Highlights
-
•
Adult rats develop ethanol-seeking habits more rapidly than adolescents.
-
•
Adolescents appear resistant to the habit promoting effects of ethanol.
-
•
Adolescent rats self-administer more ethanol than adults.
-
•
Adolescent onset ethanol self-administration results in greater self-administration in adulthood.
Keywords: Alcohol, Adolescent, Habit, Goal-directed, Contingency degradation, Self-administration
Abstract
Early age of onset alcohol drinking is significantly more likely to lead to alcohol use disorders (AUDs) than alcohol drinking that begins after the age of 18. Unfortunately, the majority of people in the United States begin drinking in adolescence. Therefore, it is important to understand how early alcohol drinking leads to increased risk for AUDs so that better treatments and prevention strategies can be developed. Adolescents perceive greater rewarding properties of alcohol, and adolescents may be more likely to form alcohol-seeking habits that promote continued use throughout the lifetime. Therefore, we compared the development of alcohol seeking habits in adolescent and adult male, Sprague-Dawley rats. Rats were trained to lever press to receive 10% ethanol + 0.1% saccharin on a schedule that promotes habit formation. Rats were tested using a contingency degradation procedure at different points in training. Adult rats formed ethanol-seeking habits with only moderate training, while adolescents remained goal-directed even with extended training. Nevertheless, adolescents consumed more ethanol than adults throughout the experiment and continued to consume more ethanol than adults when they reached adulthood. Therefore, early onset alcohol use may promote AUD formation through establishment of high levels of drinking that becomes habitual in adulthood.
1. Introduction
Early age of onset of alcohol use is one of the best predictors of future development of an alcohol use disorder (AUD). Individuals who begin drinking before the age of 15 have significantly increased odds of developing an AUD than individuals who begin drinking after the age of 19 (Grant and Dawson, 1997, Grant et al., 2006, Bratek et al., 2013, Hingson and Zha, 2009). Adolescents consume more alcohol, and perceive the positive effects of alcohol as more positive and the negative effects as less negative than adults (Anderson et al., 2010, Spear, 2000, Spear, 2014, Silveri and Spear, 1998). However, this does not necessarily explain why early age of use is more likely to correlate with AUDs later in life. Indeed, alcohol use could simply decline once people reach adulthood. One possible explanation is that alcohol use initiated at a younger age is more likely to become a habitual behavior that is insensitive to changes in alcohol's rewarding or aversive properties. Alternatively, early alcohol use may result in changes to brain structure and function such that alcohol maintains greater rewarding properties in adulthood.
In order to begin to test these possibilities, we sought to determine whether there are differences in the propensity of adolescents and adults to form ethanol-seeking habits. A stimulus–response habit is defined as an action or behavior that is insensitive to changes in the value of the outcome produced by that action. Such actions also persist even when the contingency between the action and the outcome is changed (i.e., when the response no longer produce the outcome) (Yin and Knowlton, 2006, Balleine and O’Doherty, 2010). Current theories about the persistence of substance use disorders postulate that abnormal habit formation may explain why drug use persists even when the drug is no longer rewarding or its use results in adverse consequences (O’Tousa and Grahame, 2014, Belin et al., 2013, Everitt and Robbins, 2005). Adolescent substance use is known to be risk factor for the development substance use disorders; however, it is unclear if this increased risk is due to an increased propensity to form habits. Indeed, prior studies examining the vulnerability of adolescents to develop habitual or inflexible behaviors relative to adults have had mixed results. For example, in one study, adolescent rats demonstrated more habit-like behavior than adults when the contingency between the action and outcome was degraded, but did not appear habitual when tested using an outcome devaluation procedure (Naneix et al., 2012). In addition, Simon et al. (2013), found that adolescent rats exhibited more flexible behavior in a Pavlovian conditioning task relative to adults. Therefore, it is not clear if adolescents would be more or less likely than adults to form ethanol-seeking habits.
Furthermore, prior studies only examined habit formation for food reinforcers, but responding for ethanol becomes habitual more rapidly than responding for food (Dickinson et al., 2002, Corbit et al., 2012). Thus, adolescents may form ethanol-seeking habits at a different rate than food seeking habit. Indeed, previous research has demonstrated that food habits form more quickly in females than in males, but that the opposite is true for ethanol habit formation (Quinn et al., 2007, Barker et al., 2010). Therefore, in the present study we compared the rate of habit formation for an ethanol reinforcer in adolescent and adult rats using the contingency degradation paradigm. We also compared later adult ethanol self-administration in rats with adolescent vs. adult onset ethanol exposure.
