Abstract
Objectives
This study evaluates the Paulson-Lichtenberg Frailty Index (PLFI), a self-report measure that is based on Fried’s well-established frailty phenotype. The PLFI is examined using longitudinal data from the Health and Retirement Study (HRS) database, for which it was developed.
Methods
The sample was drawn from the HRS and included 8,844 community-dwelling older adults. Frailty was measured using the PLFI’s 5-item frailty index (wasting, weakness, slowness, falls, and fatigue).
Results
In comparison to intermediate-frail or non-frail respondents, frail respondents were found to be older, more medically compromised, and less independent for ADLs and IADLs. On average, frail respondents reported worse self-rated health and had fewer years of education. Women, ethnic minorities, and those who were not partnered were also more likely to be frail. Over subsequent years, frail respondents were more likely to be hospitalized, report more loss of independence, and experience higher mortality rates.
Conclusions
The PLFI is a valid tool for assessing frailty in the HRS dataset.
Keywords: Aging, Decline, Measurement, Health Outcomes
The construct of frailty has emerged over the past decade as a marker of physiological and medical decline and has become central to research on aging. The developing frailty research describes a geriatric syndrome with wide-ranging and adverse implications for both functional independence and the spiraling costs associated with geriatric health care. Meanwhile, large, longitudinal, publicly available datasets, such as the Health and Retirement Study (HRS), have been developed to promote research on diverse aspects of aging, including late-life decline. Presently, options of well-characterized HRS-based frailty measures are limited, raising questions about how to interpret adapted frailty instruments. This paper describes the development of a new frailty measure designed specifically for use in the longitudinal HRS database and, potentially, in clinical settings.
Various conceptualizations of frailty have been proposed, including those that emphasize objective characteristics, subjective factors, aspects of mental health, cognition, and mobility. The various perspectives on frailty, however, universally characterize this construct as indicative of physiological or biomedical decline. For instance, a model advanced by Mitnitski, Song, and Rockwood (2004) conceptualizes frailty based on 40 items related to various aspects of health and health attitudes. Fried (2001) conceptualized frailty as phenotype, resulting in “loss of homeostatic capacity to withstand stressors and resulting vulnerabilities.” This same frailty characterization is applied herein. Frailty, among other markers of late-life decline, is increasingly identified as a central concept in the public discourse on long-term care and public healthcare spending. As a clinical construct, frailty has the advantage of cutting across diagnostic codes and enabling comparison of older adults with respect to physiological decline. Fried and colleagues’ (2001) landmark study describes specific and disadvantageous prognostic implications of frailty, including hospitalization, disability, morbidity, and mortality. In contrast to deficit-accumulation models, one significant advantage of syndromal models of frailty is that they delineate a stage of late-life decline that is characterized by escalated risk for these adverse outcomes. Another advantage of indices of syndromal frailty is that they tend to have relatively few items and can either be adapted or formulated as brief clinical measures with considerable clinical utility. Despite the categorical nature of Fried’s phenotypic frailty model, her work suggests a gradient of frailty by describing “intermediate-frail” respondents as those with modest but measurable adaptive impairment (Fried et al., 2001; Hirsch et al., 2006; Varadhan et al., 2009). Thus, frailty complements other clinical constructs, such as disability and medical comorbidity, in the identification of both at-risk populations and older adults who require escalation of care.
Through its use in over 1400 research articles and 120 book chapters ("Health and retirement Study Online Bibliography," 2013), the federally-funded Health and Retirement Study has contributed immeasurably to both theoretical models and empirical exploration of aging among older Americans. In recent years, parallel forms of the HRS database have begun collection in South Korea, Japan, China, India, and New Zealand. Thus, the specification of an easily-administered, self-report frailty measure may facilitate the advancement of international aging research. Additionally, such a measure may provide an efficient, face-valid index method for tracking late-life decline among clinical populations.
Subjective measures have been well represented in both the frailty literature and throughout research with older adults. For instance, Mitnitski et al. (2004) examined self-reported frailty based on a number of variables related to symptoms, illnesses, attitudes, and functions, and Schultz-Larsen and Avlund’s (2007) findings on subjective tiredness as a measurement of frailty are intriguing. Several existing HRS-based studies have examined frailty bear mention here. Notably, our research team has published two studies using the frailty index described herein (Paulson & Lichtenberg, 2013a, 2013b). Our past studies included only stroke-free women over the age of 80, and thus were not characteristic of the full HRS sample or of the broader population of older Americans represented by the HRS. The first of these studies (Paulson & Lichtenberg, 2013b) presented concluded that women with both high cerebrovascular burden and clinically significant depressive symptomatology (CES-D scores at or above the clinical cut-off) were at significantly higher risk for both prevalent frailty and incident frailty over a 4-year period. The second of these papers, (Paulson & Lichtenberg, 2013a) employed longitudinal modeling to identify a clinical trajectory relating vascular depression, frailty, and shortened remaining lifespan.
Another recent study by our colleague, Dr. Mary Elizabeth Bowen (2012), used a very similar scale to that described here. Dr. Bowen’s scale differed from the PLFI in two ways. First, she assessed low physical activity using a question regarding engagement in physical activity at least three times per week. Second, her scale also omitted an item assessing wasting, which was necessitated by her use of BMI as the separate, primary independent variable of interest. Her analysis excluded participants scoring below the 10th percentile on a wordlist recall task, and employed respondent-level sampling weights. Finally, Cigolle, Ofstedal, Tian and Blaum (2009) defined three separate HRS-based frailty indices, each reflecting a different underlying conceptualization of frailty, including a “biological syndrome/phenotype” model highly representative of Fried’s (2001) often-cited index. While this index is conceptually similar to that described below, it was operationalized using physical performance measures, which have not been consistently gathered from most respondents across most waves. One strength of the study by Cigolle et al. (2009) is their thoughtful replication of previously-published measures, making this one of the very few studies to examine statistical overlap between various frailty indices. Use of inconsistently administered physical performance measures, however, makes this scale both impractical for longitudinal research with HRS data and inconvenient in most clinical settings.
