Abstract
There is growing evidence that heightened sensitivity to unpredictable threat is a core mechanism of dysfunction in anxiety disorders. However, it is unclear whether anxiety sensitivity is also associated with sensitivity to unpredictable threat. In the present study, 131 participants completed the Anxiety Sensitivity Index–3, which includes physical concerns (PC), social concerns (SC), and cognitive concerns (CC) subscales, and a predictable vs. unpredictable threat-of-shock task. Startle eyeblink and ERP responses (N100, P300) to the acoustic startle probes were measured during the task. PC and CC were associated with heightened and attenuated, respectively, startle for the unpredictable (but not predictable) condition. CC were also associated with attenuated probe N100 for the unpredictable condition only, and PC were associated with increased P300 suppression across the predictable and unpredictable conditions. This study provides novel evidence that the different anxiety sensitivity dimensions demonstrate unique relationships with the RDoC domains “acute” and “potential” threat.
Keywords: anxiety sensitivity, event-related potentials, predictability, startle response
1. Introduction
Anxiety sensitivity (AS) is the fear of anxiety-related sensations due to their perceived physical, psychological, or social consequences (Reiss & McNally, 1985). AS was originally conceptualized as an individual difference factor that contributed to the etiology and maintenance of panic disorder (PD) (McNally, 2002). Indeed, research has shown that AS is elevated in first-degree relatives of probands with PD relative to healthy controls (van Beek & Griez, 2003) and prospectively predicts panic attacks (Maller & Reiss, 1992; Schmidt, Lerew, & Jackson, 1999), panic symptoms (Cox et al., 2008), and panic response to a CO2 challenge (Bernstein et al., 2009; Blechert et al., 2013). However, AS has also been linked to several other psychopathological behaviors and conditions (Deacon & Abramowitz, 2006; Taylor et al., 1996), including alcohol use (Allan, Albanese, Norr, Zvolensky, & Schmidt, 2014; Schmidt, Buckner, & Keough, 2007), depression (Allan, Capron, et al., 2014; Viana & Rabian, 2009), generalized anxiety disorder (GAD; Allan, Macatee, et al., 2014), and suicide (Capron, Cougle, Ribeiro, Joiner, & Schmidt, 2012; Medley, Capron, Korte, & Schmidt, 2013). Thus, AS has more recently been considered a transdiagnostic factor of psychopathology (Boswell et al., 2013).
The National Institute of Mental Health’s Research Domain Criteria (RDoC) initiative seeks to identify biobehavioral dimensions that are common across several disorders and then relate those dimensions to specific biological processes (Insel et al., 2010; Sanislow et al., 2010). AS is an ideal construct to examine using the RDoC approach given its dimensional nature (Asmundson et al., 2011; Broman-Fulks et al., 2008, 2010), genetic correlates (Taylor et al., 2008; Waszczuk et al., 2013), high heritability (Stein et al., 1999), and the aforementioned relationship with multiple psychopathologies (Deacon & Abramowitz, 2006; Taylor et al., 1996). In terms of physiological correlates, greater AS has been associated with a heightened baseline startle eyeblink electromyography (EMG) response (McMillan et al., 2012), decreased baseline startle habituation (Campbell et al., 2014), and heightened startle response in anticipation of interoceptive threat (Melzig et al., 2008).
Affective responses to threat, however, are not uniform. Predictability is an important feature of threat that has been suggested to impact defense system activation and differentiate the states of fear and anxiety (Barlow, 2000; Grillon et al., 2004; Hamm & Weike, 2005). Fear is associated with predictable threat and a more immediate fight, flight, or immobilization response. Conversely, anxiety is elicited when perceived threat is less certain (or present) and requires a sustained state of vigilance and defensive preparedness. The distinction between fear and anxiety has been well-supported by animal (Davis, 1998), psychophysiological (Grillon et al., 2004; Nelson & Shankman, 2011), and pharmacological studies (Grillon et al., 2006), and is represented by separate Negative Valence System constructs (“acute” and “potential” threat, respectively) in the RDoC matrix (NIMH, 2011). Several anxiety disorders (e.g., PD, PTSD) have been associated with an increased startle response in anticipation of unpredictable threat, although the role of predictable threat has been mixed (Grillon et al., 2008, 2009; Shankman et al., 2013). Similarly, high AS has been associated with a preference for predictable relative to unpredictable CO2 administration (Lejuez et al., 2000). However, no study has examined whether AS is associated with the startle response in anticipation of unpredictable vs. predictable threat. This is the first aim of the present study.
High levels of AS have also been associated with increased attention toward threatening stimuli (Hunt et al., 2006; Keogh et al., 2001; Lees et al., 2005). Importantly, this relationship can be examined in the context of startle methods, as the startle probe elicits event-related potential (ERP) measures of early sensory and attentional processing. Specifically, the startle probe elicited N100 is a negative deflection in the ERP signal that is maximal around frontocentral sites and occurs 100 ms after the onset of the startle probe. The probe N100 reflects early perceptual processing of auditory stimuli and is enhanced when participants are instructed to attend to the startle probe while viewing unpleasant relative to pleasant or neutral pictures (Cuthbert et al., 1998). In addition to the probe N100, the startle probe P300 is a positive deflection of the ERP signal that is maximal at centroparietal sites and occurs approximately 300 ms after the onset of the startle probe (Putnam & Roth, 1990; Roth, Dorato, & Kopell, 1984; Sugawara, Sadeghpour, Traversay, & Ornitz, 1994). The probe P300 reflects attention toward the startle probe and is reduced when viewing emotional relative to neutral pictures due to increased attention to emotional foreground stimuli (leaving less attention allocated to the probe itself) (Bradley, Codispoti, & Lang, 2006; Cuthbert, Schupp, Bradley, McManis, & Lang, 1998; Schupp, Cuthbert, Bradley, Birbaumer, & Lang, 1997). Importantly, the startle probe N100 and P300 responses do not reflect the same attentional processes and behave differently: the N100 and P300 are potentiated and reduced, respectively, in the context of threat. Thus, examining the association between AS and startle allow for the examination of both EMG and ERP responses during the same task.
In a recent investigation, Nelson et al. (in press) examined the psychometric properties of the probe N100 and P300 responses during a no, predictable, and unpredictable threat-of-shock (NPU-threat) task. The NPU-threat task contains three distinct within-subjects conditions during which participants anticipate no threat (no aversive stimulus is delivered), predictable threat (aversive stimulus is signaled by short duration cue), or unpredictable threat (aversive stimulus is not signaled). Results indicated that the probe N100 was enhanced in the unpredictable (but not predictable) condition even though participants were not specifically instructed to attend to the startle probe. These data suggest that the anticipation of unpredictable electric shock, relative to unpleasant pictures, may more readily prime early cortical processing of sensory input. In contrast, the probe P300 was attenuated during both the predictable and unpredictable conditions. In addition, the probe N100 and P300 were not correlated across threat conditions, indicating they were measuring separate attentional processes. Collectively, these results suggest that the anticipation of unpredictable threat enhances early perceptual processing and the anticipation of threat in general (irrespective of predictability) increases attention during the threatening conditions of the NPU-threat task. However, no study has examined individual differences in these ERP responses.
Utilizing data from Nelson et al. (in press), the current study examined the association between AS and startle EMG and ERP responses in anticipation of predictable and unpredictable threat. Specifically, 131 undergraduates completed the NPU-threat task and the startle eyeblink EMG response and electroencephalography (EEG) were recorded during the different threat conditions. Self-reported anxiety was also assessed at the end of the task. The current study focused on continuous variation in AS in a college student sample to (1) minimize the contribution of severe psychopathology that is more prevalent in clinical populations, and (2) limit the possibility of a restricted range of AS scores in a clinical sample. Moreover, AS was not examined using a taxometric approach (Bernstein et al., 2007), because we did not expect to have a significant number of participants in the “high risk” group to adequately examine AS a dichotomous construct. We hypothesized that AS would be associated with increased startle EMG, probe N100, and self-reported anxiety and decreased probe P300 in anticipation of unpredictable (but not predictable) threat.