2. Materials and methods
2.1. Subjects
Male Sprague-Dawley rats (Harlan, Frederick, MD) were delivered to the animal facility aged either 22 or 64 days. Rats were allowed to acclimate to the facility for 6 days before behavioral testing began on postnatal day (PND) 28, which is generally recognized as the beginning of early adolescence (Spear, 2000, Spear and Swartzwelder, 2014) or 70 (adulthood). Rats were housed two per cage for the entire experiment. Rats were maintained on a 12:12 h light–dark cycle in a temperature- and humidity-controlled environment. The rats were given ad libitum access to food and water except for periods of food restriction described below. All procedures conformed to the policies set forth by the University of Pittsburgh Institutional Animal Care and Use Committee and the National Institutes of Health Guidelines on the Care and Use of Laboratory Animals.
2.2. Behavioral testing
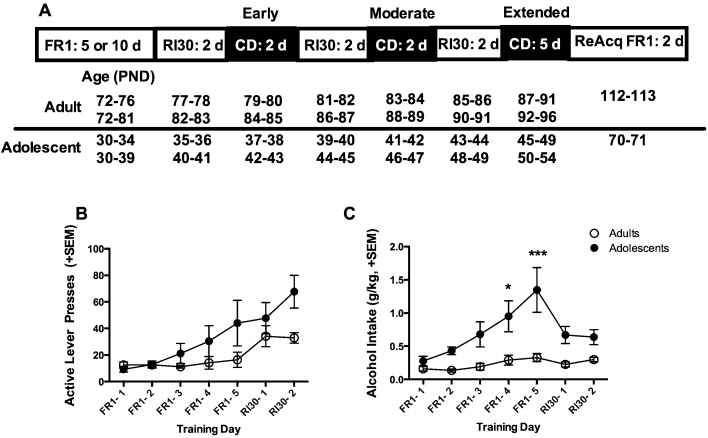
The experimental timeline and age of rats at each stage of testing are shown in Fig. 1A. Rats were food restricted beginning 3 days before behavioral testing. Rats were given sufficient daily food rations to maintain approximately 90% of their expected free-feeding body weight based on standard growth curves. Thus, adolescent rats were given sufficient food to grow and gain weight, but at a slower rate than rats fed ad libitum. The feeding procedure is based on published methods and produced no obvious detrimental effects to the animals and likely is similar to food intake that would be observed in the wild. Food restriction facilitates instrumental learning and ensured that all behavioral training and testing could occur within the age window of adolescence. In all phases of the experiment, rats were given or responded for a 10% v/v ethanol + 0.1% w/v saccharin solution made up in tap water. We used a slightly sweetened ethanol solution to best model initial ethanol drinking patterns in humans. Fig. 1 illustrates the timeline of behavioral testing and the details of each phase of testing are described below.
Fig. 1.
Acquisition of instrumental responding. (A) Experimental timeline indicating the timeline of training and testing and the age of both subgroups of adult adolescent rats that acquired self-administration at different rates. (B) Active lever presses during ethanol + saccharin self-administration on the last 5 FR1 training days for all rats and the first 2 days of RI30 training. (C) Ethanol consumption across training in g/kg. Adolescents (N = 10) consumed significantly more ethanol than adults (N = 8) on the last 2 days of FR1 training. Data are presented as the mean ± the standard error of the mean (SEM), *p < 0.05, ***p < 0.001.
2.3. Ethanol habituation
On the day before operant training began rats were habituated to the ethanol solution to avoid neophobia (i.e., fear of novelty) and facilitate acquisition of lever pressing behavior. Rats were placed in a novel cage containing one piece of standard chow for 15 min. The rats were allowed to become accustomed to the novel cage and eat the food pellet so that they would be more likely to be thirsty and sample the liquid solution. A bottle containing the ethanol solution was then placed on a wire top on each cage and the rats were allowed 30 min to sample the solution. The bottles were weighed before and after each session to verify that each rat consumed some of the solution.
2.4. Magazine training
All testing was conducted in standard operant chambers (MedAssociates, St. Albans, VT) and behavioral programs were controlled by MedPC software. All boxes contain a liquid dipper for delivery of liquid reinforcers. Two retractable levers were located on either side of a magazine (i.e., a receptacle) where reinforcers were delivered. The boxes were also equipped with a house light, stimulus lights above the levers, tone generators, and fans that allowed ventilation and produced background noise.
Rats underwent a single 30-min session of magazine training where the ethanol reinforcer was presented on a fixed-time 30-s schedule, which included the 10 s of access to the liquid dipper. The rats, therefore, had access to 60 reinforcers, and magazine entries were recorded to verify that rats learned to obtain reinforcers in the magazine.
