Abstract
Exogenous treatment with the naturally occurring peptide relaxin increases arterial compliance and reduces vascular stiffness. In contrast, relaxin deficiency reduces the passive compliance of small renal arteries through geometric and compositional vascular remodeling. The role of endogenous relaxin on passive mechanical wall properties in other vascular beds is unknown. Importantly, no studies have investigated the effects of aging in arteries of relaxin-deficient mice. Therefore, we tested the hypothesis that mesenteric and femoral arteries stiffen with aging, and this is exacerbated with relaxin deficiency. Male wild-type (Rln+/+) and relaxin knockout (Rln−/−) mice were aged to 3, 6, 12, 18, and 23 months. Passive mechanical wall properties were assessed by pressure myography. In both genotypes, there was a significant increase in circumferential stiffening in mesenteric arteries with aging, whereas in the femoral artery, aging reduced volume compliance. This was associated with a reduced ability of the artery to lengthen with aging. The predominant phenotype observed in Rln−/− mice was reduced volume compliance in young mice in both mesenteric and femoral arteries. In summary, aging induces circumferential stiffening in mesenteric arteries and axial stiffening in femoral arteries. Passive mechanical wall properties of Rln−/− mouse arteries predominantly differ at younger ages compared with Rln+/+ mice, suggesting that a lack of endogenous relaxin only has a minor effect on vascular aging.
Keywords: Relaxin knockout; Arterial stiffness; Volume compliance; Mesenteric, femoral
Introduction
Aging increases the risk of cardiovascular disease, a leading cause of morbidity and mortality in developed countries (Laurent 2012). A major risk factor for cardiovascular disease is increased vascular stiffness, which is also associated with aging (Greenwald 2007; Zieman et al. 2005). Remodeling of the vessel wall components (vascular smooth muscle cells (VSMCs) and extracellular matrix) contributes to the modulation of passive mechanical wall properties of blood vessels and influence vascular stiffness and compliance (Zieman et al. 2005). The vascular remodeling that occurs with aging has commonly been associated with increased vascular stiffness (Cecelja and Chowienczyk 2012). Outward hypertrophic remodeling (increased luminal diameter and wall thickness) is also an established characteristic of aged arteries (Greenwald 2007; Sawabe 2010). The effects of aging on the vasculature largely arise from research on larger conduit arteries. It is unclear if smaller more peripheral arteries also age in an analogous way.
Several endogenous peptides are known to limit the extent of vascular stiffening, a process that is associated with aging. The 6-kDa peptide hormone relaxin modifies arterial remodeling and reduces vascular stiffness. Both relaxin and its receptor RXFP1 are expressed in the thoracic aorta and mesenteric and small renal arteries of non-pregnant rodents (Jelinic et al. 2014; Novak et al. 2006). Mesenteric and small renal arteries of rodents treated with recombinant human relaxin (rhRLX) for 3–5 days have reduced vascular stiffness (Conrad et al. 2004; Jelinic et al. 2014; Li et al. 2005). In small renal arteries, this is associated with outward geometric remodeling, increased VSMC density and decreased total collagen content (Debrah et al. 2011). Exogenous relaxin treatment also causes outward remodeling (increasing inner diameter, wall thickness, and cross-sectional area) in cerebral parenchymal arterioles (Chan and Cipolla 2011). Conversely, vascular remodeling in the femoral and middle cerebral arteries is not altered by relaxin, suggesting that it may only alter the remodeling of specific vascular beds (Chan and Cipolla 2011; Jelinic et al. 2014). Collectively, these studies demonstrate that exogenous relaxin alters arterial remodeling to reduce stiffness, with differential effects in specific vascular beds.
Compared with the actions of exogenous relaxin, the vascular role of endogenous relaxin has been investigated to a lesser extent. Relaxin is well recognized for its role in remodeling reproductive tract tissues during pregnancy (Parry and Vodstrcil 2007). In pregnant rats, treatment with a monoclonal antibody against relaxin (MCA1) neutralizes high levels of circulating endogenous relaxin, preventing many of the renal and hemodynamic changes that occur throughout pregnancy (Conrad 2011; Debrah et al. 2006). In particular, MCA1 prevents the pregnancy-induced increase in global arterial compliance and decrease in systemic vascular resistance (Debrah et al. 2006; Novak et al. 2001). Importantly, MCA1 treatment increases uterine artery stiffness and reduces inner and outer diameters without altering collagen composition or wall thickness (Vodstrcil et al. 2012). Similarly, 8-month-old pregnant relaxin gene knockout (Rln−/−) mice also have stiffer uterine arteries; this is associated with reduced elastin and matrix metalloproteinase (types 2, 10, and 14) gene expression (Gooi et al. 2013) compared with their Rln+/+ counterparts. These studies illustrate the detrimental effects on the vasculature of a lack of circulating endogenous relaxin during pregnancy.
Altered vascular remodeling phenotypes have also been reported in non-pregnant Rln−/− mice. In 4–6-month-old male Rln−/− mice, passive compliance is reduced in the small renal arteries (Novak et al. 2006). These arteries exhibit inward geometric remodeling, reduced VSMC density, and increased total collagen content when compared with those of Rln+/+ mice (Debrah et al. 2011), suggesting that endogenous relaxin is important in the homeostasis of normal vascular remodeling in this vascular bed. Our previous work also established that mesenteric arteries of 12-month-old male Rln−/− mice have reduced volume compliance, without any geometric remodeling (Leo et al. 2014). No studies to date have identified if endogenous relaxin has a role in vascular aging nor have Rln expression and its receptor Rxfp1 been characterized in the vasculature with aging. Furthermore, the effects of aging on the passive mechanical wall properties of peripheral arteries have not been clearly characterized. Therefore, this study aimed to test, in male mice, the following hypotheses: (i) mesenteric and femoral arteries stiffen and become less compliant with aging, (ii) that the Rln−/− mouse is a model to investigate accelerated vascular aging, and (iii) relaxin and Rxfp1 gene expression decreases with aging.
Methods
Animal model
All animal experiments were conducted with approval from The Faculty of Science, University of Melbourne Animal Experimental Ethics Committee (AEC# 0911478.1). This study used the original Rln−/− mouse (Gooi et al. 2013; Zhao et al. 1999) backcrossed on a C57/BLK6J background to the F14 generation and wild-type littermates (Rln+/+) of the same strain. Genotypes were confirmed by PCR analysis of genomic DNA from ear clips as previously described (Zhao et al. 1999). All mice were bred and housed in the School of BioSciences Animal House Facilities (University of Melbourne) in a 12-h light and dark cycle at 20 °C, with standard food pellets (Barastock, VIC, Australia) and water provided ad libitum. Mice were aged to 3, 6, 12, 18, and 23 months, which was near the end of the natural lifespan for both strains. Mice were initially intended to be aged to 24 months, but 50% of both Rln+/+ and Rln−/− mice died between 23.5 and 24 months of age (unpublished data). A total of 9–10 mice were randomly allocated into each of the different age groups. However, not all animals survived to the allocated age and it was not possible to replace the older mice, so there were variable numbers in each group (n = 6–9).
Isolation of arteries
Mice were euthanized by isofluorane overdose and cervical dislocation. The mesenteric arcade and femoral arteries were isolated and immediately placed in ice cold 0.1 M phosphate-buffered saline (PBS) solution. Small mesenteric (first-order branch of the superior mesenteric artery, diameter ∼140 μm) and femoral arteries (diameter ∼160 μm) were cleared of fat and loose connective tissue. Remaining arteries were snap frozen in liquid nitrogen and stored at −80 °C for gene analysis.
Pressure myography
Isolated mesenteric and femoral arteries were transferred to a Ca2+-free physiological saline solution (PSS; mmol/l: NaCl 149, KCl 4.7, NaHCO3 1.7, KH2PO4 1.2, MgSO4 1.7, glucose 5, HEPES 10, and EGTA 2). Leak-free segments of arteries were mounted on a pressure myograph (Living Systems Instrumentation, Burlington, VT, USA) via the proximal end. The distal end of the artery was occluded and left free to allow it to lengthen passively with pressure. Arteries were incubated in Ca2+-free PSS at 37 °C for 20 min before wall parameters (vessel length, outer diameter (OD), inner diameter (ID), and wall thickness (WT)) were recorded at 10 mmHg increments from 5–120 mmHg. Wall stress and strain were calculated as described previously (Leo et al. 2014; Novak et al. 2006). Volume compliance and normalized wall parameters were calculated for each pressure increment as mentioned previously (Leo et al. 2014). The area under the curve was calculated to enable further analysis of the effects of aging on volume compliance across the entire pressurization range. The % change in length with pressure was analyzed with % length calculated using the following equation: %length = [(value at pressure)/(value at baseline)] × 100. The initial length of the mounted vessel segments were kept constant between groups.
Quantitative PCR
Expression of Rxfp1 and Rln in mesenteric arteries was analyzed using quantitative PCR. Femoral arteries were not analyzed due to limited quantities of tissue. Frozen vessels were pooled and placed in pre-chilled Wig-L-Bug® capsules with a silver ball bearing and pulverized in a Digital Wig-L-Bug® amalgamator (Dentsply-Rinn, Elgin, IL, USA). Pulverized tissues were resuspended in 1 ml TriReagent (Ambion Inc., Scoresbury, VIC, Australia), and total RNA was then extracted as described previously (Leo et al. 2014). RNA pellets were resuspended in 17 μl RNA Secure™ (Ambion). Quality and quantity of RNA were analyzed using the NanoDrop ND1000 Spectrophotometer (Thermo Fisher Scientific Australia Pty Ltd, Scoresby, VIC, Australia) with A260:A280 ratios >1.8 indicating sufficient quality for qPCR analysis. First-strand cDNA synthesis used 0.5 μg of total RNA in a 20 μl reaction containing random hexamers (50 ng/μl) and 200 units of Superscript™ III (Invitrogen, Mulgrave, VIC, Australia).
The comparative cycle threshold (2−ΔCt) method of quantitative real-time polymerase chain reaction (qPCR) was used to compare Rxfp1 and Rln expression between groups. Primers were designed to span intron/exon boundaries and avoid Rxfp1-truncates (Table 1). qPCR analysis of mesenteric arteries was performed on the AB Applied Biosystems ViiA7 PCR machine (Life Technologies, Mulgrave, VIC, Australia) using 96-well reaction plates with 10 μl volume reactions in triplicate containing SensiFASt Probe Lo-ROX master mix (Bioline) and 10 μM of primers and FAM-labelled probe. Ribosomal 18S (Rn18s) was the reference gene for qPCR analyses. Negative template controls substituting cDNA with water or RT negative controls substituting the reverse transcriptase in cDNA synthesis with water and a positive control (female mouse myometrium from day 6 of pregnancy) were included on each plate. For each sample, the mean Rn18s CT triplicate value was subtracted from the mean gene of interest triplicate CT value to normalize gene of interest expression to the reference gene. These normalized data (ΔCT) were then presented as a relative value using the 2−ΔCt method of analysis (mean ± SEM).
Table 1.
Nucleotide sequences of primers and probe used for quantitative real-time PCR experiments
| Gene/accession ID | Nucleotide sequence (5′ to 3′) | Position | Amplicon length | |
|---|---|---|---|---|
|
Rn18s
NR_003278.3 |
Fwd | GCATGGCCGTTCTTAGTTGG | 1330 | 77 bp |
| Rev | TGCCAGAGTCTCGTTCGTTA | 1377 | ||
| Probe | TGGAGCGATTTGTCTGGTTATTCCGA | 1350 | ||
|
Rxfp1
NM_212452.1 |
Fwd | GCTTCCACTAACTCCTTTGAGGC | 247 | 76 bp |
| Rev | CATGCATTGTTTGTGCCGAG | 322 | ||
| Probe | AAACTTCCGAATGCGTGGTTGGCTC | 271 | ||
|
Rln
NM_011272 |
Fwd | AGGCAAGCCACTGAAGTTGT | 273 | 62 bp |
| Rev | GTCGTATCGAAAGGCTCTGC | 315 | ||
| Probe | GCCATCCTTCATCAACAAAGA | 293 | ||
Statistical analysis
All results are expressed as the mean ± SEM, n represents the number of animals per group. Using power of analysis calculations from previous studies (Gooi et al. 2013) an n = 6 was sufficient to detect changes in passive wall mechanics in this mouse model. For example, 6 mice per group results in a 95% chance of detecting a 10% difference in stress-strain at P = 0.05. All figure formatting and statistical analyses were completed using Prism version 5.0 (GraphPad Software, San Diego, CA, USA). In the qPCR experiments where the tissues from animals were pooled, one n represents a pooled sample and not the number of animals and represents tissues from 1–2 animals. The stress-strain curves and pressurized wall parameters (WT, OD, ID, and arterial lengthening) were analyzed with repeated measures two-way ANOVA. Body weight data and volume compliance were analyzed using a two-way ANOVA, with Bonferroni post-hoc analysis. A level of P < 0.05 was considered statistically significant.
Results
Relaxin deficiency does not significantly alter body weight
There was a significant effect of age (F4,40 = 11.17, P < 0.05) on body weight in both Rln+/+ and Rln−/− mice (Table 2). Between 3 and 12 months of age, body weight increased in both genotypes. Interestingly, between 12 and 23 months, body weight was sustained in Rln−/− mice, but in 23-month-old Rln+/+ mice, there was a significant (t26 = 3.63, P < 0.05) decrease in body weight compared with 12-month-old mice.
Table 2.
Mean animal age, body weight, and wall parameters (±SEM) at baseline (5 mmHg)
| Genotype | Number | Age (months) | Body weight (g) | Mesenteric artery (μm) | Femoral artery (μm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OD | WT | ID | Number | OD | WT | ID | Number | ||||
| Rln +/+ | 8 | 3.2 ± 0.1 | 28.7 ± 0.4 | 139 ± 4 | 18 ± 1* | 103 ± 4 | 8 | 171 ± 5* | 28 ± 1 | 117 ± 6 | 6 |
| Rln −/− | 9 | 3.4 ± 0.1 | 28.5 ± 0.6 | 132 ± 5 | 16 ± 1 | 102 ± 4 | 9 | 155 ± 4 | 25 ± 1 | 104 ± 5 | 9 |
| Rln +/+ | 9 | 6.3 ± 0.0 | 32.1 ± 0.8 | 146 ± 4 | 16 ± 1 | 114 ± 4 | 9 | 168 ± 4 | 29 ± 1 | 111 ± 4 | 9 |
| Rln −/− | 8 | 6.4 ± 0.1 | 31.6 ± 0.7 | 151 ± 6 | 16 ± 1 | 119 ± 6 | 8 | 171 ± 8 | 29 ± 2 | 113 ± 7 | 8 |
| Rln +/+ | 9 | 12.6 ± 0.1 | 36.0 ± 0.7 | 143 ± 5 | 14 ± 1 | 115 ± 5 | 9 | 164 ± 7 | 25 ± 3 | 115 ± 4 | 9 |
| Rln −/− | 9 | 12.5 ± 0.1 | 35.5 ± 0.8 | 142 ± 9 | 13 ± 1 | 116 ± 8 | 9 | 160 ± 6 | 21 ± 1 | 119 ± 6 | 7 |
| Rln +/+ | 6 | 18.8 ± 0.2 | 32.9 ± 1.0 | 144 ± 9 | 16 ± 1 | 112 ± 9 | 6 | 162 ± 5 | 26 ± 2 | 109 ± 6 | 6 |
| Rln −/− | 7 | 18.4 ± 0.1 | 35.6 ± 1.0 | 154 ± 7 | 15 ± 1 | 123 ± 7 | 7 | 165 ± 4 | 28 ± 2 | 109 ± 5 | 7 |
| Rln +/+ | 7 | 23.3 ± 0.1 | 31.5 ± 0.9 | 155 ± 8 | 14 ± 1 | 127 ± 7 | 7 | 156 ± 9† | 28 ± 1† | 101 ± 10 | 7 |
| Rln −/− | 8 | 23.1 ± 0.1 | 34.0 ± 0.8 | 155 ± 9 | 17 ± 1 | 120 ± 8 | 7 | 177 ± 5 | 33 ± 2 | 110.7 ± 4 | 8 |
*Significantly greater than Rln −/− mice; †significantly less than Rln −/− mice
OD outer diameter, WT wall thickness, ID inner diameter
Relaxin deficiency alters age-dependent stiffening of mesenteric arteries
With aging, the passive inner diameter of mesenteric arteries from male Rln+/+ mice increased less with pressure (F4,34 = 5.45, P < 0.05; Fig. 1a) and the arteries became significantly (F4,44 = 6.24, P < 0.05; Fig. 1e) stiffer. Specifically, stress-strain curves of arteries from the older age groups were progressively and significantly (P < 0.05) shifted leftwards compared with that of 3-month-old mice indicating increased arterial stiffness. Consistent with this, the stress-strain curve of the arteries from 23-month-old mice was significantly (P < 0.05) shifted to the left compared to those of younger age groups analyzed, indicating increased passive stiffness in the mesenteric arteries of older mice (Fig. 1e). Aging also had a significant effect on the inner diameter pressure (F4,35 = 2.89, P < 0.05; Fig. 1b) and stress-strain (F4,44 = 2.88, P < 0.05; Fig. 1f) curves of Rln−/− mice. Post hoc analyses revealed the stress-strain curves of arteries from the older age groups were progressively and significantly (P < 0.05) shifted leftwards compared with that of 3-month-old mice indicating increased arterial stiffness. Remarkably, from 6 months of age and onwards, these arteries did not stiffen further with aging in Rln−/− mice (Fig. 1f).
Fig. 1.
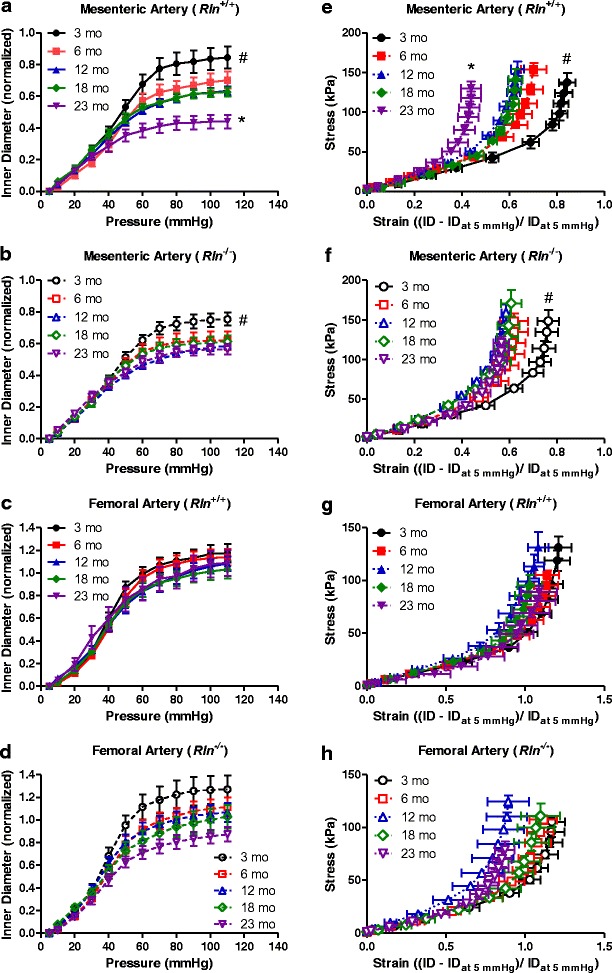
Mesenteric and not femoral arteries stiffen with aging. Normalized passive inner diameter and stress-strain relationships of mesenteric (a, b, e, and f) and femoral (c, d, g, and h) arteries of Rln +/+ (closed symbols) and Rln −/− (open symbols) mice at 3 (circle), 6 (square), 12 (triangle), 18 (diamond), and 23 (inverted triangle) months of age. Values are mean ± SEM. *Significantly (P < 0.05) different to 3–18-month-old mice, #significantly (P < 0.05) different to 6–23-month-old mice, n = 6–9
The stress-strain curves of Rln+/+ and Rln−/− mice were comparable at 3 to 18 months of age (Fig. 2a, b). Although the curves for Rln−/− mice were consistently positioned to the left of those for the Rln+/+ mice suggesting increased stiffness, this difference was not significantly different. Surprisingly, at 23 months of age, the stress-strain curve of Rln−/− mice was significantly (F1,11 = 7.69, P < 0.05) shifted to the right compared with Rln+/+ mice. This indicates that mesenteric arteries of Rln−/− mice were less stiff than those of Rln+/+ mice (Fig. 2c). This is likely due to the relatively larger inside diameter of mesenteric arteries of Rln−/− mice (Fig. 2d). Volume compliance of mesenteric arteries in 3-month-old Rln−/− mice was significantly reduced (F1,10 = 10.54, P < 0.05) when compared with 3-month-old Rln+/+ mice (Fig. 3a). Wall thickness at baseline was also significantly (P < 0.05) reduced in these 3-month-old Rln−/− mice (Table 2). Reduced volume compliance was also observed in Rln−/− mice at 3 (F1,10 = 7.88, P < 0.05) and 12 months of age (F1,10 = 5.60, P < 0.05) when compared with Rln+/+ mice of the same age (Fig. 3b). In contrast, volume compliance was comparable between genotypes at 6, 18, and 23 months of age (Fig. 3c, d). Area under the curve analysis revealed that aging did not have a significant effect on volume compliance in either Rln+/+ or Rln−/− mice (Fig. 3d).
Fig. 2.
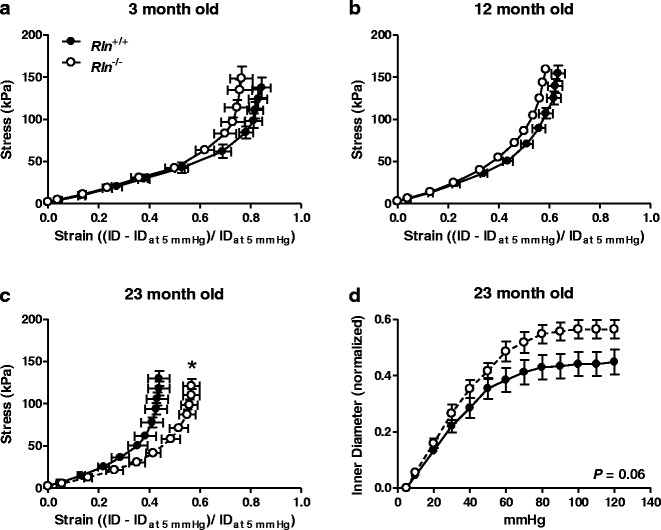
Relaxin deficiency only alters stiffness of mesenteric arteries at 23 months of age. Stress-strain relationships of mesenteric arteries from Rln +/+ (closed square) and Rln −/− (open square) mice at 3 (a), 12 (b), and 23 (c) months of age. Normalized passive inner diameter against intraluminal pressures at 23 months of age (d). Data shown in a, b, and c are reproduced from Fig. 1 to aid direct comparison of the effect of genotype on arterial wall stiffness at each age group. Values are mean ± SEM. *Significantly (P < 0.05) different to Rln +/+ mice, n = 6–9
Fig. 3.
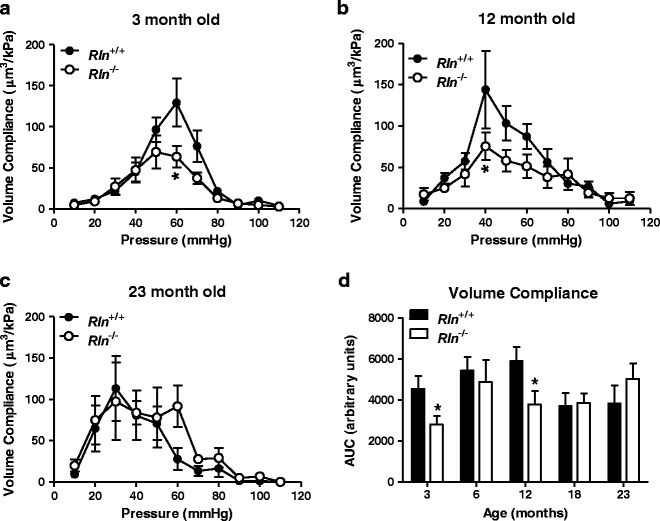
Relaxin deficiency reduces mesenteric artery volume compliance at 3 and 12 months of age. Volume compliance against intraluminal pressure of mesenteric arteries from Rln +/+ (closed square) and Rln −/− (open square) mice at 3 (a), 12 (b), and 23 (c) months of age and area under curve (AUC) analysis of both genotypes at all ages (d). Values are mean ± SEM. *Significantly (P < 0.05) less than Rln +/+ mice, n = 6–9
Relaxin deficiency alters age-dependent changes in volume compliance in femoral arteries
Inner diameter pressure and stress-strain curves for the femoral artery did not significantly alter with aging in either Rln+/+ or Rln−/− mice. This suggests that the femoral artery does not stiffen with aging in either genotype (Fig. 1c, d, g, and h). Stress-strain curves were also comparable between genotypes at all ages (individual curves not shown). Femoral artery volume compliance in Rln−/− mice was significantly reduced at 3 months of age (F1,10 = 7.63, P < 0.05) when compared with Rln+/+ mice (Fig. 4a) but was comparable between genotypes at other age groups. Area under the curve analysis revealed that aging had a significant effect on volume compliance in both Rln+/+ and Rln−/− (F4,50 = 5.132, P < 0.05). Specifically, in Rln+/+ mice volume compliance is significantly (P < 0.05) reduced at 18 and 23 months of age when compared with 3-month-old mice. In Rln−/− mice, volume compliance is only reduced at 18 months of age when compared with 6-month-old mice (Fig. 4d).
Fig. 4.
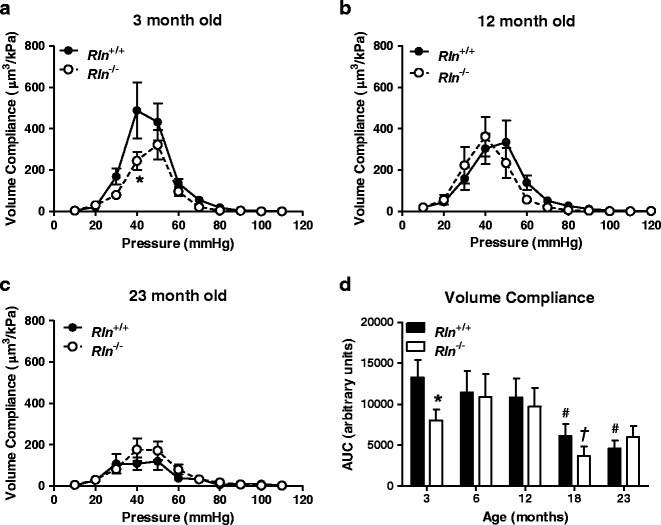
Relaxin deficiency reduces femoral artery volume compliance at 3 months of age. Volume compliance against intraluminal pressure of femoral arteries from Rln +/+ (closed square) and Rln −/− (open square) mice at 3 (a), 12 (b), and 23 (c) months of age and area under curve (AUC) analysis of both genotypes at all ages (d). Values are mean ± SEM. *Significantly (P < 0.05) less than Rln +/+ mice, #significantly less than 3-month-old Rln +/+ mice, †significantly less than 3-month-old Rln −/− mice, n = 6–9
Volume compliance correlates with arterial lengthening
To assess whether or not the effects of aging and genotypes on volume compliance were associated with differences in arterial lengthening, we analyzed % change in length with pressure. In the mesenteric arteries, % change in length was comparable between age groups in both genotypes (Fig. 5a, b). Conversely, % change in length in the femoral arteries was significantly (F4,33 = 6.29, P < 0.05) reduced with aging in Rln+/+ mice (Fig. 5c). In Rln−/− mice, aging did not have a significant effect on % change in length with pressure (Fig. 5d). Further analysis revealed that at 3 months of age, Rln−/− mice had significantly reduced % change in length when compared with Rln+/+ mice. There were no significant differences between genotypes at any other ages. As the Rln−/− mice already had reduced arterial lengthening compared with Rln+/+ mice, the changes that occured with aging were no longer significant in this genotype.
Fig. 5.
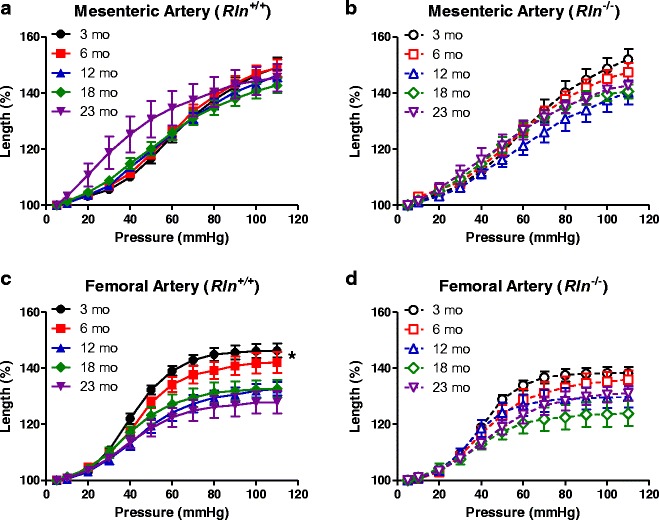
Relaxin deficiency alters arterial lengthening of femoral and not mesenteric arteries. Percentage change in length against pressure for mesenteric (a and b) and femoral (c and d) arteries of Rln +/+ (closed symbols) and Rln −/− (open symbols) mice at 3 (circle), 6 (square), 12 (triangle), 18 (diamond), and 23 (inverted triangle) months of age. Values are mean ± SEM. *Significantly (P < 0.05) greater than 12–23-month-old mice, n = 6–9
Relaxin deficiency alters Rxfp1 expression in the mesenteric artery
To further analyze the role of relaxin in the aging of arteries, Rxfp1 expression was quantified in the mesenteric arteries. Aging did not significantly alter Rxfp1 expression of either genotype. Furthermore, there was no significant (F1,66 = 2.21, P = 0.14) difference in Rxfp1 expression between Rln+/+ and Rln-/- mice at any of the ages examined (Fig. 6a). Rln expression was below the level of detection in the mesenteric arteries of both Rln+/+ and Rln−/− mice (data not shown). Housekeeping gene (Rn18S) expression was comparable between all cohorts (Fig. 6b).
Fig. 6.
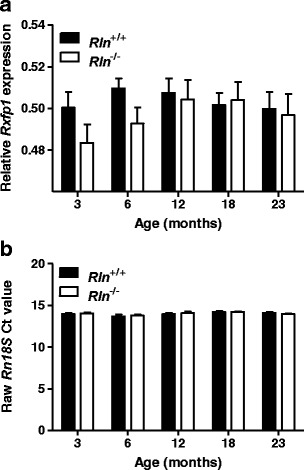
Relaxin deficiency does not significantly alter mesenteric artery Rxfp1 expression. Quantitative analysis of relaxin receptor (Rxfp1) expression (a) and raw threshold cycle (Ct) values for housekeeping gene (b, ribosomal 18S; Rn18S) in mesenteric arteries from Rln +/+ (closed square) and Rln −/− (open square) mice at 3, 6, 12, 18, and 23 months of age. Values are mean ± SEM, n = 7
Discussion
This study demonstrated that mesenteric arteries of male mice stiffen with aging. These changes in stiffness (stress-strain) occurred in the circumferential direction and were independent of both volume compliance and arterial lengthening. Remarkably, this stiffening was less pronounced in the mesenteric arteries of mice that are deficient in endogenous relaxin. Femoral arteries did not show circumferential stiffening with aging in either Rln+/+ or Rln−/− mice. Instead, the ability of the femoral arteries from Rln+/+ mice to lengthen was reduced, decreasing volume compliance with aging. Our comprehensive study demonstrated variable differences in passive mechanical wall properties of mesenteric and femoral arteries in Rln+/+ mice as they age (summarized in Table 3). Overall, we conclude that in males, a lack of endogenous relaxin has no major detrimental impact on vascular remodeling with aging, at least in the mesenteric and femoral arteries and that the relaxin-deficient mouse is not a model of accelerated vascular aging.
Table 3.
Summary of the effects of aging in mesenteric and femoral arteries of wild-type (Rln +/+) and relaxin-deficient (Rln −/−) male mice
| Artery | Genotype | Stress-strain | Volume compliance | % change in length |
|---|---|---|---|---|
| Mesenteric artery | Rln +/+ | Stiffens with age | No change | No change |
| Rln −/− | Does not stiffen further with age after 6 months | No change | No change | |
| Femoral artery | Rln +/+ | No change | ↓ with age | ↓ with age |
| Rln −/− | No change | ↓ with age | No change |
In Rln−/− mice, mesenteric arteries did not stiffen with aging. This indicates that the stiffening in these arteries occurs at an early age and does not worsen with aging. Furthermore, neither volume compliance nor arterial lengthening changed with aging suggesting that the mesenteric arteries of Rln−/− mice maintain their integrity throughout their lifespan after 6 months of age. Similarly, exogenous relaxin treatment did not alter mesenteric artery stiffness in old female rats (van Drongelen et al. 2011). Together, these data suggest that neither exogenous nor endogenous relaxin have a role in the aged mesenteric artery. The mesenteric arteries of 3- and 12-month-old Rln−/− mice exhibited reduced volume compliance, but this was not exacerbated with aging, suggesting that arteries were less compliant from an early age. Interestingly, volume was comparable between genotypes in 6-month-old mice. This may have been due to the increased variability of the 6-month-old Rln−/− mice. Furthermore, volume compliance of mesenteric arteries from Rln+/+ mice decreased with aging and matched Rln−/− mice from 18 months of age. This does not correlate with Rxfp1 expression in Rln−/− mice, which does not change with aging. The expression of other subtypes of RXFP was not assessed as endogenous relaxin has previously been shown to act only through RXFP1 to modify blood vessel structure and function (Debrah et al. 2008). There is no in vivo evidence to suggest otherwise. Overexpression of relaxin in mice deficient in the other main relaxin-like peptide (INSL3), which acts on RXFP2 to cause testicular descent, has no effect on this process (Feng et al. 2006). Furthermore, Conrad and colleagues previously demonstrated that relaxin treatment enhances small renal artery compliance in wild-type and Rxfp2 knockout mice but not Rxfp1 knockout mice (Debrah et al. 2008). This demonstrates that although relaxin may have an affinity for RXFP2, it does not act on this receptor to produce vascular effects related to passive wall stiffness. Our data suggest that relaxin plays a more important role in modulating compliance in the mesenteric artery in young animals, but this is not associated with changes in Rxfp1.
Unlike Rln+/+ mice, the femoral arteries of Rln−/− mice did not stiffen or become less compliant with aging nor was there a change in arterial lengthening. This again was likely due to the femoral arteries of Rln−/− mice being less compliant than those of Rln+/+ mice from an early age. The AUC analysis for volume compliance is bell-shaped in appearance with aging in the Rln−/− mice. Compliance increased with age up until 12 months, after which it decreased. Conversely, there is no bell-shaped curve for volume compliance AUC in Rln+/+ mice with aging. Our data contrast with previous studies that state the femoral artery is not a target of relaxin action despite the localization of RXFP1 in these arteries (Debrah et al. 2011; Jelinic et al. 2014). This may be the case for exogenous relaxin, but because the passive mechanical wall properties of the femoral artery differ between Rln+/+ and Rln−/− mice with aging, we suggest that the femoral artery is a target of endogenous relaxin. It is still uncertain whether or not this difference is solely due to a lack of endogenous relaxin or to an adaptation of living/aging without relaxin.
Similar to previous findings in aging rats (Briones et al. 2007; Laurant et al. 2004), mesenteric arteries of Rln+/+ mice stiffened with aging. Our study design differed from previous work, because the occluded end was left free and arteries were allowed to lengthen naturally with pressurization. Volume compliance of mesenteric arteries from Rln+/+ mice did not significantly change with aging, and this was largely driven by a lack of change in the ability of arteries to lengthen. Collectively, this data allowed us to conclude that with aging the ability of the mesenteric artery wall to increase in the circumferential direction with pressurization is reduced, rather than its ability to lengthen, and this is what influences increased stiffness with aging.
Femoral arteries of Rln+/+ mice did not stiffen with aging, but volume compliance was reduced at 18 and 23 months of age. This reduction in volume compliance was associated with a substantial decline in axial lengthening with aging. As there is a paucity of literature regarding the effects of aging on the passive mechanics of femoral arteries in any species, it is difficult to compare our work with those of others. One in vivo human study reported no change in femoral artery pulse wave velocity with age (35–55 years old) suggesting that arterial mechanics do not change with aging (Borlotti et al. 2012) in this vascular bed. It is important to note that only a small range over the average human lifespan was analyzed, and as with all in vivo vascular analyses, the contribution of the active components of mechanical wall properties to the measure were not removed from the analyses. A recent in situ study analyzed the passive mechanics of human femoropopliteal arteries. Aging reduced the axial but not circumferential stress of these arteries which mirrors our findings in mice (Kamenskiy et al. 2015). In the human femoropopliteal arteries, this was thought to be driven by the fragmentation and degradation of axial elastin which occurs with aging.
Our method of analysis in the mesenteric and femoral arteries, in which we analyze arterial lengthening, is an extremely useful tool when trying to assess if changes in passive mechanics are axial or circumferential in nature. Furthermore, allowing the artery to lengthen with pressure allows a more physiological change in diameter. Many biomechanical studies compare axial and circumferential mechanics (albeit isobaric), and the importance of the axial component of arterial remodeling is being realized (Humphrey et al. 2009).
In summary, our data demonstrate that with aging mesenteric arteries stiffen circumferentially, whereas femoral arteries stiffen axially in mice. A lack of endogenous relaxin had no major detrimental impact on vascular remodeling with aging because the passive mechanical wall properties of mesenteric and femoral arteries of Rln−/− mice only differed at younger ages compared with their Rln+/+ littermates. This highlights the importance of endogenous relaxin in modulating the passive mechanical properties of arteries from young mice, and that this effect is superseded by other influences and factors during the aging process. The vascular stiffness that occurs with aging at 23 months in Rln+/+ mice is not associated with decreased Rxfp1 expression. Further work is now needed to completely understand the molecular mechanisms by which endogenous relaxin modifies volume compliance of peripheral arteries.
Acknowledgments
The research was funded by an Australian Research Council Linkage Grant (LP110200543). MJ received an Australian Postgraduate Award. The authors thank Dr Dennis Stewart, the coordinator of the research partnership (Novartis Pharma AG, Basel, Switzerland), Prof Mark Elgar, Dr Chen-Huei Leo, and Dr Jackie Novak for the helpful advice with the experimental design and critical analysis of the data. We also thank Dr Jonathan Gooi, Tania Long, and Darren Cipolla for assisting in the generation and maintenance of the mouse colony used in this study.
References
- Borlotti A, Khir AW, Rietzschel ER, De Buyzere ML, Vermeersch S, Segers P. Noninvasive determination of local pulse wave velocity and wave intensity: changes with age and gender in the carotid and femoral arteries of healthy human. J Appl Physiol. 2012;113:727–735. doi: 10.1152/japplphysiol.00164.2012. [DOI] [PubMed] [Google Scholar]
- Briones AM, Salaices M, Vila E. Mechanisms underlying hypertrophic remodeling and increased stiffness of mesenteric resistance arteries from aged rats. J Gerontol Ser A Biol Sci Med Sci. 2007;62A:696–706. doi: 10.1093/gerona/62.7.696. [DOI] [PubMed] [Google Scholar]
- Cecelja M, Chowienczyk P. Arterial stiffening: causes and consequences. Arthritis Res. 2012;7:22–27. [Google Scholar]
- Chan SL, Cipolla MJ. Relaxin causes selective outward remodeling of brain parenchymal arterioles via activation of peroxisome proliferator-activated receptor-gamma. FASEB J. 2011;25:3229–3239. doi: 10.1096/fj.10-175471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP. Maternal vasodilation in pregnancy: the emerging role of relaxin. Am J Phys. 2011;301:R267–R275. doi: 10.1152/ajpregu.00156.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP, Debrah DO, Novak J, Danielson LA, Shroff SG. Relaxin modifies systemic arterial resistance and compliance in conscious, nonpregnant rats. Endocrinology. 2004;145:3289–3296. doi: 10.1210/en.2003-1612. [DOI] [PubMed] [Google Scholar]
- Debrah DO, Novak J, Matthews JE, Ramirez RJ, Shroff SG, Conrad KP. Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology. 2006;147:5126–5131. doi: 10.1210/en.2006-0567. [DOI] [PubMed] [Google Scholar]
- Debrah JE, Agoulnik A, Conrad KP. Changes in arterial function by chronic relaxin infusion are mediated by the leucine rich repeat G coupled Lgr7 receptor. Reprod Sci. 2008;15:217A. [Google Scholar]
- Debrah DO, Debrah JE, Haney JL, McGuane JT, Sacks MS, Conrad KP, Shroff SG. Relaxin regulates vascular wall remodeling and passive mechanical properties in mice. J Appl Physiol. 2011;111:260–271. doi: 10.1152/japplphysiol.00845.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Bogatcheva NV, Kamat AA, Truong A, Agoulnik AI. Endocrine effects of relaxin overexpression in mice. Endocrinology. 2006;147:407–414. doi: 10.1210/en.2005-0626. [DOI] [PubMed] [Google Scholar]
- Gooi JH, Richardson M, Jelinic M, Girling JE, Wlodek ME, Tare M, Parry LJ. Enhanced uterine artery stiffness in aged pregnant relaxin mutant mice is reversed with exogenous relaxin treatment. Biol Reprod. 2013;89:18. doi: 10.1095/biolreprod.113.108118. [DOI] [PubMed] [Google Scholar]
- Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–172. doi: 10.1002/path.2101. [DOI] [PubMed] [Google Scholar]
- Humphrey JD, Eberth JF, Dye WW, Gleason RL. Review: fundamental role of axial stress in compensatory adaptations by arteries. J Biomech. 2009;42:1–8. doi: 10.1016/j.jbiomech.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinic M, et al. Localization of relaxin receptors in arteries and veins, and region-specific increases in compliance and bradykinin-mediated relaxation after in vivo serelaxin treatment. FASEB J. 2014;28:275–287. doi: 10.1096/fj.13-233429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenskiy AV, et al. Effects of age on the physiological and mechanical characteristics of human femoropopliteal arteries. Acta Biomater. 2015;11:304–313. doi: 10.1016/j.actbio.2014.09.050. [DOI] [PubMed] [Google Scholar]
- Laurant P, Adrian M, Berthelot A. Effect of age on mechanical properties of rat mesenteric small arteries. Can J Physiol Pharmacol. 2004;82:269–275. doi: 10.1139/y04-026. [DOI] [PubMed] [Google Scholar]
- Laurent S. Defining vascular aging and cardiovascular risk. J Hypertens. 2012;30:S3–S8. doi: 10.1097/HJH.0b013e328353e501. [DOI] [PubMed] [Google Scholar]
- Leo CH, Jelinic M, Gooi JH, Tare M, Parry LJ. A vasoactive role for endogenous relaxin in mesenteric arteries of male mice. PLoS ONE. 2014;9:1–12. doi: 10.1371/journal.pone.0107382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Brookes ZLS, Kaufman S. Acute and chronic effects of relaxin on vasoreactivity, myogenic reactivity and compliance of the rat mesenteric arterial and venous vasculature. Regul Pept. 2005;132:41–46. doi: 10.1016/j.regpep.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Novak J, Danielson LA, Kerchner LJ, Sherwood OD, Ramirez RJ, Moalli PA, Conrad KP. Relaxin is essential for renal vasodilation during pregnancy in conscious rats. J Clin Invest. 2001;107:1469–1475. doi: 10.1172/JCI11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak J, et al. Evidence for local relaxin ligand-receptor expression and function in arteries. FASEB J. 2006;20:2352–2362. doi: 10.1096/fj.06-6263com. [DOI] [PubMed] [Google Scholar]
- Parry LJ, Vodstrcil LA. Relaxin physiology in the female reproductive tract during pregnancy. In: Agoulnik AI, editor. Advances in Experimental Medicine & Biology, vol 612. New York.: Springer; 2007. pp. 34–48. [DOI] [PubMed] [Google Scholar]
- Sawabe M. Vascular aging: from molecular mechanism to clinical significance. Geriatr Gerontol Int. 2010;10:S213–S220. doi: 10.1111/j.1447-0594.2010.00603.x. [DOI] [PubMed] [Google Scholar]
- van Drongelen J, et al. Ageing attenuates the vasodilator response to relaxin. Am J Phys. 2011;300:H1609–H1615. doi: 10.1152/ajpheart.00360.2010. [DOI] [PubMed] [Google Scholar]
- Vodstrcil LA, et al. Relaxin mediates uterine artery compliance during pregnancy and increases uterine blood flow. FASEB J. 2012;26:4035–4044. doi: 10.1096/fj.12-210567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Roche PJ, Gunnersen JM, Hammond VE, Tregear GW, Wintour EM, Beck F. Mice without a functional relaxin gene are unable to deliver milk to their pups. Endocrinology. 1999;140:445–453. doi: 10.1210/endo.140.1.6404. [DOI] [PubMed] [Google Scholar]
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–942. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]