AS was originally conceptualized as a unitary construct measured with the anxiety sensitivity index (ASI) (Reiss et al., 1986). However, since its inception there have been multiple revisions to the ASI and increased recognition that AS is multifaceted. In the present study, participants completed the ASI-3 (Taylor et al., 2007), the most recent version of the ASI, which consists of three factor analytically derived subscales: physical concerns (PC), cognitive concerns (CC), and social concerns (SC). The discriminant validity of these dimensions has been supported by a number of investigations that have examined the ASI-3 subscales in relation to anxiety and depression symptoms. Specifically, research has indicated that ASI-3 PC has been most consistently associated with panic, CC with depression and worry, and SC with social anxiety (Allan, Capron, et al., 2014; Kemper et al., 2012; Olthuis et al., 2014; Wheaton et al., 2012). We did not have specific hypotheses regarding which AS subscales would be associated with responding during the NPU-threat task. However, given that the aversive stimulus used in the task was a physical danger (electric shock), we hypothesized that the association between AS and these measures would be particularly strong for the PC subscale.
Finally, the ASI-3 CC subscale has been strongly associated with depression (Olthuis et al., 2014; Taylor et al., 1996). Therefore, to determine the unique association between ASI-3 CC and the anticipation of predictable and unpredictable threat, participants also completed a self-report measure of depression, and additional analyses were conducted with this measure included as a covariate. We hypothesized that the relationship between AS (and in particular the ASI-3 CC subscale) and startle EMG, probe ERPs, and self-reported anxiety would be independent of depression.
2. Method
2.1. Participants
The sample included 131 introduction to psychology students from the University of Illinois - Chicago who participated for course credit. Exclusion criteria were an inability to read or write English, history of head trauma with a loss of consciousness, or being left-handed (as confirmed by the Edinburgh Handedness Inventory; range of laterality quotient: +10 to +100; Oldfield, 1971). The sample was college-aged (M = 19.36, SD = 2.02), 64.9% female, and ethnically diverse, including 38.2% Caucasian, 28.2 % Hispanic, 22.1% Asian, and 11.5% African-American. Over the preceding 6 months 32.1% of participants reported smoking cigarettes, and over the preceding 30 days 49.6% of participants reported drinking alcohol and 14.5% reported smoking marijuana.1 No participant reported a current medical condition that impacts central nervous system functioning. Informed consent was obtained prior to participation and the research protocol was approved by the University of Illinois – Chicago Institutional Review Board.
2.2. Measures
2.2.1. Anxiety Sensitivity Index–3
The Anxiety Sensitivity Index–3 (Taylor et al., 2007) is an 18-item self-report measure of AS. Each item is rated on a 5-point Likert scale ranging from 0 (very little) to 5 (very much), with higher scores indicating greater AS. The ASI-3 consists of three subscales that contain six items each: physical concerns (PC), cognitive concerns (CC), and social concerns (SC). Cronbach’s alpha for the ASI-3 PC, CC, and SC subscales and total score were all greater than .73 (see Table 1).
Table 1.
Descriptive Statistics and Correlation Coefficients between the ASI-3 and QIDS-SR16
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| 1. | ASI-3 PC | - | .59 | .40 | .80 | .34 |
| 2. | ASI-3 CC | - | .50 | .85 | .49 | |
| 3. | ASI-3 SC | - | .79 | .42 | ||
| 4. | ASI-3 Total | - | .51 | |||
| 5. | QIDS-SR16 | - | ||||
| M | 5.18 | 4.54 | 8.54 | 18.26 | 7.02 | |
| SD | 4.08 | 4.37 | 4.64 | 10.66 | 4.00 | |
| Range | 0–20 | 0–22 | 0–24 | 1–56 | 0–17 | |
| Cronbach’s α | .75 | .82 | .74 | .87 | .73 | |
Note. All correlations were significant at p < .001. ASI-3 = Anxiety Sensitivity Index-3; CC = cognitive concerns; PC = physical concerns; SC = social concerns; QIDS-SR16 = Quick Inventory of Depressive Symptomatology-Self-Report 16-Item.
2.2.2. Quick Inventory of Depressive Symptomatology
The Quick Inventory of Depressive Symptomatology-Self-Report 16-Item (QIDS-SR16) (Rush et al., 2003) measures the nine symptoms of depression over the last week, with higher scores indicated greater depression severity. Each item was rated on a scale ranging from 0–3, with the total QIDS-SR16 score ranging from 0–27. In the present study, the average QIDS-SR16 score indicated minimal depression severity (see Table 1). Cronbach’s alpha for the QIDS-SR16 was .73.
2.3. Stimuli
Stimuli were administered using PSYLAB (Contact Precision Instruments, London, UK). Acoustic startle probes were 40-ms duration, 103-dB bursts of white noise with near-instantaneous rise time presented binaurally through headphones. Electric shocks were 400 ms in duration and administered to the wrist of the participant’s left (non-dominant) hand. Shock intensity was determined ideographically using a work-up procedure for each participant (see section 2.4).
2.4. Procedure
After electrode placement, participants were seated in an electrically shielded, sound-attenuated booth approximately 3.5 ft from a 19-in computer monitor. Participants first completed a 2.5-min baseline habituation task during which nine acoustic startle probes were administered.2 Next, shock intensity was determined using a work-up procedure where participants received increasing levels of shock, until they reached a level they described as “highly annoying but not painful” (maximum shock level was 5 mA). The average shock level was 2.25 mA (SD = 1.21).
The NPU-threat task was a variant of that used by Grillon and colleagues (Schmitz & Grillon, 2012) and included three within-subjects conditions: no shock (N), predictable shock (P), and unpredictable shock (U). Text at the bottom of the screen informed participants of the current condition by displaying “no shock” (N), “shock at 1” (P), or “shock at any time” (U). Each condition lasted 90 s, during which a 6-s visual countdown (CD) was presented five times. The inter-stimulus interval (ISI; i.e., time between CDs during the 90-s condition) ranged from 7–17 s during which only the text describing the condition was on the screen. In the N condition, no shocks were delivered. In the P condition, participants received a shock every time the CD reached 1. In the U condition, shocks were administered at any time (during CD or ISI). Startle probes were presented both during the CD (1–5 s following CD onset) and ISI (5–14 s following ISI onset). Participants did not receive instructions regarding whether they should attend to or ignore the startle probes, but rather were told that, similar to the baseline condition, they would continue to hear the startle probes during the NPU-threat task. The time intervals between shocks and subsequent startle probes were always greater than 10 s to ensure that subsequent probes were not affected by prior shocks.
The task consisted of two presentations of each 90-s condition (N, P, U), during which the CD appeared five times. Participants received startle probes during four out of the five CD and ISI presentations. Conditions were presented in one of the following orders (counterbalanced): PNUPNU or UNPUNP. All participants received 20 electric shocks (10 during P, 10 during U), and 48 startle probes (16 during N, 16 during P, and 16 during U) during the CD and ISI (with an equal number of startle probes occurring during the CD and ISI).
At the end of the task, participants rated their anxiety during each threat condition (i.e., NISI, NCD, PISI, PCD, UISI, UCD) on a scale ranging from 1 (not at all nervous/anxious) to 7 (extremely nervous/anxious).