2.5. Self-administration
Rats were trained to self-administer the oral ethanol solution in daily 30 min sessions. At the beginning of each session 2 levers were inserted into the chamber, one was designated the “active” lever and the other the “inactive” lever for each rat. Assignment of the left or right lever as the active lever was balanced across groups. The rats were initially trained to respond on a fixed ratio 1 (FR1) schedule of reinforcement where a single lever press on the active lever resulted in 10-s access to a dipper full of the ethanol solution (0.05 mL), but no other stimuli. At the end of the 10-s access period the dipper was lowered out of the magazine. Lever presses during the 10-s access period were recorded but produced no consequences. Presses on the inactive lever were recorded but produced no programmed consequences. The troughs containing the ethanol solution were weighed before and after each session and magazine entries recorded to ensure that the rats drank the solution and to calculate g/kg ethanol intake. We did not measure blood ethanol concentrations, as we did not want the stress of blood draws to affect the rate of habit formation (Dias-Ferreira et al., 2009, Gourley et al., 2012). Rats self-administered the ethanol solution for at least 5 days on the FR1 schedule until they acquired at least 10 reinforcers on two consecutive sessions.
After acquisition of responding on an FR1, rats were switched to a random interval 30 s (RI30) schedule of reinforcement. Interval schedules rapidly generate instrumental habits (Hay et al., 2013, Nevin et al., 2001) allowing for habit formation and testing during adolescence. On an RI30 schedule, the first lever press after a random interval elapses that averages 30 s in duration, but can range from 1 s to 60 s. The computer program randomly generated the intervals. In this manner, rats cannot predict when their behavioral action will generate the outcome, and responding is more likely to become independent of the action-outcome contingency. Rats received 2 RI30 training sessions before initial contingency degradation testing and were returned to the RI30 schedule for subsequent training.
2.6. Contingency degradation testing
After the last day of instrumental training, rats were given 2–4 days of contingency degradation training depending on the phase of the experiment. During this session both levers were available but responses on the levers did not generate reinforcer delivery or any other programmed consequences. Ethanol reinforcers were presented freely (non-contingently) at a rate that matched each rat's average rate of reinforcement on the previous day of training. The reinforcer was given on the fixed time schedule regardless of responding on the lever. In this manner the contingency between the action (responding on the lever) and the outcome (ethanol solution access) was degraded, and rats behaving in a goal-directed, action-outcome based manner reduce responding on the lever, while animals that have formed a habit continue to respond (Yin and Knowlton, 2006, Barker et al., 2013). Rats received 3 cycles of RI30 training and contingency degradation testing to assess the timing of habit formation across training and between groups.
2.7. Extinction test
The day following the last contingency degradation training day, rats were given a 10 min extinction test to further assess whether animals could use knowledge about changes in the action-outcome contingency to guide behavior (Yin and Knowlton, 2006, Gourley et al., 2012). In this test, both levers were extended and responses recorded, but lever presses produced no programmed consequences. No reinforcement was given during this test.
2.8. Reacquisition
After the extinction test all rats were housed in their home cage and received no behavioral training or ethanol and were given ad libitum access to food, until the adolescent group aged to adulthood (PND70). Rats were then tested on an FR1 schedule as before to determine levels of ethanol solution self-administration after adolescent-onset or adult-onset drinking. Testing on the FR1 schedule allowed for the rats to earn a substantial amount of reinforcers in the session without being limited by the time restrictions imposed by an interval schedule.
2.9. Statistical analysis
Self-administration training data were analyzed using a repeated measures two-way ANOVA. Day of training was the within-subjects factor and amount of ethanol consumed in g/kg or active lever presses was the between subjects factor. Responses on contingency degradation test days were also compared using a Two-way rm ANOVA comparing active lever responses on the last RI30 training day to responses made during the contingency degradation tests. The extinction test was analyzed by normalizing each animal's response rate on its last day of contingency degradation training and on the extinction test day to its rate of responding on the most recent RI30 training day and comparing groups by Two-way repeated measures (rm) ANOVA. All significant interactions were followed by Bonferroni's posthoc test. Alpha was set to 0.05.
3. Results
Groups of 12 adolescents and 12 adults began training. 5 adolescents and 4 adults acquired self-administration within 5 days, while an additional 5 adolescents and 4 adults acquired after 5 additional days of FR1 training. The smaller groups were each analyzed separately as the age range differed slightly for testing, but we observed the same results in both subgroups, so the data were combined and all analyses represent results from a group of 10 adolescents and 8 adults. The ages of the 2 subgroups at each stage of testing are shown in Fig. 1A. Six of the rats (2 adolescents and 4 adults) never acquired self-administration to our criteria (earning at least 10 reinforcers on FR1) and were not analyzed further. The number of rats failing to acquire is within the range often observed in rat self-administration studies in our laboratory.
3.1. Self-administration training
Both adolescent and adult rats acquired self-administration over days. Analysis of active lever presses during the last 5 days of FR1 training and the first two RI30 training days demonstrated a significant effect of day as the number of responses increased across training and, as expected, with the switch to an interval schedule of reinforcement [F(6,96) = 6.99, p < 0.001]. There was no significant interaction between age and day of training [F(6,96) = 1.461, p = 0.19] (Fig. 1B). On the other hand, analysis of gram per kilogram ethanol intake indicated main effects of day [F(6,96) = 7.79, p < 0.001] and age [F(1,16) = 8.71, p = 0.009], and their interaction [F(6,96) = 3.85, p = 0.002]. Overall, adolescents consumed more ethanol than adults and posthoc analysis indicated significant differences on the last 2 days of FR1 training (Fig. 1C). The nature of the RI30 schedule limits the amount of reinforcers rats can receive in a session, though lever-pressing behavior increases.
3.2. Contingency degradation tests
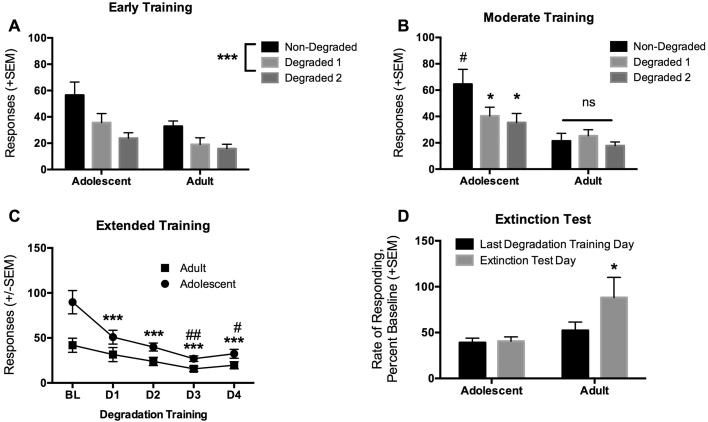
After 2 days of training on the RI30 schedule, considered an early time point, rats had their first contingency degradation tests over 2 days. We expected rats to behave in a goal-directed manner at this point in training unless they formed habits exceptionally fast. Indeed, as expected we observed a main effect of day [F(2,32) = 18.95, p < 0.001], a non-significant effect of age [F(2,32) = 4.24, p = 0.056], and no significant interaction [F(2,32) = 1.80, p = 0.18], indicating that both adolescent and adult rats significantly reduced responding after contingency degradation relative to their level of responding on the last training day, indicating goal-directed, non-habitual behavior (Fig. 2A).
Fig. 2.
Contingency degradation testing. (A) Early in instrumental training both adolescent and adult rats demonstrated significant reductions in active lever presses on two daily contingency degradation tests, indicating goal-directed behavior. ***p < 0.001 main effect of degradation in both groups. (B) After moderate training the adolescent rats continued to demonstrate goal-directed behavior on contingency degradation tests, and also consumed significantly more ethanol on the last non-degraded session relative to the adults. The adults, however, did not reduce responding on contingency degradation tests relative to the last non-degraded session, indicating the formation of habitual behavior. *p < 0.05 comparing degraded to non-degraded responding, #p < 0.05 comparing adolescent to adult responding in the non-degraded session. (C) After extended training, the adolescents again remained goal-directed on contingency degradation tests, while adults did not significantly reduce responding from baseline until day 3 of degradation of training. The adolescents did continue to reduce responding across days. By the last day of degradation training both adolescents and adults reached less than 50% of baseline responding. ***p < 0.001 indicating a significant decrease in responding on degradation days relative to baseline in the adolescents, ##p < 0.01, #p < 0.05 indicating significant decrease in responding on degradation days relative to baseline in adults. (D) In an extinction test following the last degradation training day, the adolescents did not significantly increase responding relative to the last day of degradation training, while the adults increased responding back to baseline levels, providing further evidence that the adults formed a habitual ethanol seeking behavior. *p < 0.05 comparing adult behavior on the last degradation day to the extinction test. Data are presented as the mean ± the standard error of the mean (SEM).
Rats were then returned to RI30 training for 2 additional days, reaching a moderate level of training where we expect more vulnerable groups to have formed habits. Rats again underwent contingency degradation, and interestingly, we observed significant main effects of day [F(2,32) = 8.32, p = 0.001] and age [F(1,16) = 7.37, p = 0.015], and an interaction between day and age [F(2,32) = 7.31, p = 0.002]. Posthoc analysis indicated that while adolescent rats did show a significant reduction in responding on both contingency degradation days relative to their last RI30 training day, the adult rats showed no significant reduction in responding after degradation of the contingency, which is indicative of a behavior that has become a habit (Fig. 2B). However, while the adolescents remained goal-directed, they did respond significantly more for the ethanol solution on the last RI30 training day.
Next, we set out to test whether or not adolescents would form a habit if they had extended training with an additional 2 days of RI30 training when we typically observe habit formation in normal adults responding for food. We also conducted more days of degradation training to see if the adults would eventually reduce responding, and to test them in extinction. As was observed after moderate training, we found significant main effects of day [F(4,64) = 28.89, p < 0.001] and age [F(1,16) = 7.40, p = 0.015], and their interaction [F(4,64) = 5.31, p < 0.001]. Posthoc analysis indicated that the adolescent rats reduced responding on all days of degradation training relative to their last RI30 training day. On the other hand, the adults did not significantly reduce responding on degradations days 1 or 2, but did significantly reduce responding on days 3 and 4 of degradation training (Fig. 2C). Thus, the adults continued to show habitual behavior that did adjust with enough training, while the adolescents, reduced responding on day 1 and continued to significantly reduce responding on subsequent days of degradation training, indicating that the behavior of the adolescents remained flexible, and goal-directed. Again, adolescents responded significantly more for the ethanol solution on the RI30 training day.
The day after the last degradation training day, when both the adolescents and adults were responding at about 40–50% of their RI30 baseline, we tested the rats in extinction to see if the groups would express contingency degradation learning or if behavior would return to baseline rates of responding as generally occurs in animals that have formed a habit. Indeed, when we compared normalized rates of responding on the last degradation training day to the extinction test day we found main effects of day [F(1,16) = 5.72, p = 0.029] and age [F(1,16) = 4.88, p = 0.042], and their interaction [F(1,16) = 4.80, p = 0.044]. Posthoc analysis indicated that adolescent rats responded at the same rate on the last day of degradation training and on the extinction test day, indicating retention of flexible behavior. On the other hand, the adults significantly increased their rate of responding during the extinction test, almost to 100% of their RI30 baseline, further indicating that the adults had formed an ethanol seeking habit (Fig. 2D).
3.3. Adult self-administration
Next, we were interested in whether animals that began ethanol self-administration in adolescence would continue to show higher levels of self-administration if tested in adulthood relative to rats that began ethanol self-administration in adulthood. We simply allowed all rats to remain in their home cages until the adolescents reached PND70. We then tested levels of responding on two days of ethanol self-administration on an FR1 schedule, which allows for more reinforcers to be obtained than on an interval schedule. Analysis of active lever presses on the reacquisition days indicated no significant effect of age of onset of drinking (Fig. 3A). However, there was significant main effect of age of drinking onset when we analyzed the amount of ethanol consumed in g/kg [F(1,16) = 9.71, p = 0.007], indicating that the adolescent-onset drinkers did indeed self-administer higher quantities of ethanol than adult-onset drinkers.
Fig. 3.
Adult ethanol self-administration. (A) Active lever presses for ethanol reinforcement after sufficient abstinence from ethanol to allow the adolescent group to age to adulthood (PND70). (B) Ethanol intake in the two groups that had initiated ethanol self-administration in adolescence vs. adulthood. Adolescent consumed significantly greater amounts of ethanol relative to adults on both days, **p < 0.01. Data are presented as the mean + the standard error of the mean (SEM).
4. Discussion
The present study compared ethanol habit formation in adolescent versus adult male rats using a random interval training paradigm that allowed for habit development and testing within the adolescent period. The results indicated that early in training both adolescents and adults exhibited goal-directed (non-habitual) ethanol seeking in a contingency degradation test. On the other hand, at a moderate level of random interval training, adult rats did not reduce responding when the contingency was degraded, suggesting that the adults had formed ethanol-seeking habits, while the adolescents remained goal-directed. Interestingly, even after extended training, the adolescent rats remained goal-directed and reduced their responding upon contingency degradation. In contrast, the adult animals continued to demonstrate habitual behavior. The adult group also failed to express contingency degradation learning in an extinction test in contrast to the adolescents, further indicating that only adults formed habits in this experiment. Therefore, the present data indicates that adolescents are more resistant to forming ethanol-seeking habits than adults.
Despite the fact that adults formed ethanol-seeking habits more readily than adolescents, adolescents consistently consumed higher doses of ethanol than adults. Moreover, when the rats that began ethanol self-administration in adolescence were abstained from ethanol until they reached adulthood (PND70), and were reassessed on their levels of ethanol self-administration, the adolescent onset drinkers consumed significantly more ethanol than the adult onset drinkers. Therefore, adolescents do demonstrate a greater propensity to respond for ethanol reinforcement than adults, as has been reported previously (Helms et al., 2014, Vetter et al., 2007, Doremus et al., 2005), and this ethanol seeking and consumption appears to be goal-directed rather than habitual. However, it is possible that the increased adult intake was due to adolescent exposure to a sweetened ethanol solution, which has been shown to promote acceptance of that solution in adulthood (Broadwater et al., 2013). Nevertheless, in the present experiment both adolescent and adult onset groups had prior exposure to the solution, suggesting that acceptance levels should have been similar, and that the increased self-administration in the adolescent onset group is at least partially due to greater motivation for the solution. Indeed, other studies have found that injections of ethanol in early adolescence increased ethanol drinking and operant self-administration in adulthood (Alaux-Cantin et al., 2013), and that monkeys that begin drinking in adolescence go on to consume more ethanol in adulthood (Helms et al., 2014). However, it should be noted that one study has found that adolescent home cage, unlimited access to ethanol in a two-bottle choice paradigm does not significantly increase later adult drinking (Vetter et al., 2007). The difference between the Vetter et al., study and the other studies could be the difference in limited versus unlimited access paradigms, which results in a large difference in the total amount of ethanol consumed, or to other adaptations induced by different access conditions.
Nevertheless, our results suggest that adolescent onset ethanol exposure results in neural plasticity or pharmacological adaptations that either maintains enhanced motivation for ethanol, reduces the aversive qualities of ethanol, or alters the pharmacokinetic properties of alcohol in adulthood. Regardless of the mechanism, in this scenario, individuals that begin drinking ethanol in early adolescence are predicted to consume more ethanol in adulthood, and this high level of consumption could then become problematic due to habit formation. Early onset drinking may also make the formation of habits in adulthood more likely, as adolescent ethanol exposure can promote inflexible behavior in adulthood (Gass et al., 2014).
The results of the present study should be interpreted with caution as there are several potential limitations. One possible explanation for our observed effects is that the adults responded at such lower levels than the adolescents, that it is harder to detect a reduction in responding after contingency degradation (i.e., a floor effect). While this is theoretically possible, we do not believe that it explains our findings, as in the final contingency degradation test, the adults did eventually significantly reduce their responses relative to their baseline, indicating that reductions can be observed. Furthermore, despite eventually demonstrating a reduction in behavior after contingency degradation training, adults still failed to express this learning in the extinction test, providing fairly convincing evidence that the adults were habitual, while the adolescents remained goal-directed. Another potential limitation is that the rats underwent repeated contingency degradation testing between bouts of training. While, this design did not impair our ability to detect the development of habitual behavior in the adults, it may have disproportionately affected the adolescents, impairing our ability to detect habit formation in that age group. Thus, if adolescents received continuous training for six days, habitual behavior may have been observed. We believe that this is a distinct possibility, but that the present data, nevertheless, point to a resistance of adolescents to form habits with limited training, and possibly a better ability to retain information about shifts in contingency, which is consistent with an interpretation that adolescents maintain more flexible behavioral strategies than adults (Simon et al., 2013, Sturman and Moghaddam, 2012). Finally, both adolescents and adults were shipped to our facility about a week before testing began. While we attempt to reduce shipment stress by using a vendor within a short driving distance from our facility and allowing the animals to acclimate for a week before handling, the potential differential response of pre-pubertal adolescents and adults to stressful stimuli could have impacted how shipment stress influenced habit formation (McCormick and Mathews, 2010). Due to the fact that stress facilitates habit formation in adults (Dias-Ferreira et al., 2009, Gourley et al., 2012), it will be interesting in future studies to determine how stress influences habit formation in adolescents.
The resistance of adolescent rats to habit formation even after extended training was surprising. Indeed, ethanol exposure can promote habit formation (Corbit et al., 2012) and alter dorsal lateral striatum plasticity in adults (Depoy et al., 2014), so it is unclear why the same effects would not occur in adolescence. However, the brain undergoes many developmental changes during adolescence including in the dorsal striatum and prefrontal cortical dopamine systems, which are known to regulate the development of habitual behavior (Naneix et al., 2012, Balleine and O’Doherty, 2010, Barker et al., 2013, Hitchcott et al., 2007). In addition, dorsal striatal presynaptic dopamine availability is reduced in adolescents relative to adults (Matthews et al., 2013), and dorsal striatal neural response patterns during a rewarded instrumental learning task are significantly different from adult response patterns (Sturman and Moghaddam, 2012). Furthermore, in a Pavlovian autoshaping task, adolescents exhibit more goal-oriented behavior, than cue-oriented behavior, potentially suggesting a propensity to be goal-directed (Anderson et al., 2013).
Finally, human imaging studies consistently report altered functional activity and connectivity in corticostriatal brain regions in adolescence(Geier et al., 2010, Paulsen et al., 2014, Somerville et al., 2011, Casey and Jones, 2010). These studies often report that adolescents have impaired cognitive control and enhanced responses to reward; however, most studies have not specifically compared adolescent and adult measures of behavioral flexibility. Nevertheless, increased neural responses to reward could explain both why adolescents consume more alcohol (and other substances) and maintain goal-directed behavior. It could be that adolescents are highly engaged by rewards, or goals, such that they are highly attuned to changes in reward contingency and value. In addition, the adolescent brain may be “wired” to maintain, flexible, goal-directed behavior for other reasons, including an evolutionary advantage to individuals that flexibly optimize their behavioral repertoire to their environment during adolescence.
Our results are in contrast to those reported by Naneix et al. (2012), where higher levels of responding during contingency degradation were observed in adolescents. The difference in our results could be due to differences in the instrumental training procedures; however, in the previous study the authors did show that adolescents reduced responding relative to baseline on the first contingency degradation day by around 50%, but that they maintained this level of responding rather than continuing to decrease responding over subsequent days of contingency degradation training. Thus, it appears that the adolescents were goal-directed and recognized a change in contingency, but it is unclear why they did not continue to reduce responding over subsequent days of degradation training.
Nevertheless, evidence from other published studies (Simon et al., 2013) and the results presented here, collectively suggest that adolescents behave more flexibly and are less likely to form habits than adults. In addition, our results raise the intriguing possibility that the adolescent brain is resistant to the habit promoting effects of ethanol exposure. However, while we did not observe any facilitation of habit formation with exposure to ethanol in the adolescents, the doses consumed may not have been sufficient to do so. It is possible that exposure to higher doses of ethanol or the development of ethanol dependence may have been able to promote ethanol-seeking habits in adolescents. Future experiments could examine these possibilities; however, the present data suggest that adolescent onset drinking can promote dependence in adulthood due to the establishment of high levels of alcohol consumption.
5. Conclusions
In conclusion, we report that adolescents maintain goal-directed ethanol seeking behavior even with extended training, while adults form ethanol-seeking habits with only moderate training. Nevertheless, adolescents consume significantly greater amounts of ethanol and this persists when they are tested again in adulthood. Thus, adolescent behavior may be more flexible than adults, but adolescents are also more driven by alcohol reward, which could lead to the development of dangerous habits when they reach adulthood.
Conflict of interest
The authors have no conflicts of interest to disclose.
Acknowledgements
The authors would like to acknowledge support from USPHS grant DA031745 and the Pennsylvania Department of Health.
References
- Alaux-Cantin S. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Anderson R.I., Bush P.C., Spear L.P. Environmental manipulations alter age differences in attribution of incentive salience to reward-paired cues. Behav. Brain Res. 2013;257:83–89. doi: 10.1016/j.bbr.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.I., Varlinskaya E.I., Spear L.P. Ethanol-induced conditioned taste aversion in male Sprague-Dawley rats: impact of age and stress. Alcohol. Clin. Exp. Res. 2010;34(12):2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B.W., O’Doherty J.P. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2010;35(1):48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J.M. Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. J. Neurosci.: Off. J. Soc. Neurosci. 2010;30(27):9140–9144. doi: 10.1523/JNEUROSCI.0548-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J.M., Torregrossa M.M., Taylor J.R. Bidirectional modulation of infralimbic dopamine D1 and D2 receptor activity regulates flexible reward seeking. Front. Neurosci. 2013;7:p.126. doi: 10.3389/fnins.2013.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D. Addiction: failure of control over maladaptive incentive habits. Curr. Opin. Neurobiol. 2013;23(4):564–572. doi: 10.1016/j.conb.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Bratek A. Association of early drinking onset with subsequent alcohol abuse. Psychiatria Danubina. 2013;25(Suppl. 2):S99–S101. [PubMed] [Google Scholar]
- Broadwater M., Varlinskaya E.I., Spear L.P. Effects of voluntary access to sweetened ethanol during adolescence on intake in adulthood. Alcohol. Clin. Exp. Res. 2013;37(6):1048–1055. doi: 10.1111/acer.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(12):1189–1201. doi: 10.1016/j.jaac.2010.08.017. (quiz 1285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit L.H., Nie H., Janak P.H. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biol. Psychiatry. 2012;72(5):389–395. doi: 10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoy L. Chronic alcohol alters rewarded behaviors and striatal plasticity. Addict. Biol. 2014 doi: 10.1111/adb.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Ferreira E. Chronic stress causes frontostriatal reorganization and affects decision-making. Science (New York, N.Y.) 2009;325(5940):621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Dickinson A., Wood N., Smith J.W. Alcohol seeking by rats: action or habit? Quart. J. Exp. Psychol. B: Compar. Physiol. Psychol. 2002;55(4):331–348. doi: 10.1080/0272499024400016. [DOI] [PubMed] [Google Scholar]
- Doremus T.L. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol. Clin. Exp. Res. 2005;29(10):1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Gass J.T. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2014;39:2570–2583. doi: 10.1038/npp.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C.F. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebr. Cortex (New York, N.Y.) 2010;20(7):1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley S.L. Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc. Natl. Acad. Sci. U. S. A. 2012;109(50):20714–20719. doi: 10.1073/pnas.1208342109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B.F., Dawson D.A. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. J. Subst. Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- GRANT J.D. Adolescent alcohol use is a risk factor for adult alcohol and drug dependence: evidence from a twin design. Psychol. Med. 2006;36(01):109–118. doi: 10.1017/S0033291705006045. [DOI] [PubMed] [Google Scholar]
- Hay R.A. Specific and nonspecific effects of naltrexone on goal-directed and habitual models of alcohol seeking and drinking. Alcohol. Clin. Exp. Res. 2013;37(7):1100–1110. doi: 10.1111/acer.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms C.M. The effects of age at the onset of drinking to intoxication and chronic ethanol self-administration in male rhesus macaques. Psychopharmacology. 2014;231(8):1853–1861. doi: 10.1007/s00213-013-3417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R.W., Zha W. Age of drinking onset, alcohol use disorders, frequent heavy drinking, and unintentionally injuring oneself and others after drinking. Pediatrics. 2009;123(6):1477–1484. doi: 10.1542/peds.2008-2176. [DOI] [PubMed] [Google Scholar]
- Hitchcott P.K., Quinn J.J., Taylor J.R. Bidirectional modulation of goal-directed actions by prefrontal cortical dopamine. Cerebr. Cortex (New York, N.Y.) 2007;17(12):2820–2827. doi: 10.1093/cercor/bhm010. [DOI] [PubMed] [Google Scholar]
- Matthews M. Reduced presynaptic dopamine activity in adolescent dorsal striatum. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2013;38(7):1344–1351. doi: 10.1038/npp.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C.M., Mathews I.Z. Adolescent development, hypothalamic–pituitary–adrenal function, and programming of adult learning and memory. Progr. Neuro-psychopharmacol. Biol. Psychiatry. 2010;34(5):756–765. doi: 10.1016/j.pnpbp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Naneix F. Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. J. Neurosci.: Off. J. Soc. Neurosci. 2012;32(46):16223–16232. doi: 10.1523/JNEUROSCI.3080-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin J.A. Variable-ratio versus variable-interval schedules: response rate, resistance to change, and preference. J. Exp. Anal. Behav. 2001;76(1):43–74. doi: 10.1901/jeab.2001.76-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Tousa D., Grahame N. Habit formation: implications for alcoholism research. Alcohol (Fayetteville, N.Y.) 2014;48(4):327–335. doi: 10.1016/j.alcohol.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen D.J. Effects of incentives, age, and behavior on brain activation during inhibitory control: a longitudinal fMRI study. Dev. Cogn. Neurosci. 2014 doi: 10.1016/j.dcn.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J.J. Sex chromosome complement regulates habit formation. Nat. Neurosci. 2007;10(11):1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- Silveri M.M., Spear L.P. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol. Clin. Exp. Res. 1998;22(3):670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Simon N.W. Differences in response initiation and behavioral flexibility between adolescent and adult rats. Behav. Neurosci. 2013;127(1):23–32. doi: 10.1037/a0031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Hare T., Casey B.J. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J. Cogn. Neurosci. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. Adolescents and alcohol: acute sensitivities, enhanced intake, and later consequences. Neurotoxicol. Teratol. 2014;41:51–59. doi: 10.1016/j.ntt.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear L.P., Swartzwelder H.S. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci. Biobehav. Rev. 2014;45C:1–8. doi: 10.1016/j.neubiorev.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman D.A., Moghaddam B. Striatum processes reward differently in adolescents versus adults. Proc. Natl. Acad. Sci. U. S. A. 2012;109(5):1719–1724. doi: 10.1073/pnas.1114137109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter C.S., Doremus-Fitzwater T.L., Spear L.P. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol. Clin. Exp. Res. 2007;31(7):1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H.H., Knowlton B.J. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]