Abstract
Fluctuations in circulating levels of ovarian hormones have been shown to regulate cognition (Sherwin and Grigorova, 2011. Fertil. Steril. 96, 399–403; Shumaker et al., 2004. JAMA. 291, 2947–2958), but increases in estradiol on the day of proestrus yield diverse outcomes: In vivo induction of long-term potentiation (LTP), a model of learning, is reduced in the morning, but optimal in the afternoon (Warren et al., 1995. Brain Res. 703, 26–30). The mechanism underlying this discrepancy is not known. Here, we show that impairments in both CA1 hippocampal LTP and spatial learning observed on the morning of proestrus are due to increased dendritic expression of α4βδ GABAA receptors (GABARs) on CA1 pyramidal cells, as assessed by electron microscopic (EM) techniques, compared with estrus and diestrus. LTP induction and spatial learning were robust, however, when assessed on the morning of proestrus in α4−/− mice, implicating these receptors in mediating impaired plasticity. Although α4βδ expression remained elevated on the afternoon of proestrus, increases in 3α-OH-THP (3α-OH-5α-pregnan-20-one) decreased inhibition by reducing outward current through α4βδ GABARs (Shen et al., 2007. Nat. Neurosci. 10, 469–477), in contrast to the usual effect of this steroid to enhance inhibition. Proestrous levels of 3α-OH-THP reversed the deficits in LTP and spatial learning, an effect prevented by the inactive metabolite 3β-OH-THP (10 mg/kg, i.p.), which antagonizes actions of 3α-OH-THP. In contrast, administration of 3α-OH-THP (10 mg/kg, i.p.) on the morning of proestrus improved spatial learning scores 150–300%. These findings suggest that cyclic fluctuations in ovarian steroids can induce changes in cognition via α4βδ GABARs that are dependent upon 3α-OH-THP.
Keywords: Cognition, Ovarian hormones, Allopregnanolone, GABA-A receptor, Alpha4, Delta
1. Introduction
Changes in cognitive function can be associated with fluctuations in ovarian hormones in humans – at puberty (Johnson and Newport, 1989), across the menstrual cycle (Solis-Ortiz et al., 2004), and during menopause (Sherwin and Grigorova, 2011) – but the effect of replacement hormone therapy on cognition is highly equivocal (Sherwin and Grigorova, 2011; Shumaker et al., 2004), suggesting that the dynamic effects of these hormones on cognition are not yet completely understood. Although 17β-estradiol (E2) can promote cognitive ability (Frye et al., 2007; Vedder et al., 2013) by increasing spine density (Woolley and McEwen, 1992) and NMDA receptor expression or function (Adams et al., 2001; Bi et al., 2001), fluctuations in progesterone have also been implicated in cognitive effects that can alter those produced by E2 (Sherwin and Grigorova, 2011). This interaction is also seen in the rodent, where the in vivo induction of long-term potentiation (LTP) in the CA1 hippocampus, a model of learning (Pastalkova et al., 2006), is robust during the afternoon of, but severely impaired during the morning of, proestrus (Warren et al., 1995), despite elevated circulating levels of E2 at both time-points. What sets the two times apart are the circulating levels of progesterone and its neuroactive metabolite 3α-OH-THP (3α-OH-5[α] β-pregnan-20-one), which increase to peak levels by the late afternoon of proestrus (Walf et al., 2009). Similarly, direct administration of estrogens to post-menopausal women improves memory, even in those with Alzheimer’s dementia, only when combined with naturally occurring progesterone (Sherwin and Grigorova, 2011; Yoon et al., 2003), which can be metabolized to 3α-OH-THP.
The underlying mechanism for the effect of 3α-OH-THP on memory and synaptic plasticity is not known. Unlike most ovarian steroids, which act through nuclear receptors to produce genomic effects, 3α-OH-THP acts as a modulator of the GABAA receptor (GABAR) (Bianchi and Macdonald, 2003), which is the primary source of inhibition in the CNS (Olsen and Sieghart, 2009). GABARs are pentameric membrane channels that gate a Cl− conductance, and are composed of diverse subtypes. Although α1β2γ2 has highest CNS expression (Olsen and Sieghart, 2009), expression of α4βδ GABARs, which mediate tonic inhibitory current in a number of brain regions, is regulated by fluctuations in ovarian hormones (Lovick et al., 2005; Maguire et al., 2005; Shen et al., 2010).
These receptors are the most sensitive target for 3α-OH-THP (Belelli et al., 2002; Bianchi and Macdonald, 2003), which exerts polarity-dependent effects at this receptor: increasing depolarizing current, but decreasing hyperpolarizing current via acceleration of the rate of desensitization (Shen et al., 2007). α4βδ GABAR expression increases in the female CA1 hippocampus during adolescence (Shen et al., 2010) when it impairs cognition, an outcome that 3α-OH-THP can reverse. This possibility has not been investigated as a mechanism for 3α-OH-THP-related cognitive changes in adult females.
Thus, we assessed the role of α4βδ GABARs and 3α-OH-THP in producing cognitive changes across the estrous cycle of the mouse, comparing both morning and afternoon phases of proestrus compared to other stages of the estrous cycle.
2. Results
2.1. Spatial learning across the estrous cycle
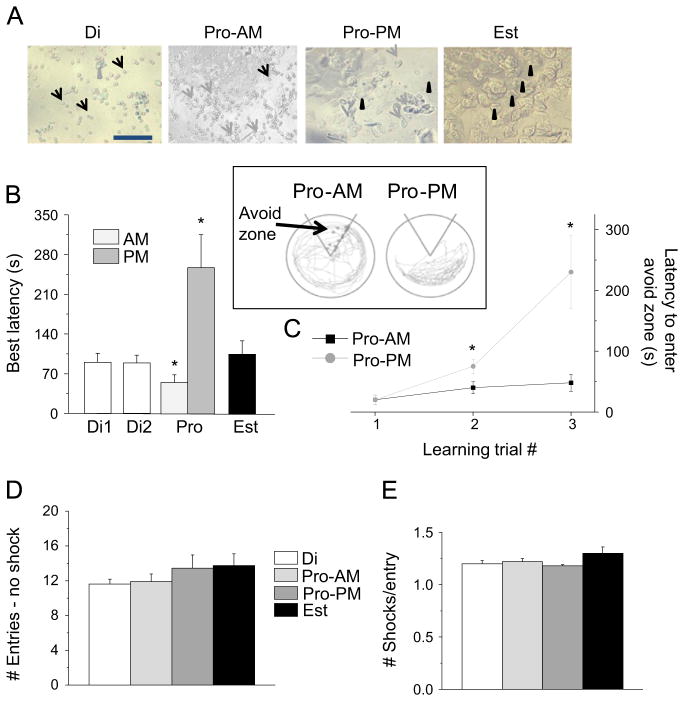
In order to investigate the mechanism for ovarian hormone-related changes in cognitive function, we initially determined that spatial learning was altered across the phases of the estrous cycle, which were verified by the vaginal cytology. To this end, we used an active place avoidance task that is dependent upon in vivo late LTP in the CA1 hippocampus (Pastalkova et al., 2006). This task requires that the animal avoid a mild shock (~0.2 mA), sub-threshold for stress steroid release (Friedman et al., 1967), within a 60° sector on a rotating arena. Because the avoidance zone does not rotate, the animal must learn to avoid the shock by distinguishing room landmarks from the arena (Fig. 1, inset). Following an initial acclimation without shock, the latency to first enter the avoidance zone, a measure of learning, was determined across three 10-min training trials. The latency to first enter the shock zone was 3-fold longer during the afternoon of proestrus (proestrus PM, Fig. 1A), suggesting superior learning, compared to other phases, when circulating ovarian steroid levels are low.
Fig. 1.
Learning was enhanced on proestrus PM. Inset, The active place avoidance task, a rotating platform with a shock (~0.2 mA, “Avoid”) zone. The latency to first enter this zone indicated learning ability. Circles within the ‘Avoid’ zone indicated incidences of shock delivered. (A) Best latencies across estrous cycle phases. ANOVA, F(4, 44)=4.9, P=0.0023, *P<0.05; n=6–11/group. Di, diestrus; Pro-AM, proestrus morning; Pro-PM, proestrus afternoon; Est, estrus. (B) The first 3 training trials for Pro-AM vs. Pro-PM. t(13)=2.46, *P=0.0287; n=6–9/group. (C) The # entries into the Avoid zone during acclimation (no shock), a measure of locomotor activity. (D) The # shocks/entry, a measure of avoidance behavior.
However, spatial learning was reduced on the morning of proestrus compared with other phases of the estrous cycle: the latency to enter the avoidance zone was nearly 80% faster on the morning of proestrus compared with the afternoon of proestrus (P<0.001), suggesting a deficit in spatial learning, which first emerged by the second training trial (Fig. 1A and B). In contrast, the number of entries into the avoidance zone during acclimation (no shock) did not vary across groups (Fig. 1C), verifying that locomotor activity was unaltered. In addition, the number of shocks received per entry into the avoidance zone did not vary across groups (Fig. 1D), signifying that the shock was equally aversive and that all animals were equally capable of escape behavior. This demonstrates that the difference in latencies among the estrous cycle phases was specifically due to differences in spatial learning ability.