2.5. EMG Recording and Processing
Startle eyeblink EMG was recorded using Neuroscan 4.4 (Compumedics, Charlotte, NC, USA) and measured from two 4-mm Ag/AgCl electrodes placed over the orbicularis oculi muscle below the right eye. EMG was recorded using a band-pass filter from DC to 200 Hz at a sampling rate of 1000 Hz. Offline, EMG data were rectified and then smoothed using a finite impulse response filter with a band-pass of 28–40 Hz. Peak amplitude of the startle response was determined in the 20–150 ms time frame following the startle probe onset relative to baseline (average baseline EMG level for the 50-ms preceding the startle probe onset). Blinks were scored as non-responses if EMG activity during the 20–150 ms post-stimulus time frame did not produce a blink peak that was visually differentiated from baseline activity. Blinks were scored as missing if the baseline period was contaminated with noise, movement artifact, or if a spontaneous or voluntary blink began before minimal onset latency and thus interfered with the probe-elicited blink response. Startle analyses were conducted using blink magnitude (i.e., averages include values of 0 for non-response trials) as this is a more conservative estimate of blink response (Blumenthal et al., 2005).
2.6. EEG Recording and Data Processing
EEG was recorded using Neuroscan 4.4 (Compumedics, Charlotte, NC, USA) and measured from Ag/AgCl electrodes in a 64-channel stretch-lycra electrode cap. The ground electrode was at the frontal pole (AFz) and the online reference was near the vertex (between Cz and CPz). Electrodes placed at the right supra- and infra-orbital sites were used to monitor vertical eye movements and electrodes placed at the right and left outer canthi were used to monitor horizontal eye movements. Electrode impedances were under 5000 Ω, and homologous sites (e.g., F3/F4) were within 1500 Ω of each other. EEG was recorded through a Neuroscan Synamp2 data acquisition system at a gain of 10K (5K for eye channels) with a band-pass of DC-200 Hz and digitized continuously at a sampling rate of 1000 Hz. Offline, EEG data were re-referenced to the average of the left and right mastoid and band-pass filtered from 0.1 to 30 Hz. Eye blink and ocular corrections were conducted using established standards (Gratton et al., 1983).
A semiautomatic procedure was employed to detect and reject artifacts. The criteria applied were a voltage step of more than 50 μV between sample points, a voltage difference of 300 µV within a trial, and a maximum voltage difference of less than 0.50 µV within 100 ms intervals. These intervals were rejected from individual channels in each trial. Visual inspection of the data was then conducted to detect and reject remaining artifacts.
2.7. Principal Components Analysis
A principal components analysis (PCA), an empirically based method of isolating and scoring ERP components, was conducted to better isolate the startle probe N100 and P300. For the PCA, the ERP was segmented for each trial beginning 200 ms before the startle probe and continuing for 1200 ms, and the baseline was the 200 ms prior to the onset of the startle probe. The ERP segment for each condition (NISI, NCD, PISI, PCD, UISI, UCD), electrode location, and participant was entered into the data matrix. Using the MATLAB ERP PCA Toolbox–Version 2 (Dien, 2010b), a temporal PCA was performed first in order to capture variance across time and to maximize the initial separation of ERP components (Dien & Frishkoff, 2005), and a promax rotation was used to rotate to simple structure in the temporal domain (Dien, Khoe, & Mangun, 2007; Dien, 2010a). Following the first rotation, a parallel test (Horn, 1965) was conducted on the resulting Scree plot (Cattell, 1966), in which the Scree plot of the actual dataset is compared to that derived from a fully random dataset. The number of factors retained is based on the largest number of factors that account for a greater proportion of variance than the fully random dataset (see Dien, 2010b for more information). Based on this criterion, 39 temporal factors were extracted for rotation and the covariance matrix and Kaiser normalization were used for the PCA (Dien, Beal, & Berg, 2005). Following the temporal PCA, a spatial PCA was performed on each temporal factor retained in the previous step in order to reduce the spatial dimensions of the datasets. Infomax was used to rotate to independence in the spatial domain (Dien et al., 2007; Dien, 2010a). Based on the results of the parallel test (Horn, 1965), four spatial factors were extracted from each temporal factor for Infomax rotation, yielding a total of 156 temporospatial factor combinations. To directly assess timing and spatial voltage distributions, the factors were translated back into voltages.
Eighteen temporospatial factor combinations accounted for more than 1% of the variance and in total accounted for 59.4% of the variance. Of the 18 factors, two resembled the temporal and spatial characteristics of the N100 and P300 (see Figure 1). Specifically, TF4SF1 resembled the N100, was maximal approximately 100 ms after the onset of the startle probe, and accounted for 3.1% of the variance. In addition, TF5SF1 resembled the P300, was maximal approximately 300 ms after the onset of the startle probe, and accounted for 3.4% of the variance. Therefore, TF4SF1 and TF5SF1 were used as the PCA-derived N100 and P300, respectively, for subsequent analyses.3
Figure 1.
Waveforms (left) and head maps (right) for the PCA-derived N100 (top) and P300 (bottom). Data were collapsed across CD and ISI phases of each threat condition. The x- and y-axes are at difference scales for the N100 and P300 figures. CD = countdown; ISI = inter-stimulus interval; ms = milliseconds; N = no threat; P = predictable threat; PCA = princal components analysis; U = unpredictable threat.
2.8. Data Analysis
Twelve participants were excluded from analyses due to equipment failure (n = 5), excessive EEG artifacts that resulted in less than 50% useable trials (n = 2), outlier startle values (n = 2) (Hoaglin & Iglewicz, 1987; Hoaglin, 1986; Tukey, 1977), or current psychotropic medication use (antidepressant, n = 2; stimulant, n = 1), leaving a final sample of 119 participants.
To examine the association between the ASI-3 and responding during the NPU-threat task, we conducted two separate ASI-3 X Condition (N, P, U) X Cue (CD vs. ISI) mixed-measures analysis of covariance (ANCOVA) models; the first model included the ASI-3 total as a mean-centered independent variable and the second had the PC, CC, and SC subscales entered as simultaneous mean-centered independent variables. NPU-threat condition order (PNUPNU vs. UNPUNP) was also included as a dichotomous covariate in both sets of analyses. Separate analyses were conducted for the startle response, N100, P300, and self-reported anxiety. One participant did not complete the self-report anxiety measure, leaving a sample of 118 participants for those analyses. All analyses were conducted in IBM SPSS Statistics, Version 22.0 (Armonk, NY, USA).
3. Results
3.1. Self-Report Questionnaires
Table 1 lists the descriptive statistics and correlation coefficients between the ASI-3 and QIDS-SR16. As expected, all measures were moderately correlated and demonstrated moderate to excellent reliability.
3.2. Startle EMG
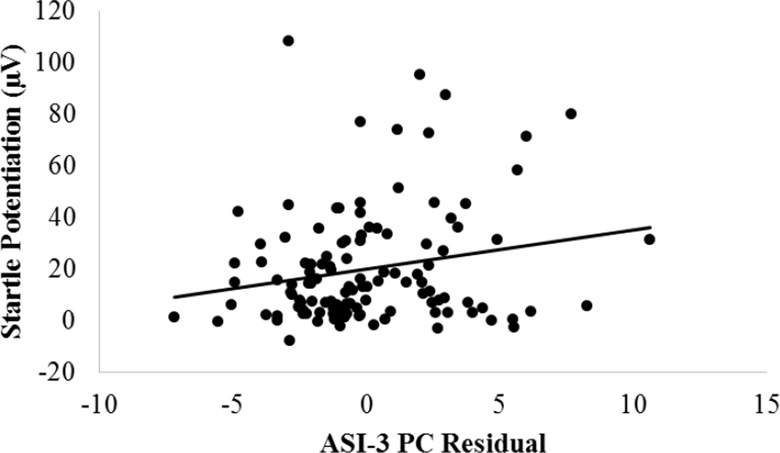
For startle EMG, there were no main effects or interactions involving ASI-3 total (ps > .24). However, there were ASI-3 PC X Condition, F(2, 228) = 5.49, p < .05, ηp2 = .05, and ASI-3 CC X Condition interactions, F(2, 228) = 7.20, p < .01, ηp2 = .06. The ASI-3 PC and CC subscales were not associated with startle during the NCD+ISI (ps < .68); therefore, the interactions were followed-up by conducting separate partial correlations between the specific ASI-3 subscale and startle potentiation for the unpredictable condition (i.e., UCD+ISI - NCD+ISI), controlling for the other ASI-3 subscales and NPU-threat task order. For the ASI-3 PC X Condition interaction, follow-up analyses indicated that greater physical concerns was associated with increased startle potentiation during the UCD+ISI, pr(114) = .20, p < .05, but not the PCD+ISI, pr(114) = −.03, ns (see top of Figure 2). In contrast, for the ASI-3 CC X Condition interaction, follow-up analyses indicated that greater cognitive concerns was associated with decreased startle potentiation during the UCD+ISI, pr(114) = −.25, p < .01, but not the PCD+ISI, pr(114) = −.09, ns (see top of Figure 3). In other words, while the ASI-3 PC and CC subscales both exhibited unique associations with startle potentiation for the unpredictable (but not predictable) condition, they demonstrated the opposite relationship (positively and negatively correlated, respectively). There were no effects for ASI-3 SC (ps > .31).