Whether examining subjective or objective aspects of frailty, a frailty-assessment tool should at the very least produce results that are consistent with those of well-established frailty models. Ideally, such an instrument might also be specific enough for use with smaller samples or individual patients, and versatile enough for use with large-sample epidemiological research. The Paulson-Lichtenberg Frailty Index (PLFI) was developed in the interest of incorporating HRS data into the ongoing study of frailty. Identification of a syndromal frailty model designed specifically for use with the HRS will contribute to longitudinal research describing late-life decline. This, in turn, will allow for the development of integrative, time-sequenced models of disease process. Such models may inform empirically supported models of integrative care for older adults.
The purpose of this study is to describe phenotypic frailty, based principally on self-report, and how this variable relates to other medical and psychological aspects of aging. Our hypothesis is that the PLFI will relate to predictor and outcome variables in ways that are essentially similar to Fried’s frailty scale. To accomplish this, many analyses presented here are simulations of those presented in Fried’s 2001 paper.
Methods
Sample
The HRS is a prospective cohort study conducted by the University of Michigan with support from the National Institute on Aging. The first wave of the HRS occurred in 1992, with a 51- to 61-year-old cohort, and, in 1998, was merged with the Asset and Health Dynamics of the Oldest Old Study (AHEAD) cohort (70 years or older). Two additional cohorts were added in 1998 to fill in the gaps between these two groups. Briefly, the HRS is a multistage probability cohort sample of U.S. households. Details as to its design and methods have previously been published (Heeringa & Conner, 1995).
For this study, we used the version of the HRS that was prepared by the RAND Center for the Study of Aging (RAND HRS). The selected portion of this publicly available, longitudinal data set includes five waves at two-year intervals from 2000 through 2008 (waves 5 through 9). All available participants with complete data at the 2000 wave were included in this study. The data set is demographically representative of the U.S. population over age 65.
Measures
Frailty
We measured frailty using Fried’s (2001) conceptualization of frailty as a phenotype. Due to differences between the HRS data and Fried’s model, we adapted the frailty index to include five symptoms: wasting, weakness, slowness, fatigue or exhaustion, and falls. The wasting criterion was met if a respondent reported loss of at least 10% of body weight over a 2-year period. The weakness criterion was met if the respondent endorsed the question, “Because of health problems, do you have any difficulty with lifting or carrying weights over 10 pounds, like a heavy bag of groceries?” Upper extremity strength is a commonly used strength measure, is known to decline with age (Newman et al., 2003), and is predictive of adaptive limitations (Fried, Ettinger, Lind, Newman, & Gardin, 1994). The slowness criterion was met if respondents answered in the affirmative to the question, “Because of a health problem, do you have any difficulty with getting up from a chair after sitting for long periods?” Though difficulties with chair rise can reflect various underlying causes including lower-body weakness (Jones, Rikli, & Beam, 1999), selection of this variable is supported by research identifying slowness in muscle recruitment as particularly detrimental to sit-to-stand task performance (Yoshioka, Nagano, Hay, & Fukashiro, 2009). The relationship between slowness and the sit-to-stand task been demonstrated in diverse clinical samples with cerebral palsy (Park et al., 2003) and Parkinson’s disease (Bishop, Brunt, Pathare, Ko, & Marjama-Lyons, 2005; Mak & Hui-Chan, 2002). The fatigue or exhaustion criterion was met if the respondent endorsed the question, “Since we last talked with you in [the last wave], have you had any of the following persistent or troublesome problems: severe fatigue or exhaustion?” And finally, the falls criterion was met if the respondent answered in the affirmative to the question, “Have you fallen down in the past 2 years?” While Fried’s frailty index includes low energy expenditure, this variable was not available in the HRS data. Instead, the frailty phenotype was modified to include falls. This substitution is consistent with findings that among older adults inactivity predict falls (Lord, A., Williams, & Anstey, 1993), and that the experience of falling leads to inactivity and deconditioning, thus compounding the problem (Hindmarsh & Estes, 1989). Additionally, the relationship between frailty and falls is well-established. None of these frailty items were drawn from the CES-D. Individuals who met at least three of the criteria were identified as frail.
The indicators of frailty selected here (weight loss, lifting/carrying 10 lbs, chair stand, fatigue, and falling) offer a conceptual and helpful representation of Fried's phenotype in the HRS dataset, however these indicators may not be an exact proxy, encompassing all of the physiological abilities indicated in the original model (wasting, weakness, slowness, fatigue, and falls). For example, an individual with weakness in the lower extremity may not be adequately represented by our indicator of weakness, which is upper body lifting and carrying. The indicators do, however, offer a best-fit of the Fried phenotype, as available in the widely used HRS dataset, and the utility of this version of a frailty scale is strengthened by the fact that these indicators are available across multiple waves of measurement (unlike some of the task-based items used in the HRS-frailty indices published by Cigolle et al. (2009).
Demographic Variables
Age was calculated at each data collection based on the respondents’ birthdate. Sex was assessed by asking whether the individual was male or female, and ethnicity by asking participants, “Do you consider yourself primarily White or Caucasian, Black or African American, American Indian, or Asian?” This variable was re-coded to indicate whether the respondent considered him or herself White/Caucasian or Minority (representing respondents who identified themselves as Black/African American, American Indian, and Asian). Calculated income included personal earnings, pension or annuity, SSI or SS disability, Social Security retirement, unemployment or Workers’ Compensation benefits, and other government-transfer income. In the interest of keeping our analyses similar to Fried and colleagues’, four annual income levels were identified: $0–$11,999; $12,000–$23,999; $24,000–$49,999; and ≥ $50,000.
Self-Reported Medical Conditions, Hospitalization, and Mortality
Medical data (hypertension, diabetes, history of cardiac disease, arthritis, pulmonary disorders, cancer, history of smoking) were collected by self-report. Hospitalization was assessed with the question, “Since [the previous data-collection month and year], have you been a patient in a hospital overnight?” Vital status was identified at each wave of data collection, as the exact date of a participant’s death within a two-year period is not included in the HRS data.
Functional Independence
The score for activities of daily living (ADLs) reflected how many of the following activities the respondent reported requiring assistance with: bathing, eating, dressing, walking across a room, and getting in or out of bed. Scores ranged from 0 to 5. Instrumental activities of daily living (IADLs) were measured by identifying which of the following the respondent required assistance with: using a telephone, taking medication, and handling money. Scores ranged from 0 to 3.
Self-Rated Health
Change in self-rated health was assessed with the question, “Compared to your health when we talked with you in [date of last wave], would you say that your health is better now, about the same, or worse?” Response options were “much better,” “somewhat better,” “same,” “somewhat worse,” and “much worse,” comprising a 5-point scale.
