Abstract
Background
There is a high prevalence of smoking among caregivers who bring their children to the pediatric emergency department (PED), and even higher rates of tobacco smoke exposure (TSE) and related morbidity among their children. The PED visit presents an opportunity to intervene with caregivers, but it is unknown whether they are more likely to quit if their child has a TSE-related illness. We sought to examine a PED-based smoking cessation intervention, and compare outcomes based on children’s TSE-related illness.
Methods
A single-arm, prospective trial, with baseline, 3 and 6 month assessments. Caregivers whose child had either a TSE-related (n=100) or non TSE-related illness (n=100) were given a brief intervention consisting of counseling, referral to the Quitline, and free NRT.
Results
Participants were: 91.5% female; 50.5% African American; 100% Medicaid recipients; 30.8 years old; child age mean 5.5; 90% highly nicotine dependent; 60.3% and 75.8% allowed smoking in the home and car, respectively. At follow-up (65% retention): 80% reported quit attempts at 3 months, and 89% between 3 and 6 months. There were significant decreases in number of cigarettes smoked, time to first cigarette, and smoking in the home and car. Quit rates were 12.2% at 3 months, 14.6% at 6 months, and 7.3% at both time points (50% biochemically confirmed). There were no significant differences in outcomes based on children’s illness.
Conclusions
A brief PED-based smoking cessation intervention resulted in quit attempts and successful quits. However, the presence of a TSE-related illness did not result in different cessation outcomes.
Keywords: smoking, tobacco, parent, secondhand smoke, tobacco smoke, tobacco control, smoking cessation
Introduction
An estimated 11 million low-income caregivers who smoke accompany their children to the pediatric emergency department (PED) annually.1–3 Children from low-income households are at higher risk for tobacco smoke exposure (TSE) and TSE-related morbidity compared to children from households above the poverty level.4–7 Compared to higher income smokers, low-income smokers are at increased risk for tobacco-related disparities such as lower cessation rates, higher smoking-related illnesses, and lower access to cessation resources. The PED is a novel, yet largely underutilized setting in which to address these disparities. Much of the care delivered in the PED is non-emergent, and these visits are characterized by long wait times. This presents a unique opportunity to help caregivers quit smoking, and thus, reduce their children’s TSE.
Our previous cessation research indicates that caregivers who visit the PED are aware of the pediatric effects of TSE, are motivated to quit, and eager to receive cessation counseling.8–11 The Health Belief Model12 posits that one’s beliefs about health problems, and the perceived benefits of action, explain engagement in health-promoting behavior, such as smoking cessation. Additionally, the model posits that a stimulus, or cue to action, such as a child’s acute illness, must also be present in order to trigger smoking cessation.13,14 Prior research evaluated whether caregivers were more likely to quit smoking if a cessation intervention was presented as a way to improve their child’s health, with largely disappointing results.15 Our prior research demonstrated that caregivers who perceive that their child’s health is at risk due to TSE are more motivated to quit, and those who believe that quitting will benefit their child’s health are more likely to quit.16,17 However, it is not known if caregivers who smoke are more likely to quit if their child is brought to the PED for a TSE-related illness and receive an intervention that includes information on the effects of TSE on their child’s health. We hypothesized that the delivery of a brief cessation intervention to caregivers during the PED visit would be more effective for those whose child had a TSE-related illness versus those who did not. The primary aim of this study was to evaluate the feasibility and overall efficacy of a brief PED-based tobacco cessation intervention among low-income caregivers who brought their child to the PED and to compare efficacy in caregivers whose child presented with and without a TSE-related illness.
Method
Participant, Screening, and Recruitment
Participants were recruited between March 2012 and June 2013 from the PED of Cincinnati Children’s Hospital Medical Center (CCHMC). A clinical research coordinator (CRC) accessed the electronic health system to screen for eligible caregivers who: presented to the PED with a child <18 years of age who was triaged in the non-urgent or urgent category and were recipients of Medicaid (used as a proxy for low-income status). These caregivers completed a screening questionnaire which assessed demographics and smoking status. Those answering yes to: “Have you smoked at least one cigarette in the past week?” were considered current smokers. Caregivers were excluded if they: were enrolled in a cessation program or using nicotine replacement therapy (NRT) or other pharmacologic cessation treatment; were non-English or non-Spanish speaking; had no working phone number or mailing address; or had plans to move within eight months. To determine if there were differences in outcomes in caregivers whose child did or did not have a TSE-related illness, we recruited an equal number (N=100) of caregivers with a child with a potentially TSE-related chief complaint as outlined by the U.S. Surgeon General (e.g., colds, ear pain, wheezing),18 and a non-TSE-related illness (N=100). Eligible caregivers provided informed consent. This study was approved by the Institutional Review Board at CCHMC.
