Abstract
Background
Mycophenolate mofetil (MMF) is an immunosuppressive agent commonly used after organ transplantation. Gastrointestinal side effects occur in approximately 45% of patients. The spectrum of histologic features associated with MMF colitis has been well described, but data on the endoscopic features is lacking. The aim of the study was to describe the endoscopic features of MMF colitis in solid organ transplant recipients (SOTRs) as well as the frequency of histologic features and identify associated risk factors.
Methods
A retrospective review of all SOTRs taking MMF and who underwent colonoscopy between 2000 and 2010 was performed. 36 cases of MMF colitis were identified and 361 patients served as controls. Descriptive statistics and data analysis looking for associated risk factors were performed.
Results
Among SOTRs taking MMF who underwent colonoscopy, MMF colitis was diagnosed in 9%. Endoscopic findings ranged from erythema (33%) to erosions/ulcers (19%). 47% of patients had a normal colonoscopy and everyone had rectal sparing. Histological findings included acute colitis-like findings (50%), inflammatory bowel disease-like characteristics (36%), ischemia-like findings (5.6%), and graft-versus-host disease-like features (8.3%). Diarrhea occurred in 83%. Kidney transplantation was associated with a higher risk of MMF colitis (OR 5.8 [2.86-11.86], P<0.0001) whereas liver transplantation was associated with a lower risk (OR 0.06 [0.03-0.16], P<0.0001).
Conclusion
MMF colitis is fairly prevalent in SOTRs taking MMF who undergo colonoscopy. Diarrhea is the most common reason for colonoscopy referral (83%) and up to 47% of patients have normal colonoscopy, suggesting the need for routine biopsies to help confirm the diagnosis.
Keywords: Colitis, colonoscopy, mycophenolate mofetil, mycophenolic acid, transplant recipients
Introduction
Mycophenolate mofetil (MMF) is an immunosuppressive drug widely used in solid organ and bone marrow transplantation as well as in autoimmune disorders like systemic lupus erythematosus. It acts by inhibiting the inosine monophosphate dehydrogenase (IMPDH) in the de novo pathway of guanine (purine) synthesis [1]. After oral administration, MMF is extensively absorbed and hydrolyzed to its active metabolite, mycophenolic acid (MPA). Oral bioavailability after administration ranges from 80% to 94%. It is metabolized in the liver, gastrointestinal (GI) tract, and kidney by UDP-glucuronyltransferases into mycophenolic acid glucuronide (MPAG). This metabolite is present in a higher concentration (20 to 100 fold) in plasma than MPA but is inactive.
Most cells are able to use both salvage and de novo pathways for purine synthesis; but lymphocytes are more dependent on the de novo pathway. MPA is a potent inhibitor of the type II isoform of IMPDH, expressed on activated lymphocytes [1]. Thus, MPA exerts its cytostatic effects by depleting guanine and deoxyguanosine nucleotides. Though this is the principal mechanism of MPA immunosuppression, other mechanisms may also contribute. It induces apoptosis of activated T-lymphocytes, which may eliminate clones of cells responding to antigenic stimuli. It suppresses glycosylation and expression of adhesion molecules, thus decreases recruitment of lymphocytes and monocytes to sites of inflammation. It also depletes tetrahydrobiopterin, a cofactor for the production of nitric oxide synthase. Hence, tissue damage by nitric oxide is averted [1,2].
MMF has multiple side effects. Those affecting the GI tract mostly occur during the first 6 months after the onset of treatment and include nausea, vomiting, abdominal pain, and diarrhea. The exact mechanism of GI toxicity is incompletely understood. Enterocytes are partially dependent on the de novo pathway of purine synthesis for proliferation, thus becoming unwanted targets of MPA. The acyl glucuronide metabolite of MPA (AcMPAG) may also have a role in inflammation and has been found in mononuclear cell cultures to stimulate the release of interleukin-6 and tumor necrosis factor-a via an increase in production of mRNA [3]. These acyl glucuronides have also been shown to be reactive electrophilic molecules which can bind to plasma proteins, lipids, and nucleic acids. These molecules lead to toxicity indirectly through formation of neoantigens and subsequent activation of the immune system, causing either a hypersensitivity reaction or an autoimmune response [4]. Anti-bacterial effect of MPA can also cause changes in the normal microbial flora in the GI tract, which promotes anaerobic growth and results in tissue damage [2].
MMF has the potential to affect both the upper and lower GI tract [5]. Colonoscopic and histological features in post-transplantation diarrhea patients who are on MMF have been studied previously. Papadimitriou et al studied 20 colonic biopsies from renal transplant patients with MMF-related diarrhea [6]. These biopsies were compared with biopsies from patients with graft-versus-host disease (GVHD), inflammatory bowel disease (IBD), and infectious colitis. MMF-induced colonic mucosal changes were found to be similar to GVHD, but different from other groups. They also found correlation between histological activity and endoscopic severity of colitis. Dalle et al [7] studied biopsies from 24 MMF-treated patients with diarrhea. 19 patients showed features resembling Crohn’s disease with crypt angularity and dilatation of crypts of varying diameter infiltrated with macrophages and neutrophils. They could not obtain any direct evidence to suggest that the diarrhea was related to the histological changes. However, the colitis in MMF-treated transplant patients is a distinct entity, which should not be overlooked by pathologists as treatment may change if misdiagnosed.
The aim of this study was to describe the macroscopic and microscopic features of MMF colitis among patients undergoing colonoscopy and to estimate the relative frequency of these findings. We also aim to identify an association between MMF colitis and multiple variables including age, gender, and type of transplant.
Patients and methods
Patients and setting
We performed a case-control study approved by the Mount Sinai Hospital Institutional Review Board. We included all solid organ transplant recipients who were taking MMF and who underwent colonoscopy for any indication between 2000 and 2010 at Mount Sinai Hospital, New York. The case group included all patients with a diagnosis of MMF colitis defined as patients taking MMF who exhibited histological features of MMF-related colitis as per previously published criteria [8]. Specifically, compatible histological features included those mimicking acute colitis, IBD, GVHD, and ischemia. Additionally, other potential etiologies, including intercurrent infections and medications were ruled out by review of the medical record where available. The control group included patients taking MMF who had histological features not consistent with MMF colitis and those were an alternative etiology for compatible histological features was found. All patients in the control group had colonic biopsies and we excluded all patients who did not undergo colonic biopsies.
Predictive variables
Recorded variables included demographics, type of transplant (liver, kidney, combined liver and kidney, and other [heart, pancreas]), indication for colonoscopy (screening for colorectal cancer/postpolypectomy surveillance, diarrhea, GI bleeding, and other [abdominal pain, weight loss, abnormal imaging studies]), time since transplantation, the dose of MMF the patient was receiving, histological and endoscopic features of each patient (normal colonoscopy, erythema, erosions/ulcers, presence of rectal involvement), and the presence of cytomegalovirus (CMV) defined as a positive CMV stain in the biopsy.
Outcomes
The primary outcome was the diagnosis of MMF colitis, defined as patients taking MMF who exhibited histologic features of MMF-related colitis as per previously published criteria [8] and who did not have an alternative etiology for these histological findings. These include histological patterns that mimic those associated with acute colitis, IBD, GVHD, and ischemia. Patterns were considered acute colitis-like if there was neutrophil-predominant lamina propria inflammation with cryptitis or crypt abscesses and preserved crypt architecture. An IBD-like pattern was defined by the presence of crypt architecture distortion with lymphoplasmacytic-predominant lamina propria inflammation. A GVHD-like pattern was defined as presence of enterocyte apoptosis without lamina propria inflammation and with no or minimal crypt architecture distortion. Patterns were considered ischemia-like if histology showed mucin-depleted crypts with preserved crypt architecture, no or minimal lamina propria inflammation, and crypt dropout. All biopsies were reviewed by expert GI histopathologists. CMV was only considered to be a cause of histologic findings if immunohistochemical stains were positive and found in association with cytopathic changes, including the presence of inclusion bodies. All patients presenting with diarrhea had stool studies before colonoscopy to exclude Clostridium difficile, Giardia, Cryptosporidium, Shigella, Salmonella, and Escherichia coli. This data was not available for patients undergoing colonoscopy for other indications such as screening/surveillance, abdominal pain, and abnormal imaging studies. Medication lists were reviewed from the medication lists in the electronic medical records and from clinical notes. Patients found to have any of the above etiologies for diarrhea were considered controls, regardless of histological findings.
Statistical analysis
Descriptive statistics were used to examine the baseline characteristics of the MMF colitis and non-MMF colitis groups. Continuous variables were compared using Student’s t-test or the Mann–Whitney U-test (for nonparametric variables). The χ2 test was used to evaluate distributions of categorical variables. Logistic regressions models were used to calculate the association between the diagnosis of MMF colitis and the studied variables. Two-sided probabilities were considered, and a values of <0.05 were considered statistically significant.
Results
Of the 3126 solid organ transplant recipients screened, 397 patients met the inclusion criteria. Of those, 36 (9%) had a histological diagnosis of MMF colitis and the rest served as controls. The mean age of patients in the MMF colitis group was 55±12 years, similar to the control group, which had a mean age of 53±12 years. 21 (58%) patients in the MMF colitis group were male, compared to 112 (31%) in the control group. The baseline characteristics and comparison between groups is shown in Table 1.
Table 1.
Baseline characteristics and histological and endoscopic findings
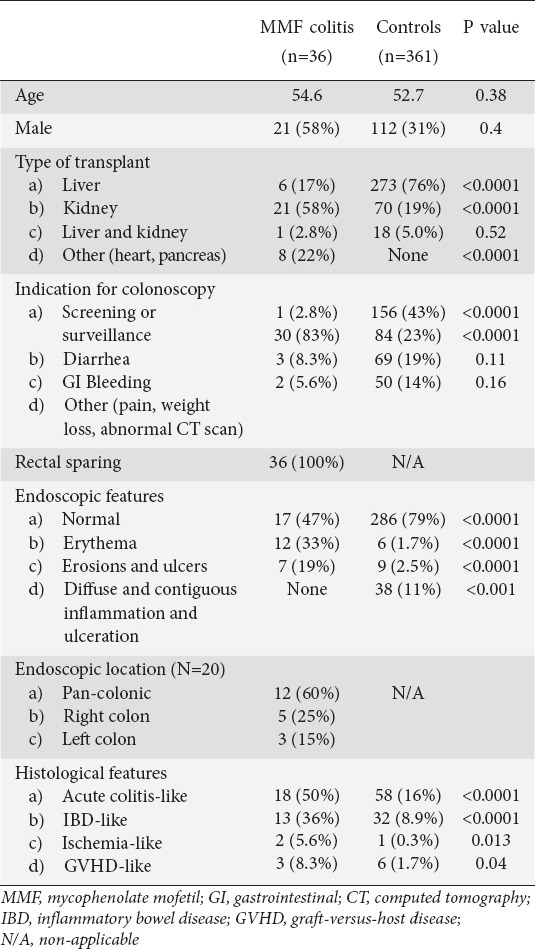
The most common indication for colonoscopy in patients with MMF colitis was diarrhea (83%), which was significantly higher than the control group (23%, P<0.0001). Other indications included screening and surveillance for colorectal cancer, GI bleeding, weight loss, abdominal pain, and abnormal imaging studies. Patients with MMF colitis were receiving a median dose of 2 g/day (range 1-2 g/day).
Variables associated with MMF colitis
Liver transplant patients were less likely to develop MMF colitis than those patients who received other organs (OR 0.06 [95%CI 0.03-0.16], P<0.0001), while kidney transplant was significantly associated with the development of MMF colitis (OR 5.8 [95%CI 2.86-11.86], P<0.0001). The variables associated with MMF colitis are shown in Table 2. In the subgroup of patients with diarrhea, this association persisted, for liver transplantation (OR 0.09 [95%CI 0.03-0.27], P<0.0001), whereas for kidney transplantation group the OR was 3.2 (95%CI 1.38-7.75, P<0.01). Patients with a combined liver and kidney transplantation showed no specific association with MMF colitis (OR 0.93 [95%CI 0.009-9.31]).
Table 2.
Odds ratio for having a diagnosis of MMF colitis by each studied variable

With regards to endoscopic findings, up to 47% of patients had a normal mucosa and the diagnosis was made with mucosal biopsies. Additionally, all of those who had abnormal endoscopic findings had sparing of the rectal mucosa. When comparing endoscopic findings, the presence of erythema (OR 7.02 [95%CI 3.13-15.7, P<0.0001]) and erosions/ulcers (OR 9.4 [95%CI 3.23-27.2, P<0.0001]) were significantly associated with MMF colitis (Fig. 1), and this was more pronounced in the subgroup with diarrhea.
Figure 1.

Colonoscopy findings in a patient with MMF colitis. (A) A 1 cm serpiginous, slightly depressed ulcer with a white exudate at the base, flat margins, and no evidence of bleeding can be seen at 3 o’ clock. The mucosa is irregular, erythematous, has no vascular pattern, and has multiple petechial lesions. (B) A 1.5 cm ulcer with a whitish exudate at the base, raised borders, and no evidence of bleeding can be seen covering most of the image
Histological findings
With respect to the histological spectrum, most patients had histological findings consistent with an acute colitis-like pattern (50%), followed by IBD-like pathologic findings (36%), ischemia-like features (5.6%), and GVHD-like abnormalities (8.3%) (Fig. 2).
Figure 2.
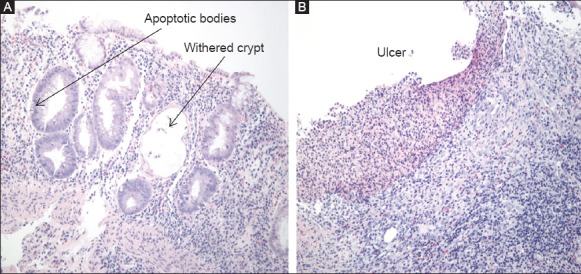
Colonic biopsies in MMF colitis. (A) IBD-like pattern with diffuse lymphoplasmacytic infiltrate, withered crypts, and apoptotic bodies. (B) Ulcer biopsy with loss of the mucosa layer and dense underlying lymphoplasmacytic infiltrate
Discussion
In this study, MMF colitis was diagnosed in 9% of patients undergoing colonoscopy and should be considered as part of the differential diagnosis of post-transplant patients that present with diarrhea. This likely overestimates the true prevalence, however, given that only patients undergoing colonoscopy were included, which comprises only 13% of patients taking MMF. Colonoscopic findings were normal in half of patients, making routine biopsies important in recognizing this entity in patients with diarrhea who are taking MMF. In patients undergoing colonoscopy for indications other than diarrhea, the absence of other causes and a high index of suspicion should determine the need for biopsies to evaluate for MMF colitis. Patients with abnormal colonoscopies had erythema and erosions/ulcers, and all had rectal sparing.
MMF has been reported to have minimal side effects compared to all new immunosuppressive agents currently used in solid organ transplant recipients. It is not nephrotoxic like cyclosporin and tacrolimus, and does not cause hyperlipidemia like sirolimus. It also induces leukopenia less frequently compared to azathioprine [9]. Nevertheless, a significant number of patients present with GI complaints. The incidence of diarrhea is reported to be highest in MMF-containing regimens (13-64%) when compared to azathioprine-based regimens (10-43%) [7]. This may require reduction of dosage or discontinuation of therapy in some cases.
The prevalence of diarrhea in our study population was 29%. In the MMF colitis group, it was the most common indication for colonoscopy (83%). Diarrhea (23%) was present in the control group as well. Diarrhea is shown to impair the quality of life in transplant patients and is a common reason for non-compliance and discontinuation of MPA therapy. Though investigators have speculated that the AcMPAG may cause erosive enterocolitis by covalently binding to villous proteins in the intestinal mucosa [4], the fixed dose versus concentration-controlled trial showed that there was no difference in plasma concentration of AcMPAG between patients with and without diarrhea [10]. Also, no association was found between occurrence of diarrhea and genetic polymorphisms in UGT2B7, responsible for production of AcMPAG [11]. Dalles et al [7] proposed several potential etiologies for the diarrhea that patients with a renal transplant receiving MMF can develop. These include superimposed infectious etiologies (CMV, Campylobacter, bacterial overgrowth), impaired mucosal restoration after mucosal injury, and an increased ratio of goblet cells to absorptive cells, with impaired absorptive function.
The mechanism responsible for the higher incidence of MMF colitis in renal compared with other organ transplant recipients is unclear, although this may be related to the fact that patients who undergo renal transplantation require heavier immunosuppression and higher doses of MMF compared to liver transplant patients. We have too little data to draw conclusions from patients who underwent pancreatic of heart transplantation. MMF has been used in kidney transplant patients to prevent acute rejection in combination with steroids and calcineurin inhibitors. MPA is metabolized by glucuronidation to an inactive compound (MPAG) that is excreted in the urine and feces. When the kidney function is poor in the initial post-operative days, and also with concomitant cyclosporin there is a two- to three-fold increase in free fraction of MPA. An increase in MPAG decreases the percentage of protein-bound MPA and increases its free fraction [12]. As the kidney function improves in these patients, the free MPA level stabilizes. Another factor that may affect MPA concentration is drug interactions. Cyclosporin co-administered with MMF significantly suppresses the enterohepatic circulation [13]. Thus there might have been prolonged contact between MPAG/MPA with intestinal cells causing colitis. In another study it was found that glucuronidation rates were higher in males compared to females [14]. This gender difference can be explained by the fact that estrogens are also metabolized by UDP glucuronyl transferase. Hence MPA could compete for the same binding sites as estrogens. This could explain the male predominance of MMF colitis if there is indeed any role of it in MMF-related GI toxicity. Though all the pancreas and heart transplant patients developed features of MMF colitis, conclusions cannot be drawn owing to the small sample size.
All relevant non-immunosuppressive causes should be evaluated first before making any changes in immunosuppressive regimen. The DIarrhea Diagnosis Act and Clinical Treatment (DIDACT) study collected data from renal transplant patients to explore the impact of several causative factors on the management of GI complications [15]. Stopping non-immunosuppressive drugs and treating infections led to remission of diarrhea in 39% of patients. In the remaining, decreasing the dose or discontinuing MMF was associated with a 65% rate of remission of diarrhea.
It has been found that the intestinal lumen contains purines, released from the nucleic acid of ingested cells during digestion. Moreover, the intestinal cells have specific transport pathways which allow the passive diffusion of these nucleosides back into the cell [16]. This finding suggests that enterocytes are not entirely dependent on the de novo pathway purine synthesis and argues against the proposition that enterocytes are susceptible to IMPDH inhibition. This also suggests that there may be other yet unknown mechanisms for GI toxicity.
The literature on macroscopic findings associated with MMF colitis is currently lacking and limited mainly to case reports and retrospective studies. In the present study we found colonoscopic findings ranging from erythema to erosions and ulcers, with approximately half of patients with normal macroscopic findings. However, the magnitude of these findings must be tempered as there is a risk of reporting bias where minor erythema/erosions may not have been reported in patients in the control group undergoing colonoscopy for non-diarrheal indications. What was particularly striking was the absence of rectal involvement in all of our cases. We found 8 case reports [17-24] and 4 case series [25-28] that included macroscopic findings, describing a total of 22 patients with MMF colitis. In these cases, 2 had normal colonoscopies while 7 had only erythema and 13 had erosions or ulcers. Of note, rectal involvement was noted in 4 cases while rectal sparing was noted in 11. In 7 cases we could not determine the presence or absence of rectal involvement. The small number of patients with normal colonoscopies may be partly explained by publication bias. We also found 4 case control studies [5,7,8,29] and 1 cohort study [30] looking mainly at microscopic findings, but that also mentioned colonoscopic findings. These pooled to a total of 85 patients with MMF colitis for whom colonoscopic findings were available. In this group, 45 had normal colonoscopies and 40 described changes ranging from mild erythema to erosions and ulcers, findings similar to our study. We were, however, unable to determine the presence of rectal involvement in those with abnormal colonoscopies. Of particular interest are findings of a study looking at 7 patients with MMF colitis [31]. In this particular study, patients had multiple biopsies (median of 12 ranging between 5-22) from both the proximal and distal colon. Unfortunately, macroscopic findings were not described. Histological findings seemed to be more severe in the right colon than the left colon. In particular there was an increased number of IBD-like changes (87.8% vs. 7.4%) and an increased number of apoptotic bodies per 100 crypts (27.7 vs. 13.7). Due to the small numbers, however, this was not statistically significant. From this study and findings in our study, it seems that mucosal involvement tends to be more significant in the proximal colon. This may be partially reflected in the paucity of rectal involvement which although absent in our study, has been previously described in at least 4 reported cases [23-26].
It is currently unclear why mucosal findings are more common in the proximal colon, although a proposed mechanism involves the enterohepatic recycling of MMF [31]. After oral administration, MMF is rapidly absorbed and de-esterified to the active metabolite, MPA. This is then glucuronidated to form MPAG, an inactive metabolite, which is partially excreted in the bile. MPAG is then deconjugated by colonic bacteria to the active compound, MPA which is again reabsorbed mainly in the proximal colon [32]. It is possible that decreased distal exposure to MPA may partially account for the paucity of distal findings on colonoscopy and rectal sparing in the present study.
Histological features found in our patient cohort included apoptosis, architectural distortion, intraglandular lymphocytes, increase in neuroendocrine cells, and mucosal vascular injury. In our study, an acute colitis-like pattern was observed at a higher frequency (50%) than GVHD (8.3%). Selbst et al found that MMF-induced changes were similar to IBD (28%), GVHD (19%), acute colitis (16%), and ischemia (3%) [8]. In GVHD, the donor’s leukocytes dominate over the recipient’s immune system. The incidence of GVHD is highest after small bowel transplantation (approximately 5%) possibly due to the presence of a large number of donor lymphocytes in the small bowel. GVHD after solid organ transplantation usually occurs 1-11 weeks post-transplantation. In a study by Gulbahce et al, four patients developed late GVHD-like features 7-34 months post-transplantation. Two of the four patients were taking MMF and the role of MMF cannot be ruled out in those cases [33]. Acute GVHD, 3 months after transplantation, has not been reported so far. The immune dysregulation caused by this drug favors proliferation of donor lymphocytes producing a GVHD-like picture. However, GVHD-like lesions may be seen in patients with CMV, HIV, and primary immunodeficiencies. On the other hand, presence of cryptitis, basal plasmocytosis, and loss of goblet cell mucin in the rectal biopsies are helpful to recognize acute colitis. The rectal biopsies have to be early (within 3 days of the onset of bloody diarrhea) to be diagnostic [34].
In a more recent study [31], pathological changes of MMF-related mucosal injury were characterized and quantified. The colonic biopsies were reviewed for the following features: crypt architectural distortion, foci of cryptitis, acute colitis-like changes, individual damaged crypts or dilated damaged crypts and apoptotic figures. IBD-like changes (crypt shortening, branching, and drop out) and focal cryptitis was found in all 7 patients. An acute colitis-like pattern was noted in only 3 patients. A mean of 3.0 foci of individual damaged crypts was present per biopsy and apoptosis was high in all cases. All cases demonstrated some degree of crypt architectural disarray, cryptitis, and apoptosis, but their sample size was too small to draw conclusions.
Our study carries a series of limitations. There are currently no universally accepted diagnostic criteria for MMF colitis, and the definition used in our study included those patients who were on MMF and developed a group of symptoms and had specific changes on colonic biopsy thought to be associated with the use of this drug. However, association does not signify causality, and a better set of criteria might include one in which discontinuation of the drug is associated with remission of this syndrome. Unfortunately, considering the retrospective nature of this study, this data was not available to us. Additionally, an even more definite criteria may include the requirement of recurrence of syndrome upon re-challenge to the drug, although this carries its own ethical implications and should not be performed routinely unless a re-challenge with MMF is the only potential therapeutic option and either causality is in doubt or the syndrome was sufficiently mild that it may be tolerated and/or managed with symptomatic treatment. Given the retrospective nature of our study, we also attempted to rule out other potential etiologies, especially infectious and drug-induced causes. However, a diagnostic workup for infectious causes was not available for patients presenting without diarrhea (17% of cases). There also exists the possibility that in patients with positive histological findings in whom an alternative etiology was found, MMF may have played a pathogenetic role in these findings.
Another limitation of our study is that, even though our goal was to determine macroscopic and microscopic features of MMF colitis, it is important to recognize that in clinical practice, most patients who develop diarrhea while on MMF will undergo dose reduction or discontinuation of this drug empirically before a colonoscopy is considered. This may induce a selection bias as the study population may represent those patients who had more severe disease, did not respond to dose reduction, or in whom an alternative diagnosis was expected. However, this is the population from which more data is needed as not all patients on MMF who develop diarrhea that resolves upon discontinuation of the drug would necessarily require further workup.
In conclusion, MMF colitis may occur in up to 9% of solid organ transplant recipients taking MMF who undergo colonoscopy. However, the pathogenesis of MMF-related diarrhea remains elusive and unclear and we lack studies that connect the histological changes in patients taking MMF with the clinical syndrome of MMF colitis. Future long-term cohort studies looking at the natural history of MMF colitis may help us better understand this largely under-recognized entity.
Summary Box.
What is already known:
Mycophenolate mofetil (MMF) colitis is a known adverse effect in solid organ transplant recipients taking MMF
Mycophenolate mofetil (MMF) colitis is a known adverse effect in solid organ transplant recipients taking MMF
Histological findings of MMF colitis resemble those of acute colitis, inflammatory bowel disease (IBD), ischemic colitis, and graft-versus-host disease (GVHD)
What the new findings are:
Endoscopic findings of MMF colitis range from erythema (33%) to erosions and ulcers (19%), and rectal sparing is almost universal
Almost half of patients may have a normal colonoscopy, suggesting the need for routine biopsies in patients undergoing colonoscopy
The most common histological findings are acute colitis-like findings (50%) and IBD-like characteristics (36%), and less commonly ischemia-like findings (5.6%) and GVHD-like features (8.3%)
Patients who underwent kidney transplantation are more susceptible to developing MMF colitis than those who underwent liver transplantation, possibly related to the higher doses of MMF used in this population
Biography
University of Miami Miller School of Medicine, Miami; Jackson Memorial Hospital, Miami; Mount Sinai School of Medicine, New York, USA
Footnotes
Conflict of Interest: None
References
- 1.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanism of action. Immunopharmacology. 2000;47:85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 2.Allison AC, Eugui EM. Mechanism of action of mycophenolate mofetil in preventing acute and chronic allograft rejection. Transplantation. 2005;80(2 Suppl):S181–S190. doi: 10.1097/01.tp.0000186390.10150.66. [DOI] [PubMed] [Google Scholar]
- 3.Wieland E, Shipkova M, Schellhaas U, et al. Induction of cytokine release by the acyl glucuronide of mycophenolic acid: a link to side effects? Clin Biochem. 2000;33:107–113. doi: 10.1016/s0009-9120(99)00101-0. [DOI] [PubMed] [Google Scholar]
- 4.King AR, Dickinson RG. Studies on the reactivity of acyl glucuronides – IV. Covalent binding of diflunisal to tissues of the rat. Biochem Pharmacol. 1993;45:1043–1047. doi: 10.1016/0006-2952(93)90248-u. [DOI] [PubMed] [Google Scholar]
- 5.Parfitt JR, Jayakumar S, Driman DK. Mycophenolate mofetil-related gastrointestinal mucosal injury: variable injury patterns, including graft-versus-host disease-like changes. Am J Surg Pathol. 2008;32:1367–1372. doi: 10.1097/pas.0b013e31816bf3fe. [DOI] [PubMed] [Google Scholar]
- 6.Papadimitriou JC, Cangro CB, Lustberg A, et al. Histologic features of mycophenolate mofetil-related colitis: a graft-versus-host disease-like pattern. Int J Surg Pathol. 2003;11:295–302. doi: 10.1177/106689690301100406. [DOI] [PubMed] [Google Scholar]
- 7.Dalle IJ, Maes BD, Geboes KP, Lemahieu W, Geboes K. Crohn’s-like changes in the colon due to mycophenolate? Colorectal Dis. 2005;7:27–34. doi: 10.1111/j.1463-1318.2004.00695.x. [DOI] [PubMed] [Google Scholar]
- 8.Selbst MK, Ahrens WA, Robert ME, Friedman A, Proctor DD, Jain D. Spectrum of histologic changes in colonic biopsies in patients treated with mycophenolate mofetil. Mod Pathol. 2009;22:737–743. doi: 10.1038/modpathol.2009.44. [DOI] [PubMed] [Google Scholar]
- 9.Feng L, Deng J, Huo DM, Wu QY, Liao YH. Mycophenolate mofetil versus azathioprine as maintenance therapy for lupus nephritis: a meta-analysis. Nephrology (Carlton) 2013;18:104–110. doi: 10.1111/nep.12006. [DOI] [PubMed] [Google Scholar]
- 10.Heller T, van Gelder T, Budde K, et al. Plasma concentrations of mycophenolic acid acyl glucuronide are not associated with diarrhea in renal transplant recipients. Am J Transplant. 2007;7:1822–1831. doi: 10.1111/j.1600-6143.2007.01859.x. [DOI] [PubMed] [Google Scholar]
- 11.van Agteren M, Armstrong VW, van Schaik RH, et al. AcylMPAG plasma concentrations and mycophenolic acid-related side effects in patients undergoing renal transplantation are not related to the UGT2B7-840G>A gene polymorphism. Ther Drug Monit. 2008;30:439–444. doi: 10.1097/FTD.0b013e318180c709. [DOI] [PubMed] [Google Scholar]
- 12.Kamińska J, Glyda M, Sobiak J, Chrzanowska M. Pharmacokinetics of mycophenolic acid and its phenyl glucuronide metabolite in kidney transplant recipients with renal impairment. Arch Med Sci. 2012;8:88–96. doi: 10.5114/aoms.2012.27287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cremers S, Schoemaker R, Scholten E, et al. Characterizing the role of enterohepatic recycling in the interactions between mycophenolate mofetil and calcineurin inhibitors in renal transplant patients by pharmacokinetic modelling. Br J Clin Pharmacol. 2005;60:249–256. doi: 10.1111/j.1365-2125.2005.02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morissette P, Albert C, Busque S, St-Louis G, Vinet B. In vivo higher glucuronidation of mycophenolic acid in male than in female recipients of a cadaveric kidney allograft and under immunosuppressive therapy with mycophenolate mofetil. Ther Drug Monit. 2001;23:520–525. doi: 10.1097/00007691-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Maes B, Hadaya K, de Moor B, et al. Severe diarrhea in renal transplant patients: results of the DIDACT study. Am J Transplant. 2006;6:1466–1472. doi: 10.1111/j.1600-6143.2006.01320.x. [DOI] [PubMed] [Google Scholar]
- 16.Arns W. Noninfectious gastrointestinal (GI) complications of mycophenolic acid therapy: a consequence of local GI toxicity? Transplant Proc. 2007;39:88–93. doi: 10.1016/j.transproceed.2006.10.189. [DOI] [PubMed] [Google Scholar]
- 17.Gorospe EC. Chronic diarrhoea from mycophenolate mofetil-induced colitis. BMJ Case Rep 2012. 2012:pii. doi: 10.1136/bcr.12.2011.5344. bcr1220115344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patra S, Vij M, Sukanya B, Kapoor D. Mycophenolate mofetil-induced colitis with graft versus host disease-like features in a liver transplant recipient. Indian J Pathol Microbiol. 2012;55:506–508. doi: 10.4103/0377-4929.107792. [DOI] [PubMed] [Google Scholar]
- 19.Childers R, Chow G, Fraig M, Asamoah V, Wong P. Education and imaging. Gastrointestinal: chronic cellcept-induced colitis. J Gastroenterol Hepatol. 2011;26:1214. doi: 10.1111/j.1440-1746.2011.06673.x. [DOI] [PubMed] [Google Scholar]
- 20.Bouhbouh S, Rookmaaker MB. Rapid resolution of persistent mycophenolate mofetil-induced diarrhoea with a single dose of infliximab. Nephrol Dial Transplant. 2010;25:3437–3438. doi: 10.1093/ndt/gfq379. [DOI] [PubMed] [Google Scholar]
- 21.Mohsin N, Jha A, Kallankara S, Asif P, Malvathu R. Rapid resolution of mycophenolate associated diarrhea with a small dose of octreotide: a case report. Transplant Proc. 2003;35:2754. doi: 10.1016/j.transproceed.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 22.Kim HC, Park SB. Mycophenolate mofetil-induced ischemic colitis. Transplant Proc. 2000;32:1896–1897. doi: 10.1016/s0041-1345(00)01482-2. [DOI] [PubMed] [Google Scholar]
- 23.Hamouda M, Mahmoudi H, Skhiri H, Elmay M. Mycophenolate mofetil-related pancolitis in a kidney transplant recipient. Exp Clin Transplant. 2012;10:501–505. doi: 10.6002/ect.2011.0200. [DOI] [PubMed] [Google Scholar]
- 24.Khoury N, Ammor M, Durrbach A, Kriaa F, Charpentier B. Colite diffuse associée à un traitement par mycophénolate mofétil: a propos d’une observation. Nephrologie. 2000;21:437–439. [PubMed] [Google Scholar]
- 25.Jakes AD, Roy A, Veerasamy M, Bhandari S. Case report: Crohn’s-like mycophenolate-induced colitis, a fallout in steroid-free regimens. Transplant Proc. 2013;45:842–844. doi: 10.1016/j.transproceed.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Phatak UP, Seo-Mayer P, Jain D, Selbst M, Husain S, Pashankar DS. Mycophenolate mofetil-induced colitis in children. J Clin Gastroenterol. 2009;43:967–969. doi: 10.1097/MCG.0b013e3181a8754d. [DOI] [PubMed] [Google Scholar]
- 27.Al-Absi AI, Cooke CR, Wall BM, Sylvestre P, Ismail MK, Mya M. Patterns of injury in mycophenolate mofetil-related colitis. Transplant Proc. 2010;42:3591–3593. doi: 10.1016/j.transproceed.2010.08.066. [DOI] [PubMed] [Google Scholar]
- 28.Golconda MS, Valente JF, Bejarano P, Gilinsky N, First MR. Mycophenolate mofetil-induced colonic ulceration in renal transplant recipients. Transplant Proc. 1999;31:272–273. doi: 10.1016/s0041-1345(98)01531-0. [DOI] [PubMed] [Google Scholar]
- 29.Liapis G, Boletis J, Skalioti C, et al. Histological spectrum of mycophenolate mofetil-related colitis: association with apoptosis. Histopathology. 2013;63:649–658. doi: 10.1111/his.12222. [DOI] [PubMed] [Google Scholar]
- 30.Maes BD, Dalle I, Geboes K, et al. Erosive enterocolitis in mycophenolate mofetil-treated renal-transplant recipients with persistent afebrile diarrhea. Transplantation. 2003;75:665–672. doi: 10.1097/01.TP.0000053753.43268.F0. [DOI] [PubMed] [Google Scholar]
- 31.Lee S, de Boer WB, Subramaniam K, Kumarasinghe MP. Pointers and pitfalls of mycophenolate-associated colitis. J Clin Pathol. 2013;66:8–11. doi: 10.1136/jclinpath-2012-200888. [DOI] [PubMed] [Google Scholar]
- 32.Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet. 1998;34:429–455. doi: 10.2165/00003088-199834060-00002. [DOI] [PubMed] [Google Scholar]
- 33.Gulbahce HE, Brown CA, Wick M, Segall M, Jessurun J. Graft-vs-host disease after solid organ transplant. Am J Clin Pathol. 2003;119:568–573. doi: 10.1309/395B-X683-QFN6-CJBC. [DOI] [PubMed] [Google Scholar]
- 34.Kumar NB, Nostrant TT, Appelman HD. The histopathologic spectrum of acute self-limited colitis (acute infectious-type colitis) Am J Surg Pathol. 1982;6:523–529. doi: 10.1097/00000478-198209000-00004. [DOI] [PubMed] [Google Scholar]