2.2. Synaptic plasticity changes across the estrous cycle
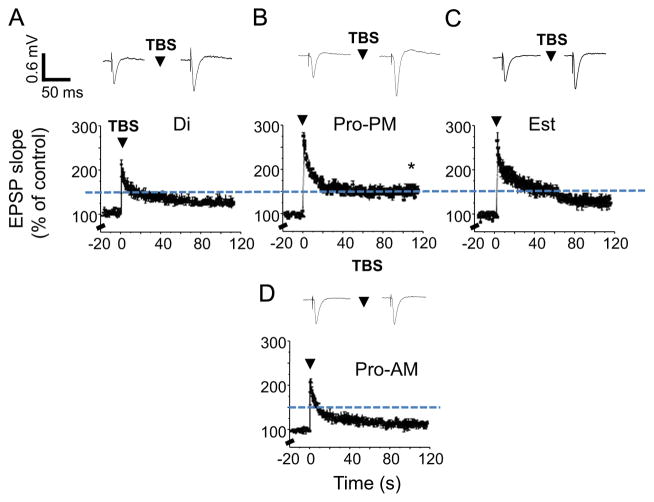
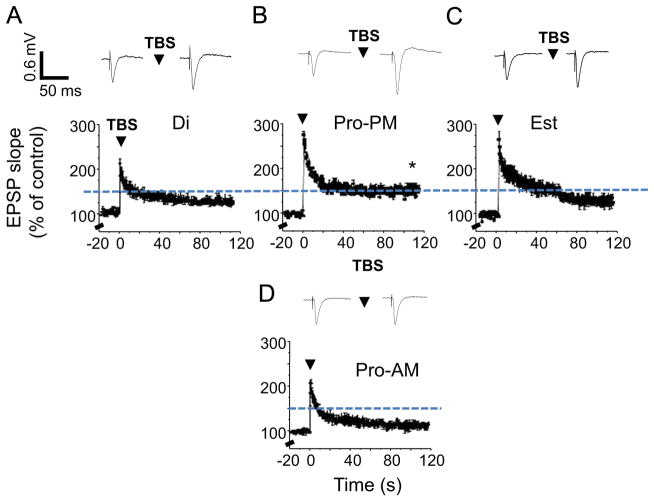
Synaptic plasticity was also facilitated on the afternoon of proestrus (145±4%, Fig. 2C) compared with estrus and diestrus (Fig. 2A and D, Table 1, P<0.05, 128±6%, 123±2.4%, respectively), assessed with LTP induced by theta burst stimulation of the Schaffer collaterals to the CA1 hippocampus, the CNS site which underlies spatial memory (Pastalkova et al., 2006). The facilitation of LTP induction on the afternoon of proestrus is consistent with previous reports (Warren et al., 1995) and may be a result of the increase in spine density and NMDA receptor function reported during proestrus (Woolley and McEwen, 1992; Adams et al., 2001). However, LTP induction was impaired on the morning of proestrus, when the average level of potentiation was reduced by 75% compared to this parameter on the afternoon of proestrus (P<0.05, Fig. 2B). In fact, this reduced level of potentiation was not quite significant (P=0.05, Table 1) suggesting that LTP was not induced on the morning of proestrus.
Fig. 2.
Synaptic plasticity is enhanced on proestrus PM. LTP was induced by theta burst stimulation (TBS) of the Schaffer collaterals to the CA1 hippocampus (arrow). LTP induction was enhanced on proestrus PM (Pro-PM, (C)) but impaired on proestrus AM (Pro-AM, (B)) compared with other stages of the estrous cycle, diestrus (Di, (A)) and estrus (Est, (D)). Graphs present the mean±SEM before and after TBS. Dashed line: mean level of potentiation for Pro-PM. Insets, Representative fEPSPs. ANOVA, F(2, 29)=5.87, *P=0.014, Pro-PM vs. Di, Est; t(12)=4.9, P=0.0004, Pro-AM vs. Pro-PM. *P<0.05, post-TBS vs. pre-TBS. **P<0.05 vs. Di, Pro-AM n=3–6/group.
Table 1.
Average values of LTP across the estrous cycle. Induction of LTP in the CA1 hippodampus by theta burst stimulation was tested statistically for each of the groups using a paired t-test comparing pre-induction (20 min) levels of the slope of the fEPSP with this parameter calculated during the final 20 min of the 2 h post-induction period.
| Estrous stage | Average potentiation | S.E.M. | n | t | P value |
|---|---|---|---|---|---|
| Diestrus | 123 | 2.5 | 8 | 5.21 | 0.00062** |
| Proestrus-AM | 111 | 4 | 6 | 2.57 | 0.05* |
| Proestrus-PM | 145 | 4 | 6 | 14.1 | 0.000032** |
| Estrus | 128 | 6 | 8 | 3.58 | 0.0089** |
Trend towards significance.
P values are considered to be statistically significant.
2.3. α4βδ GABAR expression across the estrous cycle
In order to determine a possible explanation for the deficits in synaptic plasticity and spatial learning on the morning of proestrus, we investigated the possibility that GABAR-mediated inhibition might impair plasticity at this time via increased expression of dendritic α4βδ GABARs. Expression of α4 can be regulated by E2 (Shen et al., 2005), which begins to increase the day before, and peaks on the day of proestrus (Wood et al., 2005). To test this possibility, we used electron microscopic–immunocytochemical (EM–ICC) techniques to localize and quantify the expression of α4 and δ subunits along the plasma membrane of CA1 pyramidal cells across the estrous cycle. Plasmalemmal expression of the subunits on the dendritic shaft of pyramidal cells was highest on the day of proestrus, with significantly lower levels on diestrus and estrus (Fig. 3 (label/100 μm)—A=α4: 60±5, Pro vs. 37±3.6, Di, 17±2.4, Est, P<0.0001; B=δ: 16±2, Pro vs. 7±2.3, Di, 11±2, Est, P=0.012). In contrast, although α4 and δ immunoreactivity was detected at dendritic spines, as we have demonstrated previously (Shen et al., 2010), these values did not vary across the cycle (data not shown). Administration of E2 on estrus mimicked the increase in receptor expression during the morning of proestrus (Fig. 3C and D), suggesting that the proestrous peak in E2 is the likely trigger, as has been previously suggested (Shen et al., 2005).
Fig. 3.
α4 and δ expression is increased on the plasma membrane of dendritic shafts of CA1 hippocampal pyramidal cells during proestrus. (A) Representative images of dendritic shafts (sh) in stratum radiatum across the estrous cycle, showing silver-intensified immunogold (SIG) labeling of the GABAR subunits on the plasma membrane (arrowheads) by electron microscopic immunocytochemistry (EM/ICC). Representative images from mice during diestrus (i), proestrus (ii) and estrus (iii). Scale, 500 μm. (B) Bar graph shows mean±SEM of SIG particle density per unit length of plasma membrane. ANOVA, α4, F(2, 177)=29.02, *P<0.0001; δ, F(2, 177)=3.8, *P=0.0121. n=60 pooled from 3 mice/group. (C) Western blot, hippocampal α4 and δ expression in vehicle and E2-treated mice. (D) Mean±SEM, optical density. α4, t(4)=6.2, *P=0.003; δ, t(4)=3.94, *P=0.017, n=9.
2.4. Pharmacological verification of α4βδ GABAR expression
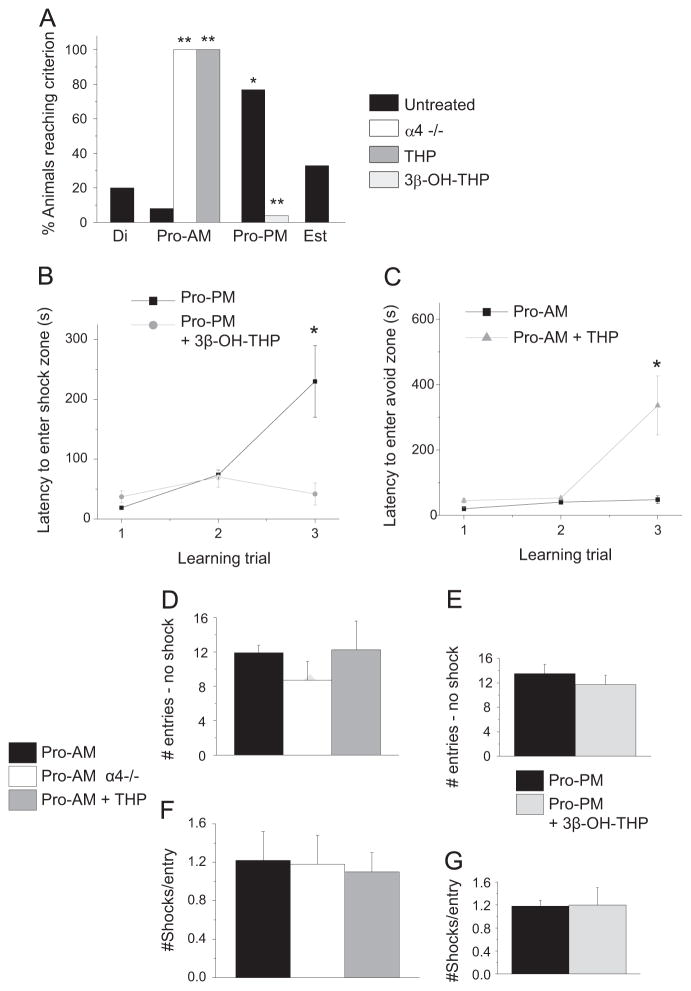
Functional expression of α4βδ GABARs was confirmed by whole cell current responses of pyramidal cells to the GABA agonist gaboxadol (Fig. 4A and B) at a concentration (100 nM) selective for δ-containing GABARs (Brown et al., 2002; Meera et al., 2011). Gaboxadol generated 25–30 pA responses during proestrus (AM and PM), which were up to 10-fold greater than observed during diestrus and estrus (3–8 pA, P<0.001), reflecting increased expression of α4βδ GABARs during both the morning and afternoon of proestrus. Similarly, responses to the GABAR antagonist SR95531 (120 μM) were significantly greater both times of the day during proestrus than on other days of the cycle, reflecting a greater inhibitory tonic current. Input resistance (Rm), calculated from the current response to a −40 mV step, was reduced by 35% during proestrus relative to estrus (Fig. 4C, P<0.05), which may be a result of the increase α4βδ GABAR expression. However, it also suggests that the gaboxadol current responses assessed on proestrus may be underestimates of the true value.
Fig. 4.
Pharmacological verification of increased α4βδ GABAR expression on proestrus. (A) Representative traces, whole cell voltage clamp recordings of CA1 pyramidal cells response to gaboxadol (GBX, 100 nM), a measure of functional δ-GABAR expression. (B) Mean±SEM. ANOVA, GBX, F(2, 10)=27, *P<0.0074; S95531, F(2, 10)=14.6, *P=0.0011. *P<0.05 vs. Di, Est, n= 4–5/group. (C) Increases in functional α4βδ GABAR expression during proestrus. Input resistance (Rm) assessed under control conditions, Pro-AM, Est. t(7)=2.42, *P=0.0461, n=4–5/group.