Figure 2.
Scatterplots depicting the association between ASI-3 PC residuals and startle potentiation during the UCD+ISI (relative to the NCD+ISI; top), P300 suppression (more negative values indicate greater suppression) during the PCD+ISI and UCD+ISI (relative to the NCD+ISI; middle), and self-reported anxiety across all conditions. ASI-3= Anxiety Sensitivity Index-3; au = arbitrary units; CD = countdown; ISI = inter-stimulus interval; N = no threat; P = predictable threat; PC = physical concerns; U = unpredictable threat.
Figure 3.
Scatterplots depicting the association between ASI-3 CC residuals and startle potentiation during the UCD+ISI (relative to the NCD+ISI; top) and N100 enhancement (more negative values indicate greater enhancement) during the UCD (relative to the NCD; bottom). ASI-3 = Anxiety Sensitivity Index-3; CC = cognitive concerns; CD = countdown; ISI = inter-stimulus interval; U = unpredictable threat.
3.3. ERPs
3.3.1. N100
For the PCA-derived N100,4 there were no main effects or interactions involving ASI-3 total (ps > .08). However, ASI-3 subscale analyses5 indicated an ASI-3 CC X Condition X Cue interaction, F(2, 228) = 4.45, p < .05, ηp2 = .04. The ASI-3 CC subscale was not associated with the N100 during the NCD or NISI (ps < .66); therefore, to follow-up the interaction separate ASI-3 CC X Cue ANCOVA models were conducted for N100 enhancement during the predictable (i.e., PCD - NCD, PISI - NISI) and unpredictable (i.e., UCD - NCD, UISI - NISI) conditions. For the predictable condition, there were no main effects or interactions involving ASI-3 CC (ps > .85). For the unpredictable condition, results indicated an ASI-3 CC X Cue interaction, F(1, 114) = 6.66 p < .05, ηp2 = .06. This interaction was followed-up by conducting separate partial correlations between ASI-3 CC (controlling for the PC and SC subscales and the NPU-threat task order) and N100 enhancement during the UISI and UCD. Results indicated ASI-3 CC was positively associated with N100 enhancement during the UCD, pr(114) = .25, p < .01, but not the UISI, pr(114) = −.01 (see bottom of Figure 3). In other words, greater cognitive concerns were associated with a decreased (i.e., less negative) N100 during the UCD. There were no associations between ASI-3 PC or SC and the N100 during any threat condition (ps > .12).
3.3.2. P300
For the PCA-derived P300,4 there was a main effect of ASI-3 total, F(1, 116) = x4.56, p < .05, ηp2 = .04, such that greater AS was associated with a smaller P300 across all conditions, pr(116) = −.19, p < .05. ASI-3 subscale analyses indicated an ASI-3 PC X Condition interaction, F(2, 228) = 3.56, p < .05, ηp2 = .03. The ASI-3 PC subscale was not associated with the P300 during the NCD or NISI (ps < .61); therefore, to follow-up the interaction separate ASI-3 PC X Cue ANCOVA models were conducted for P300 suppression during the predictable (i.e., PCD - NCD, PISI - NISI) and unpredictable (i.e., UCD - NCD, UISI - NISI) conditions. Results indicated a main effect of ASI-3 PC, F(1, 114) = 737, p < .01, ηp2 = .06, such that greater physical concerns were associated with increased P300 suppression during the PCD+ISI and UCD+ISI, pr (114) = −.25, p < .01 (see middle of Figure 2). There were no effects for ASI-3 CC or SC (ps > .12). These results suggest that the association between ASI-3 total and P300 suppression in anticipation of threat was due to the PC subscale and was evident across predictable and unpredictable contexts.
3.4. Self-Reported Anxiety
For self-reported anxiety, there was a main effect of ASI-3 total, F(1, 115) = 14.63, p < .001, ηp2 = .11, such that greater AS was associated with increased anxiety across all conditions, pr(115) = 33, p < .001. ASI-3 subscale analyses indicated a main effect of ASI-3 PC, F(1, 113), such that greater physical concerns were associated with increased anxiety across all conditions, pr (113) = .22, p < .05 (see bottom of Figure 2). There were no effects for ASI-3 CC or SC (ps > .11).6 These results suggest that the association between ASI-3 total and self-reported anxiety was due to the PC subscale and was evident across all threat contexts.
3.5. Independence of AS and Depression
Finally, we examined whether the aforementioned associations between the ASI-3 subscales and responding during the NPU-threat task were independent of depression. Specifically, we conducted identical follow-up partial correlations between the ASI-3 subscales and startle EMG, N100, P300, and self-reported anxiety but also included the QIDS-SR16 as a covariate.7 Results again indicated a significant association between ASI-3 PC and CC and startle potentiation during the UCD+ISI, pr (113) = .20, p < .05; pr(112) = −.25, p < .01, respectively; ASI-3 CC and N100 enhancement during the UCD, pr(113) = .24, p < .05; ASI-3 PC and P300 suppression during the PCD+ISI and UCD+ISI, pr (113) = −.24, p < .01; and ASI-3 PC and self-reported anxiety across all conditions, pr (112) = .22, p < .05. These results suggest that the associations between the ASI-3 subscales and responding to predictable and unpredictable threat were not better accounted for by depression.8
4. Discussion
The present study examined the association between AS and startle EMG, probe N100 and P300, and self-reported anxiety in anticipation of predictable and unpredictable threat. ASI-3 total was associated with increased P300 suppression across predictable and unpredictable threat conditions and self-reported anxiety across all conditions, but was not associated with startle EMG or probe N100 during any threat condition. However, the ASI-3 subscale analyses revealed a more complex pattern of results. Specifically, the ASI-3 PC and CC subscales were both associated with startle potentiation for the unpredictable (but not predictable) condition; however, they demonstrated the opposite relationship. Greater physical concerns were associated with heightened startle potentiation and greater cognitive concerns were associated with attenuated startle potentiation (even when both subscales were in the same model). ASI-3 CC were associated with decreased (i.e., less negative) probe N100 enhancement for the unpredictable condition only. ASI-3 PC were associated with probe P300 suppression across both threat contexts, such that greater physical concerns was associated with increased P300 suppression. ASI-3 PC were also associated with increased self-reported anxiety across all conditions. Finally, all associations between AS and responding during the threat conditions were independent of current depression symptoms. Together, these results provide novel evidence for an association between AS and affective (startle EMG, self-report anxiety) and attentional (N100, P300) indicators of threat sensitivity, and suggest that the different AS dimensions demonstrate unique relationships with these measures.
