., Righi E. & Viscoli C. Pseudomonas aeruginosa serious infections: mono or combination antimicrobial therapy? Curr Med Chem 15, 517–522 (2008). [DOI] [PubMed] [Google Scholar]
- Maschmeyer G. & Braveny I. Review of the incidence and prognosis of Pseudomonas aeruginosa infections in cancer patients in the 1990s. Eur J Clin Microbiol Infect Dis 19, 915–925 (2000). [DOI] [PubMed] [Google Scholar]
- Todeschini G. et al. Improved prognosis of Pseudomonas aeruginosa bacteremia in 127 consecutive neutropenic patients with hematologic malignancies. Int J Infect Dis 3, 99–104 (1998). [DOI] [PubMed] [Google Scholar]
- Gaynes R., Edwards J. R. & National Nosocomial Infections Surveillance S. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis 41, 848–854 10.1086/432803 (2005). [DOI] [PubMed] [Google Scholar]
- Morrow B. J. et al. Activities of carbapenem and comparator agents against contemporary US Pseudomonas aeruginosa isolates from the CAPITAL surveillance program. Diagn Microbiol Infect Dis 75, 412–416 10.1016/j.diagmicrobio.2012.12.012 (2013). [DOI] [PubMed] [Google Scholar]
- Baumgart A. M., Molinari M. A. & Silveira A. C. Prevalence of carbapenem resistant Pseudomonas aeruginosa and Acinetobacter baumannii in high complexity hospital. Braz J Infect Dis 14, 433–436 (2010). [DOI] [PubMed] [Google Scholar]
- Gasink L. B. et al. Fluoroquinolone-resistant Pseudomonas aeruginosa: assessment of risk factors and clinical impact. Am J Med 119, 526 e519–525 10.1016/j.amjmed.2005.11.029 (2006). [DOI] [PubMed] [Google Scholar]
- King A., Shannon K. & Phillips I. Resistance to imipenem in Pseudomonas aeruginosa. J Antimicrob Chemother 36, 1037–1041 (1995). [DOI] [PubMed] [Google Scholar]
- Acar J. F. Consequences of bacterial resistance to antibiotics in medical practice. Clin Infect Dis 24 Suppl 1, S17–18 (1997). [DOI] [PubMed] [Google Scholar]
- Pena C. et al. Prospective multicenter study of the impact of carbapenem resistance on mortality in Pseudomonas aeruginosa bloodstream infections. Antimicrob Agents Chemother 56, 1265–1272 10.1128/aac.05991-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbarth S., Nobre V. & Pittet D. Does antibiotic selection impact patient outcome? Clin Infect Dis 44, 87–93 10.1086/510075 (2007). [DOI] [PubMed] [Google Scholar]
- Joo E. J. et al. Risk factors for mortality in patients with Pseudomonas aeruginosa bacteremia: clinical impact of antimicrobial resistance on outcome. Microb Drug Resist 17, 305–312 10.1089/mdr.2010.0170 (2011). [DOI] [PubMed] [Google Scholar]
- Hattemer A. et al. Bacterial and clinical characteristics of health care- and community-acquired bloodstream infections due to Pseudomonas aeruginosa. Antimicrob Agents Chemother 57, 3969–3975 10.1128/AAC.02467-12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani D., Raman G., Sulham K., Gavaghan M. & Menon V. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control 3, 32 10.1186/2047-2994-3-32 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krcmery V. Jr. et al. Imipenen-resistant Ps. aeruginosa bacteraemia in cancer patients: risk factors, clinical features and outcome. Int J Clin Pharmacol Res 16, 43–49 (1996). [PubMed] [Google Scholar]
- Cofsky. The cost of antibiotic resistance: effect of resistance among Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudmonas aeruginosa on length of hospital stay. Infect Control Hosp Epidemiol 23, 106–108 10.1086/502018 (2002). [DOI] [PubMed] [Google Scholar]
- Kang C. I. et al. Risk factors for antimicrobial resistance and influence of resistance on mortality in patients with bloodstream infection caused by Pseudomonas aeruginosa. Microb Drug Resist 11, 68–74 10.1089/mdr.2005.11.68 (2005). [DOI] [PubMed] [Google Scholar]
- Marra A. R., Bearman G. M., Wenzel R. P. & Edmond M. B. Comparison of the systemic inflammatory response syndrome between monomicrobial and polymicrobial Pseudomonas aeruginosa nosocomial bloodstream infections. BMC Infect Dis 5, 94 10.1186/1471-2334-5-94 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenbach E. et al. Imipenem resistance among pseudomonas aeruginosa isolates: risk factors for infection and impact of resistance on clinical and economic outcomes. Infect Control Hosp Epidemiol 27, 893–900 10.1086/507274 (2006). [DOI] [PubMed] [Google Scholar]