2.5. LTP induction and synaptic plasticity in α4−/− mice
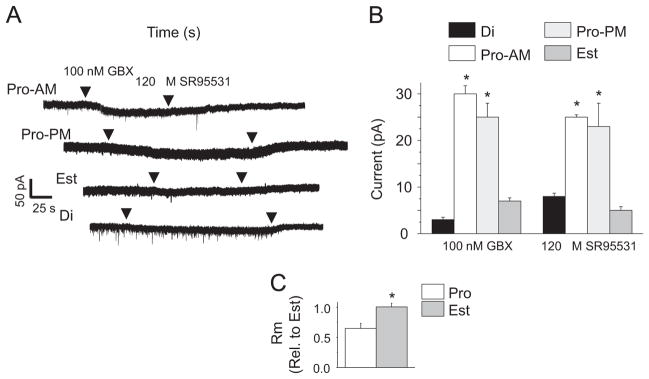
In order to determine whether the increase in dendritic expression of α4βδ on the morning of proestrus played a role in reducing synaptic plasticity, we compared LTP induction in +/+ and α4−/− mice, which are also functional knock-outs of the δ subunit (Sabaliauskas et al., 2012). Induction of LTP was significantly enhanced in the α4−/− compared to +/+ hippocampus on the morning of proestrus (Fig. 5A). Use of the α4−/− mouse also reversed the deficit in spatial learning (Fig. 5B) at this time, resulting in a nearly 20-fold increase in the latency to enter the shock zone by the third training trial (P<0.05). These data implicate the estrous cycle-regulated expression of α4βδ GABARs as mediators of the impairment in synaptic plasticity and learning on the morning of proestrus.
Fig. 5.
α4 knock-out reverses deficits in synaptic plasticity and learning during proestrus. (A) LTP, CA1 hippocampus (TBS, arrow) in α4−/− vs. +/+ on Proestrus AM (Pro-AM), t(9)=12.5, *P<0.0001; n=5–6/group. (B) Effects of α4 knock-out on latency to first enter the avoid zone, a measure of learning, across training trials in the spatial learning task on Pro-AM. t(7)=32.2, *P<0.0001 vs. Pro-AM +/+. n=3–6 mice/group.
2.6. Polarity of GABAergic current on proestrus
Because the inhibitory effects of THP at α4βδ GABARs are polarity-dependent (Shen et al., 2007), we assessed the polarity of the GABAergic current on proestrus. With a >1 gigohm seal and passing 0 current, assessment of the direction of potential change in response to a GABA agonist is possible with current clamp recordings (Perkins, 2006) because a high resistance seal produces a detectable voltage change in response to the ligand-gated current, as we have previously described (Shen et al., 2007). In addition, the polarity of the membrane potential change caused by a GABA agonist can be measured without disturbing the intracellular milieu, where a hyperpolarizing (downward) response to agonist indicates inward Cl− flux. In fact, the responses to 5 μM gaboxadol was a downward deflection when assessed on both the morning and afternoon of proestrus as well as on estrus (Fig. 6A), suggesting that the direction of Cl− flux generated by GABARs is inward (hyperpolarizing) across these days of the estrous cycle.
Fig. 6.
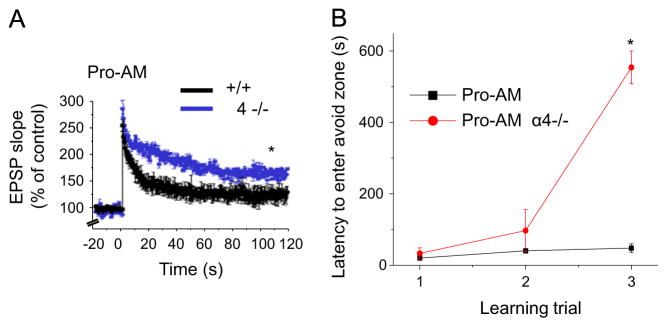
THP reduces the GABAergic tonic current recorded from CA1 hippocampal pyramidal cells during proestrus. (A) Tight seal, cell-attached, current clamp recordings reveal hyperpolarizing responses to gaboxadol (5 μM, GBX), Bic (20 μM bicuculline). Rep. of 6 cells/group (3 from proestrus AM, 3 from proestrus PM, which were the same). (B) Gramicidin perforated patch recordings of GABAergic current responses to 30 nM THP (upper panel). Bic, lower panel. (C) Mean±SEM, ANOVA, F(2, 12)=16.7, P=0.0003 *P<0.05 vs. other groups, n=5/group.
2.7. 3α-OH-THP effects on GABAergic current across the estrous cycle
We tested whether the higher levels of THP on the afternoon of proestrus could explain the improved plasticity at this time compared with the morning of proestrus, as 3α-OH-THP has been shown to reduce outward (hyperpolarizing) current at α4βδ GABARs, and to improve synaptic plasticity at puberty (Shen et al., 2007). Responses to 3α-OH-THP were assessed using gramicidin perforated patch recording techniques to maintain the Cl− gradient. 3α-OH-THP uniquely decreased the outward current by 30% on proestrus in +/+ mice (Fig. 6B and C), but had no significant effect during proestrus in α4−/− mice (Sabaliauskas et al., 2012) or in mice during estrus, when α4βδ expression is reduced in CA1 hippocampus.
2.8. 3α-OH-THP effects on spontaneous spiking across the estrous cycle
The 3α-OH-THP-mediated decrease in tonic inhibition on proestrus should impact neuronal excitability. In order to test this, we recorded from CA1 pyramidal cells in cell-attached mode to monitor spiking without altering the internal neuronal milieu. As predicted, 3α-OH-THP increased spiking by 90% in +/+ mice during proestrus, but not in α4−/− mice during proestrus or +/+ mice during estrus (Fig. 7A and B). 3α-OH-THP also increased Rm by 68% (Fig. 8C) on proestrus, in contrast to its effect on estrus, when it decreased this parameter by 35% (P<0.05).
Fig. 7.
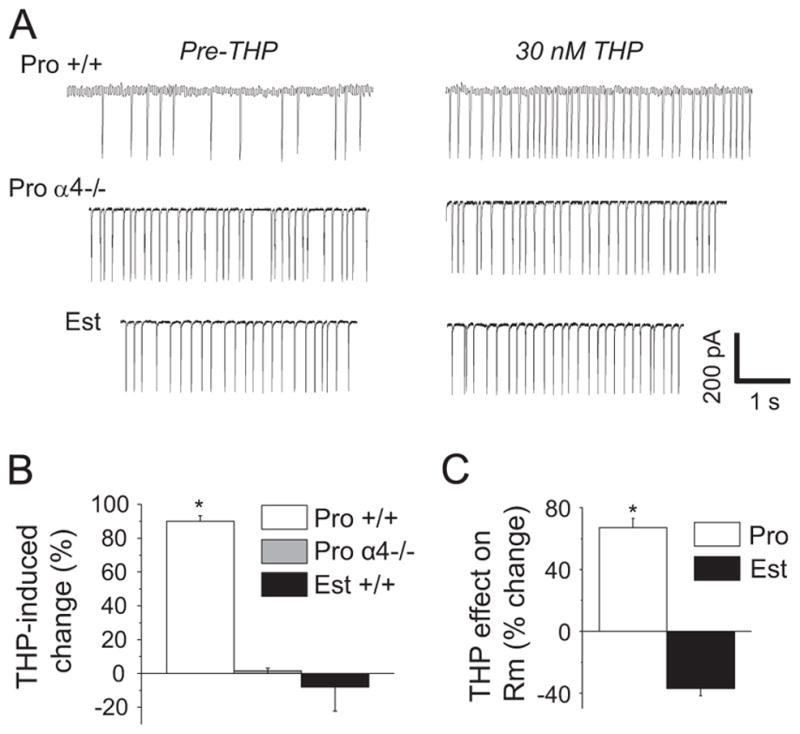
THP increases excitability of CA1 hippocampal pyramidal cells during proestrus. (A) Cell-attached voltage clamp recordings of spiking before and after 30 nM THP. (B) Mean±SEM, spiking frequency. ANOVA, F(2, 19)=69.15, P<0.0001, *P<0.05 vs. other groups, n=7–8/group. (C) THP effects on input resistance (Rm), t(8)=559, *P=0.0005, n=4–5/group. Pro, proestrus AM, Est, estrus AM.
Fig. 8.
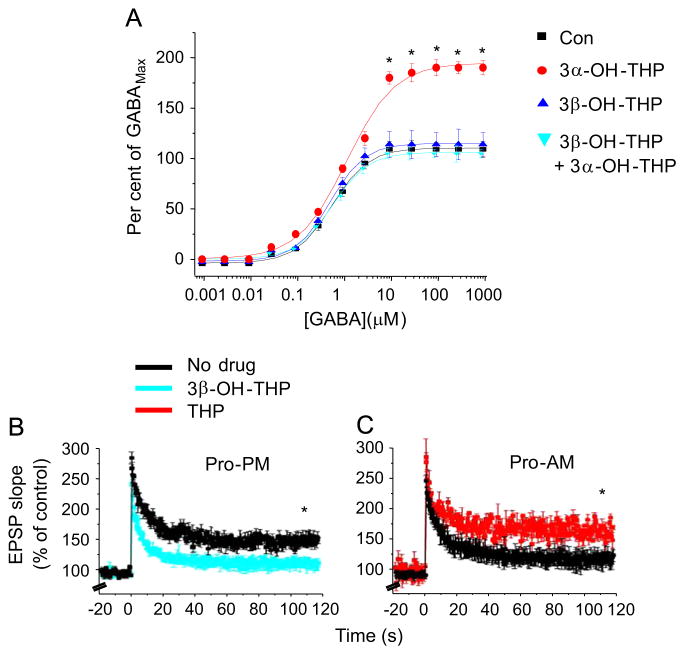
The inactive isomer of THP prevents deficits in synaptic plasticity during proestrus. (A) Effect of THP isomers as a % of maximal GABA-gated current (GABAMAX), recorded from recombinant α4βδ GABARs expressed in HEK-293 cells (whole cell voltage clamp). n=5 cells/group; *P<0.001 vs. other groups. (B) LTP, CA1 hippocampus (TBS, arrow) on Proestrus PM (Pro-PM), vehicle vs. 3β-OH-THP (30 nM), the inactive isomer of THP. t(9)=6.1, *P=0.0002, n=5–6/group. (C) LTP, Proestrus AM (Pro-AM), vehicle vs. THP (30 nM). t(9)=7.44, *P<0.0001, n=5–6/group.
