Abstract
Background
The aim of this study was to evaluate the role of interleukin-17 (IL-17) level in synovia and its relationship with the severity of knee osteoarthritis (OA).
Material/Methods
We enrolled 226 OA patients and 106 controls in this study. The symptomatic/radiation severity of OA was assessed by the Western Ontario McMaster University Osteoarthritis Index (WOMAC) pain score/Kellgren-Lawrence (KL) grading system. Serum IL-17 levels were measured by enzyme-linked immunosorbent assay (ELISA).
Results
Synovia IL-17 levels were significantly higher in OA patients compared with controls (P<0.01), and were negatively correlated with OA severity. IL-17 level gradually decreased among different phases but lacked statistical significance.
Conclusions
IL-17 might play a crucial role in the pathogenesis of OA and is closely related to pain. Blocking the IL-17 signaling pathway may delay pain related to OA.
MeSH Keywords: Chondromatosis, Synovial; Osteoarthritis, Knee; Receptors, Interleukin-17
Background
Osteoarthritis is one of the most common diseases in middle-aged and older people [1]. Its specific pathogenic factor is still not clear, which may be associated with trauma, strain, and immune response. In recent years, increasing attention has been focussed on biological factors that impact cytokines in joint synovial fluid and articular cartilage. Cytokines are an important focus in OA treatment and prevention research [2]. IL-17, specifically secreted by TH17 cells, has received much attention because it has properties similar to those of cytokines [3], and because of its regulatory function in IFN-α-induced IL-10 secretion [4] in activating T cell proliferation. IL-17 has received extensive research attention in the pathogenesis and treatment of multiple sclerosis [5–7], systemic lupus erythematosus [8,9], inflammatory bowel disease [10,11], and psoriasis [12,13]. However, little is known about its relationship with osteoarthritis. In the present study, we investigated the relationship between IL-17 level and the severity of pain and radiological manifestations in knee OA patients.
Material and Methods
Experiment time
Time and place: The experiment was performed between January 2012 and January 2014 in our hospital.
Subjects
Subject selection
The protocol of this study was approved by the ethics committee of Renmin Hospital of Wuhan University. Informed consent was obtained from all subjects. We enrolled 226 knee OA unrelated patients (group A). Clinical informations such as age, course of disease, joint X-ray stages, and joint function classification were selected. The joint X-ray severity of osteoarthritis was evaluated according to the Kellgren and Lawrence (KL) classification standard, with selected patients divided into 4 levels: Level 1, suspicious narrowed joint gap; Level 2, cleared osteophytes and narrowed joint space; Level 3, moderate multiple osteophyte, hardening joint activity space, and malformed bone contour; and Level 4, large osteophyte, severe cirrhosis, and malformed narrowed bone contour. As the control group (group B) we enrolled 106 healthy subjects age- and sex-matched to the OA patients There were no significant differences in age, sex, or body mass index (BMI) between the OA patients and healthy controls (P>0.05) (Table 1).
Table 1.
General information between OA patients and healthy control.
| Group | Healthy control (106) | OA group (226) | |||
|---|---|---|---|---|---|
| K1 (57) | K2 (66) | K3 (60) | K4 (53) | ||
| Age | 63.46±11.07 | 64.11±9.85 | 64.46±10.28 | 63.76±7.67 | 63.12±9.32 |
| Gender ratio | 61 (57.55%) | 32 (56.14%) | 42 (63.64%) | 31 (51.66%) | 29 (54.72%) |
| BMI | 22.43±2.28 | 23.10± 2.04 | 23.31±2.24 | 22.94±1.78 | 22.96±1.99 |
TOA diagnostic criteria
OA diagnosis is based on the updated diagnosis and treatment guideline of the American Academy of Orthopedic Surgeons [14] as follows:
(1) average level of knee pain during the last month; (2) X-ray examination showed osteophyte formation; (3) synovial fluid inspection in accordance with osteoarthritis; (4) patients age 40 or older; (5) morning stiffness <30 min; and (6) bone fricative and bone noise when joint activity moved. Knee joint OA can be diagnosed when the comprehensive clinical, laboratory, and X-ray result conform to item 1 + 2, 1 + 3 + 5 + 6, or 1 + 4 + 5 + 6.
Exclusion criteria
We excluded patients with suppurative, tuberculous, and rheumatoid arthritis; who used non-steroidal anti-inflammatory analgesic drugs in the last month; and who had severe cardiovascular disease, cerebrovascular disease, liver or renal insufficiency, immune system disease, malignant tumor, or other diseases.