- Eagye K. J., Kuti J. L. & Nicolau D. P. Risk factors and outcomes associated with isolation of meropenem high-level-resistant Pseudomonas aeruginosa. Infect Control Hosp Epidemiol 30, 746–752 10.1086/603527 (2009). [DOI] [PubMed] [Google Scholar]
- Lautenbach E. et al. Imipenem resistance in Pseudomonas aeruginosa: emergence, epidemiology, and impact on clinical and economic outcomes. Infect Control Hosp Epidemiol 31, 47–53 10.1086/649021 (2010). [DOI] [PubMed] [Google Scholar]
- Suarez C. et al. Influence of carbapenem resistance on mortality and the dynamics of mortality in Pseudomonas aeruginosa bloodstream infection. Int J Infect Dis 14 Suppl 3, e73–78 10.1016/j.ijid.2009.11.019 (2010). [DOI] [PubMed] [Google Scholar]
- Babu K. V. Y. et al. Study of Imipenem Resistant Pseudomonas aeruginosa and Associated Predisposing Risk Factors in a Rural Tertiary Care Hospital. J Pure Appl Microbio 5, 793–800 (2011). [Google Scholar]
- Pena C. et al. Prospective multicenter study of the impact of carbapenem resistance on mortality in Pseudomonas aeruginosa bloodstream infections. Antimicrob Agents Chemother 56, 1265–1272 10.1128/AAC.05991-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuon F. F., Gortz L. W. & Rocha J. L. Risk factors for pan-resistant Pseudomonas aeruginosa bacteremia and the adequacy of antibiotic therapy. Braz J Infect Dis 16, 351–356 10.1016/j.bjid.2012.06.009 (2012). [DOI] [PubMed] [Google Scholar]
- Lin K. Y., Lauderdale T. L., Wang J. T. & Chang S. C. Carbapenem-resistant Pseudomonas aeruginosa in Taiwan: Prevalence, risk factors, and impact on outcome of infections. J Microbiol Immunol Infect 10.1016/j.jmii.2014.01.005 (2014). [DOI] [PubMed] [Google Scholar]
- Dantas R. C., Ferreira M. L., Gontijo-Filho P. P. & Ribas R. M. Pseudomonas aeruginosa bacteraemia: independent risk factors for mortality and impact of resistance on outcome. J Med Microbiol 63, 1679–1687 10.1099/jmm.0.073262-0 (2014). [DOI] [PubMed] [Google Scholar]
- Kim Y. J. et al. Risk factors for mortality in patients with Pseudomonas aeruginosa bacteremia; retrospective study of impact of combination antimicrobial therapy. BMC Infect Dis 14, 161 10.1186/1471-2334-14-161 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos E. V. et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect 20, 416–423 10.1111/1469-0691.12363 (2014). [DOI] [PubMed] [Google Scholar]
- Kang C. I. et al. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin Infect Dis 37, 745–751 10.1086/377200 (2003). [DOI] [PubMed] [Google Scholar]
- Bjorkman J. & Andersson D. I. The cost of antibiotic resistance from a bacterial perspective. Drug Resist Updat 3, 237–245 10.1054/drup.2000.0147 (2000). [DOI] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis E. J. et al. Stimulation of innate immunity by susceptible and multidrug-resistant Pseudomonas aeruginosa: an in vitro and in vivo study. Clin Exp Immunol 135, 240–246 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Amin N. et al. Carbapenem resistance mechanisms in Pseudomonas aeruginosa: alterations of porin OprD and efflux proteins do not fully explain resistance patterns observed in clinical isolates. APMIS 113, 187–196 10.1111/j.1600-0463.2005.apm1130306.x (2005). [DOI] [PubMed] [Google Scholar]
- Wirth F. W. et al. Metallo-beta-lactamase-producing Pseudomonas aeruginosa in two hospitals from southern Brazil. Braz J Infect Dis 13, 170–172 (2009). [DOI] [PubMed] [Google Scholar]
- Garner J. S., Jarvis W. R., Emori T. G., Horan T. C. & Hughes J. M. CDC definitions for nosocomial infections, 1988. Am J Infect Control 16, 128–140 (1988). [DOI] [PubMed] [Google Scholar]
- Wells G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2014) Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (Accessed: 15th February 2015)
- Mantel N. & Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22, 719–748 (1959). [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188 (1986). [DOI] [PubMed] [Google Scholar]
- Zhang J. & Yu K. F. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 280, 1690–1691 (1998). [DOI] [PubMed] [Google Scholar]
- Egger M., Smith G. D. & Phillips A. N. Meta-analysis: principles and procedures. BMJ 315, 1533–1537 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.