2.9. 3α-OH-THP effects on spontaneous inhibitory post-synaptic currents during proestrus
In order to rule out potential direct effects on synaptic current, including presynaptic effects, 3α-OH-THP’s effect on spontaneous inhibitory post-synaptic currents (sIPSCs) was recorded with whole cell voltage clamp techniques. 3α-OH-THP had no effect on sIPSC frequency or half-width or on the amplitude distribution of the events (data not shown), suggesting a selective effect on the extrasynaptic current. These results further suggest that the steroid was not acting pre-synaptically, which would have been expected to alter sIPSC frequency.
2.10. Testing the hypothesis that 3α-OH-THP alters LTP induction across the estrous cycle
We predicted that the peak in 3α-OH-THP levels on the afternoon of proestrus, by decreasing inhibition mediated by α4βδ GABARs, would overcome this obstacle to achieving high levels of synaptic plasticity even in the slice where steroids are known to be sequestered for prolonged periods of time (Chisari et al., 2009; Puia et al., 2003). To test this we first verified that 3β-OH-THP, the inactive isomer of 3α-OH-THP, could be used as an antagonist of the active isomer, as has been suggested (Shen et al., 2007). 3β-OH-THP effectively prevented the potentiating effect of 3α-OH-THP on GABA-gated current across a range of GABA concentrations (1 × 10−9–1 × 10−3 M) in recombinant α4βδ GABARs, transiently expressed in HEK-293 cells (Fig. 8A), suggesting that it functions as an antagonist at α4βδ GABARs.
When pre-applied to the slice, 3β-OH-THP significantly (P<0.05) reduced the degree of LTP by 75% compared to vehicle on the afternoon of proestrus (Fig. 8B), suggesting a role for 3α-OH-THP in permitting robust levels of synaptic plasticity. Conversely, application of the active isomer, 3α-OH-THP, to the slice on the morning of proestrus reversed the deficit in LTP induction (Fig. 8C), resulting in levels of potentiation similar to the afternoon of proestrus. These data suggest that increased 3α-OH-THP, from either local or blood-borne sources, enhances synaptic plasticity on the afternoon of proestrus despite high levels of α4βδ GABAR expression.
2.11. 3α-OH-THP effects on spatial learning across the estrous cycle
Similar findings were obtained in the spatial learning task, where pre-administration of 3β-OH-THP on the afternoon of proestrus prevented animals from reaching learning criterion (120 s latency to first enter the avoidance zone) across 4 training trials (Fig. 9A) and decreased the latency to enter the avoidance zone by trial #3 (Fig. 9B). In contrast, pre-administration of the active isomer, 3α-OH-THP, to mice on the morning of proestrus markedly increased the percentage of animals reaching learning criterion from 8% to 100% (Fig. 9A) and increased latencies to enter the avoidance zone (Fig. 9C), suggesting that 3α-OH-THP can reverse the learning deficit at this time, as can knock-out of α4 (Figs. 5A and 9A). Neither of the steroid treatments or use of the α4−/− mouse altered the number of entries (without shock) or the number of shocks/entry (Fig. 9D–G), suggesting that the observed behavior reflects differences in spatial learning.
Fig. 9.
THP improves learning on the morning of proestrus. (A) % animals reaching criterion (120 s latency) for spatial learning. THP, 10 mg/kg, i.p. 1 h before testing. *P<0.001 vs. other groups; **P<0.001 vs. untreated +/+ cohorts, Chi square. n=3–19/group. (B) Effect of the inactive THP isomer, 3β-OH-THP, across training trials on Proestrus PM (Pro-PM, t(10)=2.34, *P=0.04 vs. Pro-PM+3β-OH-THP, n=3–9 mice/group). (C) Effects of THP across training trials on Proestrus AM (Pro-AM, t(8)=5.83, *P=0.0004 vs. Pro-AM +/+. n=4–6 mice/group). (D and E) The number of entries into the avoidance zone during acclimation (no shock), a measure of locomotor activity, across steroid treatment groups and genotype on Pro-AM (D) and Pro-PM (E). (F and G) The # shocks/entry, a measure of escape behavior, across the same groups on Pro-AM (F) and Pro-PM (G). n=3–9 mice/group.
3. Discussion
The results from this study suggest that the transient expression of α4βδ GABARs in CA1 hippocampus impairs cognition during the morning of proestrus. Because the current gated by these receptors is reduced by the progesterone metabolite 3α-OH-THP (Shen et al., 2007), which peaks in the afternoon of proestrus, the diurnal variation in synaptic plasticity and spatial learning is substantial: with lowest levels in the morning and highest levels in the afternoon of proestrus compared to the other days of the ovarian cycle, as suggested by earlier reports (Warren et al., 1995).
3.1. Effects of E2 on synaptic plasticity
E2 can increase CNS plasticity by increasing the expression (Weiland, 1992) and/or function (Bi et al., 2001; Snyder et al., 2011) of hippocampal NMDA receptors and by increasing spine density (Woolley and McEwen, 1994). Both parameters are increased on the day of proestrus (Adams et al., 2001; Bi et al., 2001; Dalla et al., 2009; Woolley and McEwen, 1992), when endogenous levels of E2 peak, assessed both in the morning and afternoon. Thus, the network of excitatory synapses mediating synaptic plasticity has the potential to provide an equivalent basis for enhanced LTP induction and spatial learning at the two time-points. However, E2 can also increase membrane expression of α4βδ GABARs, as evidenced in the present study, which would hold this optimized substrate of plasticity in check. Indeed, the role of 3α-OH-THP in reversing this impediment to plasticity is notable because it permits synaptic plasticity and spatial learning at a time in the cycle when reproductive behavior occurs, during the afternoon and evening of proestrus, thus optimizing the chances of reproduction.
3.2. Estrous cycle changes in α4βδ expression in other brain areas and conditions
In contrast to the CA1 hippocampus, α4βδ GABARs are increased in other CNS areas, such as the midbrain central grey and dentate gyrus, on late diestrus (Lovick et al., 2005; Maguire et al., 2005), following the secondary peak in circulating levels of 3α-OH-THP. In addition, declining levels of THP, found post-partum and at the onset of puberty, also trigger expression of α4βδ in hippocampus (Maguire and Mody, 2009; Shen et al., 2007), suggesting that the hormonal regulation of these receptors is complex.
3.3. Effects of E2 and progesterone on memory
Consistent with the present findings, clinical studies suggest that E2 alone does not improve memory (Espeland et al., 2013; Schmidt et al., 2013) unless accompanied by a form of progesterone which can be metabolized to THP (Sherwin and Grigorova, 2011). In fact, levels of progesterone, rather than E2, significantly correlate with improvements in verbal and spatial memory in post-menopausal women (Henderson et al., 2013). This may explain the equivocal findings of studies investigating hormone replacement therapy on cognition, when the nature of the progestin was not considered (Lethaby et al., 2008). However, use of anti-estrogens in breast cancer has been shown to result in slowing of certain cognitive functions (Lejbak et al., 2010), without overall impairment, suggesting that there is a threshold effect of E2 in facilitating the speed of cognitive events. One factor not considered in these studies is the impact of cyclic fluctuations in ovarian steroids, as most hormone replacement regimens employ static hormone administration.
3.4. Mechanisms of E2-induced facilitation of synaptic plasticity
Maximal levels of LTP in the present study were seen on the afternoon of proestrus, following increases in circulating levels of E2. The mechanisms underlying the facilitating effect of E2 are diverse and have been widely investigated. E2 increases phosphorylation of extracellular signal-regulated kinase 2 ERK2, a component of the MAP kinase (mitogen activated protein kinase) pathway, and increases tyrosine phosphorylation of NR2 subunits (Bi et al., 2001), both of which underlie its role in facilitating synaptic plasticity. These changes are seen after E2 administration as well as during the morning of proestrus (Bi et al., 2001). In addition, E2 increases the probability of glutamate release (Smejkalova and Woolley, 2010) and increases the NR2B component of excitatory synaptic currents (Snyder et al., 2011). Recent studies show that E2 also acts to promote actin polymerization within dendritic spines, which optimizes synaptic physiology (Kramar et al., 2013; Briz and Baudry, 2014). E2 activates a signaling cascade involving GTPase RhoA within the spine and inactivates the filament severing protein cofilin (Kramar et al., 2013), which would promote spine stability. Recent reports suggest that this effect of the steroid is mediated by tropomyosin-related kinase B (TrkB) receptors and brain-derived neurotrophic factor (BDNF) (Kramar et al., 2013). Taken together, these actions of E2 would form the basis of a synaptic network which would promote synaptic plasticity.
3.5. E2 and synaptic plasticity
3.5.1. Use of ovariectomized rats
In contrast to the present results, where LTP induction was impaired on the morning of the E2-dominant proestrous phase of the cycle due to increased GABAergic inhibition, administration of E2 to ovariectomized rats improves cognition (Frye et al., 2007; Vedder et al., 2013) and facilitates induction of LTP (Smith and McMahon, 2005, 2006) via an NR2B-mediated mechanism (Smith and McMahon, 2006), which likely includes the diverse mechanisms listed above. The reason for the discrepancy with the present results may be that E2-induced increases in α4βδ GABAR expression do not occur following ovariectomy. Therefore, the steroid would only trigger mechanisms serving to facilitate synaptic plasticity.
However, progesterone-induced improvements in learning in ovariectomized rats are dependent upon 3α-OH-THP (Frye et al., 2010), while other studies conducted in intact rats suggest that cognition is optimal on the night of proestrus (Walf et al., 2006), consistent with the present results.
3.5.2. Effect of different stimulation frequencies
One additional factor which may explain seemingly contradictory results among synaptic plasticity studies is the mode of LTP induction, high frequency stimulation versus theta burst stimulation (Larson and Lynch, 1986; Larson et al., 1986). In the present study theta burst stimulation did not permit optimal induction of LTP during the E2-dominant phase of the ovarian cycle, the morning of proestrus. In contrast, LTP induction on the morning of proestrus is facilitated when high frequency stimulation is employed (Bi et al., 2001). E2 administration to ovariectomized rats also optimizes LTP when it is induced by high frequency stimulation (Smith and McMahon, 2005). These two stimulation protocols differentially affect activation of the interneuron population because theta burst stimulation (Chapman et al., 1998) activates interneuron circuits, and LTP is not optimally induced, in contrast to high frequency stimulation which does not activate interneurons (Chapman et al., 1998). Thus, recruitment of interneurons with theta burst stimulation in the present study may have been the critical factor that revealed the differential effects of GABAR expression across the estrous cycle.
