Abstract
Background
Pancreatic cancer is a devastating disease with poor prognosis. It is characterized by a pronounced stromal reaction, which resists chemotherapeutics and effective tumor treatment. Pancreatic stellate cells (PSCs) are mainly responsible for this stromal reaction. Moreover, the cancer and stromal interaction seems to promote tumor proliferation. In this study, L49H37, a newly synthesized curcumin analog, was used as intervention to target the stromal compartment of pancreatic cancer.
Methods
In vitro cultures of human PSCs were exposed to curcumin and L49H37. Cell viability as well as growth promoting and survival signaling pathways were monitored by MTT, flow cytometry and western blotting.
Results
Curcumin and L49H37 effectively inhibited proliferation and induced apoptosis in PSCs. L49H37 was found to be more potent at a lower concentration than curcumin in the induction of apoptosis, as evidenced by cleaved poly (ADP-ribose) polymerase (PARP). The cells were retained in the G0/G1 phase of the cell cycle through the downregulation of p21WAF1/Cip1. L49H37 significantly decreased the phosphorylation of extracellular signal regulated kinase ½ (ERK½).
Conclusion
The results indicate that curcumin analog L49H37 exhibits more potent inhibitory effects than curcumin itself at a lower concentration, which suggests that it may have a potential for further evaluation of its use against pancreatic adenocarcinoma, either as a single agent but, more probable, as part of a combination regimen.
Keywords: Pancreatic cancer, stroma, cleaved PARP, p21WAF1/Cip1
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the 10th most common type of cancer in the world. It is a very aggressive malignant cancer derived from the exocrine part of the pancreas with a dismal prognosis. Due to late diagnosis, related to the usually quite vague symptoms, it is the fourth most common cause of cancer-related deaths in the Western society [1]. The overall 5-year survival rate among PDAC patients is less than 5%. PDAC is characterized by a pronounced desmoplastic (prominent fibrotic stroma) reaction. Normal stroma is necessary to provide a connective and functionally supportive framework in the pancreas. With the occurrence of preneoplastic lesions in the pancreas there is an enhanced stromal formation and progression to invasive carcinoma, characterized by a further increase in the dense reactive stroma [2]. The neoplastic stroma is very heterogeneous in nature. It is composed of non-cellular and cellular components, such as extracellular matrix (ECM) proteins, growth factors, cytokines, nerve fibers, various inflammatory cells, fibroblasts and activated PSCs surrounding the cancer cells, altogether considered to substantially contribute to the aggressive nature of pancreatic cancer, including the metastatic potential and the chemoresistant characteristics of PDAC [3,4].
The major cellular components of the desmoplastic reaction in PDAC are PSCs and fibroblasts. PSCs are considered as key players in the desmoplastic response in pancreatic cancer. In normal tissue they appear in quiescent fat storing phenotypes expressing intermediate cytofilamentous proteins, such as vimentin, desmin and glial fibrillary acidic protein [5]. Following pancreatic injury, such as pancreatitis or pancreatic cancer, this quiescent fat storing phenotype PSCs lose their vitamin A droplets and transform themselves into highly activated myofibroblast-like phenotypes, and start expressing α-smooth muscle actin as trans-differentiation marker. This activated form of PSCs increase their proliferation rate and start producing different growth factors, cytokines, ECM proteins, matrix metalloproteinases and tissue inhibitors of metalloproteinases [6-8]. The similar pathological features were previously described for hepatic stellate cells (HSCs). HSCs and PSCs share numerous characteristics as indicated by morphological, functional and gene expression studies [9,10].
Despite some limited improvement over the past decades in the overall treatment of pancreatic cancer, the survival rate of patients with disease progression has not significantly improved. The first-line therapy over the years against pancreatic cancer has been gemcitabine, a nucleoside analog, which inhibits tumor growth by blocking DNA synthesis mechanism, although it provides limited survival benefits. Hence, there is a need for novel therapeutics, early detection, and chemotherapy strategies to improve survival and render a curative potential in pancreatic cancer [11].