The present study adds to a growing number of anxiety phenotypes that have been associated with a heightened sensitivity to unpredictable threat. Greater physical concerns, the ASI-3 dimension most closely connected with risk for panic disorder (Schmidt et al., 1999; van Beek & Griez, 2003), was associated with heightened potentiation in anticipation of unpredictable threat only. This result is consistent with a previous investigation that found a heightened startle response in anticipation of unpredictable (but not predictable) threat was associated with a familial history (i.e., risk) of PD, independent of current anxiety (Nelson et al., 2013). However, the present study and Nelson et al. were both cross-sectional and it is unclear whether physical concerns or a heightened sensitivity to unpredictable threat predicts the development of PD, or, alternatively, whether they are concurrent risk factors. Future longitudinal studies are needed to determine the causal relationship amongst these factors.
ASI-3 CC were associated with decreased startle potentiation in anticipation of unpredictable (but not predictable) threat. There are several potential explanations for this finding. For example, experiential avoidance (i.e., the unwillingness to remain in contact with an aversive experience; Chawla & Ostafin, 2007) has been particularly associated with the ASI-3 CC subscale (Berman et al., 2010). It is therefore possible that individuals high in cognitive concerns engaged in some form of avoidance (e.g., rumination, worry) to diminish their anxiety while anticipating unpredictable threat. This interpretation is consistent with Borkovec’s cognitive avoidance theory of worry, which postulates that worry is a verbal linguistic, thought-based activity that inhibits emotional reactivity (Borkovec & Inz, 1990; Borkovec et al., 2004). Interestingly, ASI-3 CC is the subscale of AS most closely linked to depression (Olthuis et al., 2014), and depression has often been associated with a decreased emotion-modulated startle response to unpleasant stimuli (Allen et al., 1999; Kaviani et al., 2004; McTeague et al., 2009). However, in the present study the association between cognitive concerns and startle potentiation remained significant after controlling for depression. This suggests that the cognitive distress elicited by the anticipation of unpredictable threat, and not depression per se, is what contributed to diminished defense system activation. The startle response results also highlight the importance of considering the heterogeneity of anxiety in relation to emotional responding, and suggests that future studies are needed to delineate the neural mechanisms that contribute to these important differences (e.g., Shackman et al., 2013).
The present study also adds to a growing literature on AS and aberrations in attention to threat. Behavioral studies have indicated that high AS is associated with an increased attentional bias toward threat (Hunt et al., 2006; Keogh et al., 2001; Lees et al., 2005); however, this relationship has not been examined in the context of predictable and unpredictable threat. In the present study, ASI-3 PC were associated with increased P300 suppression across the predictable and unpredictable conditions. Probe P300 suppression purportedly reflects increased attention to foreground emotional stimuli and the resultant decreased orienting response to the probe (Bradley et al., 2012). The present results suggest that individuals with high physical concerns directed more attention toward the foreground stimuli (i.e., the threat cues) and away from the startle probe. In contrast, ASI-3 CC were associated with decreased (i.e., less negative) N100 enhancement for the unpredictable condition only. The probe N100 has been proposed to reflect the enhancement of salient sensory input (Näätänen & Picton, 1987). Thus, in addition to attenuated defense system activation, high cognitive concerns is also related to diminished early sensory processing in anticipation of unpredictable threat. It is important to note that ASI-3 CC were only associated with the cued (i.e., CD) but not contextual (i.e., ISI) phase of the unpredictable threat condition. Interestingly, both phases of the unpredictable threat condition were equally dangerous, and it is possible that, at least in participants with high cognitive concerns, the unpredictable threat cue was particularly distressing and elicited early attention disengagement.
The correlation between AS and the startle EMG and probe N100 and P300 measures ranged from .20−.25, indicating small effect sizes. However, there was no shared method variance between these measures, which has been shown to impact the association between two measured constructs (Campbell & Fiske, 1959). The present study provides novel evidence regarding the association between AS and sensitivity to unpredictable threat, and these results are particularly useful regarding the conceptualization of AS, future research design, and hypothesis generation. It is important to highlight the relationship between AS and anticipatory threat responding was not affected by variables that have been shown to impact aversive responding (e.g., alcohol use, smoking, etc.). However, there were several other demographic and individual difference factors (e.g., diet, exercise, menstrual cycle, sleep) that were not assessed in this sample and could influence AS and anxious responding. Future studies should measure these (and other) factors to account for a greater proportion of variance in AS and sensitivity to unpredictable threat.
The ASI-3 social concerns subscale was not associated with responding during any threat condition. It is possible that the context under which threat is measured may be differentially important for the different ASI-3 dimensions. Indeed, in the present study the NPU-threat task was administered in a sound-attenuated booth with no other people present. Future studies might consider whether being observed or different types of threat (e.g., social rejection) impact the association between the social concerns dimension of the ASI-3 and emotional responding in anticipation of predictable and unpredictable threat.
Although previous studies have been successful in targeting and reducing AS (Norr, Allan, McAtee, Keough, & Schmidt, 2014; Gallagher et al., 2013; Boswell et al., 2013; MacDonald, Koerner, & Antony, 2013; Mitchell, Capron, Raines, & Schmidt, 2014), the current results may provide further insight into possible therapeutic targets for these treatment efforts. Specifically, rather than focusing treatments on reducing sensitivity to threat more generally, interventions that target predictable and (perhaps more importantly) unpredictable threat should be considered. These treatments may want to consider increasing tolerance of unpredictability (Robichaud & Dugas, 2008), which in turn may reduce prolonged anxiety. Treatments should also consider structuring goals based on the problematic AS dimension(s) (i.e., PC, CC, SC), which, may vary from tolerating the unpredictability of threat (e.g., individuals with high physical concerns) to cognitively processing anticipatory threat cues (e.g., individuals with high cognitive concerns).
The present study supports the utility of the AS construct in relation to two separate RDoC Negative Valence System constructs—acute and potential threat (i.e., “fear” and “anxiety”, respectively). The NPU-threat task is particularly useful for this aim as it assesses both constructs. The predictable condition taps responses to an acute “fearful event” (i.e., the pending danger is occurring in a matter of moments) and the unpredictable condition taps responses to a prolonged stressful or “anxiety event” (i.e., the danger might occur, but no immediate threat is pending). Additionally, as shown in the present study, the startle variant of the NPU-threat task is particularly useful for RDoC studies as it elicits both of these constructs across multiple units of analysis (i.e., startle EMG, probe ERPs, self-report) at the same time.
The present study had several limitations that warrant consideration. First, the sample consisted of introduction to psychology students and the results may not generalize to all populations (e.g., children). Indeed, the ASI-3 means were below that of clinical samples (Kemper et al., 2012); however, they were still higher than what is typically reported in undergraduates (Wheaton et al., 2012). Future studies should attempt to replicate these findings in a mixed clinical sample. Second, all measures were collected cross-sectionally and causal relationships between AS and emotional responding to threat cannot be determined. Third, the NPU-threat task used only one type of aversive stimulus (electric shocks) and it is unclear whether a similar or different pattern of results would emerge using other threatening events or stimuli (e.g., aversive noises, social rejection, unpleasant pictures). Finally, the present study focused on examining the association between AS and the temporal predictability of threat. However, controllability is another feature of threat that overlaps substantially with predictability (Chorpita & Barlow, 1998; Mineka & Kihlstrom, 1978; Seligman & Bink, 1977), and AS has been associated with increased anxiety when there is a lack of control over interoceptive threat (Zvolensky et al., 2001). Future studies should better delineate these features of threat and examine whether they have overlapping or unique relationships with AS.