3.6. Inhibition and synaptic plasticity
It is widely known that inhibitory input can compromise induction of LTP and learning ability. This has been shown with a GABA agonist, gaboxadol (Whissell et al., 2013a), at a dose selective for α4βδ GABARs (Brown et al., 2002), as well as for amnestic, sedative drugs such as propofol, isoflurane and benzodiazepines (del Cerro et al., 1992; Nagashima et al., 2005; Saab et al., 2010) although long-term activation of α4βδ GABARs in dentate gyrus improves memory via an effect on neurogenesis (Whissell et al., 2013b). Knock-out of either α4 or δ subunits has also been shown to improve contextual-dependent fear conditioning (Moore et al., 2010; Wiltgen et al., 2005) although sex differences were observed in which trace conditioning or delay conditioning were selectively affected, in female versus male mice, respectively (Moore et al., 2010). In this case, the females also exhibited estrous cycle variability, with improved performance on estrus versus diestrus (Cushman et al., 2014). These differences were not observed in the δ −/− mouse, suggesting that the increase in α4βδ GABAR expression on late diestrus in dentate gyrus (Maguire et al., 2005), a site necessary for fear conditioning (Saxe et al., 2006), underlies these changes in learning across the estrous cycle. In addition to α4βδ GABARs, the predominant extrasynaptic GABAR population in CA1 hippocampus, α5β3γ2, reduces induction of LTP in a frequency-dependent manner (Cheng et al., 2006), and ablation of these receptors facilitates LTP and fear-conditioning (Crestani et al., 2002).
3.7. Localization of α4βδ under different hormonal conditions
The impact of proestrus-associated increases in α4βδ GABARs on synaptic plasticity is distinct from their effect at puberty, because the proestrous rise in expression was restricted to the dendritic shaft, while at puberty these receptors increase primarily on the dendritic spine (Shen et al., 2010), where they shunt depolarizing current and impair NMDA receptor activation. Thus, the ultimate impact of α4βδ expression is greater at puberty, when LTP induction is prevented, while on the morning of proestrus, potentiation was merely reduced compared to phases of the estrous cycle when α4βδ expression is lower (estrus, diestrus) as well as during the afternoon of proestrus when 3α-OH-THP levels reduce the current generated by these receptors. The mechanism for the impairment on LTP induction during the morning of proestrus is likely the shunting of back-propagating action potentials which influences synaptic plasticity (Hao and Oertner, 2012; Hardie and Spruston, 2009).
3.8. Effect of 3α-OH-THP on tonic inhibition during proestrus
3α-OH-THP reversed the impairment in synaptic plasticity and spatial learning during proestrus when α4βδ GABAR expression was increased. This steroid reduces inhibition via α4βδ GABARs when GABA-gated current is in the hyper-polarizing direction (Shen et al., 2007). 3α-OH-THP’s reduction in hyperpolarizing current in the CA1 pyramidal cell is in contrast to 3α-OH-THP’s well-known effect to enhance current in the depolarizing direction, as in the dentate gyrus granule cell, where it increases the tonic inhibitory current (Stell et al., 2003), because depolarizing GABAergic current in the dentate gyrus is a shunting inhibition (Staley and Mody, 1992). 3α-OH-THP reduces hyperpolarizing current at α4βδ GABARs due to acceleration of desensitization (Shen et al., 2007), as shown for other δ-containing GABARs (Bianchi and Macdonald, 2003). Similar effects are obtained with the isomers 3α,5α-THP and 3α,5β-THP, as well as for THDOC (Gong and Smith, 2014). These steroids reduce steady-state current as well as the current response to rapid application of agonist, suggesting that their effect is robust (Shen et al., 2007). It is dependent on a positively charged amino acid in the second intracellular loop (IL2), arginine 353 (Shen et al., 2007), suggesting that it may be a Cl− binding site, and requires phosphorylation via protein kinase C (Gong and Smith, 2014). Similar Cl− dependent events have been reported for other GABARs (Houston et al., 2009). In addition, residues in IL2 of homologous receptors have been shown to play a role in receptor desensitization (Turner and Raymond, 2005), while in others, the intracellular loop functions as a permeation pathway (Kelley et al., 2003; Miyazawa et al., 2003), suggesting a potential mechanism for 3α-OH-THP’s polarity-dependent effect.
3.9. Ovarian cycle-linked cognitive changes in primates
These findings in female mice may also be relevant for humans and non-human primates where marked cognitive changes have also been observed across the ovarian cycle. The progesterone-dominant luteal phase, when 3α-OH-THP levels are concomitantly increased, is associated with improvement in several hippocampal-dependent visuo-spatial tasks. These include spatial learning (Lacreuse et al., 2001), visuo-spatial detection on the embedded figures task (Broverman et al., 1981) and working memory used for the digit span test and Wisconsin card sort (Solis-Ortiz et al., 2004). Although working memory also requires the prefrontal cortex, the hippocampus, which has extensive connectivity with this cortical region (Kirwan and Stark, 2007; Wall and Messier, 2001), plays a critical role, as evidenced by imaging studies showing activation during these tasks (Schon et al., 2004) and reduced volume correlated with impaired performance (Frodl et al., 2006). This is likely due to its role in mismatch detection which is necessary for working memory (Schon et al., 2009).
3.10. Conclusions
In addition to proestrus-associated improvements in spatial memory, which occur prior to the onset of reproductive events, improvements in spatial memory are also seen during pregnancy in rodents (Bodensteiner et al., 2006; Frye et al., 2007), when nest building occurs (Lisk et al., 1969). Thus, changes in hippocampal function associated with the ovarian hormones may permit adaptive cognitive alterations which would be relevant for the reproductive state of the individual. The actions of THP at α4βδ GABARs may also suggest novel therapeutic hormonal strategies for hormonally-associated cognitive impairments.
4. Experimental procedures
4.1. Animal subjects
Female C57/BL6 mice (+/+and α4−/−) were housed in a reverse or standard light:dark cycle (12:12). Both sets of mice were originally supplied by G. Homanics (Univ. of Pittsburgh), and were bred on site, with +/+ mice supplemented from Jackson Laboratories (Bar Harbor, Maine). Initially, age-matched +/+ littermates of the α4−/− were used as controls, but because they were indistinguishable from C57/BL6, based on LTP results and THP responses, data from both groups were pooled. Genotyping of the tails was used to identify mice that were homozygous α4−/−. α4−/− mice are functional δ knock-downs (Sabaliauskas et al., 2012); they were used rather than δ −/− to spare the α1βδ present on interneurons (Glykys et al., 2007). Some animals were injected with THP (3α-OH-5[α]β-pregnan-20-one or [allo]pregnanolone, 10 mg/kg, intraperitoneally in oil) or its inactive isomer (Harrison et al., 1987), 3β-OH-THP (3β-OH-5β-pregnan-20-one, 10 mg/kg, intraperitoneally in oil) to block THP’s effect 30 min before behavioral testing (Shen et al., 2007). Animals were either tested in early in the light cycle (AM) in animals with a regular light:dark cycle or 1–3 h before the onset of the dark cycle (PM), where animals were placed on a reverse light:dark cycle for convenience, as well as to allow the tissue to be processed in parallel. Estrous cycle stage was determined by the vaginal cytology in animals with established regular cycles. Animals with ambiguous cytology or irregular cycles were excluded from the study. Because the data from the morning and afternoon of estrus and diestrus were similar (based on behavioral and LTP findings), these data were pooled. Procedures were in accordance with the SUNY Downstate Institutional Animal Care and Use Committee.
4.2. Western blot
Hippocampal membranes were prepared from female mice following 2 d of E2 administration (17β-estradiol, 2 μg/kg, i.p. in oil vehicle once a day) as we have described (Shen et al., 2007). Mice were injected with E2 or vehicle beginning on the day of estrus, which was verified from the vaginal cytology, and is characterized by low levels of circulating ovarian steroids (Wood et al., 2005). Protein concentrations, assessed using the Bradford assay, were in the linear range (5–10 μg). Samples were electrophoresed on a 10% NuPage Bis–Tris gel, and electrophoresed, followed by transfer of proteins to nitrocellulose membranes (Invitrogen). Following a 1 h block with 5% non-fat dry milk, membranes were incubated overnight at 4 °C with a 1:15,000 (α4), 1:5000 (δ) and 1:100,000 (glyceraldehyde-3-phosphate dehydrogenase, GAPDH) dilution of the antibody, followed by a 1:5000 dilution of horseradish peroxidase-conjugated donkey anti-rabbit IgG (α4, δ) or goat anti-mouse IgG (GAPDH) (Sigma).
α4 (67 kDa) and δ (54 kDa) bands were detected with enhanced chemiluminescence (Pierce Supersignal West Femto substrate). Optical densities were quantified with Adobe Photoshop from a scanned image. Results were standardized to a GAPDH (36 kDa) control and are presented as a ratio relative to the vehicle control.
4.3. Immunocytochemisty
4.3.1. Tissue preparation
Mice (3 per group) were anesthetized with urethane (0.1 ml 40% or 0.05–0.2 ml, 20%, IP) 1–3 h before the onset of the dark cycle (PM, except where noted) and transcardially perfused using a peristaltic pump to set the flow-rate to 30 ml/min, first with saline containing heparin (1 U/ml), followed by fixation with 4% paraformaldehyde buffered to pH 7.4 with 0.1 M phosphate buffer (PB). The brains were removed from the skulls and stored in the same fixative until sectioning. Forty micrometer coronal sections were generated using a vibratome, then stored at 4 °C in a buffer consisting of 0.01 M PB with 0.9% sodium chloride (PBS) supplemented with 0.05% sodium azide, to prevent bacterial growth.