Curcumin (diferuloyl-methane) is an active constituent extracted from the rhizomes of the turmeric plant (curcuma longa), a yellow color powder commonly used as a spice in Asian cuisines and extensively utilized in ayurvedic herbal remedies [12]. Over the past few decades many scientific and clinical studies have focused on the potential of curcumin in treating various diseases due to its pharmacological safety. Curcumin has mainly been investigated for its anti-inflammatory and anti-oxidant properties. Studies on the anticancer effects of curcumin have been conducted, including pulmonary and pancreatic adenocarcinomas, prostate and breast cancer [13]. Curcumin has potent antiproliferative and pro-apoptotic activities, which are exerted through various molecular pathways. It is a well-known inhibitor of nuclear factor-κB, JNK and ERKpathways. These signal transduction pathways also play an important role in PSCs activities [14]. Although this plant-derived curcumin has shown promising anticancer results in in vitro settings, it has poor bioavailability [15]. Hence, we hypothesized that modified curcumin analogs may improve anticancer activity. To test this hypothesis, we have synthesized L49H37, a novel synthetic analog of curcumin in the search for novel therapy alternatives in pancreatic cancer.
In this study, we compared curcumin and L49H37, for their ability of inhibiting and inducing apoptosis in PSCs in vitro at different concentrations as a strategy to target the stromal compartment of pancreatic cancer.
Materials and methods
Cell culture
A conditionally generated, immortalized human PSCs line described previously by Rosendahl et al was used for the experiments [16]. Briefly, the PSCs were maintained in RPMI1640 (catalog # 21875-034, Gibco-Life technologies, Paisley, UK) supplemented with 10% FBS (catalog # 10270, Gibco-Life technologies, Paisley, UK), Insulin-Transferrin-Selenium (catalog # 41400-045, Gibco-Life technologies, Grand Island, NY, USA) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin; catalog # 15140-122, Gibco-Life technologies, Paisley, UK) in a humidified 5% CO2 atmosphere at 33°C. Once PSCs became confluent, cells were trypsinized and reseeded in fresh flasks. After 24 h, flasks were shifted from 33°C to 37°C and grown to confluence for an additional 6 days. The thermo switching to 37°C switches off T antigen expression, thus causing minimal interference with native cell phenotype.
HL-7702, a human normal cell line was purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (catalog # GNHu 6) and were maintained in RPMI1640 supplemented with 10% FBS and antibiotics.
All experiments were performed in phenol red and serum-free DMEM/Hams Nutrient Mix F12 medium (catalog # 11039-021, Gibco-Life technologies, Paisley, UK), supplemented with penicillin and streptomycin (as above), 2 mM L-glutamine (catalog # 25030-024, Gibco-Life technologies, Paisley, UK), 1.2 mg/mL sodium bicarbonate (catalog# S5761, Sigma, Steinheim, NRW, Germany), 0.2 mg/mL BSA (catalog # A4503, Sigma, Steinheim, NRW, Germany) and 0.01 mg/mL transferrin (catalog # T1147, Sigma, Steinheim, NRW, Germany) serum-free medium (SFM).
Compounds
Preparation of curcumin stock solution
Curcumin (catalog # 28260, Fluka, Steinheim, NRW, Germany) was purchased from Sigma Aldrich. It was dissolved in dimethyl sulfoxide (DMSO) (catalog # D-5879, Sigma, St. Louis, MO, USA) and filtered through a 0.2 µm sterile polyethersulfone membrane filter (catalog # 514-0073, VWR, USA) and the stock solution was stored at 100 mM.
Synthesis of L49H37
To a stirred solution of 3,4,5-trimethoxybenzaldehyde (156.96 mg, 0.80 mmol) and 1-methylpiperidin-4-one (45.36 mg, 0.40 mmol) in ethanol (10 mL), 40% sodium hydroxide was added drop wise at room temperature. After 10 h, the resulting mixture was diluted with H2O (15 mL) and extracted with ethyl acetate. The combined organic layers were washed with brine (15 mL) and dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The residue was further purified by chromatography on silica gel to yield 150.24 mg of L49H37 [(3E,5E)-1-Methyl-3,5-bis(3,4,5-trimethoxybenzylidene)piperidin-4-one] as a yellow solid (80%). It was dissolved in DMSO and filtered through a 0.2 µm sterile polyethersulfone membrane filter and the stock solution was stored at 50 mM.