Pain assessment
The pain severity of OA was assessed by the Western Ontario McMaster University Osteoarthritis Index (WOMAC) pain score, which involves functional activity and consists of 5 questions [15] regarding: pain during horizontal motion, walking up and down stairs, sitting, standing, lying down, and pain at night. A scale of 0–4 is used for scoring answers, where 0 means no pain and higher scores represent more severe pain. The total range of WOMAC pain score was from 0 to 20. The scoring system is widely used and has good reliability and validity.
X-ray classification
We used the Kellgren and Lawrence (KL) classification for grading knee joint osteoarthritis X-ray film as follows: Level 1, suspicious narrowed joint gap; Level 2, cleared osteophytes and narrowed joint space; Level 3, moderate multiple osteophyte, hardening joint activity space, and malformed bone contour; and Level 4, large osteophyte, severe cirrhosis, and malformed narrowed bone contour. All knee joint peripheral punctures were performed after consent was provided by patients or their families, and the study was approved by our medical ethics committee.
Methods
Enzyme-linked immunosorbent assay (ELISA) was used for IL-17 content detection. The homogenate of the specimen was centrifuged for 20 min (2000–3000 r/min) and the supernatant was collected. The absorbance value at 450 nm wavelength was measured to make the standard curve, and then the IL-17 content in the specimen was calculated. The experiments were performed in triplicate.
Statistical analysis
All statistical analyses were performed using SPSS17.0 software (Chicago, IL). The Kolmogorov-Smirnov test was used to test for normality. Measurement data that had normal distribution are presented as χ̄±s, and data with non-normal distribution are presented as median (quartile). Enumeration data are presented as rate. The unpaired t-test, Mann-Whitney U test, and rank-sum test were used to compare the 2 groups. ANOVA/Kruskal-Wallis or rank-sum test were used for comparison among multiple subgroups. Spearman correlation analysis was used to assess the relationship between IL-17 level and WOMAC score or KL grading, inspection level α=0.05.
Results
Serum IL-17 level
Compared with healthy controls, IL-17 level in OA patients increased significantly. Following the upgrading of KL, IL-17 decreased slightly but this difference was not statistically significant (Table 2).
Table 2.
IL-17 level and WOMAC pain scoring.
| Group | Health control (106) | OA group (226) | |||
|---|---|---|---|---|---|
| K1 (57) | K2 (66) | K3 (60) | K4 (53) | ||
| IL-17 level | 2.17 (1.53–3.37) | 6.04 (4.82–8.96) | 6.35 (5.09–10.09) | 6.00 (4.35–8.47) | 5.85 (4.10–7.38) |
| WOMAC-pain score | 9 (6–12) | 10 (9–14) | 11 (6–18) | 14 (9–18) | |
Correlation analysis between serum IL-17 level with WOMAC pain score and KL grading
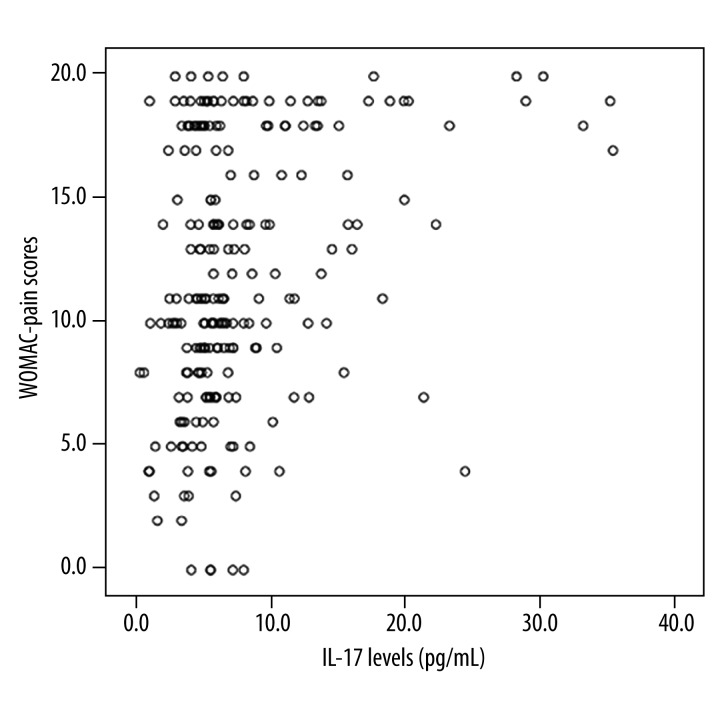
IL-17 level in synovial fluid was positively correlated with WOMAC pain score in osteoarthritis patients (r=0.279, P<0.05). However, IL-17 level in synovial fluid exhibited no correlation with KL grade (r=0.116, P=0.116). In addition, OA WOMAC pain score had no correlation with KL grade (r=0.115, P=0.115) (Figure 1).