In conclusion, the present study found an association between AS and multiple measures of affective and cognitive responding in anticipation of predictable and unpredictable threat. In an unselected sample of undergraduates, increased physical concerns were associated with enhanced defense system activation (e.g., startle EMG) in anticipation of unpredictable (but not predictable) threat. Conversely, increased cognitive concerns demonstrated the opposite relationship—they were associated with decreased defense system activation. Increased cognitive concerns were also associated with decreased perceptual processing (i.e., probe N100) in anticipation of unpredictable threat only, and increased physical concerns were associated with greater attention toward foreground threat stimuli (i.e., P300 suppression) across both predictable and unpredictable contexts and increased self-reported anxiety across all conditions. AS is a multi-faceted dimensional construct that cuts across several anxiety disorders and depression (Allan, Capron, et al., 2014; Wheaton et al., 2012). The present study provides novel evidence indicating that facets of AS demonstrates unique relationships with different Negative Valence System constructs (i.e., “acute” and “potential” threat) that have been implicated in the etiology and maintenance of many psychopathological conditions. Future research is needed to determine the neurodevelopment (Casey et al., 2014) and predictive validity and utility of these emotional and motivational systems.
Highlights.
Examined anxiety sensitivity and anticipatory threat responding
Physical concerns associated with increased startle to unpredictable threat
Cognitive concerns associated with decreased startle to unpredictable threat
Anxiety sensitivity subscales also associated with ERPs during threat conditions
Anxiety sensitivity dimensions uniquely related to acute and potential threat
Acknowledgements
This study was supported by National Institute of Health grant R01MH098093 awarded to S.A.S. and the University of Illinois -Chicago Chancellor’s Discovery Fund.
Footnotes
A history of substance use has been shown to impact aversive responding (Engelmann et al., 2011; Gorka et al., 2013). However, in the present study all associations between AS and anticipatory threat responding remained significant when cigarette, alcohol, and marijuana use were included as additional covariates (all ps < .05).
The baseline data from this sample were previously examined in a study that found AS was associated with decreased startle habituation (see Campbell et al., 2014).
Similar to the grand average ERPs reported in Nelson et al. (in press), a Condition (N, P, U) X Cue (CD vs. ISI) repeated-measures analysis of variance (ANOVA) incidated that the PCA-derived N100 differed between the threat conditions, F(2, 236) = 39.79, p < .001, ηp2 = .25, and was enhanced during the UCD+ISI relative to the NCD+ISI and PCD+ISI, F(1, 118) = 55.64, p < .001, ηp2 = .32; F(1, 118) = 41.88, p < .001, ηp2 = .26, respectively, but did not differ between the NCD+ISI and PCD+ISI, F(1, 118) = 1.63, ns, ηp2 = .01. The PCA-derived P300 also differed between the threat conditions, F(2, 236) = 16.19, p < .001, ηp2 = .12, and was attenuated during the PCD+ISI and UCD+ISI relative to the NCD+ISI, F(1, 118) = 27.38, p < .001, ηp2 = .19; F(1, 118) = 23.77, p < .001, ηp2 = .17, respectively, but did not differ between the PCD+ISI and UCD+ISI, F(1, 118) = 0.19, ns, ηp2 .01.
The PCA-derived N100 and P300 variables for each condition were highly correlated with the analogous variables reported by Nelson et al. (in press) derived from scoring the ERP grand average (i.e., average activity; Pearson’s r range: .72 to .87). Nonetheless, identical analyses for the grand average variables indicated the ASI-3 CC X Condition X Cue interaction for the N100, F(2, 228) = 1.52, p = .22, ηp2 = .03, and the ASI-3 PC X Condition interaction for the P300, F(2, 228) = 1.66, p = .19, ηp2 = .01, only (at best) approached significance. These results for grand averages further supported use of the PCA-derived ERP components as they are likely to have a better signal-to-noise ratio than grand average waveforms (Dien & Frishkoff, 2005).
There were also ASI-3 PC X Condition X Cue, F(2, 228) = 3.78, p < .05, ηp2 = .03, and ASI-3 SC X Condition X Cue interactions, F(2.228) = 4.32, p < .05, ηp2 = .04. However, follow-up analyses revealed no significant correlations between ASI-3 PC or SC and the N100 during any condition (ps > .12).
There was also a main effect of ASI-3 SC, F(1, 113) = 4.82, p < .05, ηp2 = .04, such that greater social concerns was associated with increased anxiety across all conditions, pr (113) = .20, p < .05. However, the scatterplot indicated this correlation was influenced by an outlier ASI-3 SC value (> 3 standard deviations from the mean). When this participant was excluded from analyses ASI-3 PC was still associated with anxiety, pr (112) = 21, p < .05, but ASI-3 SC was no longer related, pr (112) = .16, ns.
There were no main effects or interactions of QIDS-SR16 for any measure (ps > .19).
Prior research has indicated sex differences in the startle response to predictable and unpredictable threat, such that females demonstrate a larger sustained startle response across both threat conditions (Grillon, 2008). Therefore, we tested for sex differences for all significant associations between the ASI-3 and threat responding during the NPU-threat task. To this end, for startle potentiation to unpredictable threat, P300 suppression to predictable and unpredictable threat, N100 enhancement to unpredictable threat, and self-reported anxiety across all conditions, we conducted separate hierarchical linear regressions with startle block order, sex, and ASI-3 PC, CC, and SC subscales entered as block 1 independent variables, and ASI-3 PC X Sex, ASI-3 CC X Sex, and ASI-3 SC X Sex interaction terms entered as block 2 independent variables. Results indicated no significant ASI-3 X Sex interactions for any dependent measure (ps > .10), suggesting that the associations between AS and threat responding did not differ between females and males.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan NP, Albanese BJ, Norr AM, Zvolensky MJ, Schmidt NB. Effects of anxiety sensitivity on alcohol problems: evaluating chained mediation through generalized anxiety, depression and drinking motives. Addiction. 2015;110:260–280. doi: 10.1111/add.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan NP, Capron DW, Raines AM, Schmidt NB. Unique relations among anxiety sensitivity factors and anxiety, depression, and suicidal ideation. Journal of Anxiety Disorders. 2014;28:266–275. doi: 10.1016/j.janxdis.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Allan NP, Macatee RJ, Norr AM, Schmidt NB. Direct and interactive effects of distress tolerance and anxiety sensitivity on generalized anxiety and depression. Cognitive Therapy and Research. 2014;38:530–540. [Google Scholar]
- Allen NB, Trinder J, Brennan C. Affective startle modulation in clinical depression: Preliminary findings. Biological Psychiatry. 1999;46:542–550. doi: 10.1016/s0006-3223(99)00025-6. [DOI] [PubMed] [Google Scholar]
- Asmundson GJG, Weeks JW, Carleton RN, Thibodeau Ma, Fetzner MG. Revisiting the latent structure of the anxiety sensitivity construct: more evidence of dimensionality. Journal of Anxiety Disorders. 2011;25:138–147. doi: 10.1016/j.janxdis.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist. 2000;55:1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Berman NC, Wheaton MG, McGrath P, Abramowitz JS. Predicting anxiety: the role of experiential avoidance and anxiety sensitivity. Journal of Anxiety Disorders. 2010;24:109–113. doi: 10.1016/j.janxdis.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Zvolensky MJ, Norton PJ, Schmidt NB, Taylor S, Forsyth JP, Cox B. Taxometric and factor analytic models of anxiety sensitivity: integrating approaches to latent structural research. Psychological Assessment. 2007;19:74–87. doi: 10.1037/1040-3590.19.1.74. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Zvolensky MJ, Schmidt NB. Laboratory test of a novel structural model of anxiety sensitivity and panic vulnerability. Behavior Therapy. 2009;40:171–180. doi: 10.1016/j.beth.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Blechert J, Wilhelm FH, Meuret AE, Wilhelm EM, Roth WT. Experiential, autonomic, and respiratory correlates of CO2 reactivity in individuals with high and low anxiety sensitivity. Psychiatry Research. 2013;209:566–573. doi: 10.1016/j.psychres.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Borkovec T, Alcaine O, Behar E. Avoidance theory of worry and generalized anxiety disorder. Generalized Anxiety Disorder: Advances in Research and Practice. 2004:77–108. [Google Scholar]