The stability test for curcumin and L49H37 was performed using a reverse phase (High Performance Liquid Chromatography) HPLC (Agilent Technologies 1260 Infinity, Santa Clara, CA, USA). Briefly, 20 μL of 1 mg/mL curcumin and L49H37 (dissolved in methanol) were added to 980 μL of 0.1 M phosphate buffer (pH 7.4). Samples were incubated at 37°C for indicated times. After incubation, 200 μL of mixtures were detected by HPLC with a mobile phase of methanol and water.
MTT proliferation assay
Proliferation assay was carried out on PSCs (9×103 cells/well) in 96-well plates in growth media for 24 h before switching to SFM for a further 24 h. SFM was added to each well in order to synchronize cells to a resting stage. Cells were subsequently dosed with increasing concentrations of curcumin (0-25 µM) or L49H37 (0-5 µM) in SFM in triplicates. After 24 h of incubation, cell proliferation assay using the MTT; Cell Proliferation Kit I (catalog # 11465007001, Roche, Mannheim, BW, Germany) was performed according to the manufacturer’s instructions. The absorbance was measured on a Labsystems Multiskan Plus plate reader (test wavelength 595 nm, reference wavelength 660 nm) using the DeltaSoft JV software (BioMetallics Inc., Princeton, NJ, USA).
The HL-7702 cells were seeded (7×103 cells/well) in 96-well plate in RPMI1640 medium. The cells were maintained at 37°C in 5% CO2. Cells were incubated with L49H37 for 24 h before the MTT assay. A fresh solution of MTT (5 mg/mL) prepared in NaCl solution (0.9%) was added to each single well of the 96-well plate. After the 4 h of incubation, cells were dissolved with 180 mL of DMSO, and then analyzed in a multi-well-plate reader at 490 nm.
Cell cycle analysis by flow cytometer
PSCs were seeded in 6-well culture plates (5.5×105 cells/well) for 24 h in growth media and then replaced with SFM for a further 24 h to synchronize the cells. Cells were exposed to increasing concentrations of curcumin (0-25 µM) or L49H37 (0-5 µM) in SFM for 24 h. Both floating and adherent cells were collected, fixed in 70% cold ethanol and incubated overnight at 4°C. Thereafter, cells were stained with propidium iodide/RNAse buffer (catalog # 550825, BD Pharmingen, San Diego, CA, USA), and then cell cycle distribution was analyzed using accuri c6 flow cytometer (Catalog # SY-01, BD Biosciences, St. Ives, Cambs, UK). Approximately 20,000 events (cells) were collected for each sample and data analyzed using accuri c6 flow cytometer software. Three independent experiments were performed.
Western immunoblotting
Cells (5.5×105/well) were grown in 6-well plates for 24 h. After an additional 24 h in SFM, the cells were treated with increasing concentrations of curcumin (0-25 µM) or L49H37 (0-5 µM) in SFM for 24 h, and then lysed on ice for 10 min in lysis buffer (10 mM Tris-HCl, 50 mm NaCl, 5 mM EDTA, 30 mM sodium pyrophosphate, 50 mM sodium fluoride, 100 µM sodium orthovanadate, 1% Triton X100, pH 7.6) containing protease and phosphatase inhibitor cocktails (catalog # P8340/catalog # P5726, Sigma-Aldrich, Steinheim, NRW, Germany). The protein concentration was determined using BCA protein assay reagent kit (catalog # 23223/catalog # 23224, Pierce, ThermoScientific, Rockford, IL, USA). Lysates were dissolved in Laemmli buffer, boiled for 5 min and separated by SDS-PAGE (8%) and transferred to 0.2 μm Hybond-C extra nitrocellulose membrane (catalog # RPN303E, Amersham Biosciences, Little Chalfont, BKM, UK). The blocked membranes were probed overnight (4°C) with the following antibodies: anti-cleaved poly (ADP-ribose) polymerase (PARP) (Asp214) (catalog # 9541, Cell Signaling, 1:1000), anti-phospho-p44/p42 MAPK (Thr202-Tyr204) (catalog # 9106, Cell Signaling; 1:2000), anti-GAPDH clone 6C5 (catalog # MAB374, Millipore; 1:1000) and anti-p21WAF1/Cip1 clone CP74 (catalog # 05-345, Millipore; 1:750). Subsequently, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody and visualized by SuperSignal West Femto Substrate (catalog # 34095, ThermoScientific, Rockford, IL, USA) using LI-COR Odyssey Fc imaging system and Image studio version 3.1 software (Li-Cor, Bad Homburg, HE, Germany).