Figure 1.
IL-17 level in synovial fluid and WOMAC pain score.
Discussion
IL-17 plays an important role in autoimmune disease. Accurate and effective regulation of IL-17 signaling can prevent inflammation. The IL-17 family includes IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F. IL-17 and Th17 cells play important roles in human autoimmune disease. Previous research showed that inflammatory reaction in T cells, epithelial cells, endothelial cells, and fibroblasts may influence IL-17 expression. IL-17 can regulate host defense and chronic inflammation, which cause tissue damage and autoimmune effects [16,17]. A large number of studies have demonstrated that IL-17 is overexpressed in the synovium of rheumatoid arthritis (RA) patients. IL-17 can stimulate bone resorption and collagen destruction in vitro [18]. Neutralization of IL-17 or its receptor can alleviate the symptoms of RA in a rat arthritis model. IL-17 defects can protect the host from damage by collagen-induced arthritis in mice, and IL-17 gene therapy may cause further aggravation [19–21]. The soluble and specific inhibitor can block its effect, reducing the release of IL-6 and collagen degradation markers in the synovial membrane and bone tissue [22,23]. Thus, IL-17 in RA can cause both inflammation and bone destruction. IL-17 can promote cartilage, synovial cells, macrophages, and osteocytes to produce the inflammatory cytokines such as TNF-α, IL-1β, and IL-6. IL-17 cells can promote receptor activator of nuclear factor-κB ligand (RANK) differentiation in osteoblasts, and stimulate the degradation of matrix metalloproteinases (MMPS) and extracellular matrix, and the activity of bone resorption. In addition, IL-17 can also stimulate many chemokines, such as IL-8/CXCL8, CXCL1 (KC/GRO-α), CXCL2 (MIP2α/GRO-β), CCL20 (MIP-3α), CCL2 (MCP1), and CCL7 MCP3. These chemokines can cause aggregation of neutrophils, macrophages, and lymphocytes in the synovial membrane, which creates a cascade amplification effect, resulting in more severe joint damage [24].
The antibody fragment of IL-17 factor has been widely used for RA examination and treatment; however, the meaning of its expression in OA patients and its relationship to the severity of arthritis is still unknown. Our study explored the relationship of IL-17 level in joint fluid level with pain score and knee arthritis radiographic severity. Our results show that IL-17 level is correlated with knee osteoarthritis pain degree, but it has no obvious correlation with the severity of radiation. It may be a new potential biochemical marker to reflect the severity of osteoarthritis pain. This study has certain limitations. Firstly, the sample size in this research group is relatively small, and further research is needed with larger size and wider range of subjects for prospective longitudinal study. Secondly, we measured IL-17 level only in the joint fluid of patients with osteoarthritis in the testing; whether its expression in blood has research value still need further study. Thirdly, OA knee pain includes many types of pain, such as pain at night, pain while resting, and activities-related pain. Our study only focused on the evaluation of pain on a scale of 0–4 in a small number of patients. Lastly, only the IL-17 expression in serum was examined in our study. Other inflammatory biomarkers (e.g., C-reactive protein, and IL-1) may provide more valuable information on the role of the IL-17 signaling pathway in OA-related pain.
Conclusions
Our results show that IL-17 level is correlated with the severity of osteoarthritis knee pain and that blocking the IL-17 signaling pathway can delay osteoarthritis-related pain. These results may provide new ideas and methods for the prevention and treatment of knee pain.