- Borkovec TD, Inz J. The nature of worry in generalized anxiety disorder: A predominance of thought activity. Behaviour Research and Therapy. 1990;28:153–158. doi: 10.1016/0005-7967(90)90027-g. [DOI] [PubMed] [Google Scholar]
- Boswell JF, Farchione TJ, Sauer-Zavala S, Murray HW, Fortune MR, Barlow DH. Anxiety sensitivity and interoceptive exposure: a transdiagnostic construct and change strategy. Behavior Therapy. 2013;44:417–431. doi: 10.1016/j.beth.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Lang PJ. A multi-process account of startle modulation during affective perception. Psychophysiology. 2006;43:486–497. doi: 10.1111/j.1469-8986.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Keil A, Lang PJ. Orienting and emotional perception: facilitation, attenuation, and interference. Frontiers in Psychology. 2012;3:493. doi: 10.3389/fpsyg.2012.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman-Fulks JJ, Deacon BJ, Olatunji BO, Bondy CL, Abramowitz JS, Tolin DF. Categorical or dimensional: a reanalysis of the anxiety sensitivity construct. Behavior Therapy. 2010;41:154–171. doi: 10.1016/j.beth.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Broman-Fulks JJ, Green Ba, Olatunji BO, Berman ME, Arnau RC, Deacon BJ, Sawchuk CN. The latent structure of anxiety sensitivity-revisited. Assessment. 2008;15:188–203. doi: 10.1177/1073191107311284. [DOI] [PubMed] [Google Scholar]
- Campbell DT, Fiske DW. Convergent and discriminant validity by the multitrait-multimethod matrix. Psychological Bulletin. 1959;56:81–105. [PubMed] [Google Scholar]
- Campbell ML, Gorka SM, McGowan SK, Nelson BD, Sarapas C, Katz AC, Shankman Sa. Does anxiety sensitivity correlate with startle habituation? An examination in two independent samples. Cognition & Emotion. 2014;28:46–58. doi: 10.1080/02699931.2013.799062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron DW, Blumenthal H, Medley AN, Lewis S, Feldner MT, Zvolensky MJ, Schmidt NB. Anxiety sensitivity cognitive concerns predict suicidality among smokers. Journal of Affective Disorders. 2012;138:239–246. doi: 10.1016/j.jad.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron DW, Cougle JR, Ribeiro JD, Joiner TE, Schmidt NB. An interactive model of anxiety sensitivity relevant to suicide attempt history and future suicidal ideation. Journal of Psychiatric Research. 2012;46:174–180. doi: 10.1016/j.jpsychires.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron DW, Norr AM, Macatee RJ, Schmidt NB. Distress tolerance and anxiety sensitivity cognitive concerns: testing the incremental contributions of affect dysregulation constructs on suicidal ideation and suicide attempt. Behavior Therapy. 2013;44:349–358. doi: 10.1016/j.beth.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biological Psychiatry. 2014;76:350–353. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Cattell R. The Scree test for the number of factors. Multivariate Behavioral Research. 1966;1:245–276. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- Chawla N, Ostafin B. Experiential avoidance as a functional dimensional approach to psychopathology: An empirical review. Journal of Clinical Psychology. 2007;63:871–890. doi: 10.1002/jclp.20400. [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Barlow DH. The development of anxiety: the role of control in the early environment. Psychological Bulletin. 1998;124:3–21. doi: 10.1037/0033-2909.124.1.3. [DOI] [PubMed] [Google Scholar]
- Cox BJ, Taylor S, Clara IP, Roberts L, Enns MW. Anxiety sensitivity and panic-related symptomatology in a representative community-based sample: A 1-year longitudinal analysis. Journal of Cognitive Psychotherapy. 2008;22:48–56. [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley M, McManis M, Lang PJ. Probing affective pictures: attended startle and tone probes. Psychophysiology. 1998;35:344–347. doi: 10.1017/s0048577298970536. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Deacon B, Abramowitz J. Anxiety sensitivity and its dimensions across the anxiety disorders. Journal of Anxiety Disorders. 2006;20:837–857. doi: 10.1016/j.janxdis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Dien J. Evaluating two-step PCA of ERP data with Geomin, Infomax, Oblimin, Promax, and Varimax rotations. Psychophysiology. 2010a;47:170–183. doi: 10.1111/j.1469-8986.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- Dien J. The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. Journal of Neuroscience Methods. 2010b;187:138–145. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Dien J, Beal DJ, Berg P. Optimizing principal components analysis of event-related potentials: Matrix type, factor loading weighting, extraction, and rotations. Clinical Neurophysiology. 2005;116:1808–1825. doi: 10.1016/j.clinph.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Dien J, Frishkoff G. Principal components analysis of event-related potential datasets. In: Handy TC, editor. Event-related potentials: A methods handbook. Cambridge: The MIT Press; 2005. [Google Scholar]
- Dien J, Khoe W, Mangun GR. Evaluation of PCA and ICA of simulated ERPs: Promax vs. infomax rotations. Human Brain Mapping. 2007;28:742–763. doi: 10.1002/hbm.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Gewirtz JC, Cuthbert BN. Emotional reactivity to emotional and smoking cues during smoking abstinence: Potentiated startle and P300 suppression. Psychophysiology. 2011;48:1656–1668. doi: 10.1111/j.1469-8986.2011.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Nelson BD, Shankman SA. Startle response to unpredictable threat in comorbid panic disorder and alcohol dependence. Drug and Alcohol Dependence. 2013;132:216–222. doi: 10.1016/j.drugalcdep.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Grillon C. Greater sustained anxiety but not phasic fear in women compared to men. Emotion. 2008;8:410–413. doi: 10.1037/1528-3542.8.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JMP, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biological Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. American Journal of Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biological Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm AO, Weike AI. The neuropsychology of fear learning and fear regulation. International Journal of Psychophysiology. 2005;57:5–14. doi: 10.1016/j.ijpsycho.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hoaglin D. Performance of some resistant rules for outlier labeling. Journal of the American Statistical Association. 1986;81:991–999. [Google Scholar]
- Hoaglin DC, Iglewicz B. Fine-tuning some resistant rules for outlier labeling. Journal of the American Statistical Association. 1987;82:1147–1149. [Google Scholar]
- Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30:179–185. doi: 10.1007/BF02289447. [DOI] [PubMed] [Google Scholar]
- Hunt C, Keogh E, French CC. Anxiety sensitivity: the role of conscious awareness and selective attentional bias to physical threat. Emotion. 2006;6:418–428. doi: 10.1037/1528-3542.6.3.418. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research Domain Criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kaviani H, Gray JA, Checkley SA, Raven PW, Wilson GD, Kumari V. Affective modulation of the startle response in depression: Influence of the severity of depression, anhedonia, and anxiety. Journal of Affective Disorders. 2004;83:21–31. doi: 10.1016/j.jad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Kemper CJ, Lutz J, Bähr T, Rüddel H, Hock M. Construct validity of the Anxiety Sensitivity Index-3 in clinical samples. Assessment. 2012;19:89–100. doi: 10.1177/1073191111429389. [DOI] [PubMed] [Google Scholar]
- Keogh E, Dillon C, Georgiou G, Hunt C. Selective attentional biases for physical threat in physical anxiety sensitivity. Journal of Anxiety Disorders. 2001;15:299–315. doi: 10.1016/s0887-6185(01)00065-2. [DOI] [PubMed] [Google Scholar]