Statistical analysis
The results were expressed as mean ± S.E. (n=3) from at least three independent experiments. Statistical significance was determined by one- or two-way analysis of variance (ANOVA). A P-value of <0.05 was considered statistically significant.
Results
Stability analysis of curcumin and L49H37
Evidence suggests that the active methylene group and β-diketone moiety in curcumin structure contribute to the instability under physiological conditions, which induce rapid degradation and metabolism of curcumin. Therefore, we have chosen to delete the β-diketone moiety acquiring mono-carbonyl analog of curcumin (Fig. 1), which shows a greater metabolic stability and comparable bioactivity than curcumin (Fig. 2).
Figure 1.
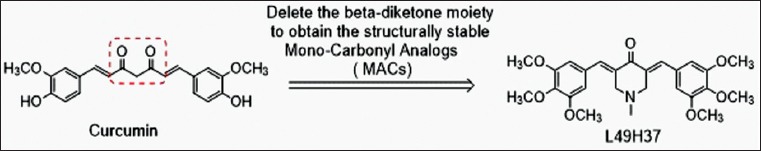
Deletion of β-diketone moiety from curcumin to acquire mono-carbonyl analog of curcumin (L49H37)
Figure 2.
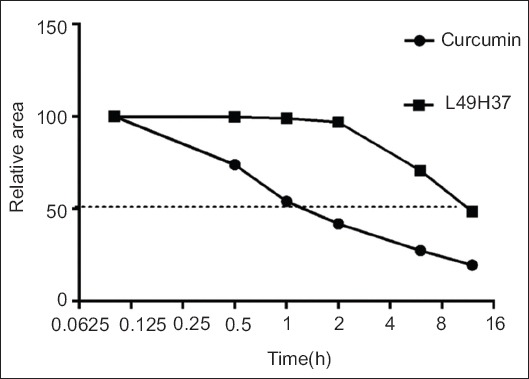
Stability analysis of curcumin and L49H37 by using HPLC system
Curcumin and L49H37 treatment reduces PSC proliferation
In the pilot experiments, we have exposed PSCs to the increasing concentrations of curcumin and L49H37. The dose response curve was obtained at different concentrations (data not shown) for curcumin and L49H37. Based on these results, we used following doses (curcumin 1, 10 and 25 µM; and L49H37 1, 2.5 and 5 µM) for the subsequent experiments. PSCs proliferation/survival was assessed by MTT assay. Exposure to curcumin (0-25 µM) and L49H37 (0-5 µM) in SFM for 48 h, inhibited the growth of PSCs with increasing concentration (Fig. 3C). The antiproliferative effect of L49H37 was much more effective than that of curcumin even at lower concentrations of the drug. The maximum growth inhibition (65%) for PSCs with curcumin was observed at the highest concentration (25 µM; Fig. 3A). It was observed that curcumin up to 25 µM did not affect the cell viability, but further increase in concentration was found to be cytotoxic (data not shown). On the other hand, 52.2% inhibition was achieved with 2.5 µM of L49H37 (Fig. 3B), which was ten times less than that of curcumin (25 µM). The further increase in concentration (5 µM) of L49H37 did not increase the degree of inhibition. The effect of L49H37 on HL-7702, a human normal cell line, was observed (Fig. 1; Supplementary data sheet - see online version).
Figure 3.

Effect of curcumin and L49H37 on proliferation of pancreatic stellate cells (PSCs). PSCs were treated with curcumin (A) at indicated concentrations (0-25 µM) in serum-free medium and L49H37 (B) at indicated concentrations (0-5 µM) for 48 h and cell proliferation was assessed by MTT assay. The PSCs were treated with control (DMSO) or different concentrations of curcumin and L49H37 (C) Scale bar = 40 µm. Results represent the mean of three independent experiments. The indicated differences are significant ** P<0.01, *** P<0.001 compared to the control (DMSO) group
Effects of curcumin and L49H37 on the cell cycle
We analyzed the effects of curcumin and L49H37 on PSCs cell cycle. The cell cycle analysis of PSCs demonstrated (Fig. 4A) that there was an accumulation of cells in G0/G1 phase upon 24 h treatment of both compounds. The two-way ANOVA with Dunnett’s multiple comparison test was performed and we found that 1 µM (81.8%; P<0.04) and 2.5 µM (83.9%; P<0.001) concentrations of L49H37 resulted in cell arrest at G0/G1 phase compared to control cells in G0/G1 phase (79%), while 25 µM of curcumin arrested the cells in G0/G1 phase (82.6%; P<0.01) (Fig. 4C). When both treatments were compared, the concentration of L49H37 required was 10 times less than that of curcumin (Fig. 4B). The cell death was also assessed by measuring cleaved PARP, a classical marker for apoptotic cells. The curcumin treatment induced apoptosis in PSCs at the highest concentration of curcumin used (25 µM), while more pronounced apoptosis was observed at 2.5-5 µM of L49H37 (Fig. 4D), which indicates that L49H37 was more potent as compared to curcumin.
Figure 4.
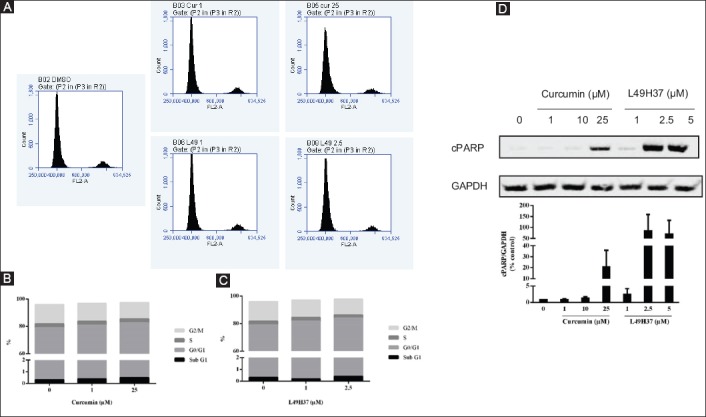
Cell cycle distribution and induction of apoptosis after 24 h of curcumin and L49H37 treatment in pancreatic stellate cells (PSCs). The cell cycle histograms (A) of PSCs after treatment with curcumin and L49H37. Percentages of cells in Sub-G1 (apoptotic), G0/G1, S and G2/M phases are shown (B and C). Western blot for cleaved PARP cleavage from cell lysates of PSCs treated for 24 h in serum-free medium with indicated concentrations of curcumin and L49H37. One representative blot of two independent experiments is shown. The values represent the mean ± standard error of 2 experiments and are expressed as percentage of the control. (D) The FACS data shown is representative of at least two independent experiments
Effect of curcumin and L49H37 treatment on phospho-ERK1/2
The effects of curcumin and L49H37 treatment were investigated on growth and survival signaling pathway of PSCs. The enhanced proliferative effect of PSCs at lower (1 µM and 10 µM) concentrations of curcumin and the lowest (1 µM) concentration of L49H37 showed increased phosphorylation of ERK1/2. However, the highest concentration (25 µM) of curcumin decreased the phosphorylation of ERK1/2. Increasing the concentrations (2.5 µM and 5 µM) of L49H37 significantly reduced the phosphorylation of ERK1/2, indicating its involvement in growth inhibition (Fig. 5).
Figure 5.
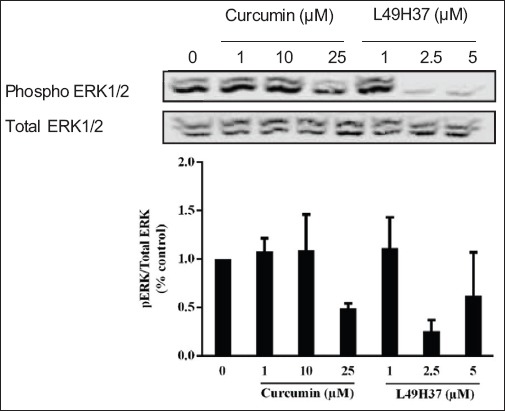
Curcumin and L49H37 downregulates phosphorylation of ERK1/2. Western blot of cell lysates collected from pancreatic stellate cells treated for 24 h in serum-free medium with indicated concentrations of curcumin and L49H37. One representative blot of two independent experiments is shown. The values represent the mean ± standard error of 2 experiments and are expressed as percentage of the control (DMSO)
Curcumin and L49H37 treatment downregulates the cell cycle regulatory protein p21WAF1/Cip1 in PSCs
After curcumin and L49H37 treatments in PSCs cell cycle analysis, it was observed that cells were accumulated in the G0/G1 phase. In this regard, we further examined the major cell cycle transition regulator p21WAF1/Cip1 after 24 h (Fig. 6). The low dose of L49H37 increased the expression levels of p21WAF1/Cip1 consistent with the increased proliferation observed at this concentration. However, p21WAF1/Cip1 was decreased with increasing concentration, which explains the increased number of cells in G0/G1 phase of the cell cycle.
Figure 6.
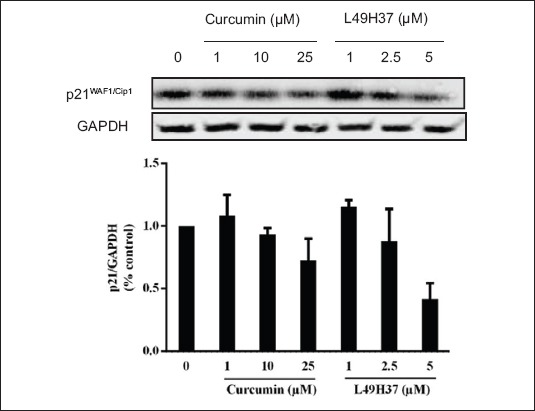
Curcumin and L49H37 downregulates p21WAF1/Cip1. Western blot of cell lysates collected from pancreatic stellate cells treated for 24 h in serum-free medium with indicated concentrations of curcumin and L49H37. GAPDH is shown as loading control. One representative blot of two independent experiments is shown. The values represent the mean ± standard error of 2 experiments and are expressed as percentage of the control (DMSO)
Discussion
There are various preclinical studies on curcumin showing that it can modulate the growth of cancer cells through regulation of multiple cell signaling pathways, thereby exerting chemopreventive as well as chemotherapeutic activities in vitro and in xenograft models [17]. In this study, we have investigated the effects of curcumin and the curcumin analog L49H37 on PSCs. Both treatments effectively inhibited proliferation and induced apoptosis in PSCs. L49H37 was found to be more potent as much as 10 times at lower concentration than curcumin in the induction of apoptosis, as evidenced by cleaved PARP. The cells were retained in the G0/G1 phase of the cell cycle through the downregulation of p21WAF1/Cip1. L49H37 significantly decreased the phosphorylation of ERK 1/2, a known regulator of cell proliferation and growth.
PDAC is characterized by a prominent desmoplastic reaction, which recently has been suggested to be involved in chemoresistance in pancreatic cancer [18]. There is increasing evidence demonstrating that the targeting of the stromal compartment of pancreatic cancer may have antitumor effects and enhance sensitivity to radiation and chemotherapy. PSCs, the principle source of the stromal reaction in pancreatic cancer, provide a growth permissive niche for cancer cells to grow and spread. There are potential ways to reduce this stromal reaction. It can be achieved by inhibiting the activation and proliferation of PSCs, or by inducing apoptosis in PSCs. This depletion of stroma may reduce cancer progression and increase drug delivery into the tumor [19,20].
In this study the in vitro antitumor activity of curcumin and its novel synthetic analog L49H37 was investigated against PSCs. It was found that the antiproliferative effect of L49H37 (2.5 µM) significantly inhibited the PSCs proliferation as compared to curcumin (25µM). This inhibition was observed at about a ten-fold lower concentration, indicating its potency. Prior to apoptotic induction, treatment of curcumin and L49H37 caused a significant cell cycle arrest in PSCs, where cells were arrested at the G0/G1 phase. In efforts to understand the molecular mechanisms for the cell cycle arrest caused by curcumin and L49H37, we examined changes at cell cycle regulatory protein levels. This cell arrest occurred through downregulation of p21WAF1/Cip1 dependent cell cycle arrest at the G0/G1 phase with increasing concentration. It was previously shown that curcumin induced apoptosis and decreased the expression of p21WAF1/Cip1 in human colon cancer [21].
It was previously shown in non–small-cell lung cancer cell lines that curcumin treatment has decreased the phosphorylation of ERK [22] and similar kind of effect was observed in MDA-MB-231 triple-negative breast cancer cell line [23]. In HSCs, curcumin has been reported to inhibit proliferation and activation by a reduction in ERK phosphorylation [24]. Furthermore, we observed that the highest (25 µM) concentration of curcumin and 2.5-5 µM of L49H37 decreased the expression of phosphorylated ERK, which inhibited the proliferation of PSCs. These results are also in line with previously published results [14].
The anti-cancer effect of curcumin has been observed in several clinical trials, including pancreatic cancer [25]. Albeit curcumin has an evident anti-cancer activity in humans, rapid degradation and low bioavailability have been put forth as limitations for therapeutic applications. To enhance metabolic stability and pharmacological potency, we synthesized the novel curcumin analog L49H37, a cyclocurcumin resulting in better growth suppressing effects and enhanced stability in vitro. Further strategies to improve the biological activity of L49H37 may include the use of drug delivery vehicles, such as nanoparticles to increase circulation times in vivo and increase accumulation in target areas [26,27].
To summarize, the present study demonstrated that curcumin and L49H37 inhibit PSC proliferation and induce apoptosis in PSCs through downregulation of p21WAF1/Cip1 dependent cell cycle arrest at the G0/G1 phase. As a single agent, curcumin exerted its effect on PSCs. The poor bioavailability and low potency of curcumin when applied in humans limits its potential clinical beneficial effects. The L49H37 analog is several times more potent than curcumin. Thus, L49H37 may be a promising therapeutic agent in pancreatic cancer as a single agent, or as part of combination chemotherapy regimens. Additional studies are warranted to further elucidate both mechanisms and effects.
Summary Box.
What is already known:
Pancreatic cancer is characterized by a pronounced stromal reaction
This stromal reaction promotes tumor progression and contributes to drug resistance
Pancreatic stellate cells (PSCs) are mainly responsible for the excess collagenous stromal deposition
What the new findings are:
In this study, L49H37, a newly synthesized curcumin analog, was used as intervention to target the stromal compartment of pancreatic cancer
The results indicate that the curcumin analog L49H37 exhibits more potent inhibitory effects against PSCs than curcumin itself at a lower concentration, and may be used as a novel treatment strategy in pancreatic cancer
Acknowledgments
The manuscript was supported by grants from the Medical Faculty at Lund University. We would like to thank Ramasri Sathanoori for her technical help with FACS, Srikanth Ranganathan and Parveen Kumar for their scientific guidance.
Biography
Lund University, Sweden; Skåne University Hospital Lund, Sweden; Wenzhou Medical University, Zhejiang, China; University of Bristol, Learning and Research Building, Southmead Hospital, UK
Appendix A. Supplementary data
Figure 1.
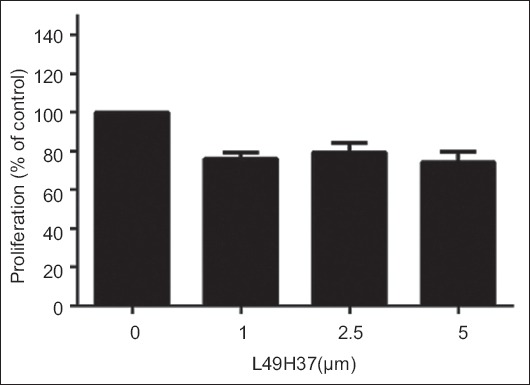
Effect of L49H37 on proliferation of HL7702. HL7702 cells were treated with L49H37 at indicated concentrations (0-5 μM) for 24 h and cell proliferation was assessed by MTT assay
Footnotes
Conflict of Interest: None
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Korc M. Pancreatic cancer-associated stroma production. Am J Surg. 2007;194:s84–s86. doi: 10.1016/j.amjsurg.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 4.Welsch T, Kleeff J, Friess H. Molecular pathogenesis of pancreatic cancer: advances and challenges. Curr Mol Med. 2007;7:504–521. doi: 10.2174/156652407781387082. [DOI] [PubMed] [Google Scholar]
- 5.Apte MV, Park S, Phillips PA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Duner S, Lopatko Lindman J, Ansari D, Gundewar C, Andersson R. Pancreatic cancer: the role of pancreatic stellate cells in tumor progression. Pancreatology. 2010;10:673–681. doi: 10.1159/000320711. [DOI] [PubMed] [Google Scholar]
- 7.Tang D, Wang D, Yuan Z, et al. Persistent activation of pancreatic stellate cells creates a microenvironment favorable for the malignant behavior of pancreatic ductal adenocarcinoma. Int J Cancer. 2013;132:993–1003. doi: 10.1002/ijc.27715. [DOI] [PubMed] [Google Scholar]
- 8.Masamune A, Shimosegawa T. Signal transduction in pancreatic stellate cells. J Gastroenterol. 2009;44:249–260. doi: 10.1007/s00535-009-0013-2. [DOI] [PubMed] [Google Scholar]
- 9.Mitra A, Satelli A, Yan J, et al. IL-30 (IL27p28) attenuates liver fibrosis through inducing NKG2D-rae1 interaction between NKT and activated hepatic stellate cells in mice. Hepatology. 2014;60:2027–2039. doi: 10.1002/hep.27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erkan M, Adler G, Apte MV, et al. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut. 2012;61:172–178. doi: 10.1136/gutjnl-2011-301220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stan SD, Singh SV, Brand RE. Chemoprevention strategies for pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2010;7:347–56. doi: 10.1038/nrgastro.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun L, Liu J, Lin SS, et al. Potent anti-angiogenic activity of B19--a mono-carbonyl analogue of curcumin. Chin J Nat Med. 2014;12:8–14. doi: 10.1016/S1875-5364(14)60002-9. [DOI] [PubMed] [Google Scholar]
- 14.Masamune A, Suzuki N, Kikuta K, Satoh M, Satoh K, Shimosegawa T. Curcumin blocks activation of pancreatic stellate cells. J Cell Biochem. 2006;97:1080–1093. doi: 10.1002/jcb.20698. [DOI] [PubMed] [Google Scholar]
- 15.Shehzad A, Wahid F, Lee YS. Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm (Weinheim) 2010;343:489–499. doi: 10.1002/ardp.200900319. [DOI] [PubMed] [Google Scholar]
- 16.Rosendahl AH, Gundewar C, Said Hilmersson K, Ni L, Saleem MA, Andersson R. Conditionally immortalized human pancreatic stellate cell lines demonstrate enhanced proliferation and migration in response to IGF-I. Exp Cell Res. 2015;330:300–310. doi: 10.1016/j.yexcr.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 17.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 18.Wilson JS, Pirola RC, Apte MV. Stars and stripes in pancreatic cancer: role of stellate cells and stroma in cancer progression. Front Physiol. 2014;5:52. doi: 10.3389/fphys.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rucki AA, Zheng L. Pancreatic cancer stroma: understanding biology leads to new therapeutic strategies. World J Gastroenterol. 2014;20:2237–2246. doi: 10.3748/wjg.v20.i9.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su CC, Lin JG, Li TM, et al. Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+and the activation of caspase-3. Anticancer Res. 2006;26:4379–489. [PubMed] [Google Scholar]
- 22.Tsai MS, Weng SH, Kuo YH, Chiu YF, Lin YW. Synergistic effect of curcumin and cisplatin via down-regulation of thymidine phosphorylase and excision repair cross-complementary 1 (ERCC1) Mol Pharmacol. 2011;80:136–146. doi: 10.1124/mol.111.071316. [DOI] [PubMed] [Google Scholar]
- 23.Sun XD, Liu XE, Huang DS. Curcumin induces apoptosis of triple-negative breast cancer cells by inhibition of EGFR expression. Mol Med Rep. 2012;6:1267–1270. doi: 10.3892/mmr.2012.1103. [DOI] [PubMed] [Google Scholar]
- 24.Smith MR, Gangireddy SR, Narala VR, et al. Curcumin inhibits fibrosis-related effects in IPF fibroblasts and in mice following bleomycin-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;298:L616–L625. doi: 10.1152/ajplung.00002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. Aaps j. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today. 2012;17:71–80. doi: 10.1016/j.drudis.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vyas A, Dandawate P, Padhye S, Ahmad A, Sarkar F. Perspectives on new synthetic curcumin analogs and their potential anticancer properties. Curr Pharm Des. 2013;19:2047–2069. [PMC free article] [PubMed] [Google Scholar]