Depressive Symptoms
A shortened, 8-item form of the original Center for Epidemiological Studies Depression Scale (CES-D) was used to evaluate depression (Radloff, 1977). Six of the eight items are negatively worded and two are positively worded. Participants are asked to respond “yes” or “no” to each item (“was depressed,” “everything was an effort,” “sleep was restless,” “was happy,” “felt lonely,” “enjoyed life,” “felt sad,” “could not get going”), based on whether or not they had experienced it during the preceding week. Scores ranged from 0 to 8, with higher scores indicating greater depressive symptoms. Using HRS data, the reliability of the 8-item CES-D measure was adequate, with high Cronbach’s alpha (.81–.83; Steffick, 2000). The 8-item CES-D has high internal consistency (α = .77) and validity (Steffick, 2000), and is broadly used in epidemiological studies of late-life depression (Beekman et al., 1997). Citing the recommended interpretation of this measure (Steffick, 2000), CES-D scores ≥3 were interpreted as indicating probable clinical depression.
Cognition
The HRS data include a brief, standardized 35-point measure of cognitive functioning that was developed for remote screening of cognitive disorders based on the Telephone Interview for Cognitive Status (TICS; Brandt, Spencer, & Folstein, 1988). It includes indices of orientation, concentration, short-term memory, mathematical skills, praxis, and language and has a maximum score of 35 points (observed range: 0–35), with higher scores reflecting better functioning. The TICS has a Cronbach’s alpha of .69, and past work has identified factors that reflect mental status and memory (Herzog & Wallace, 1997). The instrument has demonstrated high test-retest reliability and is generally sensitive to cognitive impairment (Brandt et al., 1988; Desmond, Tatemichi, & Hanzawa, 1994; Järvenpää et al., 2002; Welsh, Breitner, & Magruder-Habib, 1993). Using the cutoff score that indicates the presence of probable dementia, as proposed by Langa et al. (2008), we identified respondents with TICS scores of 10 or less as having probable cognitive impairment.
Statistical Methods
As a generalization, the statistical methods applied here were developed to assimilate those used by Fried et al. (2001) to identify the phenotype on which this model is based. Simple associations between the frailty status (non-frail, intermediate, or frail) and demographic and health characteristics of this sample were evaluated using chi-squared tests of independence. The hypotheses that increasing degrees of frailty (non-frail, intermediate, and frail) would predict (a) worsening ADL disability (defined as an increase of 1 unit or more in the ADL scale), (b) hospitalization, and (c) death over the subsequent 4 and 8 years were explored using both unadjusted and adjusted logistic regression analyses. In the unadjusted analyses, outcome variables at 4 and 8 years were predicted using only frailty status. In the adjusted analyses, outcome variables at 4 and 8 years were predicted based on frailty and controlling for age, gender, minority status, income, hypertension, diabetes, arthritis, cancer, pulmonary disease, cardiac disease, IADL disability, self-rated health, and TICS and CES-D scores. Two additional logistic regression analyses were conducted to test the hypothesis that intermediate-frailty status at baseline, in comparison to non-frail respondents, would predict a higher incidence of frailty over 4 years. The first analysis included only frailty status (non-frail versus intermediate frail) at the 2000 wave, and the second analysis controlled for the variables included in the previous analyses that predicted hospitalization, increasing ADL disability, and mortality.
Results
The sample included 8,844 respondents between the ages of 65 and 101. The mean age was 74.5 years (SD=7.04), and the mean years of education was 11.85 (SD=3.35). The sample was predominantly female (58.8%) and Caucasian (85.4%). The sample had a relatively low mean income of $16,473 (SD=$17,470). Overall, 36% of the sample had no frailty symptoms, 47% had 1 or 2 frailty symptoms, and 16% of the sample had three or more frailty symptoms (Table 2). In most age groups (65–89), women were roughly twice as likely to meet the criteria for frailty, though this difference was diminished among respondents aged 90 and over (Table 3).
Table 2.
Prevalence of frailty phenotype components in percentages for men and women
| Total (N=8,844) |
Men (n=3,644) |
Women (n=5,200) |
|
|---|---|---|---|
| Frequency of Frailty Components | % | % | % |
| Chair Rise | 40.6% | 34.6% | 44.7% |
| Weakness | 29.0% | 15.6% | 38.4% |
| Falls | 27.2% | 23.6% | 29.7% |
| Fatigue | 18.0% | 13.6% | 21.1% |
| Wasting | 5.7% | 4.0% | 6.9% |
| Number of Frailty Components Present | |||
| 0 | 36.2% | 46.2% | 29.2% |
| 1 | 28.7% | 28.8% | 28.7% |
| 2 | 18.9% | 15.2% | 21.6% |
| 3 | 11.1% | 7.0% | 14.0% |
| 4 | 4.5% | 2.6% | 5.9% |
| 5 | 0.5% | 0.2% | 0.7% |
Table 3.
Prevalence of frailty by sex and age group
| Men | Women | |||
|---|---|---|---|---|
| Overall | (n=3644) | (n=5200) | ||
| Age Group |
(n) | % Frail | 10.0% | 20.6% |
| 65–69 | 2,730 | 11.9% | 7.0% | 15.5% |
| 70–74 | 2,108 | 12.7% | 6.7% | 17.4% |
| 75–79 | 1,861 | 15.5% | 10.5% | 19.1% |
| 80–84 | 1,420 | 18.1% | 12.5% | 21.3% |
| 85–89 | 541 | 35.5% | 22.1% | 43.3% |
| 90+ | 184 | 52.7% | 41.2% | 57.1% |
As shown in Table 1, frail respondents were more likely to be older, female, and have no partner, less education, and lower income. Frailty was associated with worse self-rated health and was more common among elders with arthritis, cancer, hypertension, pulmonary disease, diabetes, and cardiac disease. Frailty was also more common among respondents with 3 or more chronic diseases and among respondents with IADL or ADL disability. Frailty was also more common among those with TICS scores indicating cognitive impairment and CES-D scores suggesting clinically significant depressive symptomatology.
Table 1.