- Lees A, Mogg K, Bradley BP. Health anxiety, anxiety sensitivity, and attentional biases for pictorial and linguistic health-threat cues. Cognition & Emotion. 2005;19:453–462. doi: 10.1080/02699930441000184. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Eifert GH, Zvolensky MJ, Richards JB. Preference between onset predictable and unpredictable administrations of 20% carbon-dioxide-enriched air: implications for better understanding the etiology and treatment of panic disorder. Journal of Experimental Psychology Applied. 2000;6:349–358. doi: 10.1037//1076-898x.6.4.349. [DOI] [PubMed] [Google Scholar]
- Maller RG, Reiss S. Anxiety sensitivity in 1984 and panic attacks in 1987. Journal of Anxiety Disorders. 1992;6:241–247. [Google Scholar]
- McMillan KA, Asmundson GJG, Zvolensky MJ, Carleton RN. Startle response and anxiety sensitivity: subcortical indices of physiologic arousal and fear responding. Emotion. 2012 doi: 10.1037/a0029108. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Anxiety sensitivity and panic disorder. Biological Psychiatry. 2002;52:938–946. doi: 10.1016/s0006-3223(02)01475-0. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Strauss CC, Bradley MM. Fearful imagery in social phobia: Generalization, comorbidity, and physiological reactivity. Biological Psychiatry. 2009;65:374–382. doi: 10.1016/j.biopsych.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medley AN, Capron DW, Korte KJ, Schmidt NB. Anxiety sensitivity: a potential vulnerability factor for compulsive hoarding. Cognitive Behaviour Therapy. 2013;42:45–55. doi: 10.1080/16506073.2012.738242. [DOI] [PubMed] [Google Scholar]
- Melzig CA, Michalowski JM, Holtz K, Hamm AO. Anticipation of interoceptive threat in highly anxiety sensitive persons. Behaviour Research and Therapy. 2008;46:1126–1134. doi: 10.1016/j.brat.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Mineka S, Kihlstrom JF. Unpredictable and uncontrollable events: a new perspective on experimental neurosis. Journal of Abnormal Psychology. 1978;87:256–271. doi: 10.1037//0021-843x.87.2.256. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Nelson BD, Hajcak G, Shankman SA. Event-related potentials to acoustic startle probes during the anticipation of predictable and unpredictable threat. Psychophysiology. doi: 10.1111/psyp.12418. in press. [DOI] [PubMed] [Google Scholar]
- Nelson BD, McGowan SK, Sarapas C, Robison-Andrew EJ, Altman SE, Campbell ML, Shankman SA. Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology. Journal of Abnormal Psychology. 2013;122:662–671. doi: 10.1037/a0033982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Shankman SA. Does intolerance of uncertainty predict anticipatory startle responses to uncertain threat? International Journal of Psychophysiology. 2011;81:107–115. doi: 10.1016/j.ijpsycho.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIMH. NIMH Research Domain Criteria (RDoC) 2011 Retrieved from http://www.nimh.nih.gov/research-priorities/rdoc/nimh-research-domain-criteria-rdoc.shtml.
- Oldfield RC. The assesment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olthuis JV, Watt MC, Stewart SH. Anxiety Sensitivity Index (ASI-3) subscales predict unique variance in anxiety and depressive symptoms. Journal of Anxiety Disorders. 2014;28:115–124. doi: 10.1016/j.janxdis.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Putnam LE, Roth WT. Effects of stimulus repetition, duration, and rise time on startle blink and automatically elicited P300. Psychophysiology. 1990;27:275–297. doi: 10.1111/j.1469-8986.1990.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Reiss S, McNally RJ. The expectancy model of fear. In: Reiss S, Bootzin RR, editors. Theoretical issues in behavior therapy. Longdon, Englnd: Academic Press; 1985. pp. 107–121. [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour Research and Therapy. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Robichaud M, Dugas MJ. A Cognitive-Behavioral Treatment Targeting Intolerance of Uncertainty. Worry and its Psychological Disorders: Theory, Assessment and Treatment. 2008:289–304. [Google Scholar]
- Roth WT, Dorato KH, Kopell BS. Intensity and task effects on evoked physiological responses to noise bursts. Psychophysiology. 1984;21:466–481. doi: 10.1111/j.1469-8986.1984.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Keller MB. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey Ma, Heinssen RK, Cuthbert BN. Developing constructs for psychopathology research: research domain criteria. Journal of Abnormal Psychology. 2010;119:631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Buckner JD, Keough ME. Anxiety sensitivity as a prospective predictor of alcohol use disorders. Behavior Modification. 2007;31:202–219. doi: 10.1177/0145445506297019. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Lerew DR, Jackson RJ. Prospective evaluation of anxiety sensitivity in the pathogenesis of panic: replication and extension. Journal of Abnormal Psychology. 1999;108:532–537. doi: 10.1037//0021-843x.108.3.532. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nature Protocols. 2012;7:527–532. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Birbaumer N, Lang PJ. Probe P3 and blinks: Two measures of affective startle modulation. Psychophysiology. 1997;34:1–6. doi: 10.1111/j.1469-8986.1997.tb02409.x. [DOI] [PubMed] [Google Scholar]
- Seligman MEP, Bink YM. The safety signal hypothesis. In: Davis H, Hurmitz HMB, editors. Operant-Pavolvian interactions. Hillsdale, NJ: Erlbaum; 1977. pp. 165–187. [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Davidson RJ, Kalin NH. Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6145–6150. doi: 10.1073/pnas.1214364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, Gorka SM. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2013;122:322–338. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Jang KL, Livesley WJ. Heritability of anxiety sensitivity: A twin study. American Journal of Psychiatry. 1999;156:246–251. doi: 10.1176/ajp.156.2.246. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Sadeghpour M, Traversay JD, Ornitz EM. Prestimulation-induced modulation of the P300 component of event related potentials accompanying startle in children. Electroencephalography and Clinical Neurophysiology. 1994;90:201–213. doi: 10.1016/0013-4694(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Taylor S, Jang KL, Stewart SH, Stein MB. Etiology of the dimensions of anxiety sensitivity: a behavioral-genetic analysis. Journal of Anxiety Disorders. 2008;22:899–914. doi: 10.1016/j.janxdis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Taylor S, Koch WJ, Woody S, McLean P. Anxiety sensitivity and depression: How are they related? Journal of Abnormal Psychology. 1996;105:474–479. doi: 10.1037//0021-843x.105.3.474. [DOI] [PubMed] [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, Cardenas SJ. Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychological Assessment. 2007;19:176–188. doi: 10.1037/1040-3590.19.2.176. [DOI] [PubMed] [Google Scholar]
- Tukey JW. Exploratory Data Analysis Analysis. 1977;Vol. 2 [Google Scholar]
- Van Beek N, Griez E. Anxiety sensitivity in first-degree relatives of patients with panic disorder. Behaviour Research and Therapy. 2003;41:949–957. doi: 10.1016/s0005-7967(02)00129-8. [DOI] [PubMed] [Google Scholar]
- Viana AG, Rabian B. Fears of cognitive dyscontrol and publicly observable anxiety symptoms: depression predictors in moderate-to-high worriers. Journal of Anxiety Disorders. 2009;23:1126–1131. doi: 10.1016/j.janxdis.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Waszczuk MA, Zavos HMS, Eley TC. Genetic and environmental influences on relationship between anxiety sensitivity and anxiety subscales in children. Journal of Anxiety Disorders. 2013;27:475–484. doi: 10.1016/j.janxdis.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton MG, Deacon BJ, McGrath PB, Berman NC, Abramowitz JS. Dimensions of anxiety sensitivity in the anxiety disorders: evaluation of the ASI-3. Journal of Anxiety Disorders. 2012;26:401–408. doi: 10.1016/j.janxdis.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Eifert GH, Lejuez CW. Offset control during recurrent 20% carbon dioxide-enriched air induction: relation to individual difference variables. Emotion. 2001;1:148–165. doi: 10.1037/1528-3542.1.2.148. [DOI] [PubMed] [Google Scholar]