Footnotes
Source of support: Departmental sources
References
- 1.Ruiz-Romero C, Blanco FJ. Proteomics role in the search for improved diagnosis, prognosis and treatment of osteoarthritis. Osteoarthritis Cartilage. 2010;18(4):500–9. doi: 10.1016/j.joca.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 3.van der Waart AB, van der Velden WJ, Blijlevens NM, Dolstra H. Targeting the IL17 pathway for the prevention of graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(6):752–59. doi: 10.1016/j.bbmt.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Touzot M, Cacoub P, Bodaghi B, et al. IFN-alpha induces IL-10 production and tilt the balance between Th1 and Th17 in Behcet disease. Autoimmun Rev. 2014 doi: 10.1016/j.autrev.2014.12.009. pii: S1568-9972(14)00309-7. [DOI] [PubMed] [Google Scholar]
- 5.Sexton M, Cudaback E, Abdullah RA, et al. Cannabis use by individuals with multiple sclerosis: effects on specific immune parameters. Inflammopharmacology. 2014;22(5):295–303. doi: 10.1007/s10787-014-0214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elain G, Jeanneau K, Rutkowska A, et al. The selective anti-IL17A monoclonal antibody secukinumab (AIN457) attenuates IL17A-induced levels of IL6 in human astrocytes. Glia. 2014;62(5):725–35. doi: 10.1002/glia.22637. [DOI] [PubMed] [Google Scholar]
- 7.Kallaur AP, Oliveira SR, Colado Simão AN, et al. Cytokine profile in relapsingremitting multiple sclerosis patients and the association between progression and activity of the disease. Mol Med Rep. 2013;7(3):1010–20. doi: 10.3892/mmr.2013.1256. [DOI] [PubMed] [Google Scholar]
- 8.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129(3):311–21. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanasescu C, Balanescu E, Balanescu P, et al. IL-17 in cutaneous lupus erythematosus. Eur J Intern Med. 2010;21(3):202–7. doi: 10.1016/j.ejim.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Katz LH, Kopylov U, Fudim E, et al. Expression of IL-2, IL-17 and TNF-alpha in patients with Crohn’s disease treated with anti-TNF antibodies. Clin Res Hepatol Gastroenterol. 2014;38(4):491–98. doi: 10.1016/j.clinre.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Yu P, Shen F, Zhang X, et al. Association of single nucleotide polymorphisms of IL23R and IL17 with ulcerative colitis risk in a Chinese Han population. PLoS One. 2012;7(9):e44380. doi: 10.1371/journal.pone.0044380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiricozzi A. Pathogenic role of IL-17 in psoriasis and psoriatic arthritis. Actas Dermosifiliogr. 2014;105(Suppl 1):9–20. doi: 10.1016/S0001-7310(14)70014-6. [DOI] [PubMed] [Google Scholar]
- 13.Raychaudhuri SP. Role of IL-17 in psoriasis and psoriatic arthritis. Clin Rev Allergy Immunol. 2013;44(2):183–93. doi: 10.1007/s12016-012-8307-1. [DOI] [PubMed] [Google Scholar]
- 14.Sasek C. An update on primary care management of knee osteoarthritis. JAAPA. 2015;28(1):37–43. doi: 10.1097/01.JAA.0000458853.38655.02. [DOI] [PubMed] [Google Scholar]
- 15.Singh S, Kumar D, Sharma NR. Role of hyaluronic Acid in early diagnosis of knee osteoarthritis. J Clin Diagn Res. 2014;8(12):Lc04–7. doi: 10.7860/JCDR/2014/11732.5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinert-Hartwall L, Honkanen J, Salo HM, et al. Th1/Th17 plasticity is a marker of advanced beta cell autoimmunity and impaired glucose tolerance in humans. J Immunol. 2015;194(1):68–75. doi: 10.4049/jimmunol.1401653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanabe S, Yamashita T. Repulsive guidance molecule-a is involved in Th17-cell-induced neurodegeneration in autoimmune encephalomyelitis. Cell Rep. 2014;9(4):1459–70. doi: 10.1016/j.celrep.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 18.Roeleveld DM, Koenders MI. The role of the Th17 cytokines IL-17 and IL-22 in Rheumatoid Arthritis pathogenesis and developments in cytokine immunotherapy. Cytokine. 2014 doi: 10.1016/j.cyto.2014.10.006. pii: S1043-4666(14)00550-X. [DOI] [PubMed] [Google Scholar]
- 19.Zheng Y, Sun L, Jiang T, et al. TNFalpha promotes Th17 cell differentiation through IL-6 and IL-1beta produced by monocytes in rheumatoid arthritis. J Immunol Res. 2014;2014:385352. doi: 10.1155/2014/385352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Baarsen LG, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16(4):426. doi: 10.1186/s13075-014-0426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu F, Li Y, Zheng L, et al. Toll-like receptors expressed by synovial fibroblasts perpetuate Th1 and th17 cell responses in rheumatoid arthritis. PLoS One. 2014;9(6):e100266. doi: 10.1371/journal.pone.0100266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roşu A, Mărgăritescu C, Stepan A, et al. IL-17 patterns in synovium, serum and synovial fluid from treatment-naive, early rheumatoid arthritis patients. Rom J Morphol Embryol. 2012;53(1):73–80. [PubMed] [Google Scholar]
- 23.Sarkar S, Justa S, Brucks M, et al. Interleukin (IL)-17A, F and AF in inflammation: a study in collagen-induced arthritis and rheumatoid arthritis. Clin Exp Immunol. 2014;177(3):652–61. doi: 10.1111/cei.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]