Abstract
Myeloid cells are key drivers of physiological responses to pathogen invasion or tissue damage. Members of the C-type lectin receptor (CLR) family stand out among the specialized receptors utilized by myeloid cells to orchestrate these responses. CLR ligands include carbohydrate, protein and lipid components of both pathogens and self, which variably trigger endocytic, phagocytic, pro-inflammatory or anti-inflammatory reactions. These varied outcomes rely on a versatile system for CLR signaling that includes tyrosine based motifs that recruit kinases, phosphatases or endocytic adaptors, as well as non-tyrosine based signals that modulate the activation of other pathways or couple to the uptake machinery. Here, we review the signaling properties of myeloid CLRs and how they impact the role of myeloid cells in innate and adaptive immunity.
Keywords: C-type lectins, innate immunity, endocytosis, pattern recognition, myeloid cells
Introduction
Myeloid cells are the cornerstones of the innate immune system. They play a key role in fighting infection, clearing bacteria, protozoa and viruses or releasing soluble mediators that damage metazoan parasites. Myeloid cells, in particular dendritic cells (DC), also initiate adaptive immune responses that help clear the pathogen and protect from re-infection. However, not all myeloid cell functions are related to infection. Macrophages (MØ) and neutrophils participate in clearance of dead cells, promote tissue repair following sterile injury and are involved in lipid scavenging, processes that occur independently of pathogenic insult. DCs regulate T cell tolerance to self antigens and maintain numbers and functional competence of lymphocytes. In other words, myeloid cells play a key role in the maintenance of homeostasis, participating in the normal physiological processes that underlie it and responding to any perturbations that impact it. To perform these functions, myeloid cells rely on a plethora of receptors and associated signaling pathways that allow them to continuously survey tissues, decode the infectious or non-infectious nature of any alterations and mount a coordinate response designed to restore tissue homeostasis. Receptors for cytokines, chemokines, lipids and other mediators constitute one means by which myeloid cells detect deviation from normality. They allow myeloid cells to respond to signals made by other cells that have sustained or been exposed to insult. Myeloid cells additionally possess the capacity to sense insults directly, through receptors that detect tissue damage or microbial or viral presence. These receptors prominently include members of the C-type lectin superfamily. Some myeloid C-type lectin receptors (CLRs) detect the molecular signatures of microbes, while others recognize damaged cells, oxidized lipids and other self alterations indicative of abnormality. CLRs then signal to engage the endocytic and phagocytic machinery of the phagocyte, thereby promoting the uptake of microbes, viruses or abnormal body constituents. Signals from CLRs can additionally promote microbicidal activity or can markedly change the transcriptome of the phagocyte thereby reprogramming its function, including rendering DC competent to prime an adaptive immune response. Finally, signals from myeloid CLRs can synergize with, antagonize or modulate signals from other receptors, thereby fine tuning the response to infection or damage. Here we discuss the signaling functions of myeloid CLRs and how they, together with Toll-like receptors (TLRs), Nod-like receptors (NLRs) and other innate immune receptors, confer myeloid cells with a formidable repertoire with which to assess their environment and shape the response to pathogen invasion or to abnormal self.
C-type lectins
The C-type lectin-like domain (CTLD) (1) is a conserved structural motif arranged as two protein loops stabilized by two disulfide bridges at the base of each loop (2). The second loop is more flexible than the first and generally contains the ligand binding site (1). CTLD-containing proteins, known as C-type lectins, are identified computationally on the basis of conserved CTLD residues. They constitute a superfamily of upwards of 1000 proteins classified into seventeen sub-groups (I – XVII) based on domain organization and phylogeny (2). Ca2+-dependent carbohydrate binding is the most common CTLD function in vertebrates, giving the name to the family. In those instances, the CTLD provides lectin (i.e., carbohydrate binding) activity and is therefore known as a carbohydrate recognition domain (CRD). Four Ca2+ binding sites are found in CRD structures but site 2 is key for carbohydrate recognition. This site contains two aminoacids with long carbonyl side chains separated by a cis-proline. The carbonyl side chains coordinate Ca2+, form hydrogen bonds with individual monosaccharides and determine binding specificity: a “EPN” (Glu-Pro-Asn) motif confers specificity for mannose-based ligands whereas a “QPD” (Gln-Pro-Asp) motif is typical of galactose-specific CRDs. However, not all CTLDs bind carbohydrates and calcium and many specifically recognize proteins, lipids or even inorganic ligands. The multitude of C-type lectin ligands is consistent with structural studies demonstrating the versatility of the CTLD scaffold, which allowed divergent evolution away from carbohydrate binding (2).
CLRs expressed by myeloid cells
In this review, we focus on integral membrane C-type lectins, otherwise known as CLRs, that are expressed prominently in monocytes, MØ, granulocytes and dendritic cells (DC) and possess the ability to signal to induce or modulate gene transcription, promote endocytosis, control microbicidal activity and/or otherwise alter myeloid cell function. Table 1 summarizes the attributes of the CLRs covered in this review; the list is not exhaustive. Some of these CLRs, such as Dectin-1 and Dectin-2, preferentially bind to microbial organisms and, therefore, function as “pattern recognition receptors” (PRRs) in the original sense of the term (3). Others, such as Lox-1 or DNGR-1, primarily respond to self-ligands such as damaged or altered self, including dead cell corpses. Yet others, such as Mincle or DC-SIGN, have well-established ligands of microbial and self origin and may mediate distinct responses to each.
Table 1.
Selected transmembrane mouse and human CLRs expressed in myeloid cells.
| CLR group | Common name(s) |
Gene name |
Signalling motifs |
Signalling pathways & proteins |
Endocytic activity |
Functional effects | Ligand specificity | Ligand origin | References |
|---|---|---|---|---|---|---|---|---|---|
| II CRD Ca2+ dep Type II |
DC-SIGN, CLEC4L(Hs) |
CD209 (Hs) | YxxL LL EEE | ManLAM: LSP1, KSR1, CNK RHOA, LARG, RAS, Src kinases, PAKs RAF1 Salp15: MEK-RAF1 Fucose: LSP1 Abs: Ca2+, PLCγ, PI3K, Akt, ERK1/2 |
Abs: late endosomes– lysosomes Viruses: early endosomes |
ManLAM:↑ TLR-stimulated IL-10, IL-8, IL-6, IL-12 Salp15:↓ TLR-induced IL-6, IL-12, TNF-α Fucose: ↑ IL-10, ↓ IL-6, IL-12 |
High mannose and fucose (LeX, LeY, LeA, LeB) | - HIV-1, measles, dengue, SARS, CMV, filoviruses - Mycobacterium spp, Lactobacilli spp, H. pylori, E. coli - C. albicans - Leishmania spp. - Ixodes saliva Salp15, Schistosoma egg antigen, Arah1 - ICAM-2, ICAM-3, CEACAM-1, Mac1, CEA |
(144, 149-154, 214) |
| SIGNR1 (Mm) | Cd209 b (Mm) | DDDE Y | RAF1, SYK? | Lysosomes | ManLAM: ↑ SOCS1, ↓ IL-12 Man(51)BSA: oral tolerance, ↑ IL-10 ↑ zymosan-induced TNF-α ↑ ROS to Candida Abs: ↑IL-12 and TNF-α |
Dextran Mannan Fucose | - HIV-1 - Mycobacterium spp., Streptococcus spp. - zymosan, C. albicans |
(64, 155-157, 160, 161, 163, 215) | |
| SIGNR3 (Mm) | Cd209 d (Mm) | YxxI | HemITAM-SYK | Yes | ↑ Mycobacteria-induced TNF-α | High mannose and fucose | - M. tuberculosis | (20, 64, 192, 193) | |
| L-SECtin | CLEC4 G (Hs) Clec4g (Mm) |
YxxV/L EE | ND | Yes | Negative regulation of liver T cells | GlcNAc | - Filoviruses, Coronaviruses | (190, 191) | |
| BDCA-2, DLEC, CD303, CLECSF7 (Hs) |
CLEC4 C (Hs) | Tmb K EEE | FcR-γ chain-ITAM-SYK LYN,BTK, BLNK, PLCγ2 Abs: Src; Ca2+ |
Clathrin internalization to late endosome– lysosome |
↓ TLR-induced type I IFN & TRAIL secretion ↑ TLR induced IL-10 |
gp120 | HIV-1 | (77, 80-82, 85) | |
| DCAR (Mm) | Clec4b 1 (Mm) | Tmb R | FcR-γ chain-ITAM-SYK Abs: FcRγ;Ca2+, PTyr |
ND | Activating | ND | ND | (78) | |
| mDCAR1(Mm) | Clec4b 2 (Mm) | Tmb R | FcR-γ chain-ITAMSYK? | Early and late endosomes |
↑ IL-12, ↓ IL-10 in CD40L-CpG activated DC | ND | ND | (79) | |
| DCIR, CLECS- F6, LLIR (Hs) |
CLEC4 A (Hs) | IxYxxV | SHP-1, SHP-2 | Clathrin internalization to endosomes |
↓ IL-12 & TNF-α in TLR-8 actv. DC ↓ IFN-α in TLR-9 actv. PDC |
ND | - HIV-1 | (99, 100, 104, 105, 107, 108) | |
| Dcir1, DCIR, Clecf6 (Mm) |
Clec4a 2 (Mm) | IxYxxV | SHP-1, SHP-2 | Clathrin | ↓ BCR signals (chimera) ↓ GM-CSF Stat5 phosphorylation and DC expansion |
ND | ND | (100, 101) | |
| Dcir2 (33D1) (Mm) |
Clec4a 4 (Mm) | IxYxxV | ND | Clathrin-Late endosome- lysosome |
ND | ND | (102, 103) | ||
| Dectin-2 | CLEC6 A (Hs) Clec4n (Mm) |
Tmb R | FcR-γ chain-ITAM-SYK, CARD9, Src kinases | Yes | ↑ TNF-α, IL-1RA and IL-6 ↑ IL-1β, IL-23 ↑ Cysteinyl Leukotrienes ROS, NALP3 ↑ IL-4, IL-10 UV-induced tolerance |
High mannose, α-mannans | - M.tuberculosis - C. albicans, S. cerevisiae, P. brasiliensis, H. capsulatum, M. audouinii, T. rubrum, C. neoformans - house dust mite allergens, S. mansoni eggs extracts - Ligand on CD4+ CD25+ T cells |
(68-72, 74) | |
| MCL, CLECS- F8 |
CLEC4 D (Hs) Clec4d (Mm) |
ND | ND | Yes | ND | ND | ND | (181) | |
| Mincle | CLEC4 E (Hs) Clec4e (Mm) |
Tmb R | FcR-γ chain-ITAM-SYK, Src kinases CARD9 | ND | ↑ TNF-α, IL-6, CXCL2, CXCL1 production | α-mannose, glycolipids, SAP-130. | - M. tuberculosis - C. albicans, Malasezzia spp. - dead cells |
(87-91) | |
| Langerin, CLEC4K |
CD207 (Hs) Cd207 (Mm) |
P-rich | ND | Birbeck granules (Rab11+ recycling endosomes) |
HIV-1 endocytosis for degradation M. leprae uptake for presentation |
High mannose, Fucose (LeY and LeB) and GlcNAc | - HIV-1 - M. leprae - Candida spp., Saccharomyces spp., Malassezia furfur - Type I pro-collagen |
(164, 167, 168) | |
| MGL, CD301 (Hs) |
CLEC10A (Hs) | YxxF LL | ND | Late endosome- lysosome |
binds to CD45 to inhibit T cells | Terminal GalNAc | - Filoviruses, influenza - S. mansoni - CD45 (T cells), MUC-1, gangliosides, tumor cells (Tn & Tf antigens, core2) |
(172, 216) | |
| Mgl1, Mgl, CD301a (Mm) |
Clec10a (Mm) | YxxL | ND | Late endosome- lysosome |
anti-inflammatory: gut bacteria ↑ IL-10 | LeX, LeA Terminal galactose, GalNAc, | - Streptococcus spp. Lactobacillus spp - Sialoadhesin, apoptotic bodies |
(171, 175, 176, 216) | |
| Mgl2, CD301b (Mm) |
Mgl2 (Mm) | YxxF | ND | Late endosome- lysosome |
anti-inflammatory | Terminal GalNAc | Similar to MGL? | (216) |
| CLR group | Common name(s) |
Gene name | Signalling motifs |
Signalling pathways & proteins |
Endocytic activity |
Functional effects | Ligand specificity | Ligand origin | References |
|---|---|---|---|---|---|---|---|---|---|
| V NK cell receptor like Non-CRD Ca2+ indep Type II |
MDL-1, CLECSF5 |
CLEC5A (Hs) Clec5a (Mm) |
Tmb K | DAP10 – PI3K DAP12-ITAM-SYK Abs: DAP-12;Ca2+ |
ND | ↑ TNF-α Positive modulator of RANKL osteoclastogenesis | ND | Dengue virus Role in osteoclastogenesis: endogenous ligand? | (93-95) |
| DCAL-1 (Hs) |
CLECL1 (Hs) | Tmb K? | Abs: JNK, p44/42 MAPK | ND | ↑class II HLA-DR (T cell costimulation) | ND | - Ligand on CD4+ CD45RA+ T cells | (178) | |
| Ly49Q (Mm) |
Klra17 (Mm) | VxYxxV | Abs: SHP-1, SHP-2, phosphoproteins, cytoskeleton | Controls intracellular trafficking of TLR9 and CpG |
↑ TLR-9 and TLR7-induced IL-12, IFN-α Inhibition of ITAM, TLR, Src and PI3K signalling Inhibition of adhesion |
MHC class I | cells | (116, 119, 122) | |
| MICL, DCAL-2, KLRL1, CLL1 |
CLEC12A (Hs) Clec12a (Mm) |
VxYxxL | SHP-1, SHP-2 Abs: ERK, p38 |
Yes | Abs:↑ CCR7, IL-6, IL-10, MIP-3β, TNF ↓ TLR-mediated IL-12, TNF ↑ CD40L-mediated IL-12 Chimeras: inhibitory role |
ND | - endogenous ligands in bone marrow, thymus, heart, spleen and kidney. | (109, 112, 114) | |
| CLEC-2 | CLEC1B (Hs) Clec1b (Mm) |
YxxL LL | HemITAM-SYK; Src and Tec kinases. PLCγ2, LAT, SLP76; RAC-1, VAV-1/3 | Yes | ↑ TNF-α ↑ LPS-induced IL-10 |
-Podoplanin - rhodocytin |
- lymphatic endothelial cells, lymph node stroma, tumor cells, HIV-1 - snake venom |
(17, 40, 41, 43, 45) | |
| DNGR-1 | CLEC9A (Hs) Clec9a (Mm) |
YxxL | HemITAM-SYK | Early endosomes (cross- presentation?) |
Necrotic cargo cross-presentation | ND | - dead cells | (18, 19, 54, 55) | |
| CLEC12B, MAH |
CLEC12B (Hs) Clec12b (Mm) |
VxYxxL | SHP-1, SHP-2 | ND | Inhibition of positive ITAM-derived signals | ND | ND | (115) | |
| CLEC-1 | CLEC1A (Hs) Clec1a (Mm) |
YxxT DDD TmbR? | Requires an adaptor for membrane expression: FcRγ chain? | ND | ND | ND | ND | (40, 177) | |
| Dectin-1, β-GR, CLECSF12 |
CLEC7A (Hs) Clec7a (Mm) |
YxxL DED | HemITAM-SYK Bcl10-Malt1-CARD9, PLCγ2 ERK, p38, JNK NIK RAF-1 | Late endosome– lysosome |
↑ IL-10, IL-2, IL-6, IL-23, ROS, NALP3 ↑ TLR-stimulated TNF, IL-12 |
β-1,3 glucans | - Mycobacteria spp. - P. carinii, C. albicans, A. fumigatus, Penicillium marneffei, Coccidioides posadasii and Histoplasma capsulatum - Ligand on T cells |
(6, 13-16, 25, 27, 28, 32, 36, 37) | |
| LOX-1 | OLR1 (Hs) Olr1 (Mm) |
DDL/LL | ARHGEF1, ROCK2, RhoA, Rac, ROS | early endosomes / cross- presentation? |
Role in cross-presentation. Pathogen recognition, antigen capture. | Hsp-70, oxidized lipids? | - E.coli, S. aureus - oxLDL, oxidized lipids, apoptotic/ aged cells, red blood cells |
(183, 185, 187) |
| CLR group | Common name(s) |
Gene name | Signalling motifs |
Signalling pathways & proteins |
Endocytic activity |
Functional effects | Ligand specificity | Ligand origin | References |
|---|---|---|---|---|---|---|---|---|---|
| VI MMR family multiple CRD Ca2+ dep Type I |
MR, MMR, CD206 |
MRC1 (Hs) Mrc1 (Mm) |
FxxxxY LL | CDC42, RHOB, PAKs, ROCK1 | Early endosomes (cross- presentation) |
↑ CD80, CD86, IL-10, IL1RA ↓ TLR-mediated TNF-α and IL-12 Inhibition of response to Pneumocystis, ↓ IL-1β, IL-6 and TNF-α LPS + apoptotic cells: ↑ TNF-α ↓ IL-10 |
High mannose, Fucose, sLeX, GlcNAc | - HIV-1, Dengue - M. tuberculosis, M. kansasii, F. tularensis, K. pneumoniae, S. pneumoniae - P. carinii, C. albicans, C. neoformans - Leishmania spp. -glycosylated allergens - Lysosomal hydrolases, thyroglobulin, L-selectin, MUC-1, apoptotic cells |
(61, 124, 132-134, 137, 139) |
| DEC205, CD205 |
LY75 (Hs) Ly75 (Mm) |
FxxxxY EDE | ND | Late endosome– lysosome |
Pathogen receptor and antigen presentation | PLA (Y. pestis) K12 (E. coli) |
- HIV-1 - Y. pestis, E. coli - apoptotic cells, oxLDL |
(138, 139, 141-143, 217) |
CLRs were selected on the basis of expression in myeloid cells; the list is not exhaustive and refers to the CLRs covered in the review. CLR protein and gene designations are based on NCBI database nomenclature.
Abbreviations: Ab, antibody; dep, dependent; β-GR, β-glucan receptor; Hs, Homo sapiens; IL, interleukin; Mm, Mus musculus; ND, not determined; PTyr, Tyr phosphorylation; ROS, Reactive oxygen species; Tmb, transmembrane.
Interest in myeloid CLRs comes from their ability to regulate homeostasis, which in turn is dictated by their signaling properties. The latter have only recently begun to be deciphered and cut across earlier CLR classifications. We have therefore championed the grouping of myeloid receptors based on signaling similarities (4, 5) and adopt this principle here as an underlying structure (Fig. 1). Nevertheless, to facilitate cross-referencing, we refer to CLR structural classifications throughout (Table 1 and Fig. 1). We focus exclusively on CLRs from mouse (Mus musculus; Mm) and human (Homo sapiens; Hs) and, unlike our previous reviews (4, 5), discuss CLRs individually to allow the reader to “zoom in” on receptors of specific interest. At the same time, as developed more extensively elsewhere (4, 5), we attempt to highlight myeloid CLR similarities and differences and to extract some of the emerging principles that come from recent studies uncovering their signaling properties.
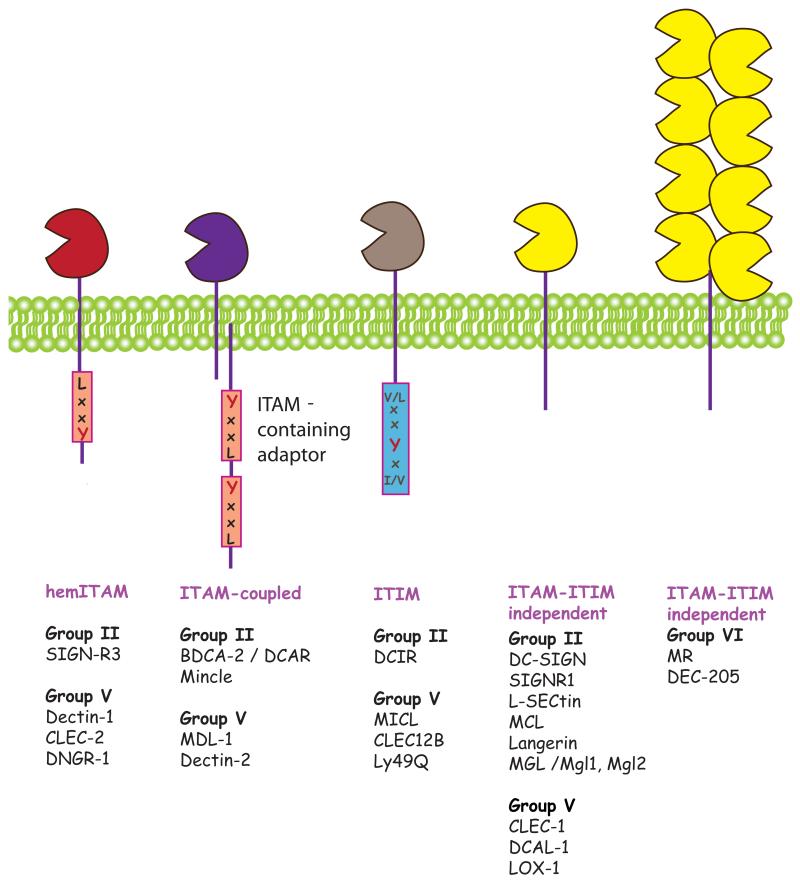
Figure 1. Signaling families of myeloid CLRs.
Myeloid CLRs can be grouped independently of structure into four groups, based on cytoplasmic signaling motifs and the binding of early adaptors, kinases or phosphatases,: a) hemITAM-coupled CLRs signal via Syk through a single tyrosine-based motif in their tail. b) ITAM-coupled CLRs signal via Syk through association with ITAM-bearing adaptors as FcRγ chain or DAP-12. c) ITIM-containing CLRs possess an ITIM motif that can recruit phosphatases SHP-1 and SHP-2. d) ITAM-ITIM-independent CLRs do not signal through Syk or phosphatases although they may contain tyrosine-based motifs involved in endocytosis.
With these considerations in mind, we have selected for our discussion the following CLRs belonging to C-type lectin structural groups II, V, and VI and expressed in myeloid cells (Table 1 and Fig. 1).
• Group II: type II transmembrane CLRs containing a short cytoplasmic tail, a transmembrane domain, an extracellular stalk region, and a single CTLD possessing Ca2+ and carbohydrate binding activity (i.e., a CRD). The length of the stalk region, involved in oligomerization, varies among members. Several sub-groups can be defined on the basis of gene clustering (2).
• Group V: type II transmembrane CLRs with a short cytoplasmic tail, a transmembrane domain and an extracellular stalk region followed by a single CTLD lacking typical Ca2+ and carbohydrate binding motifs. All of these CLRs except MDL-1 are clustered on chromosome 12p13 in human and 6F3 in mouse, the “natural killer receptor gene complex” that encodes CLRs expressed in NK cells. However, the receptors discussed here are those expressed primarily by myeloid cells.
• Group VI: type I transmembrane proteins with an extracellular domain comprising an N-terminal ricin-like domain, a fibronectin type 2 domain, followed by 8 or 10 CTLDs. The extracellular domain is linked to a transmembrane regions and a short cytoplasmic domain. Two CLRs from this group are expressed in myeloid cells: mannose receptor and DEC-205.
Signaling properties of myeloid CLRs
Based on cytoplasmic signaling motifs and signaling potential, myeloid CLRs can be grouped independently of structure into the following broad categories (Table 1):
• Syk-coupled CLRs: Syk has emerged as a major tyrosine kinase involved in the early signaling by a subset of CLRs. CLR coupling to Syk can be indirect, through the adaptors Fc receptor γ chain (FcRγ) or DAP-12, which bear classical Syk-recruiting ITAM motifs, or direct via a single tyrosine-based motif, termed hemITAM, found in the cytoplasmic domain of some CLRs (6). Phosphorylation of the tyrosine(s) in the ITAM or hemITAM motifs generates docking sites for the SH2 domains of Syk, which undergoes a conformational change permitting auto-phosphorylation and activation. Active Syk can then bind directly to SLP-65/SLP-76, Vav, PI3K or PLCγ, which in turn coordinate many downstream signaling pathways leading to myeloid cell activation (7). We consider separately the hemITAM-based CLRs (Dectin-1, CLEC-2, DNGR-1 and SIGN-R3) and the ITAM-coupled CLRs (Dectin-2, hBDCA-2, mDCAR, mDCAR1, Mincle and MDL-1).
• CLRs with ITIM domains: a distinct group of CLRs expresses ITIM motifs that recruit phosphatases and thereby negatively regulate signaling through kinase-associated receptors, notably the Syk-coupled CLRs. ITIM-bearing CLRs generally do not have any activity per se but will modulate myeloid cell activation when triggered together with activatory receptors. Myeloid CLRs included in this group are hDCIR, mDcir1, mDcir2, MICL, MØ antigen H and Ly49Q.
• CLRs without ITAM or ITIM domains: this catch-all designation encompasses CLRs without a clear ITAM or ITIM motif, including mannose receptor, DEC-205, DC-SIGN, SIGNR1, Langerin, hMGL, mMgl1, mMgl2, CLEC-1, DCAL-1, MCL, LOX-1 and LSECtin. These CLRs have endocytic activity and can mediate the capture of antigenic cargo for processing and presentation to T cells (8). Nevertheless, triggering of these receptors in isolation does not induce obvious signs of myeloid cell activation although it can, in some cases, modulate the outcome of signaling by other receptors. For some CLRs (e.g., DC-SIGN), the signaling pathway involved in modulation has been elucidated but for most it is unknown.
1. HemITAM-based CLRs
1.1. Dectin-1 (Hs: CLEC7A; Mm: Clec7a)1
Dectin-1 is the paradigm for a CLR able not only to promote ligand uptake, as well as engage signaling cascades that drive innate and adaptive immunity. Mouse Dectin-1 is expressed in myeloid cells, including DCs, monocytes, MØ, neutrophils, as well as in a subset of γδ T cells (9, 10). The human ortholog shows a similar expression pattern but is additionally expressed in B cells, eosinophils and mast cells (11, 12). Dectin-1 is a PRR for β-1,3-linked glucans present in the cell wall of fungi, some bacteria and plants (13) (14). However, Dectin-1 does not have a typical CRD and binding to β-glucans is calcium-independent. In addition to β-glucans, Dectin-1 is reported to bind an unidentified self ligand expressed in T cells (15).
Dectin-1 is able to directly recruit and activate Syk upon binding to agonist ligands (6, 16) (Fig. 2). ITAM motifs are effectively a tandem repeat of YxxL/I sequences (where x designates any aminoacid) but the tail of Dectin-1 possesses only one tyrosine within an YxxL motif and this tyrosine is necessary to mediate signaling via Syk (6). The Dectin-1 motif was consequently termed “hemITAM” and has since been described in the other members of the family, CLEC-2, DNGR-1 and SIGN-R3 ((17-20) and reviewed in (4, 5)). A conserved “DEDG” sequence precedes the YxxL in both Dectin-1 and CLEC-2 but not in DNGR-1 or SIGN-R3, which also signal via Syk. Therefore, a consensus sequence for the hemITAM is still missing and the motif is defined empirically by the ability of a given CLR to directly recruit Syk via a single tyrosine.
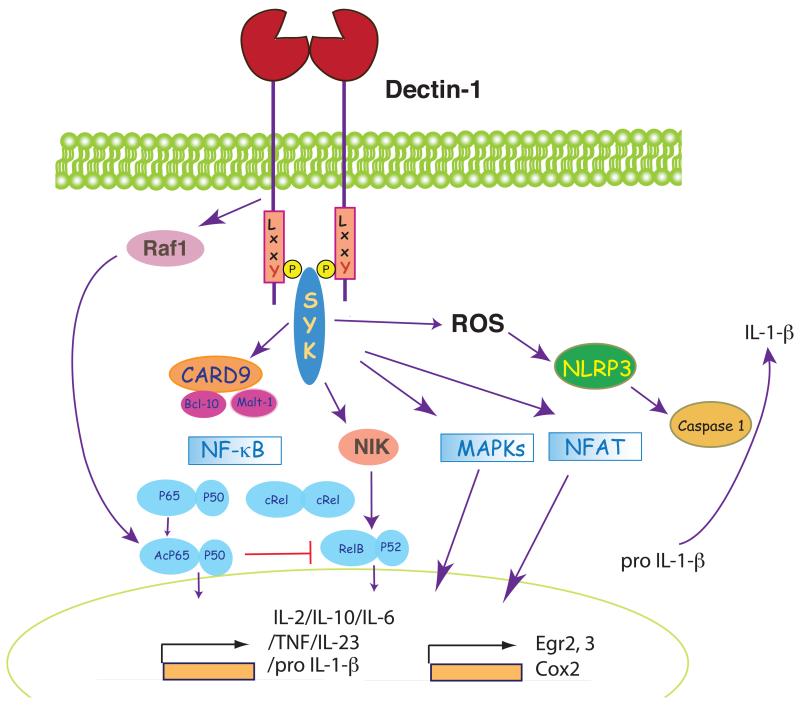
Figure 2. Dectin-1 as a model hemITAM-coupled receptor.
Following binding to agonist ligands, Dectin-1 recruits Syk through a phospho-tyrosine in the hemITAM motif. Syk induces production of ROS that acts as microbicidal agent and contributes to the activation of the NALP3 inflammasome, leading to processing of pro-IL-1β. Syk also leads to activation of NF-κB at different levels. First, Syk recruits CARD9/Bcl-10 to activate the canonical p65/p50 pathway and Malt-1, which activates c-Rel in human DC. Independent of CARD9, Syk also leads to activation of NIK and the non-canonical RelB pathway. Finally, Dectin-1 engagement also leads to Syk-independent activation of Raf-1, which results in acetylation of p65/p50 and modulation of NF-κB activity in part through inhibition of the RelB module. Syk also activates the p38, ERK and JNK cascades, as well as NFAT, which regulate gene transcription in cooperation with NF-κB.
Dectin-1 signaling through Syk in myeloid cells likely occurs through formation of dimers as for CLEC-2 (see below) and shares similarities with antigen receptor signaling in lymphocytes. As for the latter, signaling is affected by disruption of lipid microdomains (21) and NF-κB-dependent transcriptional activity is a major outcome. However, unlike lymphocytes that utilize CARMA1 to couple Syk or ZAP-70 signaling to NF-κB, myeloid cells use the adaptor CARD9 (Fig. 2). Syk triggers the recruitment of CARD9 to the membrane (22, 23) or to phagosomes containing ingested fungal particles (24). The CARD9/Bcl10/Malt-1 module then activates the IκB kinase (IKK) complex for canonical NF-κB signaling: IKK phosphorylates IκB and promotes its degradation, allowing for NF-κB family members to translocate to the nucleus (25)(Fig. 2). CARD9 and Bcl10 activate all canonical NF-κB subunits whereas Malt-1 specifically activates c-Rel in human DC, which preferentially induces IL-1β and IL-23 p19 (26). Dectin-1 can also activate the non-canonical NF-κB pathway (RelB) in an NF-κB-inducing kinase (NIK)-dependent but CARD9-independent fashion (27) (Fig. 2).
Activation of NF-κB underlies the pro-inflammatory program of myeloid cell activation (Fig. 2). Consistent with this fact, Dectin-1-Syk signaling induces DC maturation and secretion of cytokines, including IL-2, IL-10, IL-6, TNF-α and IL-23, rendering DC fully competent to direct priming of CD4+ T helper cells, CD8+ cytotoxic T cells and antibody responses (28, 29). Notably, the CD4+ T cells response includes both a Th1 and Th17 component, the latter characterized by the expansion of Th17 cells, as well as Foxp3+ regulatory T cells producing IL-17 (30).
Human Dectin-1 can also induce a second signaling pathway mediated by the serine-threonine kinase Raf-1 (Fig. 2), known for its role in the DC-SIGN signaling pathway (see below). This is independent of Syk but converges with Syk-coupled pathways at the level of NF-κB (27). Whereas Syk signals result in activation of the canonical and non-canonical NF-κB pathways, Raf-1 activation results in selective phosphorylation and permits subsequent acetylation of the NF-κB p65 subunit, as in DC-SIGN signaling (see below) (27). Acetylated p65 can become transcriptionally active in partnership with p50 or can sequester Syk-induced RelB into RelB-p65 inactive dimers that do not bind to DNA (27). Overall, Raf-1 activation enhances the expression of some Syk-dependent cytokines in human DC, including IL-10, IL-12 p35, IL-12/23 p40, IL-6 and IL-1β, but negatively regulates the RelB-dependent cytokines, including IL-23 p19 (27). This potentiates IL-12 p70 production by human DCs and favors induction of Th1 responses downstream of Dectin-1 (27).
The ability of Dectin-1 signaling to drive NF-κB activation is myeloid cell type-dependent. DC derived from bone marrow progenitors under the aegis of GM-CSF in vitro are easily activated by Dectin-1 agonists, whereas this is not the case for DC derived using Flt3L or for M-CSF-derived MØ (24). Much of this difference may be attributable to levels of expression of CARD9 and/or other signaling components, which may be limiting in certain myeloid cell types. However, pre-treatment of MØ with GM-CSF or IFN-γ primes for response to Dectin-1 engagement without affecting CARD9 expression suggesting that a combination of limiting activatory and inhibitory factors is involved (24). The nature of the ligand also dictates the extent to which Dectin-1 signaling results in induction of a pro-inflammatory gene program: large particulate ligands that induce “frustrated phagocytosis” result in increased inflammatory responses, suggesting that endocytosis attenuates Dectin-1 signaling (22, 23). Dectin-1 can bind soluble and particulate β-glucans, but a “synapse-like” structure excluding the inhibitory phosphatases CD45 and CD148 forms only upon interaction with particulate ligand (31). Such large scale phosphatase exclusion may prolong Dectin-1 signaling via Syk and facilitate the activation of NF-κB when myeloid cells come into contact with large β-glucan-containing particles. Coupling myeloid cell activation to encounter with particulate ligands may be a means of ensuring that microbicidal responses occur only when the cell is in contact with the pathogen and not in response to shed microbial components (31).
In addition to NF-κB, Dectin-1 signaling results in activation of p38, ERK and JNK cascades (28, 32) and NFAT (33) (Fig. 2). This is similar to antigen receptor signaling in lymphocytes but different from TLR signaling, which does not appreciably induce Ca2+ elevations and NFAT activation. The Syk-NFAT axis imparts a unique pattern to myeloid cell activation by Dectin-1 agonists that combines induction of typical pro-inflammatory cytokines (TNF-α, IL-6, IL-12/23 p40) with high levels of IL-2, IL-10 in DC and COX-2 and PGE-2 in MØ (6, 28, 33). NFAT activation by Dectin-1 – Syk is critically dependent on PLCγ2, which regulates Ca2+ signaling and activation of the ERK and JNK pathways in DCs (34, 35).
Apart from inducing transcriptional responses, Syk activation in myeloid cells can impact migration, phagocytosis or microbicidal activity (7). Upon interaction of zymosan with Dectin-1 in mouse DC, Syk activates the Rho GTPases Cdc42 and Rac-1, and triggers pseudopod extension around the particle (6, 25, 36). Interestingly, particle uptake mediated by Dectin-1 is not dependent on Syk kinase in MØ despite requiring the key tyrosine in the hemITAM motif, as well as the tri-acidic motif upstream (6, 16, 36). However, reactive oxygen species (ROS) generation within MØ phagosomes is Syk-dependent (16, 25). ROS have a direct microbicidal role in the phagosome but also can impact IL-1β secretion by activating the NLRP3 inflammasome, which in turns activates caspase-1 and permits processing of pro-IL-1β (37) (Fig. 2). IL-1β is essential for antifungal immunity and NLRP3 deficient mice have been shown to be highly susceptible to fungal infection (37) although the role of caspase-1 and of the inflammasome in human anti-fungal responses remains controversial (38, 39). Nevertheless, the connection between Syk signaling and IL-1β processing constitutes an example of how Syk-coupled CLRs can affect myeloid cell function independent of a role in regulation of gene expression.
1.2. CLEC-2 (Hs: CLEC1B; Mm: Clec1b)
CLEC-2 mRNA was detected in many myeloid cell types (40) and later shown to be highly expressed in platelets (17). Mouse CLEC-2 is expressed by peripheral blood neutrophils and activated monocytes (41), liver Kupffer cells (42), DCs, NK cells and B cells (43). It is a target of the Malayan pit viper venom toxin, rhodocytin, which induces tyrosine phosphorylation and Syk, Src and Tec kinases and PLCγ-2 activation, leading to platelet aggregation and coagulation (17, 44). CLEC-2 also possesses a self ligand, the mucin podoplanin (45). Podoplanin is a cell surface glycoprotein found on lymphatic endothelium, stroma of secondary lymphoid organs and some cancer cells. Podoplanin can also become incorporated in the envelope of HIV-1 produced by cultured cells, leading to virus binding to CLEC-2 (46). Notably, the interaction of platelet-expressed CLEC-2 with podoplanin expressed on lymphatic endothelial cells has emerged as key for the separation of blood and lymphatic vessels during embryonic development. Indeed, mice lacking podoplanin or mice lacking CLEC-2, Syk or SLP76 in platelets and megakaryocytes display aberrant vascular connections between blood and lymphatic vessels, which result in bleeding into the latter (42, 47, 48).
CLEC-2 signaling in platelets has been an excellent model to study hemITAM function. Contrary to the conventional model for ITAM activation in which Src family kinases phosphorylate the ITAM tyrosines to allow Syk recruitment, rhodocytin induces CLEC-2 phosphorylation in platelets independently of Src kinases but dependent on Syk itself (49). This is reminiscent of a model proposed for B cell receptor signaling where receptor clustering and local phosphatase exclusion are sufficient to induce a low level of ITAM phosphorylation, which is then sustained and propagated by the kinase activity of Syk itself (50). Signal initiation is also dependent on translocation of CLEC-2 to lipid rafts, actin polymerization, Rac1 activation and release of ADP and thromboxane A(2) (51).
An important issue addressed by studying CLEC-2 is how a single tyrosine hemITAM can serve as a docking site for a tandem SH2 kinase such as Syk. Mutation in either Syk SH2 domain blocks responses induced by CLEC-2 or Dectin-1 (44 and unpublished observations), suggesting that both domains are engaged during productive signaling. Although this may reflect binding of one SH2 to the hemITAM and of another to an unidentified partner, an attractive hypothesis is that dimerization of hemITAM-bearing CLR molecules allows two YxxL motifs to come together and form a “pseudo-ITAM” in trans (52). Interestingly, stoichiometric analyses indicates that CLEC2 pre-exists as a dimer, which is drawn into larger complexes upon ligand binding (52, 53).
Despite the ability to signal via Syk and, potentially, act in a manner analogous to Dectin-1, the function of CLEC-2 in myeloid cells remains unclear. A chimeric Dectin-1 receptor bearing the intracellular tail of CLEC-2 induces ligand-dependent Syk signaling and production of TNF-α but not ROS when transfected into a MØ cell line (41). In contrast, antibody-mediated cross-linking of CLEC-2 induces Syk signaling and activates NFAT but not NF-κB in DC. As a consequence, CLEC-2 crosslinking with antibodies does not induce detectable DC activation although it markedly augments production of IL-10 and IL-2 when combined with a TLR stimulus (43). The NFAT axis may therefore be the most characteristic feature of CLR-Syk signaling in myeloid cells as it can be observed even in conditions when NF-κB activation is not apparent (43).
1.3. DNGR-1 (Hs: CLEC9A; Mm: Clec9a)
Mouse DNGR-1 (DC, NK-lectin group receptor-1) is expressed selectively at high levels by CD8α+ DC (18, 54, 55) and tissue-resident CD103+ CD11b− DC (unpublished observations), and at lower levels by plasmacytoid DC. In human, DNGR-1 expression appears restricted to BDCA-3+ DC, one of the factors that led to the recent identification of these cells as the putative human equivalents of mouse CD8α+ DC (56-59). The selective expression in mouse and human CD8α+-like DC, together with its endocytic capacity, make DNGR-1 an appealing receptor for targeting antigens to DCs (54, 55, 60). Interestingly, DNGR-1-bound antibodies are directed to non-lysosomal compartments (19) that may overlap with endosomes targeted by mannose receptor where crosspresentation of antigens is facilitated (61) (see below). Consistent with that notion, antigens coupled to anti-DNGR-1 antibodies are efficiently crosspresented to CD8+ T cells by CD8α+ DC (54). Antigens targeted to DNGR-1 can also be efficiently presented by MHC class II molecules, resulting in prolonged CD4+ T cell responses and effective help for humoral immunity (55, 62, 63).
No microbial ligand has yet been identified for DNGR-1 but the receptor binds in a Ca2+-independent manner to an unidentified ubiquitous self ligand that is normally sequestered inside healthy cells but exposed upon loss of cell membrane integrity (19). The latter occurs naturally upon primary or secondary necrotic cell death and necrotic corpses bear exposed DNGR-1 ligands, which trigger hemITAM-dependent DNGR-1 signaling via Syk in CD8α+ DC (19). The DNGR-1 signal contributes to priming of CTL against antigens carried by the dead cells but the mechanism involved is poorly understood (19). One possibility is that DNGR-1 functions to activate CD8α+ DC in response to contact with cell corpses, analogous to the activatory function of Dectin-1 upon contact with fungal organisms. However, ligation of DNGR-1 with antibodies does not result in DC activation (54, 55). Similarly, DC activation is not seen upon engagement of chimeric receptors comprising a Dectin-1 ectodomain fused to DNGR-1 (unpublished observations) although activation was reported for MØ cell lines transduced with similar constructs (18). The dedicated role of DNGR-1 in crosspriming to dead cell-associated antigens may therefore reflect an activity other than DC activation. One hypothesis currently under consideration is that DNGR-1 may have a function in handling of necrotic cargo, retaining it in a non-lysosomal compartment that favors cross-presentation ((19) and unpublished observations).
1.4. Mouse SIGNR3 (Cd209d)
SIGNR3 binds mannose-containing mycobacterial surface proteins (64). Unlike Dectin-1, CLEC-2 or DNGR-1, SIGNR3 belongs to C-type lectin group II and is part of a cluster of mouse SIGNR genes highly homologous to human DC-SIGN. Both mouse SIGNR3 and human DC-SIGN possess a YxxL/I motif in the intracellular domain but human DC-SIGN does not couple to Syk and is not considered a hemITAM-bearing CLR (8, 44). In contrast, mouse SIGN-R3 has been reported to signal via a Syk-dependent pathway that results in induction of pro-inflammatory cytokines in mouse MØ in response to Mycobacterium tuberculosis or its mannosylated lipoarabinomannan (ManLAM) component (20). Syk-dependent SIGN-R3 signaling depends on the integrity of the tyrosine residue within the YxxI intracellular motif (20). Therefore, mouse SIGNR3 might constitute an additional hemITAM-bearing Syk-coupled CLR located outside the cluster that encodes the other members of the family. Other mouse SIGNR receptors do not appear to signal via Syk (see below).
2. ITAM-coupled CLRs
2.1. Dectin-2 (Hs: CLEC6A; Mm: Clec4n)
Dectin-2 is expressed in MØ, monocytes and several DC subtypes (15, 65, 66). Dectin-2 has affinity for high-mannose structures and binds α-mannans in fungal cell walls (67, 68). It can additionally recognize mannose-bearing glycans in extracts of house dust mite (69) although whether the ligands are derived from the organism in question or its commensal fungi has not been established. Independently of fungi, Schistosoma mansoni egg extracts also trigger Dectin-2 activity in myeloid cells (70) and a self ligand is reported to be expressed in CD4+CD25+ T cells (71).
Dectin-2 lacks a clear intracellular signaling motif but associates with the ITAM-bearing FcRγ chain (72). The association with FcRγ is required for surface expression of Dectin-2 and the FcRγ ITAM is subsequently required for signaling following Dectin-2 engagement (66)(Fig. 3). In a MØ cell line, ligation of Dectin-2 induces tyrosine phosphorylation of FcRγ, Src-dependent activation of NF-κB and production of TNF-α and IL1RA (72). Antibody crosslinking of Dectin-2 in DCs induces Syk recruitment to the phosphorylated tyrosines in the FcRγ ITAM motif and permits CARD9-dependent activation of NF-κB (66)(Fig. 3). In response to fungal ligands, Syk activated by Dectin-2/FcRγ signaling regulates IκBα kinase phosphorylation whereas CARD9 mediates IκBα kinase-NEMO ubiquitination, suggesting that Syk and CARD9 act in concert, and not sequentially as in Dectin-1 signaling (73). A further difference from Dectin-1, which activates all NF-κB subunits, is that Dectin-2 selectively activates the NF-κB subunit c-Rel, at least in human DC, through the recruitment of Malt1, which results in the expression of Th17 polarizing cytokines IL-1β and IL-23 (26). Dectin-2 signaling in mouse DC further triggers activation of the ERK, JNK and p38 MAPK pathways (66).
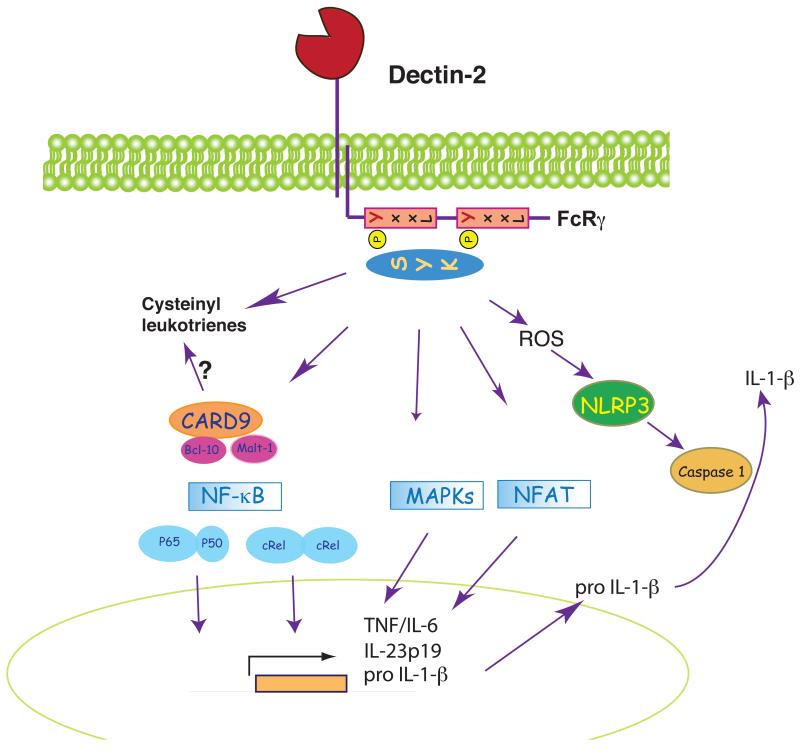
Figure 3. Dectin-2 as a model ITAM-coupled receptor.
Dectin-2 associates with the FcRγ chain via an arginine residue located in the transmembrane region. Upon Dectin-2 triggering, Syk is recruited to the phosphorylated ITAM of FcRγ and coordinates activation of many of the same pathways as for Dectin-1 signaling (see legend to Fig. 2 and text for discussion of differences between Dectin-1 and Dectin-2). Dectin-2 / Syk signaling in response to allergens can also lead to synthesis of cysteinyl leukotrienes, which are involved in allergic inflammation and induction of Th2 responses.
Like Dectin-1, Dectin-2 belongs to the selective group of CLRs that links pathogen recognition to adaptive immunity. In fact, Dectin-2 rather than Dectin-1 is the predominant Syk-coupled receptor in the response of DC to Candida albicans and in the induction of Th17-based immunity to the organism in mouse models (66, 68). Aside from transcriptional outcomes, Dectin-2 signaling also promotes endocytosis and cargo uptake, facilitating fungal cell clearance and/or presentation of fungal antigens (72). In addition, the activation of Dectin-2 / Syk signaling in response to Schistosoma triggers ROS and potassium efflux, leading to NALP3 activation and processing of pro-IL-1β (70), analogous to the response of Dectin-1 to fungi (37).
An unexpected facet of Dectin-2 biology has come from the study of allergic responses. Allergenic extracts of house dust mites or the mold Aspergillus fumigatus bind Dectin-2 to trigger Syk-dependent arachidonic acid metabolism and rapid production of cysteinyl leukotrienes (69) (Fig. 3). These lipid mediators mediate eosinophilic and neutrophilic pulmonary inflammation and facilitate allergic Th2 responses (74). Thus, in addition to the induction of cytokines that facilitate Th17 responses to fungi, the Dectin-2 pathway induces pro-inflammatory lipids that promote a Th2 response to some allergens. It remains to be determined whether these two outcomes are controlled by the nature of the ligand or whether, in fact, Dectin-2 signaling always induces a mixed Th2/Th17 response, which is then shaped and selected through the action of other innate immune receptors. It is interesting to note that β-glucans have also been implicated in allergic responses (75), suggesting that Dectin-1 (or other β-glucan receptors) could, in some circumstances, also favor Th2-biased immunity.
2.2. Human BDCA-2 (Hs: CLEC4C, CD303), mouse DCAR (Mm: Clec4b1) and mouse mDCAR1 (Mm: Clec4b2)
Human BDCA-2, its putative mouse ortholog DCAR and the related mDCAR1 are encoded in a gene cluster that also includes the genes for Dectin-2, Mincle and the DCIRs (see above and below). BDCA-2 expression is restricted to human plasmacytoid DC and the receptor is often used as a marker for those cells (76, 77). Mouse DCAR is expressed in DC, monocytes, MØ and B cells (78); mDCAR1 is expressed in DC and CD11b+ cells in a tissue-specific fashion (79). The ligands for these receptors are not well characterized, although binding of gp120 to BDCA-2 has been reported (80).
All these receptors possess an intracellular lysine or arginine next to the transmembrane region that promotes association with FcRγ (78, 81, 82). BDCA-2 signals through FcRγ/Syk, Lyn, Btk, BLNK and PLCγ2 in plasmacytoid DC but whether this results in activation of the cells remains unclear (83): plasmacytoid DC do not express CARD9 but CARMA1, which may impact NF-κB activation by CLR/Syk signaling (84). Suppression of type I IFN and TRAIL secretion is the major phenotype observed upon triggering of BDCA-2 and TLR receptors in plasmacytoid DC (81, 82, 85). Interestingly, plasmacytoid DC from systemic lupus erythematosus patients have reduced BDCA2 expression and increased IFN-α production, suggesting that downregulation of BDCA2 could be a marker for plasmacytoid DC activation and disease severity (86).
Not much is known about the BDCA-2-like receptors in mouse. Cross-linking of DCAR leads to signaling via the FcRγ chain ITAM, calcium mobilization and tyrosine phosphorylation (78). Antibody triggering of mDCAR1 causes increased secretion of IL-12 and reduced IL-10 in mouse CD8α+ DC activated by CD40L and CpG DNA (a TLR9 agonist) (79). Consistent with a possible activatory role in DC, targeting of antigens to mDCAR1 in vivo induces cellular and humoral responses even in the absence of adjuvants (79). Further understanding of the role of BDCA-2-like CLRs in mouse and human will be greatly facilitated by definition of their ligands.
2.3. Mincle (Hs: CLEC4E; Mm: Clec4e)
Mincle (Macrophage-inducible C-type lectin) is expressed at low levels on MØ and neutrophils but is strongly increased after MØ exposure to inflammatory cytokines or TLR agonists (87). Mincle has an arginine residue in the transmembrane region permitting association with FcRγ chain (87). Ca2+-dependent α-mannose-containing Mincle ligands are found in Malasezzia fungal species, some Candida strains and in mycobacteria (88-90). Indeed, Mincle is the receptor for the mycobacterial glycolipid trehalose-6,6′-dimycolate (TDM), long known as a potent innate immune stimulus and adjuvant (91, 92). Stimulation of MØ with TDM leads to NF-κB activation via the FcRγ/Syk/CARD9 pathway and results in production of pro-inflammatory cytokines and chemokines such as TNFα, CXCL2, CXCL1 and IL-6, as well as in nitric oxide production (91, 92).
Mincle is also a MØ sensor for damaged cells and recognizes the self ribonucleoprotein SAP-130 in necrotic corpses (87). The recognition of SAP-130 does not require calcium or the canonical CRD residues, indicating a distinct binding site (87). As for TDM, SAP-130 recognition by Mincle also results in signaling via FcRγ-Syk-CARD9 and in production of TNFα and CXCL2. This pro-inflammatory response attracts neutrophils to damaged tissues, a “sterile inflammation” reaction that is likely to be important for tissue repair (87). This contrasts with the possible involvement of Mincle in host defense against pathogenic fungi or mycobacteria, which would be designed to promote immunity. Consistent with the latter, Mincle is necessary for the generation of Th1/Th17-based immunity following vaccination using trehalose dibehenate (a synthetic analogue of TDM) as an adjuvant (92). It remains unclear whether Mincle signals differentially when engaged by SAP-130 vs. microbial-derived ligands or whether the difference between “sterile inflammation” and response to infection is in fact dictated by engagement of additional receptors.
2.4. MDL-1 (Hs: CLEC5A; Mm: Clec5a)
Myeloid DAP12-associating lectin-1 (MDL-1)is expressed in monocytes, MØ and osteoclasts (93-95). MDL-1 has a short cytoplasmic region that associates noncovalently through a transmembrane lysine with the adaptors DAP12 or DAP-10, which bear ITAM or YINM motifs, respectively (93-95). Phosphorylation of the tyrosine in the DAP10 YINM sequence mediates coupling to phosphatidylinositol 3-kinase, which potentiates signaling induced via DAP-12/Syk. The DAP10 association with MDL-1 depends almost entirely on DAP12 in osteoclasts and bone marrow-derived MØ, generating MDL-1-DAP12/DAP10 trimolecular complexes with mixed ITAM/YINM motifs. Crosslinking of MDL-1 with antibodies results in Ca2+ signaling and acts as a positive modulator of RANKL-induced osteoclastogenesis (93, 95). In addition, MDL-1 triggering increases joint inflammation whereas blockade or ablation of MDL-1 markedly ameliorates disease in mouse models or arthritis (96).
The impact of MDL-1 blockade on arthritis implies the existence of an unidentified self ligand. MDL-1-dependent pro-inflammatory responses to this ligand may, in a non-pathological setting, promote tissue repair, similar to the role of Mincle in sterile inflammation. In a further similarity to Mincle, MDL-1 also possesses a pathogen-derived ligand. MDL-1 binds Dengue virus, which induces the phosphorylation of the DAP-12 ITAM and causes TNF-α production by MØ (94). Blockade of the MDL-1-Dengue virus interaction in a mouse infection model suppresses the secretion of pro-inflammatory cytokines but not IFN-α by MØ and prevents the severe inflammatory reaction that is characteristic of Dengue disease (94). Thus, recognition of Dengue virus by MDL-1 could to be a major driver of the hemorrhagic and plasma leakage shock syndrome characteristic of lethal infection in humans (94).
3. ITIM-based CLRs
3.1 Human DCIR (Hs: CLEC4A), Mouse Dcir1 (Mm: Clec4a2) and Dcir2 (Mm: Clec4a4)
DCIR shows specificity for mannose and fucose-based glycans (97, 98). Human DCIR is expressed in monocytes, monocyte-derived DC, MØ, granulocytes, B cells and DCs (97, 99). There are four homologues in mouse (Dcir1 to 4), but only Dcir1 and Dcir2 bear the ITIM sequence (79). Dcir1 is expressed in B cells, monocytes/MØ, and dendritic cells (100, 101). Mouse Dcir2 is recognized by the monoclonal antibody 33D1, which stains mouse CD8− DCs (102, 103). Antigens coupled to 33D1 are directed to late endosome-lysosomal compartments of CD8− DCs and efficiently presented by MHC class II molecules (103). In humans, targeting of antigens to DCIR in vitro allows crosspresentation to CD8+ T cells by different human DC subsets including Langerhans cells (LC), blood myeloid DCs and plasmacytoid DCs (99).
The ligands for DCIR receptors are not characterized although human DCIR has been reported to bind HIV-1 (104). Signaling through the ITIM, however, has been studied. A phosphotyrosine peptide encompassing the human DCIR ITIM associates with phosphorylated SHP-1 and nonphosphorylated SHP-2 (105). Ligation of human DCIR with HIV-1 in a B cell line co-expressing CD4 led to activation of SHP-1, SHP-2, but also Syk and Src kinases, which together controlled HIV-1 internalization and led to PKC-α, p38 and Erk1/2 activation (106). More typically, ITIM signaling inhibits that which is initiated by ITAMs. Consistent with that notion, Dcir1 inhibits BCR signaling upon co-ligation of BCR and a chimeric receptor containing extracellular FcγR-IIB and intracellular tail of Dcir1 (100) (Fig. 4). A negative regulatory role for Dcir1 in vivo is inferred from the phenotype of deficient mice, which have increased numbers of DC and develop autoimmunity (101). In vitro, DCIR1-deficient bone marrow cells display a higher degree of STAT5 phosphorylation in response to GM-CSF and differentiate more efficiently into DCs, suggesting that one of the functions of DCIR1 may be to limit DC expansion (101) (Fig. 4).
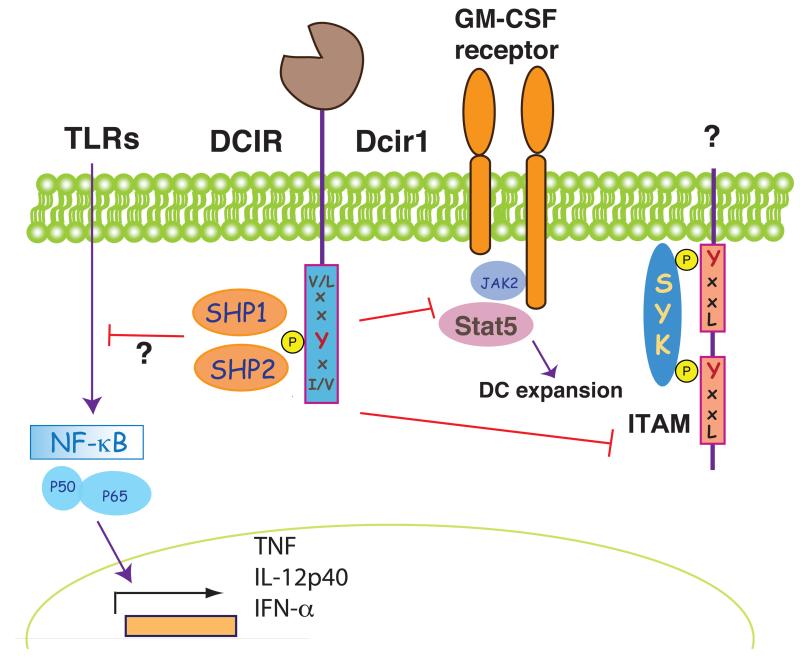
Figure 4. DCIR and Dcir1 as model ITIM-containing CLRs.
The phosphorylation of the tyrosine in the ITIM domain allows binding of SHP1 and SHP-2 phosphatases. Human DCIR inhibits signaling to NF-κB and modulates the pattern of genes activated by TLR although it is unclear whether this is a direct effect. Dcir1 also inhibits ITAM signaling by unidentified receptors and impairs recruitment and activation of Stat5 by the GM-CSF receptor, limiting DC expansion.
Crosslinking of DCIR with antibodies inhibits TLR8-driven production of IL-12 and TNF-α by myeloid DCs and TLR9-induced IFN-α production by human plasmacytoid DCs (107, 108) (Fig. 4). The mechanism of inhibition is not likely to be attributable to dephosphorylation, as TLR signaling does not trigger a phosphotyrosine cascade. The negative effects of DCIR ligation could therefore imply an indirect effect of ITIM signaling on the activity of Syk-coupled receptors that synergize with TLRs (Fig. 4).
DCAR and DCIR share 91% amino-acid sequence identity in the CTLD region. It is likely that they bind the same ligand and form a myeloid paired activating/inhibitory receptor complex in similar fashion to the NK cell paired receptors. Fine regulation of the outcome of receptor engagement in this system can be achieved by controlling of the relative expression of the activating and inhibitory partners. In this regard, DCIR expression is downregulated in response to signals inducing DC activation, such as CD40 ligand, LPS, TNF-α or TLR9-triggering (97, 107). The phenomenon of activation-induced downregulation is also seen for other inhibitory receptors (see below).
3.2 MICL/DCAL-2 (Hs: CLEC12A; Mm: Clec12a)
Human MICL (Myeloid inhibitory C-type lectin receptor, also known as DCAL-2, KLRL-1 or CLL-1) is expressed in granulocytes, monocytes, MØ and DCs (109-113). Mouse MICL is expressed in myeloid cells, but also in B cells and CD8+ T cells in peripheral blood, and NK cells in the bone marrow (113, 114). The endocytic capacity of MICL and its high expression on myeloid cells have led to its use as an antigen targeting receptor (113). Similar to other inhibitory CLRs, expression is down-regulated following activation with some TLR agonists (109, 114). MICL ligands have not been identified, although self ligands in mouse bone marrow, thymus, heart, spleen and kidney have been detected (114).
Following activation, the phosphorylated ITIM of MICL recruits SHP-1 and SHP-2, but not SHIP-1 (109, 110). A chimera comprising the Dectin-1 ectodomain fused to part of the MICL stalk, transmembrane domain and cytoplasmic tail inhibits TNF-α production in response to zymosan in a MØ cell line co-expressing wild type Dectin-1 (109). This shows that MICL could potentially act as an inhibitory receptor for Syk-coupled CLRs. However, MICL ligation with antibodies has been reported to result in p38 MAPK and ERK phosphorylation, upregulation of CCR7 and production of low levels of IL-6, IL-10, TNF-α and MIP-3β in human cells (112). In contrast, others have not seen cell activation after antibody crosslinking of mouse MICL (113, 114) Anti-MICL also has differing effects when combined with other stimuli, variably inhibiting IL-12 and TNF-α production induced by LPS and zymosan but increasing cytokine production induced by CD40L (112). Although antibody crosslinking differs from ligand stimulation, these results suggest that the effect of ITIM-bearing CLRs may not always be as simple as recruitment of phosphatases and inhibition of Syk signaling (see below).
3.3 CLEC12B/MØ Antigen H (Hs: CLEC12B; Mm: Clec12b)
MØ antigen H or CLEC12B was identified in a screen for receptors similar to NKG2D, an NK and T cell activatory CLR that recognizes cell surface ligands upregulated following DNA damage (115). However, CLEC12B is not expressed on T or NK cells but on MØ and does not bind NKG2D ligands. CLEC12B contains an ITIM that is able to recruit SHP-1 and SHP-2 and inhibit ITAM signaling in an experimental setup (115). Its ligand specificity is unknown.
3.4. Mouse Ly49Q (Mm: Klra17)
The Ly49 family is expressed predominantly in T or NK cells, but Ly49Q is absent from NK cells and, instead, expressed in Ly6C/G+ myeloid precursors, immature monocytes and plasmacytoid DCs (116-118). Ly49Q expression decreases during monocyte maturation but, in both monocytes and GM-CSF bone marrow-derived DC, can be induced following activation with IFN-γ and IFN-α, respectively (116, 118). In contrast, expression increases during plasmacytoid DC differentiation (117). As for other members of the Ly49 family, Ly49Q binds to MHC class I (119).
Antibody-mediated crosslinking of Ly49Q on activated MØ induces phosphorylation of the ITIM and mediates recruitment of SHP-1 and SHP2, protein phosphorylation and cytoskeletal rearrangements (116). The recruitment of SHPs may result in inhibition of ITAM or TLR-induced signaling, as discussed above for DCIR ligation. In contrast, the class I MHC-Ly49Q interaction in cis enhances plasmacytoid DC cytokine production in response to TLR9 and TLR7 agonists by regulating endosomal dynamics (120, 121). Ly49Q-deficient plasmacytoid DC produce lower levels of IL-12 p70 and type I IFN in response to TLR7 and TLR9 stimulation and Ly49Q-deficient mice infected with mouse cytomegalovirus show a limited TLR9-driven anti-viral response (121).
In neutrophils, Ly49Q signals through SHP-1 in the steady state to inhibit Src and PI3 kinases and prevent focal adhesion complex formation, thereby decreasing neutrophil adhesion (122). In contrast, in the presence of inflammatory stimuli, Ly49Q recruits SHP-2 and Src to membrane rafts, which results in rapid neutrophil polarization and tissue infiltration (122). Thus, Ly49Q impacts morphology and migratory capacity through the spatiotemporal regulation of membrane rafts and raft-associated signaling molecules (122). Ly49Q illustrates how an ITIM coupled receptor can indirectly affect the action of other receptors, resulting in an activation readout. Moreover, it also highlights the balance between tonically bound SHP-1, which plays a major inhibitory role in signaling, versus induced association with SHP-2, which can act as a positive regulator (122). Thus, ITIM signaling plays a complex role in regulating myeloid cell function, with both activatory and downregulatory outcomes that depend on cellular context and the activity of other receptors. As discussed above, similar complexity has been noted for ITAM-coupled receptors.
4. ITAM/ITIM-independent CLRs
4.1 Mannose Receptor/CD206 (Hs: MRC1; Mm: Mrc1)
Despite being sometimes called “MØ mannose receptor”, MR expression is not restricted to MØ, and MR is also found on human monocyte-derived DCs, a subset of mouse DCs, and in some epithelial, mesangial and smooth muscle cells (123). MR contains eight CRDs, with CRDs 4-8 mediating binding to high mannose, fucose, N-acetylglucosamine and sulfated glycan structures found on the surface of many microorganisms and self molecules (124). Like other CLRs, MR has been used as a candidate for antibody-mediated antigen delivery to DCs but a natural role for MR in presentation of mannose-bearing ligands has been uncovered using the model antigen, ovalbumin (OVA). MR deficiency selectively impairs the crosspresentation on MHC class I but not the presentation by MHC class II of soluble OVA offered to DC (125). This is because OVA bound to MR is routed to a specialized EEA1+ Rab5+ endosomal compartment that favors cross-presentation whereas OVA taken up independently of MR (e.g., by pinocytosis) is directed towards late endosomes/lysosomes, which are not propitious for MHC class I crosspresentation but allow for MHC class II presentation (61). OVA crosspresentation additionally depends on polyubiquitination of a single lysine residue in the tail of MR, which somehow facilitates antigen translocation to the cytosol via recruitment of the endoplasmic reticulum protein p97 (126).
MR has a tyrosine-based motif in its intracellular tail that promotes the delivery of mannosylated ligands to early endosomes (127). Mutation of the tyrosine reduces but does not completely block endocytosis (128). MR has also been reported to mediate phagocytosis, for example of Pneumocystis by human alveolar MØ, a process dependent on Cdc42 and RhoB activation and actin polymerization (129). However, when expressed in non-professional phagocytes (e.g., CHO cells), MR does not mediate phagocytosis of particulate ligands (130). These results raise the possibility that MR may not be able to directly signal to mobilize the actin cytoskeleton but, rather, facilitates phagocytosis by other receptors present in DC or MØ. In this regard, DC-SIGN can collaborate with MR in the uptake of Candida particles and both receptors are found in yeast-containing phagosomes (131).
The ability of MR to signal is not formally demonstrated but has been inferred from observations on how its engagement influences myeloid cell activity. Ligation of MR in immature monocyte-derived DC induced the production of IL-10 and IL-1Ra, leading to the suggestion that the receptor could induce an anti-inflammatory program upon interaction with ligands expressed on apoptotic cells (132). However, MR-deficient MØ make less TNF-α and more IL-10 than wild type controls in response to treatment with LPS and apoptotic cells (133). In addition, in a mouse model of glomerulonephritis, MR deficiency leads to reduced pathology associated with a decrease of MØ Fc-receptor mediated functions, including phagocytosis and oxidative burst activity (133). Therefore, an anti-inflammatory role of MR in self recognition is not clear. In contrast, such a role has been noted in response to microbial ligands. The MR-dependent uptake of unopsonized Pneumocystis by human alveolar MØ mentioned above is accompanied by IL-8 and MMP-9 production, but not IL-1β, IL-6 or TNF-α; blockade or silencing of MR leads to production of the inflammatory cytokines whereas ligation of MR reduces LPS-induced TNF-α release (134). ManLAM from mycobacteria also interacts with MR to promote PPARγ activation and prevent phagosome-lysosome fusion, both of which promote the intracellular survival of the Mycobacteria (135, 136). Thus, MR binding of certain pathogens may reflect immune subversion rather than host defense. In addition to pathogens, MR can recognize glycosylated allergens and contributes to the Th2 polarization bias induced by DC exposed to such allergens (137). In sum, MR engagement clearly affects myeloid cell properties but it remains unclear to what extent this reflects direct signaling by the receptor or indirect effects where MR favors signaling by other receptors at the cell surface or in endosomes.
4.2 DEC-205/CD205 (Hs: LY75; Mm: Ly75)
Mouse DEC-205 is expressed at high levels in CD8α+ DC and at lower levels in B cells, MØ, T cells, and granulocytes. Human DEC-205 is expressed widely. The intracellular domain of DEC-205 has a tyrosine-based internalization signal and a tri-acidic lysosomal targeting signal. Following ligation with antibodies, DEC-205 is internalized and targets a late endosomal/lysosomal compartment that allows the processing of cargo for MHC presentation (138). In the case of MHC class II, DEC-205 targeting results in a 100-fold increase in antigen presentation compared to targeting of MR (139). DEC-205 targeting also allows efficient delivery of antigens for crosspresentation by mouse CD8α+ DC (60) and has been proposed as a useful platform for the development of both prophylactic and therapeutic vaccines (140). DEC-205 possesses ten CRDs and IgG fusion proteins made with the paired CRDs 3+4 or 9+10 are able to bind to apoptotic cells (141). In addition, DEC-205 also mediates oxLDL uptake, similar to LOX-1 and other scavenger receptors (142). As for ligands derived from pathogens, DEC-205 binds to plasminogen activator (PLA) of Yersinia pestis and E. coli K12, a key molecule involved in infection of MØ (143). Interestingly, the blockade of PLA-DEC-205 interaction with antibodies prevented infection of alveolar MØ and reduced the dissemination of Y. pestis in mice (143). Thus, binding to PLA does not appear to reflect a function of DEC-205 in host defense but, rather, exploitation of the receptor by the pathogen, as noted above for MR.
4.3 Human DC-SIGN (Hs: CD209)
Human DC-specific ICAM-grabbing non-integrin (DC-SIGN) is expressed predominantly by human myeloid DCs. Its single CRD has a highly conserved Glu-Pro-Asn (EPN) motif that mediates binding to internal mannose branched structures and terminal di-mannoses, and also fucose-bearing glycans (Lewis (Le) antigens: LeX, LeY, LeA, LeB) (144, 145). DC-SIGN ligands are broadly expressed in pathogen, allergen and self molecules (see Table 1).
DC-SIGN bears di-leucine, tri-acidic and tyrosine-based motifs for internalization in its cytoplasmic tail. Similar to other CLRs, DC-SIGN mediates endocytosis of soluble cargo for antigen presentation and has been used as an antigen targeting receptor for DCs (146). DC-SIGN has additionally been found in phagosomes, suggesting a role in particle uptake (147). Indeed, when expressed in non-phagocytic HeLa cells, DC-SIGN promotes uptake of E. coli independent of the intracellular tyrosine (148), possibly through activation of Rho-GTPases (149). Ligation of DC-SIGN with antibodies re-localizes it to late endocytic and lysosomal compartments (146). However, it is retained in early endosomes upon uptake of HIV-1 (150), suggesting that cargo-dependent signaling dictates endocytic fate.
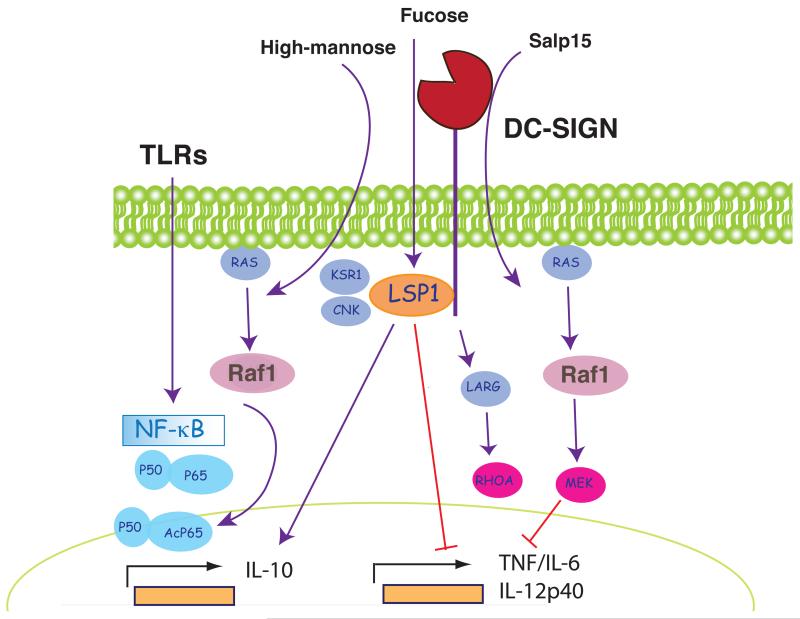
Apart from endocytosis, DC-SIGN also signals for modulation of gene transcription. Crosslinking of DC-SIGN with antibodies induces ERK1/2 and Akt phosphorylation, but not p38 MAPK activation, and potentiates IL-10 gene transcription (151). However, the pathways stimulated by actual DC-SIGN ligands appear to be different from those elicited by antibody crosslinking. DC-SIGN associates with the proteins LSP1, KSR1 and CNK in a tyrosine-independent fashion (152) (Fig. 5). Upon binding of ManLAM from M. tuberculosis, this “signalosome” promotes activation of LARG and RHOA, which act as upstream activators of Raf-1 (149, 152, 153) (Fig. 5). As noted above for Dectin-1 signaling, activation of Raf-1 leads to phosphorylation of the NF-κB p65 subunit on Ser276. This phosphorylation allows binding of the histone acetyl-transferases CREB-binding protein (CBP) and p300 to p65, resulting in acetylation of p65 (153) (Fig. 5). Acetylation of p65 increases its DNA binding affinity and enhances transcriptional output, particularly from the IL-8, IL-12, IL-6 and IL-10 promoters (152, 153). It is important to note that DC-SIGN cannot activate NF-κB by itself and only modulates p65 activity induced by another receptor. Thus, a signature of DC-SIGN engagement in human DC is failure to induce cytokines but marked potentiation of some TLR-induced cytokines such as IL-10 (151-153).
Figure 5. DC-SIGN as a model ITAM-ITIM-independent receptor.
Binding of different ligands results in recruitment of different effectors to the tail of DC-SIGN. High mannose ligands such as ManLAM promote binding of LSP1, KSR1 and CNK in a tyrosine-independent fashion. This complex triggers the small GTPase Ras, which activates Raf-1. Raf-1 activation culminates in acetylation of NF-κB p65 and alters the pattern of gene expression induced through TLR signaling. Fucose-based ligands promote binding of LSP1 only, which acts in a Raf-1-independent fashion to enhance TLR-dependent synthesis of IL-10 and to decrease production of pro-inflammatory cytokines. Finally, another DC-SIGN agonist, Salp15, activates a Raf-1/MEK pathway that promotes TNF and IL-6 mRNA decay and decreases production of IL-12 by impairing chromatin remodeling.
The nature of the ligand appears to regulate DC-SIGN signaling via Raf-1. High mannose ligands from Candida or HIV-1 behave like ManLAM and induce Raf-1 activation. In contrast, fucose-based ligands, such as Lewis antigens in the LPS of Helicobacter pylori, cause dissociation of the “signalosome” leaving DC-SIGN associated only with LSP1 (Fig. 5). LSP1-dependent signals generated in response to fucose-based DC-SIGN ligands synergize with signals from TLRs and result in enhanced production of IL-10 but decreased IL-12 and IL-6 by human DC independent of Raf-1 (152). Salp15, an immunomodulatory protein produced by the salivary glands of Ixodes scapularis ticks, also contains a ligand for DC-SIGN. Salp15 inhibits TLR2- and TLR4-induced production of IL-12, IL-6 and TNF-α. Similar to ManLAM, modulation of TLR-responses is dependent on the activation of Raf-1 (154) (Fig. 5). However, in contrast to the activation of Raf-1 pathway by DC-SIGN interaction with ManLAM, Salp15-induced signaling leads to activation of MEK, and not to phosphorylation or acetylation of p65 NF-κB. MEK signaling enhances degradation of IL-6 and TNF-α mRNAs, while decreased production of IL-12 results from impaired nucleosome remodeling at the IL-12 p35 promoter (154) (Fig. 5). In this way, Salp15 acts as an immunosuppressive molecule in tick saliva that can facilitate transmission of tick-borne pathogens such as Borrelia burgdorferi. In sum, signalling by DC-SIGN, whether for endocytosis or modulation of gene expression, is markedly dependent on the nature of the ligand. Whether the two outcomes are connected, namely whether different signalosome modules assemble at different sub-cellular locations, remains to be determined.
4.4 Mouse SIGNR1 (Mm: Cd209b)
SIGNR1 is encoded by the CD209b gene and is expressed in MØ and lamina propria DCs (155-157). Confusingly, mouse DC-SIGN (encoded by the CD209a gene) is sometimes also called SIGN-R1. Not much is known about the function of mouse DC-SIGN although its recent identification as a marker of mouse monocyte-derived DC is likely to spark renewed interest in the receptor (158).
SIGNR1 binds complex mannose and fucose structures in bacteria and fungi (64, 155, 156). SIGNR1 has intracellular tri-acidic motifs and a tyrosine outside a known consensus motif. SIGNR1 cooperates with Dectin-1 in the non-opsonic recognition of zymosan by MØ and contributes to the production of TNF-α (156). The latter may be due to potentiation of TLR signaling, as SIGNR1 associates physically with the TLR4-MD2 complex and, in transfectants, enhances TLR4 oligomerization and the degradation of IκB driven by LPS (159). In addition, SIGNR1 can also potentiate production of ROS driven by Dectin-1 / Syk signaling in response to Candida albicans (160). Therefore, SIGNR1 appears widely involved in immune responses to pathogens in cooperation with other innate immune receptors. As regards to direct signaling for gene expression, antibody-mediated crosslinking of SIGNR1 on peritoneal MØ increases phosphorylation of IKK, leading to NF-κB activation and production of IL-12 and TNF-α (161). However, SIGNR1 also has an anti-inflammatory role and contributes to IL-10 production by MØ in response to mycobacterial ManLAM, which also induces SOCS1 via a pathway sensitive to Raf-1 and Syk chemical inhibitors (162, 163). Moreover, in a model of anaphylaxis, mannoside-bearing antigen targets lamina propria DCs expressing SIGNR1 and induces the expression of IL-10, which promotes the generation of IL-10-secreting T cells that suppress the reaction (157). Thus, targeting SIGNR1 in lamina propria DC could potentially be used as a strategy to prevent food allergies.
4.5 Langerin (Hs: CD207; Mm: Cd207)
Human Langerin is expressed exclusively in LC, whereas mouse Langerin is additionally expressed by CD8α+ DC and many CD103+ CD11b− DC (164, 165). The Langerin CRD has affinity for high mannose, fucose (LeY, LeB), N-acetyl glucosamine and β-glucans, which allows Langerin to bind many microorganisms, including mycobacteria, fungi and HIV-1, as well as self ligands exposed by apoptotic cells (166-168).
Langerin recycles through early endosomal compartments and endocytosis is regulated by a proline-rich motif in the receptor tail (164). Langerin expression in heterologous cells promotes the formation of Birbeck granules, a Rab11+ recycling endosomal compartment characteristic of LC (164). Langerin and CD1a co-traffic through Birbeck granules and Langerin facilitates presentation of CD1a-restricted Mycobacterium leprae antigens to T cells by LC (168). Langerin can also bind and promote internalization of intact HIV-1 particles, leading to virus degradation (167) (see below). In summary, ligand-dependent Langerin signaling appears to control endosomal trafficking in LC; whether it additionally regulates DC activation remains unknown.
4.6 MGL (Hs: CLEC10A), Mgl1 (Mm: Clec10a) and Mgl2 (Mm: Mgl2)
Human macrophage galactose C-type lectin (MGL) is expressed in subsets of DCs and MØ and is used as a marker of alternative MØ activation because it is induced in response to IL-4 and/or IL-13 (169) (170). The presence of a QPD sequence in the CRD confers MGLs with specificity for galactose or its derivative, N-acetyl-galactosamine (GalNAc). MGL and Mgl2 recognize glycans containing GalNAc moieties whereas Mgl1 binds preferentially to galactose-containing LeX and LeA glycans (171). These glycans can be present in pathogens such as Schistosoma, filoviruses or influenza virus, but are also found as O-linked glycosylation structures on self proteins. These include neo-antigens in tumor cells (Tn-antigen, Tf-antigen, core 2), CD45 in T cells, gangliosides, sialoadhesin or molecules exposed in apoptotic bodies (4).
The intracellular tail is similar in the three MGLs from the two species and displays a tyrosine-based and a di-leucine endocytosis motif. MGL mediates uptake of antigens in a tyrosine-dependent manner and targets them to the phagolysosomal compartment (172). GalNAc-coupled antigens bind to Mgl2-expressing mouse DC and are directed to both early and late endosomal compartments, resulting in both presentation to CD4+ T cells and crosspresentation to CD8+ T cells (173, 174). MHC class II presentation of the GalNAc conjugates is Mgl2-dependent although this has not been established for crosspresentation (173, 174).
Signaling by MGLs is poorly understood. In a few mouse models, Mgl1 and Mgl2 engagement is anti-inflammatory. For example, in inflammatory bowel disease induced using dextran sulfate, which damages gut epithelium, the recognition of carbohydrates on invading gut commensal bacteria (including Streptococcus spp and Lactobacillus spp) by Mgl1 induces IL-10 production by lamina propria MØ (175). Notably, Mgl1-deficient mice show more severe inflammation than wild-type controls (175). Apoptotic bodies, which often are anti-inflammatory, contain ligands for Mgl1 and, in the absence of the receptor, there is deficient removal of such bodies and early death in the developing embryo (176). In summary, MGLs may play an anti-inflammatory role in response to exogenous or self ligands but, as is often the case with myeloid CLRs, to what extent this involves direct signaling by the receptors remains to be established.
4.7 CLEC-1 (Hs: CLEC1A; Mm: Clec1a)
CLEC-1 is expressed at the cell surface on DCs and endothelial cells, similar to LOX-1 (40, 177). An arginine residue in close apposition with the predicted transmembrane region is found in both mouse and human CLEC-1, suggesting that it could mediate association with a signaling adaptor such as FcRγ chain. The requirement for association with an adaptor could explain why CLEC-1 is retained intracellularly when expressed into non-myeloid cells (40, 177). CLEC-1 has also a conserved intracellular tyrosine but it is not found within a consensus motif and may be irrelevant for signaling. CLEC-1 ligands are unknown.
4.8 Human DCAL-1 (Hs: CLECL1)
DC-associated lectin-1 (DCAL-1) expression is restricted to human monocyte-derived DCs and B cells (178), with no mouse ortholog identified. Using a DCAL-1 fusion protein, a ligand was detected on CD4+ CD45RA+ T cells (178).
DCAL-1 has a long intracellular tail but no clear signaling motif: it contains two tyrosines, several serines and threonines, and a lysine in close apposition to the transmembrane region. Antibody-mediated crosslinking of DCAL-1 on monocyte-derived DC induced phosphorylation of JNK and p44/42 MAP kinase, which resulted in increased HLA-DR expression without affecting levels of costimulatory molecules (179). Interestingly, protein phosphorylation was not found in tonsillar B cells upon DCAL-1 ligation (179), suggesting possible differences in adaptors or components of the signaling pathway in lymphoid versus myeloid cells, as discussed above for other CLRs.
4.9. MCL (Hs: CLEC4D; Mm: Clec4d)
Macrophage-restricted C-type lectin (MCL) is expressed in resting MØ in both mouse and human (180, 181). The ligands for this receptor are not known. MCL is encoded in the Dectin-2 cluster of CLRs, all of which bear CRDs with classical EPN residues required for mannose binding. MCL conserves the calcium coordination sites but not the exact EPN motif, meaning that its glycan specificity is difficult to predict.
The intracellular tail of MCL is short and without a clear internalization motif. The receptor also lacks a positively charged residue in or next to the transmembrane domain. However, MCL is rapidly internalized following ligation with antibodies (181) suggesting that it might associate with a partner chain that signals for endocytosis.
4. 10. LOX-1 (Hs: OLR1; Mm: Olr1)
Lectin-like oxidized LDL receptor (LOX-1) is predominantly expressed on endothelial cells, where it has been extensively studied in the context of atherosclerosis. LOX-1 is also expressed in immature myeloid DCs, monocyte-derived DCs, monocytes, MØ and B cells (182). The classic ligand for LOX-1 is oxidized low-density lipoprotein (oxLDL) but oxidized lipids present in apoptotic or aged cells that resemble oxLDL can also bind LOX-1 (183). LOX-1 is additionally reported to bind heat shock proteins, which could also be exposed in apoptotic cells (184).
Mouse LOX-1 but not human bears a di-leucine motif in its intracellular tail. However, a novel DDL endocytosis motif was identified in human LOX-1 that can transfer endocytic activity when transplanted to other receptors (185). Additional signaling could be mediated via a tyrosine present in human LOX-1 but not conserved in mouse. LOX-1 mediates uptake of oxLDL and apoptotic/aged cells (183). In endothelium, LOX-1 induces RhoA and Rac1 activation and signals for ROS production. Moreover, engagement of LOX-1 by oxLDL in endothelium can trigger activation of NK-κB, resulting in upregulation of endothelin-1, MCP-1 and adhesion molecules (186). This effect of LOX-1 could possibly be mediated through association with a signaling adaptor.
The role of LOX-1 in DCs is not well characterized and it is not clear whether it can also signal to regulate gene expression in response to oxidized lipids. Similar to other CLRs from C-type lectin group V, LOX-1 is an endocytic receptor that can be targeted in mouse DCs for antigen crosspresentation and induction of CD8+ T cell responses (187). In addition, LOX-1 can bind HSP-70, which ferries antigens for cross-presentation (187). Blockade of LOX-1 induced on human monocyte-derived DC by treatment with type I IFN inhibits apoptotic cell uptake and decreases CD8+ T cell cross-priming against apoptotic cell-associated antigens (188). In summary, the scavenger role of LOX-1 combined with its capacity to target the antigens to an adequate compartment makes it potentially an important player in crosspresentation of cell-associated antigens by DCs.
4.11. LSECtin (Hs: CLEC4G; Mm: Clec4g)
Liver sinusoidal endothelial cell lectin (LSECtin) is expressed in liver and lymph node sinusoidal endothelial cells, and also on monocyte-derived MØ, Kupffer cells and DC (189-191). The CRD is highly homologous to that of DC-SIGN, but LSECtin is restricted in binding to glycans containing terminal GlcNAcβ1-2Man disaccharides (192, 193). Such glycans are truncated complex N-linked glycosylation structures found on some pathogen molecules, including the glycoproteins of filoviruses and coronaviruses, and on self proteins such as CD44 (192, 194). LSECtin can negatively regulate T cells and limit T cell-driven pathology in a hepatitis model (191). The intracellular tail of LSECtin contains a tyrosine and a di-acidic motif, both of which regulate endocytosis induced by antibody-mediated crosslinking (190) and may contribute to virus uptake (195). Apart from its endocytic capacity, little is known about the signaling capacity of LSECtin.
Myeloid CLRs as pathogen receptors
Many pathogens possess atypical glycans that serve as ligands for myeloid CLRs (Table 1). As described above, many myeloid CLRs possess cytoplasmic motifs that engage the endocytic and phagocytic machinery of the cell and promote the internalization of bound pathogens, in some cases leading to their degradation, as well as to antigen retrieval and presentation to T cells. Independent of endocytosis, some CLRs also signal to potentiate myeloid cell microbicidal activity and contribute to innate and adaptive immunity. As such, it is often thought that pathogen-binding CLRs act as PRRs and play a role in host defense from infection. Actual evidence for this notion has been slow in coming, in part because loss of a given CLR seldom increases susceptibility to infection. This is to be expected from the fact that pathogens are complex structures and the host response to infection involves the compensatory activities of many different innate immune receptors (196). Nevertheless, recent developments indicate that some myeloid CLRs and their associated signaling pathways do perform unique and non-redundant roles in protection from infection (5).
Perhaps the best example comes from the study of fungal infection. Fungal pathogens such as Candida albicans are recognized by multiple myeloid CLRs, including Dectin-1, Dectin-2, MR, DC-SIGN, SIGNR1 and Mincle, as well as TLRs, NLRs and other receptors. Nevertheless, ablation of CARD9 in mice is sufficient to abrogate both innate resistance and the induction of a Th17 but not a Th1 response to experimental Candida infection (25, 28). The anti-Candida Th17 response has emerged as critical for protection from mucocutaneous candidiasis in humans - patients with deficiencies in Th17-based immunity suffer from chronic versions of the disease (197). Interestingly, paucity of Th17 cells and chronic mucocutaneous candidiasis has also been found in one family with a mutation in CARD9 that leads to loss of protein expression (198), as well as in a family bearing a polymorphism in Dectin-1 that leads to truncation of the extracellular domain (199). The Dectin-1 polymorphism is also associated with invasive fungal infections following stem cell transplantation (200-202). These findings indicate that, at least in some instances, Dectin-1 and CARD9 are indispensable for Th17-dependent human resistance to fungal disease, underscoring the importance of CLR signaling in host protection.
Despite the fungal example, in some instances CLR binding appears to benefit the pathogen rather than the host. A case in point is the exploitation of myeloid CLRs by HIV-1. HIV-1 gp120 binds to DC-SIGN, which leads to endocytosis and diversion of some of the virions to an early endosomal compartment where they are protected from degradation (150). In addition, HIV-1 induces the formation of a DC-SIGN/LARG/RhoA complex that promotes actin cytoskeleton mobilization and formation of a synapse with T cells, which facilitates subsequent transmission of the preserved intact virus (149). HIV-1 gp120 also targets BDCA-2 in plasmacytoid DC and interferes with TLR9- but not TLR7-mediated responses (80). Thus, by targeting myeloid CLRs, HIV-1 increases its infectivity for T cells and subverts immunity. However, some CLR-HIV-1 interactions can be protective to the host: Langerin-mediated uptake of HIV-1 by LCs directs the virus to Birbeck granules and causes its degradation (167).