Baseline association of demographic and health characteristics with frailty, in percentages
| Factor | Total | Not Frail |
Intermediate | Frail | X2 Value |
p Value | |
|---|---|---|---|---|---|---|---|
| 8,844 | 3,202 | 4,216 | 1,426 | ||||
| Age | |||||||
| 65–74 | 54.7% | 63.1% | 52.8% | 41.5% | 338.27 | <.001 | |
| 75–84 | 35.2 | 32.0 | 36.6 | 38.2 | |||
| 85+ | 10.1 | 4.9 | 10.7 | 20.3 | |||
| Sex | |||||||
| Female | 58.8 | 47.4 | 62.0 | 75.0 | 344.00 | <.001 | |
| Male | 41.2 | 52.6 | 38.0 | 25.0 | |||
| Race | |||||||
| Caucasian | 85.4 | 86.3 | 86.1 | 81.8 | 25.62 | <.001 | |
| African American | 11.8 | 10.7 | 11.8 | 14.7 | |||
| Other | 2.7 | 3.0 | 2.2 | 3.6 | |||
| Education | |||||||
| ≤9th grade | 20.2 | 16.3 | 20.0 | 29.5 | 164.42 | <.001 | |
| 10–11th grade | 11.2 | 10.2 | 11.0 | 13.8 | |||
| HS Grad/GED | 34.0 | 33.4 | 35.6 | 30.9 | |||
| >12 years | 34.6 | 40.0 | 33.5 | 25.9 | |||
| Income | |||||||
| <12K | 50.0 | 43.1 | 50.4 | 64.2 | 228.61 | <.001 | |
| 12–<24K | 32.0 | 33.3 | 32.8 | 26.5 | |||
| 24–50K | 14.3 | 18.4 | 13.4 | 8.1 | |||
| >50K | 3.7 | 5.2 | 3.5 | 1.1 | |||
| Self-Assessed Health | |||||||
| Excellent | 10.3 | 17.7 | 7.9 | 0.8 | 1,899.02 | <.001 | |
| Very Good | 28.6 | 39.4 | 26.6 | 10.2 | |||
| Good | 32.2 | 30.8 | 35.9 | 24.1 | |||
| Fair | 20.4 | 10.6 | 22.5 | 35.8 | |||
| Poor | 8.6 | 1.4 | 7.2 | 29.2 | |||
| Partnered | |||||||
| No | 42.6 | 34.1 | 44.0 | 57.3 | 224.77 | <.001 | |
| Yes | 57.4 | 65.9 | 56.0 | 42.7 | |||
| Prevalent Disease at Baseline | |||||||
| Arthritis | 64.2 | 48.1 | 69.8 | 84.2 | 667.17 | <.001 | |
| Cancer | 15.5 | 13.5 | 16.0 | 18.5 | 20.27 | <.001 | |
| Hypertension | 55.3 | 48.8 | 55.6 | 68.7 | 158.03 | <.001 | |
| Pulmonary | 10.3 | 6.0 | 10.4 | 19.5 | 193.99 | <.001 | |
| Diabetes | 17.1 | 11.9 | 17.7 | 27.0 | 161.57 | <.001 | |
| Cardiac | 28.1 | 19.9 | 28.5 | 45.7 | 324.21 | <.001 | |
| Number of Chronic Diseases | |||||||
| 0 | 11.2 | 18.4 | 8.8 | 2.1 | 1,000.83 | <.001 | |
| 1 | 28.1 | 35.9 | 26.9 | 14.2 | |||
| 2 | 31.2 | 28.9 | 33.0 | 30.9 | |||
| 3 | 20.2 | 13.3 | 22.1 | 29.8 | |||
| 4+ | 9.4 | 3.5 | 9.2 | 23.0 | |||
| Self-Reported Disability | |||||||
| ≥ IADL task | 7.7 | 2.2 | 6.9 | 22.2 | 564.62 | <.001 | |
| ≥ ADL task | 18.2 | 1.8 | 17.0 | 58.3 | 2,120.47 | <.001 | |
| Any Disability | 21.3 | 3.7 | 20.9 | 61.7 | 1,980.22 | <.001 | |
| Cognitive Function | |||||||
| TICS: 0–10 | 4.4 | 2.7 | 4.0 | 9.5 | 113.48 | <.001 | |
| TICS: 11–35 | 95.6 | 97.3 | 96.0 | 90.5 | |||
| Depressive Symptoms | |||||||
| CESD: 0–2 | 75.5 | 89.4 | 75.1 | 45.7 | 1,020.05 | <.001 | |
| CESD: ≥3 | 24.5 | 10.6 | 24.9 | 54.3 | |||
The 4-year incidence of new cases of frailty was identified by excluding frail participants at baseline (Year 2000). Incidence of new cases of frailty in 2004 was 16.8%; these incidence rates are likely underestimates, however, as they exclude respondents who died between 2000 and 2004 or did not respond in 2004. In the unadjusted analyses (Table 4), incident frailty in 2004 was significantly predicted by intermediate-frailty status in 2000 (exp(B)=3.92). In the adjusted analyses, incident frailty was significantly predicted by greater age, female gender, diabetes, arthritis, pulmonary disease, cardiac disease, worse self-rated health, cognitive impairment, clinically significant depressive symptomatology, and intermediate-frailty status (exp(B)=2.38; results in Table 4).
Table 4.
Results of three logistic regression analyses predicting frailty in 2004 based ondemographic, health, disability, cognitive and mood variables, and intermediate frailty status in 2000. N=5,889.
| Unadjusted | Adjusted | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Step 1 | Step 2 | |||||||||||
| B | SE | Wald | Exp(B) | B | SE | Wald | Exp(B) | B | SE | Wald | Exp(B) | |
| Age | .06 | .01 | 100.13 | 1.06 | .06 | .01 | 84.49*** | 1.06 | ||||
| Gender | .59 | .09 | 48.33** | 1.81 | .51 | .09 | 34.06*** | 1.66 | ||||
| Minority | −.13 | .11 | 1.43 | .87 | −.10 | .11 | .79 | .90 | ||||
| Income | .00 | .00 | .82 | 1.00 | .00 | .00 | .88 | 1.00 | ||||
| Smoking | .25 | .13 | 3.61 | 1.28 | .25 | .13 | 3.72 | 1.29 | ||||
| Htn | .15 | .08 | 3.67 | 1.16 | .15 | .08 | 3.52 | 1.16 | ||||
| Diabetes | .39 | .10 | 15.47** | 1.48 | .37 | .10 | 13.26*** | 1.44 | ||||
| Arthritis | .50 | .08 | 34.75** | 1.65 | .37 | .09 | 18.83*** | 1.45 | ||||
| Cancer | .12 | .10 | 1.24 | 1.12 | .11 | .10 | 1.10 | 1.12 | ||||
| Pulmonary | .43 | .13 | 11.96** | 1.54 | .41 | .13 | 10.76** | 1.51 | ||||
| Cardiac | .35 | .09 | 17.00** | 1.42 | .33 | .09 | 14.46*** | 1.39 | ||||
| IADLs | .26 | .13 | 4.24* | 1.30 | .21 | .13 | 2.83 | 1.24 | ||||
| S-R Hlth | .37 | .04 | 73.46** | 1.44 | .30 | .04 | 47.17*** | 1.35 | ||||
| TICS | −.03 | .01 | 17.07** | .97 | −.04 | .01 | 19.76*** | .96 | ||||
| CES-D | .13 | .02 | 39.58** | 1.14 | .11 | .02 | 25.88*** | 1.12 | ||||
| Int Frailty | 1.37 | .08 | 267.24** | 3.92 | .87 | .09 | 91.73*** | 2.38 | ||||
| Constant | −2.50 | .07 | 1174.44** | .08 | −8.16 | .58 | 197.94** | .00 | −7.85 | .58 | 180.67*** | .00 |
p<.05;
p<.001.
Htn=hypertension; IADLs=Instrumental Activities of Daily Living; S-R Hlth=Self-rated health; TICS=Telephone Interview for Cognitive Status; CES-D=Center for Epidemiological Studies Depression measure; Int Frailty=Intermediate frailty
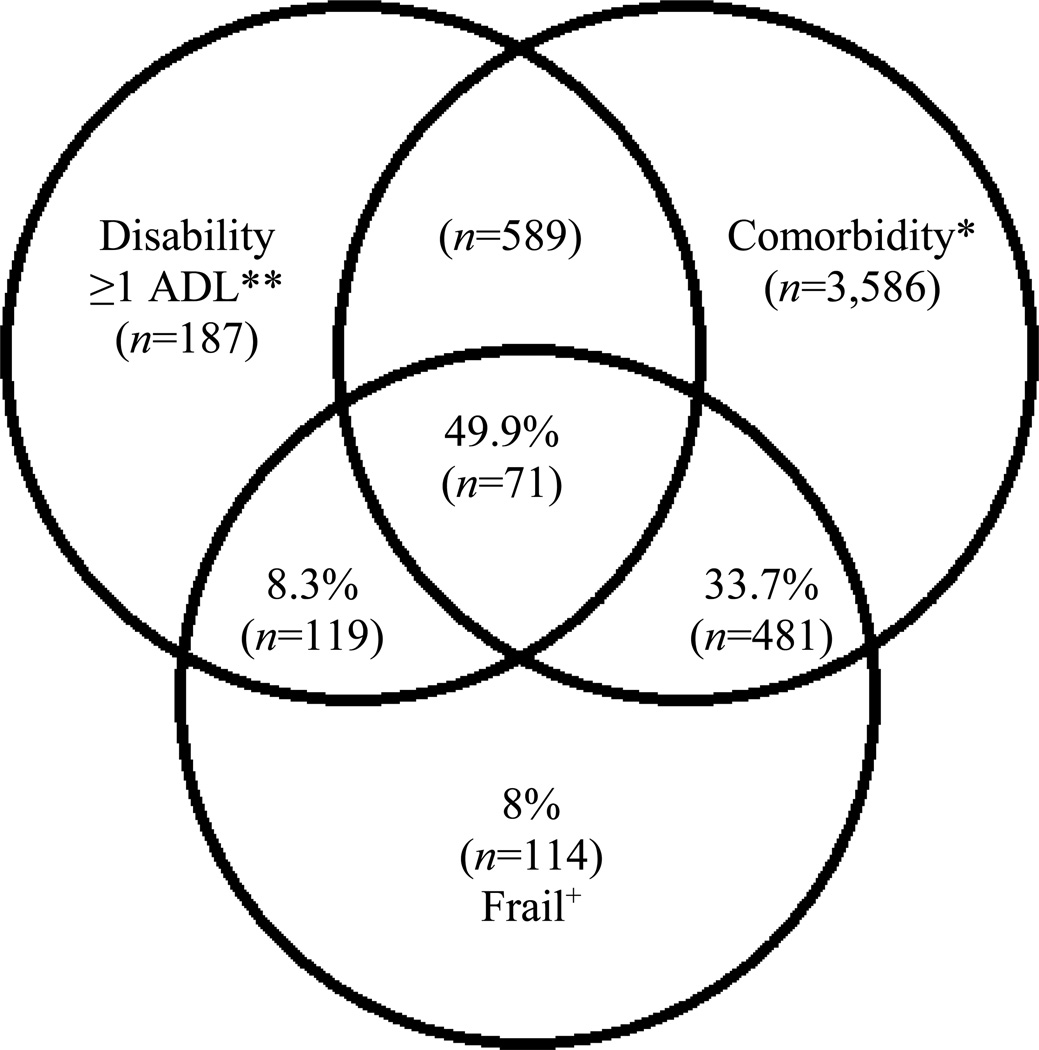
Of the 677 respondents who reported difficulty with 1 or more IADL activity, 46.7% were identified as frail. Of the 1,607 respondents who reported difficulty completing 1 or more ADL activity, 51.7% were identified as frail (Table 5). The overlap between frailty, ADL disability, and comorbidity is illustrated in Figure 1. Of those respondents identified as frail, half also reported difficulty with at least 1 ADL activity and at least 2 comorbidities. Of the remaining 50.1% of frail respondents, roughly two thirds reported at least 2 comorbidities but not ADL disability. These results support past findings that frailty largely overlaps with disability. The proposed model also supports the distinction between frailty and disability, and suggests that many frail elders can have a complex presentation with both high comorbidity and ADL disability.
Table 5.
Prevalence of IADL and ADL disability by frailty status
| Not Frail |
Intermediate | Frail | |
|---|---|---|---|
| Distribution in sample | 36.2% | 47.7% | 16.1% |
| Distribution among those with difficulty in | |||
| ≥1 IADL Task (n=677) | 10.2% | 43.1% | 46.7% |
| ≥1 ADL Task (n=1607) | 3.7% | 44.6% | 51.7% |
Figure 1.
Venn diagram displaying extent of overlap of frailty with ADL disability and comorbidity (≥2 diseases). Total represented: 5,788 (63.4% of total sample) participants who had comorbidity and/or disability and/or frailty. Each subgroup n indicated in parentheses.
+Frail: overall n=1426. *Comorbidity: overall n=5,368 with 2 or more of the following: arthritis, cancer, hypertension, pulmonary disease, diabetes, or cardiac disease. **Disability: overall n=1,607 with ADL disability; of these, 119 were frail.
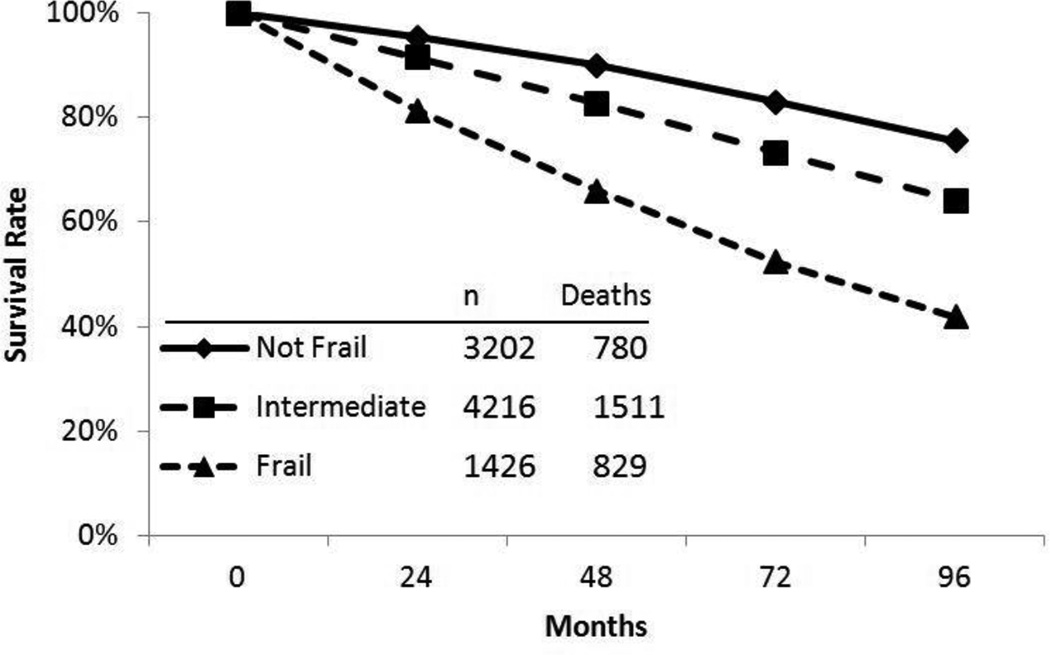
A series of logistic regression analyses, shown in Table 6, was performed to describe how the frailty construct predicted mortality over 4 and 8 years. Two logistic regressions found that compared to non-frail respondents, intermediate-frail respondents were 1.88 times more likely to die within 4 years and 1.74 more likely to die within 8 years. When adjusted for age, gender, minority status, income, hypertension, diabetes, arthritis, cancer, pulmonary disease, cardiac disease, IADL disability, self-rated health, and TICS and CES-D scores, these odds ratios were reduced to 1.38 and 1.25, respectively. Compared to non-frail respondents, frail respondents were 4.64 times more likely to die within 4 years and 4.31 times more likely to die within 8 years. When covariate adjusted as described above, those odds ratios were reduced to 2.17 and 1.94, respectively. The relationship between frailty status and mortality was also found to be statistically significant (Mantel-Cox Χ2 = 589.1, p < .001) using a Kaplan-Meier survival analysis (Figure 2). After 96 months (8 years), 76% of respondents identified as non-frail at baseline were still living (95% CI = 8.80 – 8.96), compared to 64% of intermediate-frail participants (95% CI = 8.15 – 8.32) and only 42% of frail participants (95% CI = 6.67–7.00).
Table 6.
Results of logistic regression analyses predicting worsening disability, hospitalization, and death within 4 and 8 years. Unadjusted analyses predict outcome variables based on frailty status alone. Adjusted analyses include age, gender, minority status (White=0, Minority=1), income, hypertension, diabetes, arthritis, cancer, pulmonary disease, cardiac disease, IADL disability, self-rated health, Telephone Interview for Cognitive Status, and Center for Epidemiological Studies-Depression measure
| Odds Ratios over 4 Years | Odds Ratios over 8 Years | |||
|---|---|---|---|---|
| Intermediate | Frail | Intermediate | Frail | |
| Worsening ADL | ||||
| Disability* | ||||
| Unadjusted | Exp(B)=2.31 | Exp(B)=5.73 | Exp(B)=2.29 | Exp(B)=4.79 |
| CI=1.97–2.71 | CI=4.74–6.93 | CI=2.02–2.59 | CI=4.07–5.64 | |
| Covariate Adjusted | Exp(B)=1.67 | Exp(B)=2.66 | Exp(B)=1.69 | Exp(B)=2.29 |
| CI=1.40–1.99 | CI=2.11–3.35 | CI=1.47–1.93 | CI=1.88–2.79 | |
| Hospitalization* | ||||
| Unadjusted | Exp(B)=1.50 | Exp(B)=2.94 | Exp(B)=1.43 | Exp(B)=2.40 |
| CI=1.34–1.67 | CI=2.52–3.44 | CI=1.29–1.58 | CI=2.06–2.81 | |
| Covariate Adjusted | Exp(B)=1.23 | Exp(B)=1.80 | Exp(B)=1.23 | Exp(B)=1.60 |
| CI=1.09–1.39 | CI=1.49–2.17 | CI=1.10–1.37 | CI=1.33–1.92 | |
| Death* | ||||
| Unadjusted | Exp(B)=1.88 | Exp(B)=4.64 | Exp(B)=1.74 | Exp(B)=4.31 |
| CI=1.63–2.16 | CI=3.96–5.45 | CI=1.57–1.92 | CI=3.78–4.92 | |
| Covariate Adjusted | Exp(B)=1.38 | Exp(B)=2.17 | Exp(B)=1.25 | Exp(B)=1.94 |
| CI=1.18–1.61 | CI=1.76–2.67 | CI=1.10–1.41 | CI=1.62–2.32 | |
All exp(B) values are associated with Wald X2 values, with p<.001
Figure 2.