Assessments
Demographics were collected at baseline: caregiver and child age, gender, race/ethnicity, and education level. We also assessed caregivers’ smoking behavior, nicotine dependence and prior quit attempts. To determine nicotine dependence, we used the Heavy Smoking Index (HSI) which is a validated, 2-item self-report measure of nicotine dependence (range 0–8) derived from the Fagerstrom Test for Nicotine Dependence.19,20,21 Caregivers were assessed for level of readiness to quit using the Contemplation Ladder (range 0–10).22 Caregivers also reported smoking at home and in the car. To assess caregivers’ perception of their child’s smoking related health risks, we used measures adapted from Wagener et al.23 which assess perceived child health risk due to caregiver smoking, and perceived child health benefit to caregiver cessation (range 1–7). Caregivers were asked if they believed that their child’s PED visit was due to a TSE-related illness (Yes/No). Finally, caregivers were queried regarding their acceptability of the cessation intervention (5-point Likert scale with responses ranging from “Strongly disagree” to “Strongly agree”).
The CRC conducted phone follow-up at 3 and 6 months after baseline to assess participants’ smoking behavior, nicotine dependence, quit attempts, readiness to quit, smoking in the home or car, and smoking abstinence (caregivers were asked to choose the response that best represented the amount they smoked “During the last 7 days.” Options were: “I smoked every day”, “I smoked once in a while”, and “I haven’t smoked at all, not even a puff”. Only those who selected the latter category were considered abstinent). The primary outcome measures were repeated point prevalence of smoking at 3 and 6 months, and prolonged abstinence at 6 months. Secondary outcomes included number of quit attempts, level of readiness to quit, participant retention, and use of cessation resources. Self-reported abstinence was verified during home visits with exhaled carbon monoxide testing using a Bedfont MicroSmokerlyzer™ machine with > 6 ppm as the cutoff indicating a positive smoking result.24
Smoking Cessation Intervention
Caregivers received a brief (10–15 minutes) counseling session by the CRC while their child was waiting to be evaluated by the physician. This dedicated CRC was trained by the PI (MMG) prior to the study and observed delivering the counseling for the first 10 participants. The counseling was based on the 5A’s of the PHS Clinical Practice Guideline for Treatment of Tobacco Dependence,25 highlighting the effects of TSE on children’s health. Participants responding positively to: “Do you want to quit in the next six months?” were given a description of the Ohio Quitline (QL), and then asked about their interest in being referred. For those participants responding negatively, the CRC used the 5R’s counseling technique to increase readiness to quit,25 and then gave an expanded description of the QL services. All participants were offered the opportunity to have a direct phone or fax referral to the QL during the PED visit. Those not wishing to be contacted by the QL were offered written QL cessation brochures. Counselors at the QL attempted to contact referred participants up to 3 times over a 7-day period. Caregivers contacted by the QL were provided stage-appropriate information and/or counseling. In the PED, participants who expressed an interest in receiving NRT were screened for eligibility. Eligible participants were given vouchers to be redeemed at CCHMC’s pharmacy for a free, 2-week supply of NRT (choice of gum, patch, or lozenge) with directions on proper use delivered by the CRC. Participants received gift cards of $10, $20, and $30, at baseline, 3 months, and 6 month follow-up, respectively; a bonus $10 gift card was received for completion of both follow-ups.
Analyses
Univariate analyses identified potential outliers and examined distributional properties of the dependent and independent variables. Bivariate analysis examined the relationships between dependent and independent measures. As we had measurements recorded over time, the statistical analysis used a method to account for these multiple measurements and also for any missing timepoints. The technique used is called a mixed model and uses generalized estimating equations, allowing examination of changes over time. The subject identifier is used in the regression model and is included as a random effect, as it is unique to each participant. Based on the literature, six pre-determined covariates (caregiver age, race and education, HSI, child age, and TSE-related illness)26–28 were included in the multivariable analyses, regardless of statistical significance. Adjusted odds ratios (AOR) and 95% CI’s are reported from the final models. All analyses were conducted using SAS® version 9.3 (SAS Institute, Cary, NC), PROC GENMOD was used for the repeated measures analysis. An a priori level of p<0.05 was considered statistically significant.