4.3.2. Immunocytochemistry of the α4 and δ subunits of GABARs (Aoki et al., 2000)
Free-floating sections were permeabilized by freeze-thawing, in which sections are cryoprotected using dimethyl sulfoxide. To retrieve antigenicity, sections were treated with 1% hydrogen peroxide-PBS at room temperature (30 min). Sections were blocked in PBS with 1% bovine serum albumin and 0.05% sodium azide (PBS/BSA/Azide) for 30 min, then incubated overnight at room temperature with either the α4 (2 ng/ml, SC-7523, Santa Cruz) or δ (0.5 ng/ml, a generous gift from W. Sieghart) primary antibodies in PBS/BSA/Azide. On the following day, sections were incubated in 0.8 nm colloidal gold-conjugated secondary antibodies (horse anti-goat IgG, diluted 1:100 for α4, goat anti-rabbit IgG, diluted 1:100 for δ) (EMSciences, Inc). Sections were fixed with glutaraldehyde (2%, 10 min, while free-floating in PBS), then rinsed in 0.2 M citric acid buffer pH 6.5 prior to silver intensification reaction, performed using the Silver IntensEM kit (KPL, Inc.) for 10–20 min, and was terminated by a brief citrate buffer rinse. Ultrastructure and SIG particles were preserved using osmium-free post-fixation (Phend et al., 1995).
Sections were dehydrated using 30–100% ethanol and 100% acetone, then infiltrated in EMBED812, and flat embedded between sheets of Aclar plastic. The infiltrated and embedded sections were ultrathin-sectioned at a setting of 70–90 nm thickness. Control sections were immunoprocessed in parallel and identically with experimental sections, except that the primary antibody was omitted from the overnight-incubation step. Any SIG immunolabel seen in these control tissue samples was established as the background level.
4.3.3. Electron microscopic image acquisition and analysis (Sarro et al., 2008)
So as to ensure unbiased sampling, the electron microscopists were kept blind of the estrous cycle phase of the animals throughout the procedures of tissue processing, image acquisition, and neuropil analyses. Moreover, measurements of the proportion of spines labeled and the level of immunoreactivity within spines and dendritic shafts were conducted by analyzing these profiles strictly in the order that they were encountered, so as to maintain unbiased sampling of the synaptic neuropil. Details of the EM–ICC procedure are described in a previous publication (Sabaliauskas et al., 2012). In brief, the surface-most portion of vibratome sections from each animal was probed for the presence of SIG product using the JEOL 1200XL transmission electron microscope. This area of the tissue received maximum exposure to the antibodies and is therefore most likely to be reflective of protein levels. For analysis of dendritic spine labeling, non-overlapping images of at least 100 spines per animal from the proximal one-third of the stratum radiatum of CA1 hippocampus were acquired. This region of the stratum radiatum receives input from the CA3 Schaffer collaterals, and therefore most closely parallels the area of CA1 analyzed physiologically. Images were digitally captured using a Hamamatsu CCD camera at a magnification of 40,000 × (AMT, Inc). At this magnification, approximately 19 μm2 of neuropil is sampled per micrograph. For SIG immunocytochemical analysis, a minimum of 399 μm2 of neuropil was sampled per animal for both α4 and δ. Excitatory synapses on dendritic spines were identified by the presence of a thick post-synaptic density (PSD) apposed to and parallel to the plasma membrane of the presynaptic terminal, so identified by the presence of numerous transparent vesicles. Those spines that had clear evidence of membraneously expressed SIG particles were considered to be positively labeled for either the α4 or δ subunit and were quantified as the number of SIG-labeled spines per 10 spines encountered. Altogether, at least 10 groups of 10 spines were sampled per animal. Additionally, the quantity of SIG labeling was also calculated by determining the number of SIG particles contained within each SIG cluster of an immunopositive spine, and calculated per synapse counted. For any one comparison (e.g., the proportion of spines immunolabeled for the α4 subunit), the number of spines analyzed per animal was kept strictly constant.
4.4. Recombinant receptors
4.4.1. Transfection
Plasmids obtained from J. Bracamontes (rat β2), P. Whiting (human δ) and N.L. Harrison (mouse α4) were propagated in Escherichia coli DH5α and prepared using Qiagen Maxi- or Midi-prep kits. (Rat, mouse and human β2 subunits are identical.) All receptors were transfected at a 1:1:1 ratio. HEK-293 cells were maintained in medium (DMEM:Ham’s F-12 1: 1) supplemented with 10% fetal calf serum at 37 °C in a humid 5% CO2 atmosphere. Cells were transfected using a nucleofector with a protocol optimized for HEK-293 cells (Amaxa/Lonza, Walkersville, MD) (Kuver et al., 2012) and co-transfected with enhanced green fluorescent protein (ratio of DNA to eGFP was 6:1) for visualization. α4-containing GABARs required 2–3 d for maximal levels of expression.
4.4.2. Recombinant receptors: Electrophysiology
4.4.2.1. Whole cell patch clamp recording
GABA-gated currents were recorded from transfected HEK-293 cells at room temperature (20–22 °C) at a holding potential of −50 mV (Shen et al., 2007). The bath solution contained (in mM): NaCl 120, CsCl 5, CaCl2 2, MgCl2 1, Hepes 10 and glucose 25,pH 7.4, 320 mOsmol. The pipet solution contained (in mM): N-methyl-D-glucamine chloride 120, Cs4 BAPTA 5 (Calbiochem), Mg-ATP 5, and an ATP regeneration system (20 mM Tris phosphocreatine and creatine kinase). Patch pipets (filament-capillary tubes, Sutter Instruments, Novato, CA) were fabricated from borosilicate glass using a Flaming–Brown puller to yield open tip resistances of 3–5 MΩ. Cells were recorded in the presence of 1 μM ZnCl2 to block current from binary receptors which may have formed (Meera et al., 2011). Incorporation of the δ subunit was detected by La3+ inhibition (Saxena et al., 1997). The effects of THP isomers (3α-OH-THP, 3β-OH-THP) on recombinant α4βδ GABARs were determined across a range of GABA concentrations (1 × 10−9−1 × 10−3 M), applied with or without steroid using a solenoid-controlled micropipette array 50 μm from the cell to deliver agonist/steroid for approximately 400–500 ms exposure times with 200–250 ms onset of application (Smith et al., 1998). THP isomers were also bath applied 30 s prior to tests of steroid effects. Currents were recorded using an Axopatch 1D amplifier (Axon Instruments) filtered at 2 kHz (four-pole Bessel filter) and detected at 10 kHz (pClamp 7.1).
4.4.2.2. Data analysis
Analysis of peak current was accomplished with pClamp 10.1 (Axon Instruments, Union City, CA) and Origin (Microcal, Piscataway, NJ) software packages. In all cases, 2–3 current traces were averaged for each agonist or agonist+THP group. Mean values were plotted as a semi-logarithmic function as a percentage of the maximal current generated by GABA (In/IMAX × 100), where In is the current for each concentration of agonist, and IMAX is the maximal current generated by GABA. The curve was fitted by the least squares method as a sigmoidal function using the logistic equation, , where I is the current for the indicated concentration x, A1 is the minimum current, A2 is the maximal current at a saturating agonist concentration, x is the concentration of agonist, x0 is the EC50 (concentration of agonist needed to produce a response that is 50% of the maximal response) and p is the Hill coefficient.
4.5. Slice electrophysiology
4.5.1. Hippocampal slice preparation (Shen et al., 2007)
Mice were rapidly decapitated; the brains were removed and cooled using an ice cold solution of artificial cerebrospinal fluid (aCSF) containing (in mM): NaCl 124, KCl 5, CaCl2 2, KH2PO4 1.25, MgSO4 2, NaHCO3 26, and glucose 10, saturated with 95% O2, 5% CO2 and buffered to a pH of 7.4. Following sectioning at 400 μm, slices were incubated for 1 h in oxygenated aCSF.
4.5.2. Recording of GABAergic tonic current (Shen et al., 2007)
Pyramidal cells in the CA1 hippocampal slice were visualized using a differential interference contrast (DIC)-infrared upright microscope, and recorded using whole cell patch clamp procedures in voltage clamp mode at 26–30 °C. Patch pipets were fabricated from borosilicate glass using a Flaming–Brown puller to yield open tip resistances of 2–4 MΩ. Pipet solution (in mM): 140 CsCl, 5 HEPES, 5 EGTA, 2 Mg-ATP, 0.5 CaCl2–H2O, 0.5 Li-GTP, pH 7.2, 290 mOsm. 5 mM QX-314 was added to block voltage-gated Na+ channels and GABAB receptors. The aCSF contained 50 μM kynurenic acid, to block glutamatergic excitatory current, as well as 1 μM TTX to isolate the post-synaptic component.
Recordings were conducted with a 2 kHz 4-pole Bessel filter at a 10 kHz sampling frequency using an Axopatch 200B amplifier and pClamp 9.2 software. Electrode capacitance and series resistance were monitored and compensated; access resistance was monitored throughout the experiment, and cells discarded if the access resistance increased more than 10% during the experiment. Recordings were carried out at a −60 mV holding potential, and the tonic current was assessed by the change in holding current in response to 100 nM gaboxadol (THIP), a GABAR agonist, which, at this concentration, is selective for δ-containing GABAR (Brown et al., 2002; Meera et al., 2011). The GABAergic nature of the current was verified by block with 120 μM SR95531 or 20 μM bicuculline. Both drugs were bath applied continuously in sequential order following 5–10 min of baseline recordings without drugs. In most cases, the data represent one recording/animal.
4.5.3. Estimation of the direction of GABAergic current
Cell-attached, tight seal recordings in current clamp mode can reveal the membrane potential of the cell (Perkins, 2006). Thus, we recorded from CA1 hippocampal pyramidal neurons in the slice using cell-attached >1 GΩ current clamp recordings (pipet solution, 150 mM NaCl) with no additional current injection. The bath contained 100 μM kynurenic acid, 2 μM CGP 55845 and 1 μM TTX to pharmacologically isolate the post-synaptic GABAR tonic current. After stable recordings were obtained, the GABA agonist gaboxadol (5 μM) was added to the bath. A downward deflection indicated a hyperpolariz-ing response, which reflects outward current (inward flux). The GABAergic nature of the response was verified with addition of a GABA antagonist.
4.5.4. Perforated patch recordings
In order to detect effects of THP on the tonic current without disturbing the internal Cl− milieu, gramicidin perforated patch recordings were carried out (Shen et al., 2007). Following standard slice preparation (see above), CA1 hippocampal pyramidal cells were patched with pipets containing 25 μg/ml gramicidin (mixed by sonication) in addition to (in mM): 140 KCl, 0.1 MgCl2, 0.07 CaCl2, 10 HEPES and 0.1 EGTA, pH 7.3, 300 mOsm. The pipet was front-filled with gramicidin-free solution to permit successful tight-seal formation. After >1 GΩ seals were formed, access resistance was periodically checked as the steady-state response to a 10 mV hyperpolarizing step. Recording commenced after a stable access resistance <60 MΩ had been achieved, indicating a stable level of perforation. The holding current was recorded before and after bath application of 30 nM THP in the presence of 1 μM TTX and 1 μM GABA to isolate the post-synaptic response. SR95531 (200 nM) was included to block synaptic current (Stell and Mody, 2002), 50 nM L-655,708 to block α5-GABARs and 2 mM kynurenic acid to block glutamatergic excitatory current.
4.6. Monitoring of spontaneous spiking
In some cases, cell-attached recordings were conducted from hippocampus in voltage clamp mode (at a −60 mV holding potential) to monitor spontaneous spiking, and the effect of THP. In this case, the pipet contained 140 mM NaCl, but the aCSF had no additional components.
4.7. LTP studies (Shen et al., 2010)
Hippocampal slices were placed between nylon nets in a submerged chamber of an upright microscope. Field EPSPs (fEPSPs) were recorded extracellularly from the stratum radiatum of CA1 hippocampus using an aCSF-filled glass micropipet (1–5 mΩ) in response to stimulation of the Schaffer collateral–commissural pathway using a pair of insulated tungsten bipolar electrodes. The intensity of the stimulation was adjusted to produce 50% of the maximal response. LTP was induced using theta burst stimulation (Larson et al., 1986) (TBS, 8–10 trains of 4 pulses at 100 Hz, delivered at 200 ms intervals, repeated 3 × at 30 s intervals) which is a physiological stimulation pattern (Larson and Lynch, 1986). fEPSP responses were recorded at 30 s intervals with an Axoprobe-1A amplifier and pClamp 10.1 for 20 min before and 120 min after TBS (producing 1–4 mV EPSPs). In some cases, 30 nM THP or 30 nM 3β-OH-THP (the inactive isomer of THP, used as a blocker of the active isomer), were bath-applied to the slice. The strength of synaptic excitatory responses was assessed by measuring the slope (initial 20–80%) of the fEPSP rising phase. Data are expressed as a % of the average response from the 20 min control period for each slice, and are averaged for all slices (mean±SEM) across the time-course of the experiment. A statistically significant potentiation at 120 min was determined by averaging the final 20 values for fEPSP slope compared with the average control values using the paired t-test (with a criterion of P<0.05).
4.8. Drugs
All drugs except for the steroids and QX-314 were from Sigma Chemical Co. (St. Louis, MO). Steroids were obtained from Steraloids (Newport, RI), while QX-314 was from Calbiochem (Billerica, MA). The active isomers of THP, 3α-OH-5β-pregnan-20-one (pregnanolone) and 3α-OH-5α-pregnan-20-one (allopregnanolone) were used interchangeably because they produced similar results. In some cases, the inactive isomer of THP (Harrison et al., 1987), 3β-OH-THP (3β-OH-5β-pregnan-20-one) was also used to block the effect of the active isomer (s) (Shen et al., 2007).
4.9. Spatial learning task
Acquisition of spatial learning was assessed with an active place avoidance task, described previously (Shen et al., 2010). This task was selected because it is hippocampal-dependent (Cimadevilla et al., 2001) and depends on in vivo late-LTP (Pastalkova et al., 2006) in the CA1 hippocampus. The task also produces minimal stress, as evidenced by a lack of change in corticosterone levels in response to the minimal shock delivered (<0.2 mA) (Friedman et al., 1967). After an initial 10 min habituation to the rotating disk (40 cm dia, 1 rpm), mice were trained for 3 10-min trials/h to avoid foot shock (60 Hz, 500 ms) in a 60° sector of the disk (Inset, Fig. 1). The time to first enter the avoidance zone for each trial was assessed as an indicator of learning acquisition, and 120 s was set as the learning criterion (Pastalkova et al., 2006). In some cases, additional trials were administered if the animals did not reach the learning criterion of taking 120 s or longer to first enter the avoidance zone. All animals were assessed for their ability to display robust escape behavior (#shocks/entry ≤1.3) in order to be included in the study.
4.10. Statistics
All data are presented as the mean±the standard error of the mean (SEM). Data were shown to fit a normal distribution using the Kolmogorov–Smirnov test for normality. A statistically significant potentiation at 120 min in the LTP study was determined by averaging EPSP slope in the final 20 min for each recording of a particular estrous phase to compare with average control values for each cell using the paired t-test (comparing before and after TBS, with a criterion of P<0.05). Comparisons of the degree of potentiation (final 20 min, LTP study) across estrous cycle stages were analyzed with a one-way analysis of variance (ANOVA). Post-hoc comparisons for the ANOVA were made with a post-hoc Tukey’s test. For the behavioral studies, a one-way ANOVA was performed to assess statistical significance of latency to enter the avoidance zone, followed by a post-hoc Tukey’s test. In comparisons of the number of animals to reach criterion per group, positive and negative outcomes were coded with a numerical value of 1 or 0, respectively, before statistical analysis with a Chi Square analysis. For EM-immunocytochemistry analysis, a one-way ANOVA was used, followed by a post-hoc Tukey’s test. For all tests, the level of significance was determined to be P<0.05.
Acknowledgments
The authors are grateful to G. Homanics (U. Pittsburgh, Pittsburgh, PA) for supplying the α4+/− mouse breeding pairs and for genotyping the animals, to W. Sieghart (Med. U. Vienna, Austria) for his generous gift of the δ antibody and to A. Fenton (NYU, NY, NY) for the use of the active place avoidance apparatus. We also thank N. Zeak for helpful technical assistance. The work in this study was supported by grants from the U.S. National Institutes of Health: DA09618, AA12958 and MH100561 to S.S.S. and MH091445, NS066019, NS047557, NEI Core grant EY13079 and GM097634 to C.A. as well as a grant from the Klarman Foundation Grant Program in Eating Disorders Research to C.A.
Footnotes
Uncited reference
References
- Adams MM, Morrison JH, Gore AC. N-methyl-D-aspartate receptor mRNA levels change during reproductive senescence in the hippocampus of female rats. Exp Neurol. 2001;170:171–179. doi: 10.1006/exnr.2001.7687. [DOI] [PubMed] [Google Scholar]
- Aoki C, Rogrigues S, Kurose H. Use of electron microscopy in the detection of adrenergic receptors. 2000:535–563. doi: 10.1385/1-59259-684-3:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA (A) receptors. Neuropharm. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci USA. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA (A) receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodensteiner KJ, Cain P, Ray AS, Hamula LA. Effects of pregnancy on spatial cognition in female Hooded Long-Evans rats. Horm Behav. 2006;49:303–314. doi: 10.1016/j.yhbeh.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Briz V, Baudry M. Estrogen regulates protein synthesis and actin polymerization in hippocampal neurons through different molecular mechanisms. Front Endocrinol (Lausanne) 2014;5:22. doi: 10.3389/fendo.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broverman DM, Vogel W, Klaiber EL, Majcher D, Shea D, Paul V. Changes in cognitive task performance across the menstrual cycle. J Comp Physiol Psychol. 1981;95:646–654. doi: 10.1037/h0077796. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha (4)beta (3)delta GABA (A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CA, Perez Y, Lacaille JC. Effects of GABA (A) inhibition on the expression of long-term potentiation in CA1 pyramidal cells are dependent on tetanization parameters. Hippocampus. 1998;8:289–298. doi: 10.1002/(SICI)1098-1063(1998)8:3<289::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Cheng VY, et al. Alpha5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci. 2006;26:3713–3720. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Krishnan K, Bandyopadhyaya AK, Wang C, Taylor A, Benz A, Covey DF, Zorumski CF, Mennerick S. The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: evidence for a low-affinity interaction. J Neurophysiol. 2009;102:1254–1264. doi: 10.1152/jn.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadevilla JM, Wesierska M, Fenton AA, Bures J. Inactivating one hippocampus impairs avoidance of a stable room-defined place during dissociaiton of arena cues from room cues by rotation of the arena. Proc Natl Acad Sci. 2001;98:3532–3536. doi: 10.1073/pnas.051628398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha-5 GABA (A) receptors. Proc Natl Acad Sci. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman JD, Moore MD, Olsen RW, Fanselow MS. The role of the delta GABA (A) receptor in ovarian cycle-linked changes in hippocampus-dependent learning and memory. Neurochem Res. 2014 doi: 10.1007/s11064-014-1282-6. [DOI] [PubMed] [Google Scholar]
- Dalla C, Whetstone AS, Hodes GE, Shors TJ. Stressful experience has opposite effects on dendritic spines in the hippocampus of cycling versus masculinized females. Neurosci Lett. 2009;449:52–56. doi: 10.1016/j.neulet.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Cerro S, Jung M, Lynch G. Benzodiazepines block long-term potentiation in slices of hippocampal and piriform cortex. Neuroscience. 1992;49:1–6. doi: 10.1016/0306-4522(92)90071-9. [DOI] [PubMed] [Google Scholar]
- Espeland MA, et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173:1429–1436. doi: 10.1001/jamainternmed.2013.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SB, Ader R, Grota LJ, Larson T. Plasma corticosterone response to parameters of electric shock stimulation in the rat. Psychosom Med. 1967;29:323–328. doi: 10.1097/00006842-196707000-00003. [DOI] [PubMed] [Google Scholar]
- Frodl T, et al. Reduced hippocampal volume correlates with executive dysfunctioning in major depression. J Psychiatry Neurosci. 2006;31:316–323. [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Walf AA. Mnemonic effects of progesterone to mice require formation of 3alpha,5alpha-THP. NeuroReport. 2010;21:590–595. doi: 10.1097/WNR.0b013e32833a7e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA-A receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Gong QH, Smith SS. Characterization of neurosteroid effects on hyperpolarizing current at alpha4beta2delta GABA receptors. Psychopharmacology (Berlin) 2014 doi: 10.1007/s00213-014-3538-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Oertner TG. Depolarization gates spine calcium transients and spike-timing-dependent potentiation. Curr Opin Neurobiol. 2012;22:509–515. doi: 10.1016/j.conb.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Hardie J, Spruston N. Synaptic depolarization is more effective than back-propagating action potentials during induction of associative long-term potentiation in hippocampal pyramidal neurons. J Neurosci. 2009;29:3233–3241. doi: 10.1523/JNEUROSCI.6000-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure–activity relationships for steroid interaction with a g-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1987;241:346–353. [PubMed] [Google Scholar]
- Henderson VW, et al. Cognition, mood, and physiological concentrations of sex hormones in the early and late postmenopause. Proc Natl Acad Sci USA. 2013;110:20290–20295. doi: 10.1073/pnas.1312353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston CM, Bright DP, Sivilotti LG, Beato M, Smart TG. Intracellular chloride ions regulate the time course of GABA-mediated inhibitory synaptic transmission. J Neurosci. 2009;29:10416–10423. doi: 10.1523/JNEUROSCI.1670-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JS, Newport EL. Critical period effects in second language learning: the influence of maturational state on the acquisition of English as a second language. Cogn Psychol. 1989;21:60–99. doi: 10.1016/0010-0285(89)90003-0. [DOI] [PubMed] [Google Scholar]
- Kramár EA, Babayan AH, Gall CM, Lynch G. Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton. Neuroscience. 2013;239:3–16. doi: 10.1016/j.neuroscience.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley SP, Dunlop JI, Kirkness E, Lambert JJ, Peters JA. A cytoplasmic region determines single-channel conductance in 5-HT3 receptors. Nature. 2003;424:321–324. doi: 10.1038/nature01788. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Overcoming interference: an fMRI investigation of pattern separation in the medial temporal lobe. Learn Mem. 2007;14:625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuver A, Shen H, Smith SS. Regulation of the surface expression of alpha4beta2delta GABA (A) receptors by high efficacy states. Brain Res. 2012;1463:1–20. doi: 10.1016/j.brainres.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Verreault M, Herndon JG. Fluctuations in spatial recognition memory across the menstrual cycle in female rhesus monkeys. Psychoneuroendocrinology. 2001;26:623–639. doi: 10.1016/s0306-4530(01)00017-8. [DOI] [PubMed] [Google Scholar]
- Larson J, Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986;232:985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;386:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Lejbak L, Vrbancic M, Crossley M. Endocrine therapy is associated with low performance on some estrogen-sensitive cognitive tasks in postmenopausal women with breast cancer. J Clin Exp Neuropsychol. 2010;32:836–846. doi: 10.1080/13803391003596389. [DOI] [PubMed] [Google Scholar]
- Lethaby A, Hogervorst E, Richards M, Yesufu A, Yaffe K. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database Syst Rev. 2008;23:CD003122. doi: 10.1002/14651858.CD003122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA, Griffiths JL, Dunn SM, Martin IL. Changes in GABA (A) receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience. 2005;131:397–405. doi: 10.1016/j.neuroscience.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Mody I. GABA (A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2009;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA (A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SA. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. 2012:e4389. doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Wallner M, Otis T. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA-A receptors. J Neurophysiol. 2011;106:2057. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;424:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- Moore MD, Cushman J, Chandra D, Homanics GE, Olsen RW, Fanselow MS. Trace and contextual fear conditioning is enhanced in mice lacking the alpha4 subunit of the GABA (A) receptor. Neurobiol Learn Mem. 2010;93:383–387. doi: 10.1016/j.nlm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K, Zorumski CF, Izumi Y. Propofol inhibits long-term potentiation but not long-term depression in rat hippocampal slices. Anesthesiology. 2005;103:318–326. doi: 10.1097/00000542-200508000-00015. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Perkins KL. Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J Neurosci Methods. 2006;154:1–18. doi: 10.1016/j.jneumeth.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phend KD, Rustioni A, Weinberg RJ. An osmium-free method of epon embedment that preserves both ultrastructure and antigenicity for post-embedding immunocytochemistry. J Histochem Cytochem. 1995;43:283–292. doi: 10.1177/43.3.7532656. [DOI] [PubMed] [Google Scholar]
- Puia G, Mienville JM, Matsumoto K, Takahata H, Watanabe H, Costa E, Guidotti A. On the putative physiological role of allopregnanolone on GABA (A) receptor function. Neuropharmacology. 2003;44:49–55. doi: 10.1016/s0028-3908(02)00341-6. [DOI] [PubMed] [Google Scholar]
- Saab BJ, Maclean AJ, Kanisek M, Zurek AA, Martin LJ, Roder JC, Orser BA. Short-term memory impairment after isoflurane in mice is prevented by the alpha5 gamma-aminobutyric acid type A receptor inverse agonist L-655,708. Anesthesiology. 2010;113:1061–1071. doi: 10.1097/ALN.0b013e3181f56228. [DOI] [PubMed] [Google Scholar]
- Sabaliauskas N, Shen H, Homanics GE, Smith SS, Aoki C. Knockout of the gamma-aminobutyric acid receptor subunit alpha4 reduces functional delta-containing extrasynaptic receptors in hippocampal pyramidal cells at the onset of puberty. Brain Res. 2012;1450:11–23. doi: 10.1016/j.brainres.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro EC, Kotak VC, Sanes DH, Aoki C. Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GABA-A receptors in the auditory cortex. Cerebral Cortex. 2008;18:2855–2867. doi: 10.1093/cercor/bhn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, Neelands TR, Macdonald RL. Contrasting actions of lanthanum on different recombinant gamma-aminobutyric acid receptor isoforms expressed in L929 fibroblasts. Mol Pharmacol. 1997;51:328–335. doi: 10.1124/mol.51.2.328. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Keenan PA, Schenkel LA, Berlin K, Gibson C, Rubinow DR. Cognitive performance in healthy women during induced hypogonadism and ovarian steroid addback. Arch Womens Ment Health. 2013;16:47–58. doi: 10.1007/s00737-012-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, Lopresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J Neurosci. 2004;24:11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Quiroz YT, Hasselmo ME, Stern CE. Greater working memory load results in greater medial temporal activity at retrieval. Cerebral Cortex. 2009;19:2561–2571. doi: 10.1093/cercor/bhp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at alpha4-beta2-delta GABA-A receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases delta GABA-A receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for a4bd GABA-A receptors in shaping learning deficits at puberty in mice. Science. 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB, Grigorova M. Differential effects of estrogen and micronized progesterone or medroxyprogesterone acetate on cognition in postmenopausal women. Fertil Steril. 2011;96:399–403. doi: 10.1016/j.fertnstert.2011.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker SA, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30:16137–16148. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci. 2005;25:7780–7791. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 2006;26:8517–8522. doi: 10.1523/JNEUROSCI.5279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JMH, Li X. GABAA receptor a4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–929. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Snyder MA, Cooke BM, Woolley CS. Estradiol potentiation of NR2B-dependent EPSCs is not due to changes in NR2B protein expression or phosphorylation. Hippocampus. 2011;21:398–408. doi: 10.1002/hipo.20756. [DOI] [PubMed] [Google Scholar]
- Solis-Ortiz S, Guevara MA, Corsi-Cabrera M. Performance in a test demanding prefrontal functions is favored by early luteal phase progesterone: an electroencephalographic study. Psychoneuroendocrinology. 2004;29:1047–1057. doi: 10.1016/j.psyneuen.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Mody I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J Neurophysiol. 1992;68:197–212. doi: 10.1152/jn.1992.68.1.197. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABA-A receptors. Proc Natl Acad Sci. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA (A) conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JH, Raymond JR. Interaction of calmodulin with the serotoin 5-hydroxytryptamine2A receptor. A putative regulator of G protein coupling and receptor phosphorylation by protein kinase C. J Biol Chem. 2005;280:30741–30750. doi: 10.1074/jbc.M501696200. [DOI] [PubMed] [Google Scholar]
- Vedder LC, Smith CC, Flannigan AE, McMahon LL. Estradiol-induced increase in novel object recognition requires hippocampal NR2B-containing NMDA receptors. Hippocampus. 2013;23:108–115. doi: 10.1002/hipo.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res. 2009;196:254–260. doi: 10.1016/j.bbr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PM, Messier C. The hippocampal formation—orbitomedial prefrontal cortex circuit in the attentional control of active memory. Behav Brain Res. 2001;127:99–117. doi: 10.1016/s0166-4328(01)00355-2. [DOI] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- Weiland NG. Estradiol selectively regulates agonist binding sites on the N-methyl-D-aspartate receptor complex in the CA 1 region of the hippocampus. Endocrinology. 1992;131:662–668. doi: 10.1210/endo.131.2.1353442. [DOI] [PubMed] [Google Scholar]
- Whissell PD, Eng D, Lecker I, Wang D-S, Martin LJ, Orser BA. Acutely increasing d-GABA-A receptor activity impairs memory and inhibits synaptic plasticity in the hippocampus. Front Neural Circuits. 2013a doi: 10.3389/fncir.2013.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whissell PD, Rosenzweig S, Lecker I, Wang DS, Wojtowicz JM, Orser BA. deltaGABA receptors promote memory and neurogenesis in the dentate gyrus. Ann Neurol. 2013b doi: 10.1002/ana.23941. http://dxdoi.org/10.1002/ana.23941. [DOI] [PubMed]
- Wiltgen BJ, Sanders MJ, Ferguson C, Homanics GE, Fanselow MS. Trace fear conditioning is enhanced in mice lacking the delta subunit of the GABAA receptor. Learn Mem. 2005;12:327–333. doi: 10.1101/lm.89705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PA, Bove K, You S, Chambers A, Hrushesky WJ. Cancer growth and spread are saltatory and phase-locked to the reproductive cycle through mediators of angiogenesis. Mol Cancer Ther. 2005;4:1065–1075. doi: 10.1158/1535-7163.MCT-05-0028. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BK, Kim DK, Kang Y, Kim JW, Shin MH, Na DL. Hormone replacement therapy in postmenopausal women with Alzheimer’s disease: a randomized, pospective study. Fertil Steril. 2003;79:274–280. doi: 10.1016/s0015-0282(02)04666-6. [DOI] [PubMed] [Google Scholar]