Survival curve estimates (unadjusted) over 96 months of follow-up by frailty status at baseline: Frail (3 or more criteria present); Intermediate (1 or 2 criteria present); Not frail (0 criteria present).
Logistic regression analyses were then completed to evaluate how frailty predicted worsening ADL disability, indicated by an ADL scale score increase of at least 1, over 4 and 8 years (Table 6). Over 4 years, intermediate-frail respondents were 2.31 times more likely to report more ADL (covariate adjusted odds ratio = 1.67) and frail respondents were 5.73 times more likely to experience increasing ADL disability (covariate adjusted odds ratio = 2.66) compared to non-frail respondents. When these analyses were extended over 8 years, respondents who were categorized as intermediate frail at baseline were 2.29 times more likely to experience worsening ADL disability (covariate adjusted odds ratio = 2.66) and respondents identified as frail at baseline were 4.79 times more likely (covariate adjusted odds-ratio = 2.29) to experience worsening ADL disability compared to non-frail respondents.
A third set of logistic regression analyses (Table 6) was performed to examine how frailty predicted future hospitalization within 4 and 8 years, respectively. Compared to non-frail respondents, those who were identified as intermediate frail at baseline were 1.5 times more likely to be hospitalized (covariate adjusted odds-ratio = 1.23) and frail respondents were 2.94 times more likely to be hospitalized (covariate adjusted odds-ratio = 1.80) within 4 years. Within 8 years, intermediate-frail participants were 1.43 times more likely to be hospitalized (covariate adjusted odds-ratio = 1.23) and frail participants were 2.40 times more likely to be hospitalized (covariate adjusted odds-ratio = 1.60).
Conclusions
The results of this study robustly demonstrate that the PLFI is a useful tool for research on frailty among community-dwelling older Americans. This study found that frailty, as measured with the PLFI, is more common both among women and with increasing age. The PLFI identified higher rates of frailty among those with less education and lower household income, greater medical burden, more ADL and IADL dependence, worse self-rated health and cognition, and more depressive symptomatology. These findings also support past work that has identified intermediate frailty as a primary risk factor for frailty. In comparing these results to those reported by Fried et al (2001), both studies identified about 47% of the samples as intermediate frail, respectively. Fried et al (2001) described 7% of their sample as frail, and by comparison, about 16% of the present sample was characterized as frail using the PLFI. This discrepancy is likely attributable to differences between these samples. The HRS sample included a larger proportion of respondents over age 85 (10.1% in HRS versus 3.6% in Fried’s sample). Additionally, by comparison to the average respondent in Fried’s sample, the mean HRS respondent had fewer years of education, less reported income, and worse self-rated health. Compared to frail participants in Fried’s (2001) study, participants who were identified as frail using the PLFI endorsed higher rates of both ADL disability and medical burden. Using her largely objective frailty measure, Fried reported that the 84-month mortality rate among individuals identified as frail was 43%; using the self-report PLFI, the 84-month mortality rate for individuals identified as frail was 51%. This suggest that, broadly speaking, individuals identified as frail based on the PLFI may be somewhat more medically compromised than those identified as frail using Fried’s frailty measure. Despite these relatively modest differences between complementary indices, our results strongly identify the PLFI as a valid measure of frailty.
Emerging frailty research reflects the importance of better understanding this often debilitating and financially costly late-life syndrome. Considerable research has been devoted to identifying the prognostic implications of frailty. By contrast, there remain many interesting, timely, and empirically meaningful unanswered questions concerning predictors of frailty and how frailty can be integrated into broader models of late-life decline (Paulson & Lichtenberg, 2013a). The HRS data include a broad range of longitudinally collected information, making it an excellent resource for modeling these patterns of late-life change. The PLFI, accordingly, was designed both to promote the examination of frailty using HRS data and as an accessible, 5-item frailty measure for future data collection. At this time, however, we are unaware of any self-report measures, except for the PLFI, that conceptually replicate Fried’s frailty phenotype. In general, subjective measures are easy to administer to large samples and may provide information that complements that found using objective indices (Jahedi & Mendez, 2012; Jylha, 2009).
One limitation of this study is that HRS data do not include grip strength, walking speed, or kilocalorie expenditure; this precludes direct comparison of the PLFI and Fried et al.’s (2001) frailty index, on which the PLFI is based. In an effort to facilitate comparisons between the two indices, however, we performed a series of analyses that largely paralleled those used by Fried’s group. These results broadly describe relationships between frailty and demographic variables, medical variables, disability, and longevity that are consistent with those reported by Fried et al. A second limitation of this study is that currently, HRS data do not include measures to facilitate comparisons between many aspects of biometric functioning, as were used to investigate Fried’s model (Varadhan et al., 2009; Walston et al., 2002). Selected frailty indicators in this study should not be viewed exhaustive indices of underlying domains. For instance, future physiology research should examine how well subjective difficulties for lifting 10 pound weights corresponds to global muscular weakness. Certainly, physical weakness associated with adverse aging trajectories can take many forms. Measuring all facets of weakness, for instance, is not feasible using HRS data, and this is one unavoidable limitation of this measure. Nonetheless, the proposed frailty instrument does facilitate examination of frailty over time in both a large, demographically-representative dataset, and in clinical practice. Future research might also examine how genetic information, such as Apolipoprotein E, predicts frailty trajectories. Among other intriguing questions, the PLFI may enable researchers to examine how these variables are related in large samples, demonstrating both the prospective benefits of large, publicly available data sets and the utility of the PLFI.
In addition to facilitating frailty research using HRS data, the PLFI could be used in primary care or other clinical settings. While frailty is not specific to any particular disease process, it broadly describes patient functioning in a way that is easily understood by all members of diverse treatment teams. The PLFI may reveal additional information about an individual’s level of functioning that is informative for treatment planning. For instance, the PLFI’s sensitivity to declining independence and increased risk of hospitalization may indicate a need for the identification of long-term care options or preparation for informal caregiving. Finally, the use of HRS data in this study enables comparison of individual patients to a demographically representative sample of community-dwelling older Americans.
Acknowledgments
Funding
This work was generously supported by the Blue Cross Blue Shield of Michigan Foundation (1680.SAP); and by the T32 grant-supported NIH Pre-Doctoral Training Program in Aging and Urban Health at the Institute of Gerontology (T-32 AG00275-06).
Footnotes
Neither Dr. Paulson nor Dr. Lichtenberg have any conflicts of interest to report.
References
- Beekman ATF, Deeg DJH, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression Scale (CES-D): Results from a community-based sample of older subjects in the Netherlands. Psychological Medicine. 1997;27(1):231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- Bishop M, Brunt D, Pathare N, Ko M, Marjama-Lyons J. Changes in distal muscle timing may contribute to slowness during sit to stand in Parkinson's disease. Clinical Biomechanics. 2005;20(1):112–117. doi: 10.1016/j.clinbiomech.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Bowen ME. The relationship between body weight, frailty, and the disablement process. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2012;67(5):618–626. doi: 10.1093/geronb/gbs067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1988;1:111–117. [Google Scholar]
- Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: The Health and Retirement Study. Journal of the American Geriatrics Society. 2009;57(5):830–839. doi: 10.1111/j.1532-5415.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- Desmond DW, Tatemichi TK, Hanzawa L. The Telephone Interview for Cognitive Status (TICS): Reliability and validity in a stroke sample. International Journal of Geriatric Psychiatry. 1994;9:803–807. [Google Scholar]
- Fried LP, Ettinger WH, Lind B, Newman AB, Gardin J. Physical disability in older adults: A physiological approach. Journal of Clinical Epidemiology. 1994;47(7):747–760. doi: 10.1016/0895-4356(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, McBurnie MA. Frailty in older adults: Evidence for a phenotype. Journal of Gerontology: Medical Sciences. 2001;56A(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Health and retirement Study Online Bibliography. [Retrieved May 23, 2014];2013 2014, from http://hrsonline.isr.umich.edu/index.php?p=biblio. [Google Scholar]
- Heeringa SG, Conner J. Technical description of the Health and Retirement Study sample design: HRS/AHEAD documentation report DR-002. Ann Arbor: University of Michigan; 1995. [Google Scholar]
- Herzog AR, Wallace RB. Measures of cognitive functioning in the AHEAD Study. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 1997;52B(Special Issue):37–48. doi: 10.1093/geronb/52b.special_issue.37. [DOI] [PubMed] [Google Scholar]
- Hindmarsh JJ, Estes EH. Falls in older persons: Causes and interventions. Archives of Internal Medicine. 1989;149(10):2217–2222. [PubMed] [Google Scholar]
- Hirsch C, Anderson ML, Newman A, Kop WJ, Jackson S, Gottdiener J, Fried LP. The association of race with frailty: The Cardiovascular Health Study. Annals of Epidemiology. 2006;16(7):545–553. doi: 10.1016/j.annepidem.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Jahedi S, Mendez F. On the advantages and disadvantages of subjective measures. Fayetteville: University of Arkansas; 2012. [Google Scholar]
- Järvenpää T, Rinne JO, Räihä I, Koskenvuo M, Löppönen M, Hinkka S, Kaprio J. Characteristics of two telephone screens for cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2002;13:149–155. doi: 10.1159/000048646. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Research Quarterly for Exercise and Sport. 1999;70(2):113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- Jylha M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Social Science and Medicine. 2009;69(3):307–316. doi: 10.1016/j.socscimed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Langa KM, Larson EB, Karlawish JH, Cutler DM, Kabeto MY, Kim SY, Rosen A. Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimers and Dementia. 2008;4(2):134–144. doi: 10.1016/j.jalz.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SR, A WJ, Williams P, Anstey KJ. An epidemiological study of falls in older community-dwelling women: the Randwick falls and fractures study. Australian Journal of Public Health. 1993;17(3):240–245. doi: 10.1111/j.1753-6405.1993.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Mak MKY, Hui-Chan CWY. Switching of movement direction is central to parkinsonian bradykinesia in sit-to-stand. Movement Disorders. 2002;17(6):1188–1195. doi: 10.1002/mds.10257. [DOI] [PubMed] [Google Scholar]
- Mitnitski AB, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2004;59A(6):627–632. doi: 10.1093/gerona/59.6.m627. [DOI] [PubMed] [Google Scholar]
- Newman AB, Haggerty CL, Goodpaster B, Harris T, Kritchevsky S, Nevitt MC, Visser M. Strength and muscle quality in a well-funcitoning cohort of older adults: The health, aging and body composition study. Journal of the American Geriatrics Society. 2003;51:323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- Park ES, Park C, Lee HJ, Kim DY, Lee DS, Cho S. The characteristics of sit-to-stand transfer in young children with spastic cerebral palsy based on kinematic and kinetic data. Gait and Posture. 2003;17(1):43–49. doi: 10.1016/s0966-6362(02)00055-3. [DOI] [PubMed] [Google Scholar]
- Paulson D, Lichtenberg PA. Vascular depression and frailty: A compound threat to longevity. Aging and Mental Health. 2013a;17(7):901–910. doi: 10.1080/13607863.2013.799115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson D, Lichtenberg PA. Vascular depression: An early warning sign of frailty. Aging and Mental Health. 2013b;17(1):85–93. doi: 10.1080/13607863.2012.692767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Schultz-Larsen K, Avlund K. Tiredness in dailty activities: A subjective measure for the identification of frailty among non-disabled community-living older adults. Archives of Gerontology and Geriatrics. 2007;44(1):83–93. doi: 10.1016/j.archger.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Steffick DE. Documentation of affective functioning measures in the health and retirement study. HRS Documentation Report DR-005. Ann Arbor: Survey Research Center at the Institute for Social Research, University of Michigan; 2000. [Google Scholar]
- Varadhan R, Chaves PHM, Lipsitz LA, Stein PK, Tian J, Windham BG, Fried LP. Frailty and impaired cardiac autonomic control: New insights from principal components aggregation of traditional heart rate variability indices. Journal of Gerontology: Medical Sciences. 2009;64A(6):682–687. doi: 10.1093/gerona/glp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Fried LP. Fraitly and activation of the inflamation and coagulation systems with and without clinical comorbidities. Archives of Internal Medicine. 2002;162(20):2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1993;6:103–110. [Google Scholar]
- Yoshioka S, Nagano A, Hay DC, Fukashiro S. Biomechanical analysis of the relation between movement time and joint movement development during a sit-to-stand task. Biomedical Engineering Online. 2009;8(27):1–9. doi: 10.1186/1475-925X-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]