A final example is provided by Mycobacterium tuberculosis, which is recognized by MR, DC-SIGN, Dectin-1 and Mincle, in addition to members of the TLR and NLR families (91, 147, 203-206). CLR interactions with M. tuberculosis show feature of both host defense and exploitation by the pathogen. For example, engagement of MR by ManLAM can help M. tuberculosis reach its initial phagosomal niche, thereby enhancing survival in human MØ (203). In addition, DC-SIGN signaling via Raf-1 enhances production of IL-10 that dampens immunity against the pathogen (153). In contrast, the CARD9 pathway promotes pathogen elimination and, in fact, is non-redundant for resistance to Mycobacteria in mice (207). However, Dectin-1 deficient mice showed little reduction in bacterial burden (208) and mice deficient in SIGN-R3 (which may also signal via CARD9) display only slightly elevated susceptibility to infection with the pathogen (20). Mincle might therefore be the dominant CLR in CARD9-dependent resistance to M. tuberculosis, consistent with its role in sensing TDM (91, 92). In sum, host CLR – pathogen interactions can be beneficial to either party and the function of CLRs in host defense needs to be assessed on an individual basis and cannot be inferred from the capacity of a given CLR to bind to a pathogen.
Myeloid CLRs as receptors for self ligands
Much of the biology of myeloid CLRs may have little to do with infection and is, instead, related to the role of myeloid cells in maintaining homeostasis in the steady state. Indeed, most CLRs, even those that recognize pathogens, have self ligands and function in cell adhesion, migration and intercellular communication. For example, DC-SIGN expressed on DC binds ICAM2, which regulates traffic through endothelium, to ICAM-3, which favors interactions with T cells, or to CEACAM-1 and Mac-1 in neutrophils, which promotes DC activation (209). The endocytic activity of MR is important for clearance of self glycans, as MR-deficient mice have marked increases in circulating levels of glycoproteins (210). In addition, as highlighted throughout this review, some CLRs recognize apoptotic and necrotic cells or their products such as oxidized lipids, heat shock proteins or ribonucleoproteins. These CLRs may serve to detect cell damage and play a role in tissue clearance and repair. Finally, myeloid CLRs, much like receptors on NK cells and innate lymphocytes, can recognize alterations in self that could be indicative of abnormality such as cell transformation. For example, LeX and LeY antigens detected by DC-SIGN are rare in normal colon epithelium, but are found on carcinoembryonic antigen expressed in colorectal cancer cells (211). MGL binds O-linked GalNAc residues that are expressed as neo-antigens in tumor cells (212). Podoplanin, the self ligand for CLEC-2, is expressed in some tumor cells (213). Therefore, it is possible that CLRs are involved in tumor immune surveillance by myeloid cells, if such a thing exists. However, as is the case with pathogens, the interaction between CLRs and tumors need not be protective to the host and needs to be also seen from the perspective of the tumor. In this regard, pro-inflammatory signaling by CLRs could favor tumor angiogenesis and metastasis, whereas anti-inflammatory signaling by CLRs such as DC-SIGN or MGL could contribute to tumor-dependent immune suppression.
Conclusions
Myeloid CLRs have multiple roles that critically depend on their signaling activity. These can involve gene regulation events (e.g., induction of pro-inflammatory cytokines) or transcription-independent outcomes such as endocytosis and phagocytosis, synthesis of microbicidal effector molecules (e.g., ROS), inflammasome activation or changes in cell adhesion and migration. The outcome of CLR signaling depends not only on the receptor in question but also on the nature of the ligand, ligand architecture and density, rates of receptor internalization and trafficking to distinct intracellular compartments. As such, myeloid CLRs are a versatile toolbox used by a plastic group of cells to carry out a multitude of jobs in host defense and maintenance of homeostasis. The study of myeloid CLRs is hampered by their sheer diversity and the range of signaling strategies that they employ. Nevertheless, some common principles of CLR signaling and function are beginning to emerge. These include the reliance on di-leucine, tri-acidic or tyrosine-based motifs for endocytosis, the capacity to recycle through the early endocytic pathway vs. target late endosomal degradative compartments, the ability to mobilize the actin cytoskeleton and promote migration or phagocytosis, the use of Syk, SHPs or Raf-1 as signaling nodes to induce or modulate gene transcription and, finally, the ability to cooperate positively or negatively with other receptors in regulating myeloid cell function. Much remains to be understood about myeloid CLRs and the continued study of their signaling properties will help clarify which receptors are sufficient, which are necessary and which are otherwise opposing or modulating myeloid cell activation in infectious and non-infectious situations. In turn, this will lead to more refined applications in vaccination and immunotherapy, as well as help understand the role of myeloid cells in innate immunity, pathology and homeostasis.
Acknowledgements
We are grateful to Fabiola Osorio, Diego Mourão-Sá and Angel L. Corbí for critical review of the manuscript. Work in the CRS laboratory is funded by Cancer Research UK, a prize from Fondation Bettencourt-Schueller, and a grant from the European Research Council (ERC Advanced Researcher Grant AdG-2010-268670). DS is the recipient of a Ramón y Cajal fellowship (RYC-2009-04235) from Spanish Ministry of Innovation and Science. Work in the DS laboratory is funded by Fundación Centro Nacional de Investigaciones Cardiovasculares “Carlos III” (CNIC), and grants from the Spanish Science and Innovation Ministry (SAF-2010-15120) and from the European Research Council (ERC Starting Independent Researcher Grant 2010, ERC-2010-StG 260414).
Abbreviations
- CLR
C-type lectin receptor
- CRD
Carbohydrate recognition domain
- CTLD
C-type lectin domain
- DC
Dendritic cell
- FcRγ
Crystalizable fragment receptor γ
- GalNac
N-acetyl-galactosamine
- ITAM
Immunoreceptor tyrosine-based inhibitory motif
- ITIM
Immunoreceptor tyrosine-based inhibitory motif
- Le
Lewis antigen
- LC
Langerhans cell
- ManLAM
Mannosylated lipoarabinomannan
- MØ
Macrophage
- PRR
Pattern recognition receptor
- ROS
Reactive oxygen species
- TDM
Trehalose-6,6′- dimycolate
Footnotes
Gene names for each CLR are indicated in parentheses throughout this review. See also Table I.
References
- 1.Drickamer K. C-type lectin-like domains. Curr Opin Struc Biol. 1999;9:585–90. doi: 10.1016/s0959-440x(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 2.Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 3.Janeway CA. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nat Immunol. 2006;7:1258–65. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- 5.Osorio F, Reis e Sousa C. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity. 2011;34:651–64. doi: 10.1016/j.immuni.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, Reis e Sousa C. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–17. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Mócsai A, Ruland J, Tybulewicz VLJ. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geijtenbeek T, Gringhuis S. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–79. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, Wong SY. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol. 2002;169:3876–82. doi: 10.4049/jimmunol.169.7.3876. [DOI] [PubMed] [Google Scholar]
- 10.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–30. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Willment JA, Marshall ASJ, Reid DM, Williams DL, Wong SYC, Gordon S, Brown GD. The human beta-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur J Immunol. 2005;35:1539–47. doi: 10.1002/eji.200425725. [DOI] [PubMed] [Google Scholar]
- 12.Olynych TJ, Jakeman DL, Marshall JS. Fungal zymosan induces leukotriene production by human mast cells through a dectin-1-dependent mechanism. J Allergy Clin Immunol. 2006;118:837–43. doi: 10.1016/j.jaci.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 14.Palma AS, Feizi T, Zhang Y, Stoll MS, Lawson AM, Diaz-Rodriguez E, Campanero-Rhodes MA, Costa J, Gordon S, Brown GD, Chai W. Ligands for the beta-glucan receptor, Dectin-1, assigned using “designer” microarrays of oligosaccharide probes (neoglycolipids) generated from glucan polysaccharides. J Biol Chem. 2006;281:5771–9. doi: 10.1074/jbc.M511461200. [DOI] [PubMed] [Google Scholar]
- 15.Ariizumi K, Shen GL, Shikano S, Xu S, Ritter R, Kumamoto T, Edelbaum D, Morita A, Bergstresser PR, Takashima A. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J Biol Chem. 2000;275:20157–67. doi: 10.1074/jbc.M909512199. [DOI] [PubMed] [Google Scholar]
- 16.Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106:2543–50. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki-Inoue K, Fuller GLJ, García A, Eble JA, Pöhlmann S, Inoue O, Gartner TK, Hughan SC, Pearce AC, Laing GD, Theakston RDG, Schweighoffer E, Zitzmann N, Morita T, Tybulewicz VLJ, Ozaki Y, Watson SP. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–9. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 18.Huysamen C, Willment JA, Dennehy KM, Brown GD. CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J Biol Chem. 2008;283:16693–701. doi: 10.1074/jbc.M709923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sancho D, Joffre O, Keller A, Rogers N, Martínez D, Hernanz-Falcón P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanne A, Ma B, Boudou F, Tailleux L, Botella H, Badell E, Levillain F, Taylor ME, Drickamer K, Nigou J, Dobos KM, Puzo G, Vestweber D, Wild MK, Marcinko M, Sobieszczuk P, Stewart L, Lebus D, Gicquel B, Neyrolles O. A murine DC-SIGN homologue contributes to early host defense against Mycobacterium tuberculosis. J Exp Med. 2009;206:2205–20. doi: 10.1084/jem.20090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu S, Huo J, Gunawan M, Su I-H, Lam K-P. Activated dectin-1 localizes to lipid raft microdomains for signaling and activation of phagocytosis and cytokine production in dendritic cells. J Biol Chem. 2009;284:22005–11. doi: 10.1074/jbc.M109.009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosas M, Liddiard K, Kimberg M, Faro-Trindade I, McDonald JU, Williams DL, Brown GD, Taylor PR. The induction of inflammation by dectin-1 in vivo is dependent on myeloid cell programming and the progression of phagocytosis. J Immunol. 2008;181:3549–57. doi: 10.4049/jimmunol.181.5.3549. [DOI] [PubMed] [Google Scholar]
- 23.Hernanz-Falcón P, Joffre O, Williams DL, Reis e Sousa C. Internalization of Dectin-1 terminates induction of inflammatory responses. Eur J Immunol. 2009;39:507–13. doi: 10.1002/eji.200838687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodridge HS, Shimada T, Wolf AJ, Hsu Y-MS, Becker CA, Lin X, Underhill DM. Differential use of CARD9 by Dectin-1 in macrophages and dendritic cells. J Immunol. 2009;182:1146–54. doi: 10.4049/jimmunol.182.2.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gross O, Gewies A, Finger K, Schäfer M, Sparwasser T, Peschel C, Förster I, Ruland J. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature. 2006;442:651–6. doi: 10.1038/nature04926. [DOI] [PubMed] [Google Scholar]
- 26.Gringhuis SI, Wevers BA, Kaptein TM, van Capel TMM, Theelen B, Boekhout T, de Jong EC, Geijtenbeek TBH. Selective C-Rel activation via Malt1 controls anti-fungal T(H)-17 immunity by dectin-1 and dectin-2. PLoS Pathog. 2011;7:e1001259. doi: 10.1371/journal.ppat.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gringhuis SI, Den Dunnen J, Litjens M, Van Der Vlist M, Wevers B, Bruijns SCM, Geijtenbeek TBH. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol. 2009;10:203–13. doi: 10.1038/ni.1692. [DOI] [PubMed] [Google Scholar]
- 28.Leibundgut-Landmann S, Gross O, Robinson M, Osorio F, Slack E, Tsoni S, Schweighoffer E, Tybulewicz V, Brown G, Ruland J, Reis E Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–8. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 29.Leibundgut-Landmann S, Osorio F, Brown G, Reis E Sousa C. Stimulation of dendritic cells via the Dectin-1 / Syk pathway allows priming of cytotoxic T cell responses. Blood. 2008;112:4971–80. doi: 10.1182/blood-2008-05-158469. [DOI] [PubMed] [Google Scholar]
- 30.Osorio F, Leibundgut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G, Reis e Sousa C. DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol. 2008;38:3274–81. doi: 10.1002/eji.200838950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, Bose N, Chan ASH, Magee AS, Danielson ME, Weiss A, Vasilakos JP, Underhill DM. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011;472:471–5. doi: 10.1038/nature10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slack EC, Robinson MJ, Hernanz-Falcón P, Brown GD, Williams DL, Schweighoffer E, Tybulewicz VL, Reis e Sousa C. Syk-dependent ERK activation regulates IL-2 and IL-10 production by DC stimulated with zymosan. Eur J Immunol. 2007;37:1600–12. doi: 10.1002/eji.200636830. [DOI] [PubMed] [Google Scholar]
- 33.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178:3107–15. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 34.Xu S, Huo J, Lee K, Kurosaki T, Lam K. Phospholipase Cgamma 2 is critical for dectin-1-mediated Ca2+ flux and cytokine production in dendritic cells. J Biol Chem. 2009;284:7038–46. doi: 10.1074/jbc.M806650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tassi I, Cella M, Castro I, Gilfillan S, Khan WN, Colonna M. Requirement of phospholipase C-gamma2 (PLCgamma2) for Dectin-1-induced antigen presentation and induction of TH1/TH17 polarization. Eur J Immunol. 2009;39:1369–78. doi: 10.1002/eji.200839313. [DOI] [PubMed] [Google Scholar]
- 36.Herre J, Marshall ASJ, Caron E, Edwards AD, Williams DL, Schweighoffer E, Tybulewicz V, Reis e Sousa C, Gordon S, Brown GD. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood. 2004;104:4038–45. doi: 10.1182/blood-2004-03-1140. [DOI] [PubMed] [Google Scholar]
- 37.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschläger N, Endres S, Hartmann G, Tardivel A, Schweighoffer, Tybulewicz, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–36. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 38.Mencacci A, Bacci A, Cenci E, