Abstract
Background
The incidence of diastolic dysfunction (DD) approaches 40% in patients with cirrhosis. However, the clinical impact of DD remains a subject of considerable debate. Surgery in patients with cirrhosis is innately hazardous. Diastolic heart failure has been linked to increased mortality after transjugular intrahepatic porto-systemic shunt surgery (TIPSS). To date, none of the commonly accepted preoperative risk assessment models applied to patients with liver disease incorporates DD. We aimed to examine the relationship between DD and postoperative outcomes in patients with cirrhosis undergoing abdominal surgery.
Methods
Patients with cirrhosis who underwent abdominal surgery between January 2000 and December 2011 were included if they had preoperative echocardiography done within 3 months of surgery. The echocardiographic images were reviewed using flow and tissue Doppler techniques to identify the presence of DD. Outcomes analyzed included one-year mortality and postoperative complications.
Results
A total of 140 patients were included in the study of which 63 patients (45%) met pre-established criteria for DD. Those with DD were older (P < 0.005) and less likely to have an isolated viral etiology of cirrhosis (P<0.05). The one-year mortality rate was 22.2% (14/63) in patients with DD and 20.8% (16/77) in those without DD (P=0.42). Postoperative complications were not statistically different in the two groups.
Conclusion
DD is common in patients with cirrhosis. In patients with cirrhosis undergoing TIPS and/or abdominal surgery, the presence of DD does not increase post-procedure complications or one-year mortality.
Keywords: Diastolic dysfunction, cirrhosis, liver transplantation, postoperative mortality, surgical risk
Introduction
In the final 2 years of life, nearly 10% of patients with hepatic cirrhosis require a surgical procedure of some kind [1]. As the number of patients diagnosed with advanced liver disease continues to increase, the number of cirrhotics requiring surgery is expected to rise in tandem [2]. Surgery in these patients remains innately hazardous, and is associated with an elevated postoperative morbidity and mortality that is commonly attributed to the presence of cirrhosis itself. Worsening liver disease or progression to liver failure is commonly reported in the postoperative period [1,3]. Either Child Pugh classification or model for end-stage liver disease (MELD) score provides a reasonably precise estimation of perioperative mortality in these patients [1]. Mortality rates for patients undergoing surgery increases with each level of Child Pugh class: 10% for those with Child class A, 30% for those with Child class B, and 76-82% for those with Child class C cirrhosis [1,4]. A more recent study noted that increased perioperative mortality also correlated with the MELD score: MELD <10 = 9%, MELD 10-15 = 19% and MELD >15 = 54% [5]. Other reported risk factors for adverse postoperative outcome include American Society of Anesthesiologists (ASA) score >3; emergent procedure; intraoperative blood transfusion; intraoperative blood loss >150 mL; presence of ascites; total bilirubin level >1.5 mg/dL; and albumin level <3 mg/dL [6]. While the reasons for this elevated risk remain incompletely categorized, interest has focused upon circulatory changes brought on by surgery or anesthesia, with resulting impairment in hepatic vascular flow [2].
Cirrhotic cardiomyopathy is a condition comprising a constellation of cardiac abnormalities, which include myocardial hypertrophy, decreased ventricular compliance and normal systolic function at rest, but systolic incompetence under conditions of pharmacological or physical stress [7,8]. While diastolic dysfunction (DD) is relatively common in patients with advanced cirrhosis, the clinical impact of this condition remains a subject of considerable debate [9]. Proponents argue that cirrhotic cardiomyopathy predicts worsening outcomes in a variety of settings, including post-transjugular intrahepatic porto-systemic shunt (TIPS) insertion [9,10], and liver transplantation [11]. Recent studies have shown that the presence of DD is a sensitive marker for type-1 hepatorenal syndrome (HRS) development in cirrhotics and may be associated with substantial hemodynamic alterations in patients undergoing orthotopic liver transplantation (OLT) [12,13]. Skeptics however remain doubtful whether cirrhotic cardiomyopathy produces any clinical consequences [9]. To date, none of the commonly accepted preoperative risk assessment models applied to patients with liver disease incorporates DD. Accordingly, we sought to assess the clinical relevance of DD as a predictor of adverse outcomes in patients with liver disease who undergo TIPS and abdominal surgery including liver transplantation.
Patients and methods
Patient selection
All cirrhotic patients between the ages 19 and 65 years, who had undergone TIPS or abdominal surgery at Hartford Hospital from 2000 to 2011 and had a transthoracic echocardiogram within 3 months prior to the procedure, were assessed to be included in the study. Patients had to be in sinus rhythm during echocardiography. Exclusion criteria included past history of ischemic or structural heart disease, cardiac surgery, use of supplemental oxygen at rest, end-stage renal disease requiring hemodialysis, morbid obesity (body mass index >30 kg/m2), obstructive sleep apnea requiring the need for nocturnal non-invasive ventilation, estimated left ventricular ejection fraction (LVEF) <45%, severe mitral or aortic stenosis or regurgitation by American Society of Echocardiography (ASE) criteria and constrictive pericarditis.
Echocardiographic studies
All echocardiographic images were obtained from Siemens Sequoia 512, Philips IE33 or Philips 7500 sonographs, translated into digitized DICOM format and transferred to an offline analysis system (Agfa Heartlab, Hackensack, NJ). Echocardiographic variables including LV end-systolic and end-diastolic volumes and left atrial (LA) diameter were measured and LV mass index was calculated using the standard ASE approved formula [14].
LVEF was measured from apical 4 and 2-chamber views using Simpson’s biplane method [14]. LA volume was calculated using the biplane method of discs [14]. E and A wave velocities were measured using spectral Doppler with the sample volume placed at the tips of the mitral leaflets. Deceleration time (DT) was measured as the time required for the diastolic pressure gradient between LA and LV to fall to 0 mm Hg from its peak. Pulmonary vein (PV) flow was interrogated using spectral Doppler with the sample volume placed in the left upper PV from the apical 4-chamber view. Peak PV flow in systole (PVs) and diastole (PVd) were measured. All images were reviewed by a cardiologist board certified in echocardiography (VR, DIS). DD was defined by the presence of any 2 of the following Doppler findings: a) Grade 1: DT >250 msec, E/A ratio <1, medial A’>E’ PVs/PVd ratio >1.5; and b) Grades 2 and 3: E/A >1 DT <160 msec, medial E/E’ >15, PVs/PVd ratio <0.3, medial E’ <7 cm/sec [15]. Tricuspid annular plane excursion (TAPSE), a validated measure of right ventricular (RV) function, was measured using standard techniques [15].
Data collection
Data was collected by retrospective review of the patients’ electronic medical record. Data gathered included patient demographics, baseline clinical descriptors, nature of surgery, measures of overall morbidity and mortality, and full echocardiographic and Doppler descriptors of systolic and diastolic function as outlined above. The primary outcome was all-cause mortality. The minimum required duration of follow up was 12 months. In the absence of follow up, mortality data was obtained by searching the social security database or calling the patients primary care physician. If these methods failed to ascertain outcome, the patient was labeled as ‘lost to follow up’. Adverse intraoperative and postoperative events were evaluated as secondary outcomes. These included bleeding, hypotension, renal failure, length of stay, recurrent ascites and readmission during the period of follow-up. Intraoperative bleeding and hypotension were defined as episodes that required blood transfusion or use of vasopressors. Length of stay was considered prolonged if it exceeded 4 days for non-transplantation abdominal surgery and 10 days for liver transplantation.
Statistical analysis
All data are presented as mean ± standard deviation unless otherwise specified. Means were compared using an unpaired t test. Proportions were compared using a chi square test. Analysis was performed using SPSS 19 software (IBM North America, New York, NY). A P value of less than 0.05 was considered statistically significant.
Results
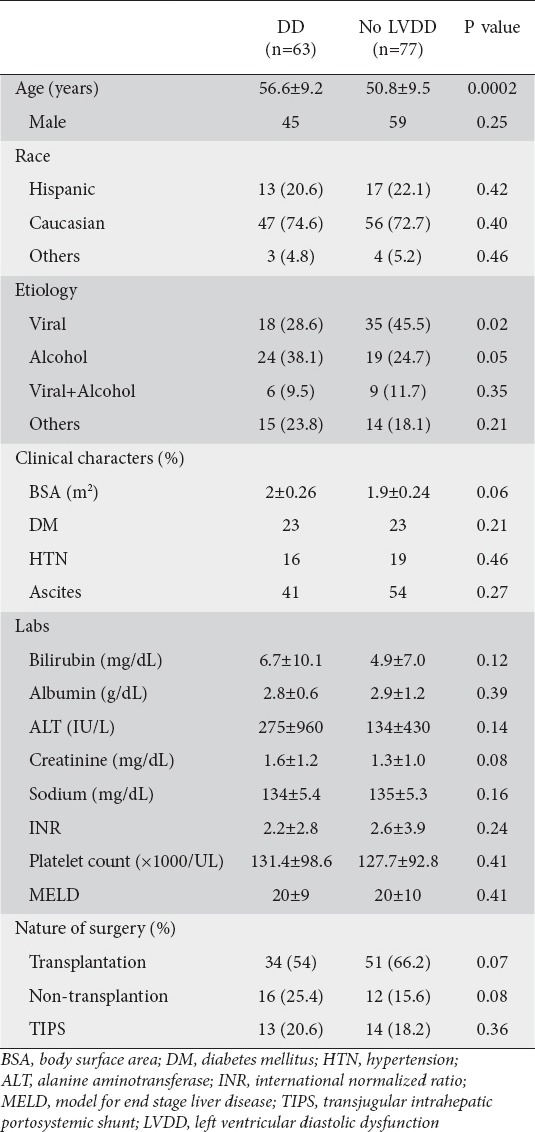
A total of 140 patients met study criteria of which 63 (45%) met criteria for DD (Table 1); of these; 14% had Grade II or Grade III DD. Those with DD were older (P<0.005) and less likely to have an isolated viral etiology of chronic liver disease (P<0.05) (Table 1), but there were no other significant difference between patients with and without DD. Twenty-eight patients [16 with DD and 12 without DD (Table 1)] underwent non-transplantation surgery, including laparoscopy/laparotomy, mostly for cholecystomy and or lysis of adhesions (n=17), splenorenal or other vascular shunt (n=8), and abdominal hernia repair (n=3).
Table 1.
Patient characteristics divided by the presence and absence of criteria for diastolic dysfunction
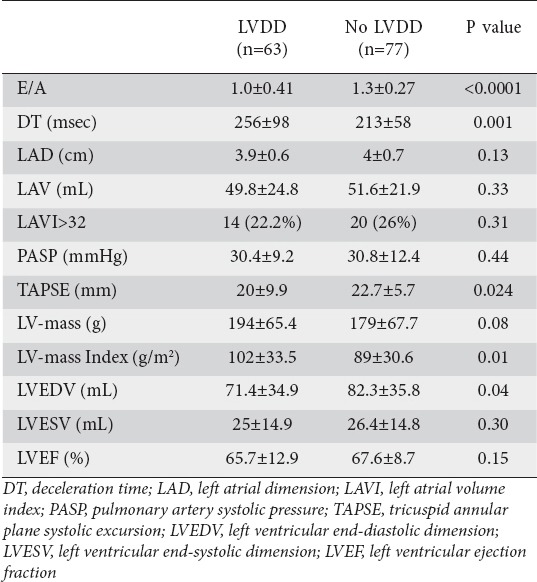
In patients with DD, E/A ratio was significantly reduced, DT and LV mass index were significantly increased, and end-diastolic dimension was significantly reduced compared to those patients with normal diastolic function (Table 2). There was no difference in mean LA volume or in the proportion of patients with increased LA volume in either group (Table 2). RV systolic function, as measure by TAPSE, was significantly impaired in patients with DD (Table 2).
Table 2.
Echocardiography parameters in patients with and without diastolic dysfunction
A total of 30 patients (21%) died within one year following their procedure, and 110 patients (79%) experienced at least one endpoint. Serum sodium and hematocrit were significantly lower in those who died (Table 3). Creatinine was significantly higher, and mean albumin was significantly lower in patients with any adverse outcome (Table 3). There were no other differences between patients with and without endpoints. Overall one-year mortality rate was 22% (14/63) in patients with DD and 21% (16/77) in those without (P=0.42). There was a trend towards higher length of hospital stay (P=0.05) and readmission rate (P=0.05) in patients with DD (Fig. 1). Postoperative complications including ascites, jaundice, and encephalopathy or wound infections were not statistically different between these two groups.
Table 3.
Clinical characteristics and echocardiography parameters in patients with and without adverse outcomes
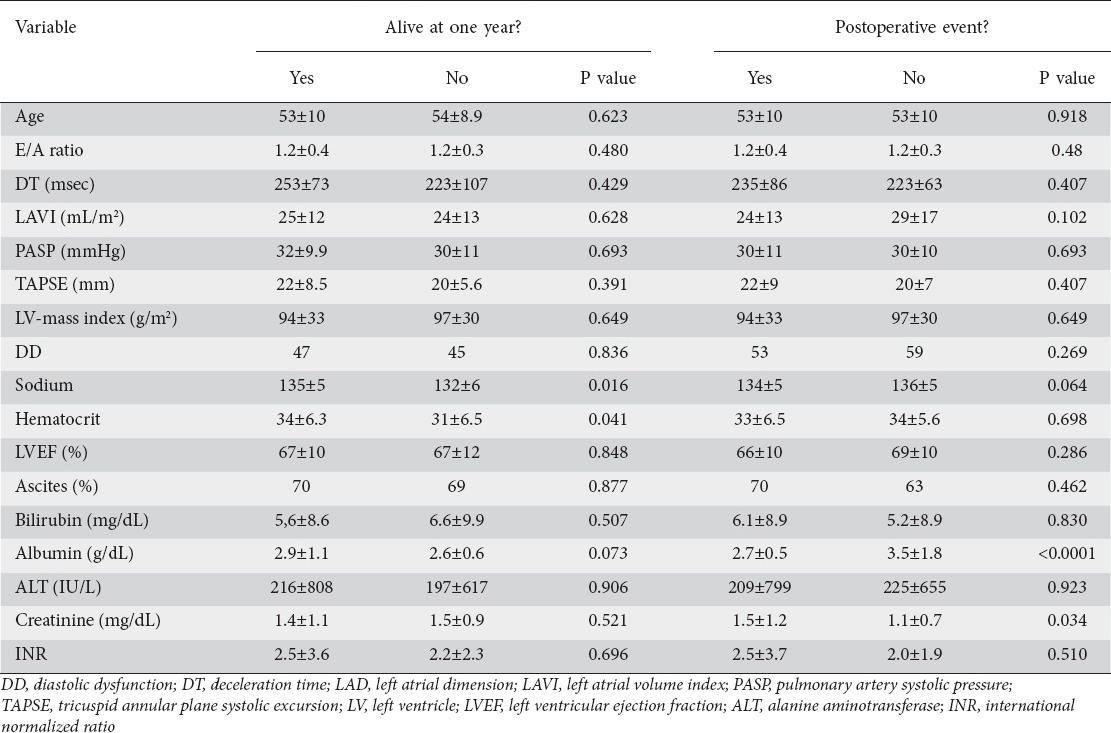
Figure 1.
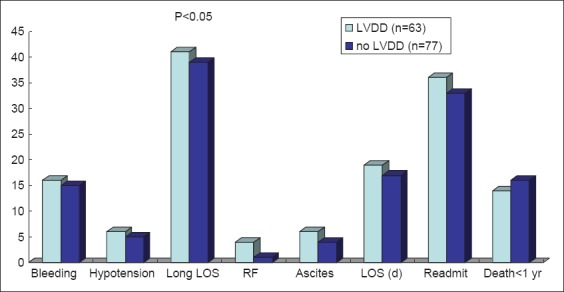
Outcomes in patients with and without diastolic dysfunction
LOS, length of stay; RF, renal failure; LVDD, left ventricular diastolic dysfunction
Outcomes by type of surgery were examined. In patients who underwent TIPS (n=27), there was no difference in mortality when comparing patients with and without DD (23 vs. 50%; X2 = 0.083, P=0.773). The presence of DD did not affect one-year mortality in patients who underwent liver transplantation (12 vs. 10%; X2 = 0.082, P=0.773), or those who underwent non-transplantation abdominal surgery (10 vs. 17%; X2 = 0.261, P=0.609).
Discussion
In this retrospective study of 140 patients with cirrhosis undergoing various abdominal surgeries, we found no difference in any indicator of DD between those patients who experienced an adverse postoperative outcome, including one-year mortality, and those who did not. Preoperative risk assessment for cirrhotic patients undergoing liver transplantation surgery can be challenging. The significance of DD in preoperative cardiac risk assessment in this cohort is unclear. In a retrospective study analyzing outcomes in 306 patients undergoing OLT, those with DD (100 / 306) were noted to have significantly higher incidence of post reperfusion syndrome requiring intraoperative inotropic support (29% vs. 11%; P<0.01) [13]. However there was no difference in mortality between the two groups [13].
In most studies evaluating DD in cirrhotics, diagnosis has been based solely upon E/A ratio <1 using 2D-Doppler echocardiography [9,10]. E/A ratio, however, has important limitations, including load and age dependence in patients otherwise free of heart disease [15,16]. Current guidelines for evaluation of diastolic function are far more comprehensive, and include measurement of DT, PV flow, and tissue Doppler imaging (TDI) [15]. In addition, inclusion of ancillary findings such as indexed LV mass and LA volume, which were included in our study increase the accuracy of diagnosis. Because TDI parameters were only available in a minority of our patients (n = 63), we could not include them for every patient, but the addition of DT and PV flow pattern for all patients represents a significant advance over previous reports. The prevalence of DD in our cohort falls within the previously reported range of 40% to 56% [9,10], and is also consistent with a more recent study that included TDI as a diagnostic criterion [12].
Also in contrast to previous studies, incidence of DD did not differ in any subset of our cohort. Previous studies have noted a higher incidence of DD in cirrhotics with ascites [17], and significant improvement in diastolic function after abdominal paracentesis [18]. We did not find any association between ascites and DD in our study population. Abnormal diastolic function has been reported to precede systolic dysfunction in patients with alcoholic cirrhosis [19], in a dose-dependent fashion [19]. In our study, patients with DD were more likely to have alcoholic cirrhosis compared to a viral etiology (48% vs. 36%) but the difference was not statistically significant.
In cirrhotics, the surgical risk depends on both disease severity as categorized by the MELD score [20], and type and urgency of surgery [21]. Other factors that have been known to predict increased operative mortality include advanced age, ASA class, and hypoalbuminemia [22,23], a finding with which our data are consistent. Recent studies have also found DD to be a marker of advanced cirrhosis and HRS [12]. The increased renal dysfunction in patients with adverse postoperative outcomes in our study patients is again consistent with those findings. Patients with normal and abnormal diastolic function, however, had comparable MELD scores.
Our study has several limitations. Firstly, this study represents a retrospective analysis. However, given the high prevalence of DD in our patient population any clinically significant predictor of adverse outcome would have been conspicuous despite subtle confounding resulting from retrospective observation. Secondly, TDI data was not uniformly available for all patients. However, the addition of DT and PV data represents a significant advance over previous studies in this field. Finally, the small number of patients undergoing non-transplantation abdominal surgery in our cohort makes it difficult to interpret the clinical impact of DD in this patient population. Based on our limited data it appears that DD does not impact postoperative outcomes in these patients. Given the common notion that DD adversely affects surgical outcomes in patients with cirrhosis, we decided to include commonly performed procedures in cirrhotics including TIPS, transplantation and non-transplantation abdominal surgeries. Although this makes the study population heterogeneous, our results can aid decision-making in clinical practice till more studies are available in specific surgical cohorts. Given the relative rarity of elective non-transplantation abdominal surgery in patients with cirrhosis, a large multicenter study is warranted to assess outcomes of any one specific surgical procedure.
As previously mentioned, we believe the estimation of DD by E/A ratio alone is subject to several limitations and might explain in part the lack of a difference in our cohort where previous studies data have suggested that a clinically relevant effect might be present. Although DD is well known to impact outcomes in non-cirrhotic patients, it is also true that cirrhosis imparts a complex series of hemodynamic changes that make it a unique disease state. One example of this is the pronounced reduction in systemic vascular resistance which may serve as an intrinsic afterload reduction, which may mitigate in part the possible consequence of impaired diastole.
To conclude, DD is common in patients with cirrhosis and normal LV systolic function, but was not associated with increased risk of mortality after TIPS, OLT or non-transplantation abdominal surgery in this study. Further prospective studies using TDI are warranted to study the clinical significance of DD as a predictor of adverse postoperative outcomes in patients with liver disease.
Summary Box.
What is already known:
Diastolic dysfunction is common in patients with chronic liver disease
Clinical impact of diastolic dysfunction in patients with cirrhosis is widely debated
What the new findings are:
The presence of diastolic dysfunction is not associated with increased one-year mortality in patients with cirrhosis undergoing transjugular intrahepatic porto-systemic shunt or abdominal surgeries including liver transplantation
Biography
University of Connecticut Health Center, Farmington; Hartford Hospital, Hartford, CT, USA
Footnotes
Conflict of Interest: None
References
- 1.O’Leary JG, Yachimski PS, Friedman LS. Surgery in the patient with liver disease. Clin Liver Dis. 2009;13:211–231. doi: 10.1016/j.cld.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Malik SM, Ahmad J. Preoperative risk assessment for patients with liver disease. Med Clin North Am. 2009;93:917–929. doi: 10.1016/j.mcna.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda Y, Kanda T, Kosugi S, et al. Gastric cancer surgery for patients with liver cirrhosis. World J Gastrointest Surg. 2009;1:49–55. doi: 10.4240/wjgs.v1.i1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansour A, Watson W, Shayani V, et al. Abdominal operations in patients with cirrhosis: still a major surgical challenge. Surgery. 1997;122:730–735. doi: 10.1016/s0039-6060(97)90080-5. [DOI] [PubMed] [Google Scholar]
- 5.Neeff H, Mariaskin D, Spangenberg HC, et al. Perioperative mortality after non-hepatic general surgery in patients with liver cirrhosis: an analysis of 138 operations in the 2000s using child and MELD scores. J Gastrointest Surg. 2011;15:1–11. doi: 10.1007/s11605-010-1366-9. [DOI] [PubMed] [Google Scholar]
- 6.Telem DA, Schiano T, Goldstone R, et al. Factors that predict outcome of abdominal operations in patients with advanced cirrhosis. Clin Gastroenterol Hepatol. 2010;8:451–457. doi: 10.1016/j.cgh.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Finucci G, Desideri A, Sacerdoti D, et al. Left ventricular diastolic dysfunction in liver cirrhosis. Scand J Gastroenterol. 1996;31:279–284. doi: 10.3109/00365529609004879. [DOI] [PubMed] [Google Scholar]
- 8.Grose RD, Nolan J, Dillon JF, et al. Exercise-induced left ventricular dysfunction in alcoholic and non-alcoholic cirrhosis. J Hepatol. 1995;22:326–332. doi: 10.1016/0168-8278(95)80286-x. [DOI] [PubMed] [Google Scholar]
- 9.Rabie RN, Cazzaniga M, Salerno F, et al. The use of E/A ratio as a predictor of outcome in cirrhotic patients treated with transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 2009;104:2458–2466. doi: 10.1038/ajg.2009.321. [DOI] [PubMed] [Google Scholar]
- 10.Cazzaniga M, Salerno F, Pagnozzi G, et al. Diastolic dysfunction is associated with poor survival in cirrhotic patients with transjugular intrahepatic portosystemic shunt. Gut. 2007;56:869–875. doi: 10.1136/gut.2006.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Møller S, Henriksen JH. Cardiovascular complications of cirrhosis. Postgrad Med J. 2009;85:44–54. doi: 10.1136/gut.2006.112177. [DOI] [PubMed] [Google Scholar]
- 12.Ruíz-Del-Árbol L, Achécar L, Serradilla R, et al. Diastolic dysfunction is a predictor of poor outcomes in patients with cirrhosis, portal hypertension and a normal creatinine. Hepatology. 2013;58:1732–1741. doi: 10.1002/hep.26509. [DOI] [PubMed] [Google Scholar]
- 13.Xu ZD, Xu HT, Li WW, Zou Z, Shi XY. Influence of preoperative diastolic dysfunction on hemodynamics and outcomes of patients undergoing orthotopic liver transplantation. Int J Clin Exp Med. 2013;6:351–357. [PMC free article] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereaux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1441–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Silverman DI, Manning WJ. Jones and Bartlett: Massachusetts; 2011. The complete guide to echocardiography. [Google Scholar]
- 17.Valeriano V, Funaro S, Lionetti R, et al. Modification of cardiac function in cirrhotic patients with and without ascites. Am J Gastroenterol. 2000;95:3200–3205. doi: 10.1111/j.1572-0241.2000.03252.x. [DOI] [PubMed] [Google Scholar]
- 18.Pozzi M, Carugo S, Boari G, et al. Functional and structural cardiac abnormalities in cirrhotic patients with and without ascites. Hepatology. 1997;26:1131–1137. doi: 10.1002/hep.510260507. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Solà J, Nicolás JM, Paré JC, Sacanella E, Fatjó F, Cofán M, Estruch R. Diastolic function impairment in alcoholics. Alcohol Clin Exp Res. 2000;24:1830–1835. [PubMed] [Google Scholar]
- 20.Befeler AS, Palmer DE, Hoffman M, Longo W, Solomon H, Di Bisceglie AM. The safety of intra-abdominal surgery in patients with cirrhosis: model for end-stage liver disease score is superior to Child-Turcotte-Pugh classification in predicting outcome. Arch Surg. 2005;140:650–654. doi: 10.1001/archsurg.140.7.650. [DOI] [PubMed] [Google Scholar]
- 21.Farnsworth N, Fagan SP, Berger DH, Awad SS. Child-Turcotte-Pugh versus MELD score as a predictor of outcome after elective and emergent surgery in cirrhotic patients. Am J Surg. 2004;188:580–583. doi: 10.1016/j.amjsurg.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 22.Teh SH, Nagorney DM, Stevens SR, et al. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology. 2007;132:1261–1269. doi: 10.1053/j.gastro.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 23.Harrington AN, Chu EW, Garg M, Divino CM. Serum markers for predicting abdominal surgery outcomes in patients with cirrhosis. J Gastrointest Surg. 2013;17:696–701. doi: 10.1007/s11605-013-2157-x. [DOI] [PubMed] [Google Scholar]
- 24.Csikesz NG, Nguyen LN, Tseng JF, Shah SA. Nationwide volume and mortality after elective surgery in cirrhotic patients. J Am Coll Surg. 2009;208:96–103. doi: 10.1016/j.jamcollsurg.2008.09.006. [DOI] [PubMed] [Google Scholar]