Results
Participant Characteristics
A total of 1809 caregivers were approached; 865 (47.8%) screened positive for current smoking but 355 (41%) refused further study screening. Of the remaining 510, 399 (78.2%) were current smokers with Medicaid. Of these, 252 (63.1%) were eligible; 212 (84.1%) consented; 12 were duplicates and were removed; thus 200 (79.4%) received the intervention and were included in the analysis (Figure 1). The sociodemographics, smoking behavior, and TSE-related characteristics of the study population at baseline are presented in Table 1.
Figure 1.
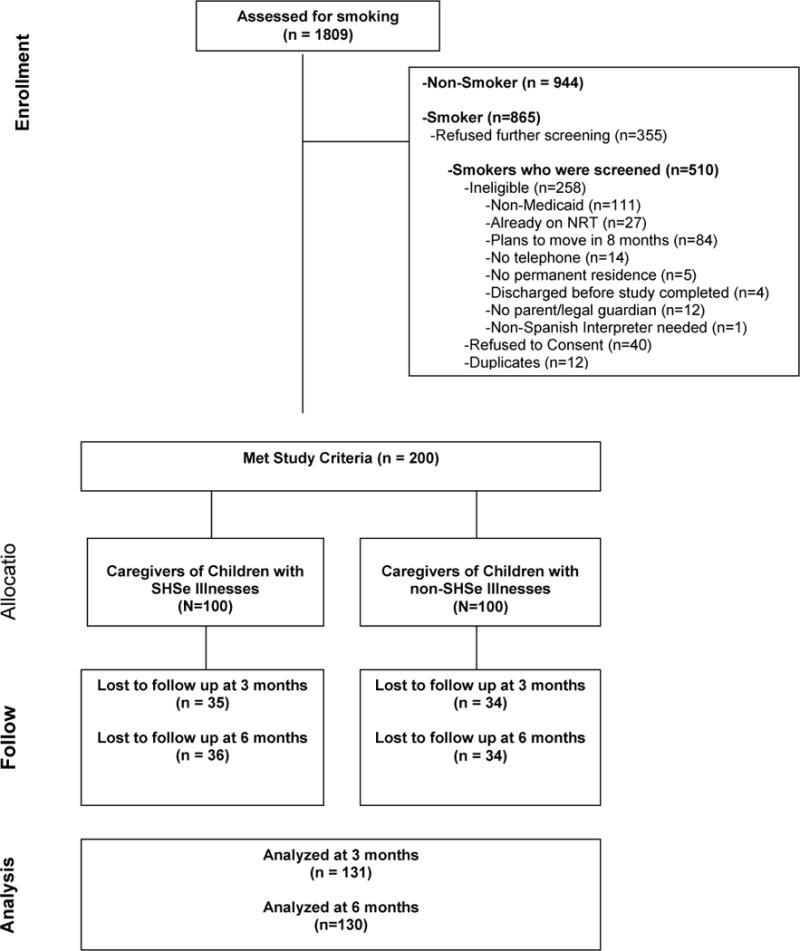
Flow of participants in the study
Table 1.
Population Demographics, Caregiver Smoking Belief, and Caregiver’s Beliefs
| Total Sample (N=200) N (%) |
TSE-Related Illness (N=100) N (%) |
Non-TSE-Related Illness (N=100) N (%) |
p Values | |
|---|---|---|---|---|
|
Parent age (mean, SD) <30 years ≥30 years |
30.8, 8.0 95 (48.2) 102 (51.8) |
29.3, 7.2 56 (57.1) 42 (42.9) |
32.3, 8.5 39 (39.4) 60 (60.6) |
0.01 0.01 |
| Child age (mean, SD) | 5.5, 5.0 | 4.0, 4.4 | 7.0, 5.3 | <0.0001 |
| Parent gender-female | 183 (91.5) | 90 (90.0) | 93 (93.0) | NS |
|
Parent Race White African American Asian, American Indian, Unspecified |
87 (43.7) 101 (50.8) 11 (5.5) |
43 (43.0) 52 (52.0) 5 (5.0) |
44 (44.4) 49 (49.5) 6 (6.1) |
NS |
|
Parent Education Less Than College Some College and above |
124 (62.3) 75 (37.7) |
61 (61.0) 39 (39.0) |
63 (63.6) 36 (36.4) |
NS |
|
Number of cigarettes/day 1–10 >10 |
100 (50.0) 100 (50.0) |
54 (54.0) 46 (46.0) |
46 (46.0) 54 (54.0) |
NS |
|
Minutes to first cigarette upon awakening 1–5 ≥6 |
100 (50.0) 100 (50.0) |
44 (44.0) 56 (56.0) |
56 (56.0) 44 (44.0) |
NS |
| Heavy smoking Index ≥ 4* | 179 (90.0) | 89 (89.0) | 90 (90.0) | NS |
| Motivation to quit (Range 0–10; Mean, SD) | 7.1, 2.3 | 7.1, 2.1 | 7.0, 2.5 | NS |
| Smoking not allowed inside the home | 79 (39.7) | 47 (47.0) | 32 (32.3) | 0.03 |
| Smoking not allowed inside the car | 29 (24.2) | 13 (23.6) | 16 (24.6) | NS |
| Caregiver’s Belief of child disease ≥ 50% likely if they do not stop smoking | 55 (27.6) | 28 (28.0) | 27 (27.3) | NS |
| Caregiver’s Belief that Child’s Health would get “much better” or “completely better” if they quit smoking | 87 (43.5) | 42 (42.0) | 45 (45.0) | NS |
| Caregiver’s Belief that Child’s PED visit is due to TSE | 14 (7.0) | 12 (12.0) | 2 (2.0) | <0.006 |
TSE-Related Characteristics
Of the total sample, 43.5% believed that their child’s health would improve if they stopped smoking but only 27.6% believed that their child would develop a serious disease if they continued smoking, and there were no differences in these beliefs if the child presented with TSE-related illness. When caregivers whose children presented with TSE-related complaints were compared to those whose children presented with a non-TSE-related complaint, they were more likely to: be younger, have a younger child, not allow smoking inside the home, and believe that their child’s visit was due to TSE. There were no other statistically significant differences in demographic or smoking-related measures related to TSE (Table 1).
Participant Retention
The CRC obtained questionnaires from 131 (65.5%) participants at 3-months, and 130 (65%) at 6-months; 109 (54.5%) were completed at both 3 and 6 months; 48 (24%) were lost to follow-up at both time points. The primary reasons we were unable to reach participants were no answer or the wrong telephone number given. Those who completed both 3- and 6-months were more likely to have older children (mean, SD 6.4, 5.2 years vs. 4.5, 4.6 years, p=0.008) and to be older than 30 years old (62.6% vs. 93.4%, p<0.001).
Changes in Caregiver Attitudes about TSE
There were no differences at 3- or 6-months in caregivers’ belief that their child’s health would worsen if they continued smoking, or that their child’s health would improve if they quit, comparing those whose child did and did not present with a TSE-related illness.
Changes in Smoking Behaviors
Of the 131 caregivers that we assessed at 3 months, 16 (12.2%) caregivers reported that they had quit at 3 months (9/16 [56%] completed the CO test; 7/16 [44%] biochemically confirmed). Of the 130 caregivers that we assessed at 6 months, 19 (14.6%) reported that they had quit at 6 months (8/19 [42%] completed the CO test; 6/19 [32%] biochemically confirmed). Of the 109 caregivers that we assessed at both 3 and 6 months, 8 (7.3%) reported that they had quit (5/8 [62.5%] completed both CO tests; 4/8 [50%] biochemically confirmed). The eight reporting quitting at both time points are included in the 16 and 19 that reported quitting at 3 months and 6 months, respectively. In addition, 80% of caregivers made a quit attempt between baseline and 3 months, and 89.2% made a quit attempt between 3 and 6 months. Using an intent to treat analysis, sixteen (8%) of caregivers reported that they had quit at 3 months, 19 (9.5%) reported that they had quit at 6 months, and eight (4%) reported that they had quit at both 3- and 6-month follow-ups.
As shown in Table 2, there were statistically significant changes from baseline to both 3- and 6-months in cigarettes smoked, time to first cigarette, and HSI. Whites had higher odds of smoking <10 cigarettes/day than African Americans (Adjusted Odds Ratio (AOR) 0.11, 95% CI 0.06–0.2), but also higher odds of smoking their first cigarette within five minutes of waking compared to African Americans (AOR 1.9, 95% CI 1.1–3.3). Additionally, Whites had higher odds of having HSI > 4 compared to African Americans (AOR 3.4, 95% CI 1.9–6.2), and caregivers older than age 30 had higher odds of an increased HSI compared to younger caregivers (AOR 2.2, 95% CI 1.1–4.2). There were significant increases in the number of participants who did not allow smoking in the home over time from baseline (39.7%) to 3 months (60.3%), (p<0.01), but not from baseline (39.7%) to 6 months (53.9%); Whites had higher odds of banning smoking in the home (AOR 1.8, 95% CI 1.1–3.1, p<0.05), compared to African Americans, as did non-heavy smokers compared to heavy smokers (AOR 1.8, 95% CI: 1.8, 1.0–3.1, p<0.05), and caregivers who had younger children (AOR 1.08, 95% CI: 1.01–1.14, p<0.05 for each year decrease in age). Similarly, there were decreases in the number of caregivers who smoked in their cars over time; caregivers with younger children had higher odds of not smoking in the car (AOR 1.19, 95% CI: 1.08–1.30, p<0.001 for each year decrease in age).
Table 2.
Changes in Smoking Characteristics
| Baseline (n=200) | 3 Month (n=131) | 6 Month (n=130) | P-value for Unadjusted/Adjusted* Gee Analysis over Time | Specific Difference in Adjusted Gee was present in Baseline to 3 months, 3months to 6 months, or Baseline to 6 months | |
|---|---|---|---|---|---|
|
Number of cigarettes/day 0–10 >10 |
100 (50.0) 100 (50.0) |
120 (91.6) 11 (8.4) |
117(90.0) 13 (10.0) |
<0.0001/<0.0001 |
Base to 3mo. (p<0.0001) Base to 6mo. (p<0.0001) |
|
Time to first cigarette (minutes) ≤ 5 ≥ 6 |
100 (50.0) 100 (50.0) |
22 (16.8) 109 (83.2) |
19 (14.6) 111 (85.4) |
<0.0001/<0.0001 |
Base to 3mo. (p<0.0001) Base to 6mo. (p<0.0001) |
| Heavy smoking ≥ 4** | 179 (90.0) | 54 (41.2) | 43 (33.1) | <0.0001/<0.0001 | Base to 3mo. (p<0.0001) Base to 6mo. (p<0.0001) |
| Motivation to quit (Range 0–10; Mean, SD) | 7.1, 2.3 | 7.6, 1.8 | 7.6, 2.0 | 0.01/0.3 | |
| Motivation to quit ≥ 7 (Range 0–10) | 110 (55.0) | 89 (77.4) | 87 (78.4) | <0.0001/0.001 | Base to 3mo. (p=0.001) Base to 6mo. (p=0.004) |
| Smoking not allowed inside the home | 79 (39.7) | 79 (60.3) | 70 (53.9) | <0.0001/0.023 | Base to 3mo. (p=0.008) |
| Smoking not allowed inside the car (N=120)*** | 29 (24.2) | 28 (40.0) | 32 (43.8) | 0.004/0.009 | Base to 3mo. (p=0.01) Base to 6mo. (p=0.002) |
Adjusted for second hand smoking related illness, heavy smoking, race, parent age group, parent education, and child age. However, heavy smoking was not used as a covariate in the model for number of cigarettes/day or time to first cigarette.
Heavy Smoking Index is a validated, 2-item self-report measure of nicotine dependence derived from the Fagerstrom Test for Nicotine Dependence (FTND);20,21,22 please see text.
80 caregivers did not own cars
There were no statistically significant differences in caregiver age, gender, education, prior quit attempts, or readiness to quit in those who reduced smoking at follow-up. However, a higher percentage of White caregivers reported smoking fewer cigarettes compared to all other races both at 3 months (68% vs. 40%, p<0.01) and at 6 months (69% vs. 40%, p<0.01). There were no differences in quit rates or reduction in smoking when we compared those who did and did not bring their child in for a TSE-related illness.
Use of Cessation Resources
Four participants accepted direct and 103 accepted fax QL referral. Of those who accepted fax referral, 25% reported on follow-up assessments that they had received QL counseling. All who expressed an interest in receiving NRT (N=184, 92%) were screened for NRT eligibility; 169 (92%) were eligible and received NRT vouchers. Of those given the voucher, 79 (47%) redeemed it; 41 (52%) received the patch, 29 (37%) the gum, and 9 (11%) the lozenge.
Consumer Satisfaction
Of the 198 (99%) caregivers who completed the acceptability survey, the majority strongly agreed or agreed that they were “satisfied” with the intervention (n=187, 94%), that the intervention was “useful” (n=187, 94%) and “interesting” (n=189, 95%). Additionally, the majority said they would “recommend this study to a friend or family member” (n=190, 96%), and that “smoking advice should be given in the PED” (n=182, 92%).
Discussion
To our knowledge, this is the first study to test the effects of a brief cessation intervention including NRT in the PED setting. Although we hypothesized that caregivers who brought their children in for a TSE-related illness would have higher cessation rates, our results did not support this hypothesis. Nor did we see differences in beliefs about the effects of smoking or the benefits of quitting on their child. Similar to our previous studies, only 28% of caregivers in our study whose child had a TSE-related illness believed that their smoking was putting their child’s health at risk, and 44% of caregivers believed that quitting would benefit their child.17 Our findings support previous primary care or hospital-based research that found no clear evidence of cessation success with caregivers whose children have a TSE-related or even a general “ill child” visit.29–32 However, a recent meta-analysis indicates that interventions designed to achieve cessation among caregivers for the sake of their children appear to increase quit rates, especially when interventions include the use of medications.15 Our lack of findings may be due to non-standardized information about how TSE relates to their child’s illness included in our intervention. Additionally, our lack of findings may be due to our broad definition of a TSE- related illness which included illnesses that are not as commonly recognized by caregivers as being associated with TSE, such as colds and ear infections. There were encouraging results in cessation, number of cigarettes smoked, nicotine dependency, readiness to quit, and reported TSE in the home and car. Moreover, similar to previous research, we found that caregivers who had younger children were more likely to have imposed smoking bans and may be more likely to perceive harm from smoking23 and to quit after an intervention.16
The current study is important for several reasons. First, it documents the feasibility and acceptability of a PED-based smoking cessation intervention for caregivers who are not the patient and are not expecting to be targeted in an intervention for themselves. We achieved high recruitment rates (84%) and the overwhelming majority (94%) found the intervention acceptable. Second, this study highlights the need for interventions that provide education for caregivers about the effects of smoke exposure on children. Caregivers did not understand the effects of TSE on their children or the benefits of quitting on their children’s health. Third, our results suggest that this intervention may be effective in reducing smoking, and increasing cessation among caregivers identified in the PED. Finally, this study highlights methodological issues related to evaluating a cessation intervention targeting caregivers recruited from the PED setting. Although the majority of caregivers welcomed the vouchers for NRT, only 47% actually picked up the NRT from the on-site pharmacy (which was just down the hall from the PED). It may have been better to provide caregivers with NRT during the PED visit. Additionally, we had difficulty with participant retention despite multiple contacts, generous incentives, and the option of home visits to caregivers who reported abstinence. Such difficulties with retention are common in low income populations.33
A number of limitations should be considered when interpreting these results. First, this was a small sample, limiting the overall power of the study. However, despite the sample size, a number of significant and important findings were detected related to cessation in this population that has not been extensively studied. In addition, the sample was drawn exclusively from a population of low-income smokers who presented to a Midwestern, tertiary care PED at one children’s hospital, which limits generalizability. On a related note, our sample was largely female which is due to fact that female caregivers are much more likely to bring their children to the PED than males.34 Greater efforts are needed to recruit and enroll male caregivers who smoke into PED-based interventions. Additionally, due to the low socioeconomic status of our PED population, we experienced a high attrition rate at follow up. Finally, no control condition was included to determine whether or not changes were due to the intervention or simply to the changing smoking patterns of caregivers; however based on our previous research in this setting, we know that it is unlikely that these caregivers would have been assisted in quitting without this intervention.28,35,36 Future PED-based efficacy trials should include a control arm and a larger sample of caregivers. Despite these limitations, results from our research may guide future research on conducting cessation interventions for low-income caregivers in the PED and other acute-care settings.
Conclusion
The results of our pilot study are promising, and suggest the need for further research in this area. The intervention model was viable and acceptable to caregivers, and there was preliminary evidence of efficacy. However, it is necessary to conduct full-scale randomized control effectiveness trials. Additionally, future studies need to improve retention rates in this transient, low-income population. Our findings did not show differences in cessation between caregivers whose children have/did not have TSE-related illnesses. Future research should focus on improving and testing the TSE intervention component. Encouragingly, our brief intervention prompted a substantial number of quit attempts, reduced cigarette consumption, increased smoking bans, and reduced smoking prevalence among this underserved population.
Acknowledgments
This study was funded by the National Institutes of Health National Cancer Institute grant K22CA163747 (to Dr. Mahabee-Gittens).
Funding Source: National Cancer Institute/National Institutes of Health K22CA163747 (Dr. Mahabee-Gittens)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURE: The authors have indicated that they have no financial relationships relevant to this article to disclose.
CONFLICT OF INTEREST: The authors have indicated that they have no conflicts of interest relevant to this article.
CLINICAL TRIAL REGISTRATION NUMBER: NCT01728038
References
- 1.Mahabee-Gittens EM, Gordon JS. Review of Adult Smoking Cessation Interventions Conducted in the Emergency Department and Application to the Pediatric Emergency Department Setting. US Respiratory Disease. 2008;4:125–8. [Google Scholar]
- 2.Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. National health statistics reports. 2008:1–38. [PubMed] [Google Scholar]
- 3.Bernstein SL, Cannata M. Nicotine dependence, motivation to quit, and diagnosis in emergency department patients who smoke. Addict Behav. 2006;31:288–97. doi: 10.1016/j.addbeh.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Fagan P, Shavers VL, Lawrence D, Gibson JT, O’Connell ME. Employment characteristics and socioeconomic factors associated with disparities in smoking abstinence and former smoking among U.S. workers. J Health Care Poor Underserved. 2007;18:52–72. doi: 10.1353/hpu.2007.0119. [DOI] [PubMed] [Google Scholar]
- 5.Moolchan ET, Fagan P, Fernander AF, et al. Addressing tobacco-related health disparities. Addiction. 2007;102(Suppl 2):30–42. doi: 10.1111/j.1360-0443.2007.01953.x. [DOI] [PubMed] [Google Scholar]
- 6.Fagan P, Moolchan ET, Lawrence D, Fernander A, Ponder PK. Identifying health disparities across the tobacco continuum. Addiction. 2007;102(Suppl 2):5–29. doi: 10.1111/j.1360-0443.2007.01952.x. [DOI] [PubMed] [Google Scholar]
- 7.Fagan P, Augustson E, Backinger CL, et al. Quit attempts and intention to quit cigarette smoking among young adults in the United States. Am J Public Health. 2007;97:1412–20. doi: 10.2105/AJPH.2006.103697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahabee-Gittens EM, Gordon J. Acceptability of tobacco cessation interventions in the pediatric emergency department. Pediatr Emerg Care. 2008;24:214–6. doi: 10.1097/PEC.0b013e31816a8d6f. [DOI] [PubMed] [Google Scholar]
- 9.Mahabee-Gittens EM, Huang B. ED environmental tobacco smoke counseling. Am J Emerg Med. 2005;23:916–8. doi: 10.1016/j.ajem.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Mahabee-Gittens M. Smoking in parents of children with asthma and bronchiolitis in a pediatric emergency department. Pediatr Emerg Care. 2002;18:4–7. doi: 10.1097/00006565-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Mahabee-Gittens M, Gordon J, Krugh M, Henry B, Leonard T. A smoking cessation intervention plus proactive quitline referral in the pediatric department: a pilot study. Nicotine & Tobacco Research. 2008;10:1745–51. doi: 10.1080/14622200802443494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenstock IM. The health belief model and personal health behavior. In: Becker MH, editor. The Health Belief Model and Preventive Health Behavior. Thorofare, NJ: Slack; 1974. pp. 27–59. [Google Scholar]
- 13.Velicer WF, Rossi JS, Diclemente CC, Prochaska JO. A criterion measurement model for health behavior change. Addict Behav. 1996;21:555–84. doi: 10.1016/0306-4603(95)00083-6. [DOI] [PubMed] [Google Scholar]
- 14.Crittenden KS, Manfredi C, Cho YI, Dolecek TA. Smoking cessation processes in low-SES women: the impact of time-varying pregnancy status, health care messages, stress, and health concerns. Addict Behav. 2007;32:1347–66. doi: 10.1016/j.addbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baxi R, Sharma M, Roseby R, et al. Family and carer smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochrane database of systematic reviews (Online) 2014;3:CD001746. doi: 10.1002/14651858.CD001746.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Mahabee-Gittens EM, Collins BN, Murphy S, et al. The parent-child dyad and risk perceptions among parents who quit smoking. Am J Prev Med. 2014;47:596–603. doi: 10.1016/j.amepre.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanis J, Byczkowski T, Mahabee-Gittens EM. Motivation to quit smoking in parental smokers in the pediatric emergency department. Pediatr Emerg Care. 2014;30:546–51. doi: 10.1097/PEC.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The health consequences of involuntary exposure to tobacco smoke : A Report of the Surgeon General. U.S. Dept of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Office of the Surgeon General; 2006. at http://www.cdc.gov/tobacco/data_statistics/sgr/2006/ [PubMed] [Google Scholar]
- 19.Piper ME, McCarthy DE, Baker TB. Assessing tobacco dependence: a guide to measure evaluation and selection. Nicotine Tob Res. 2006;8:339–51. doi: 10.1080/14622200600672765. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Rios M, Santiago-Perez MI, Alonso B, Malvar A, Hervada X, de Leon J. Fagerstrom test for nicotine dependence vs heavy smoking index in a general population survey. BMC Public Health. 2009;9:493. doi: 10.1186/1471-2458-9-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chabrol H, Niezborala M, Chastan E, de Leon J. Comparison of the Heavy Smoking Index and of the Fagerstrom Test for Nicotine Dependence in a sample of 749 cigarette smokers. Addict Behav. 2005;30:1474–7. doi: 10.1016/j.addbeh.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10:360–5. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- 23.Wagener TL, Gregor KL, Busch AM, McQuaid EL, Borrelli B. Risk perception in smokers with children with asthma. J Consult Clin Psychol. 2010;78:980–5. doi: 10.1037/a0021094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CC, Chang CH, Tsai YC, et al. Utilizing exhaled carbon monoxide measurement with self-declared smoking cessation: enhancing abstinence effectiveness in Taiwanese outpatients. The clinical respiratory journal. 2015;9:7–13. doi: 10.1111/crj.12096. [DOI] [PubMed] [Google Scholar]
- 25.Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update Clinical practice guideline. Vol. 5 Rockville, MD: US Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- 26.Winickoff JP, Healey EA, Regan S, et al. Using the postpartum hospital stay to address mothers’ and fathers’ smoking: the NEWS study. Pediatrics. 2010;125:518–25. doi: 10.1542/peds.2009-0356. [DOI] [PubMed] [Google Scholar]
- 27.Bock BC, Becker BM, Niaura RS, Partridge R, Fava JL, Trask P. Smoking cessation among patients in an emergency chest pain observation unit: outcomes of the Chest Pain Smoking Study (CPSS) Nicotine Tob Res. 2008;10:1523–31. doi: 10.1080/14622200802326343. [DOI] [PubMed] [Google Scholar]
- 28.Mahabee-Gittens EM, Gordon JS, Krugh ME, Henry B, Leonard AC. A smoking cessation intervention plus proactive quitline referral in the pediatric emergency department: a pilot study. Nicotine Tob Res. 2008;10:1745–51. doi: 10.1080/14622200802443494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Priest N, Roseby R, Waters E, et al. Family and carer smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochrane database of systematic reviews (Online) 2008:CD001746. doi: 10.1002/14651858.CD001746.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Wilson S, Farber H, Knowles SB, Lavori PW. A Randomized Trial of Parental Behavioral Counseling and Cotinine Feedback for Lowering Environmental Tobacco Smoke Exposure in Children With Asthma. CHEST Original Research Tobacco Cessation & Preventions. 2011;139:581–90. doi: 10.1378/chest.10-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakefield M, Banham D, McCaul K, et al. Effect of feedback regarding urinary cotinine and brief tailored advice on home smoking restrictions among low-income parents of children with asthma: a controlled trial. Prev Med. 2002;34:58–65. doi: 10.1006/pmed.2001.0953. [DOI] [PubMed] [Google Scholar]
- 32.Groner JA, Ahijevych K, Grossman LK, Rich LN. The impact of a brief intervention on maternal smoking behavior. Pediatrics. 2000;105:267–71. [PubMed] [Google Scholar]
- 33.Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC medical research methodology. 2014;14:42. doi: 10.1186/1471-2288-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alpern ER, Stanley RM, Gorelick MH, et al. Epidemiology of a pediatric emergency medicine research network: the PECARN Core Data Project. Pediatr Emerg Care. 2006;22:689–99. doi: 10.1097/01.pec.0000236830.39194.c0. [DOI] [PubMed] [Google Scholar]
- 35.Mahabee-Gittens EM, GJ Missed Opportunities to Intervene with Caregivers of Young Children Highly Exposed to Secondhand Tobacco Smoke. Prev Med. 2014 doi: 10.1016/j.ypmed.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahabee-Gittens EM, Stone L, Gordon JS. Pediatric Emergency Department Is a Promising Venue for Adult Tobacco Cessation Interventions. Nicotine Tob Res. 2013 doi: 10.1093/ntr/ntt069. [DOI] [PMC free article] [PubMed] [Google Scholar]


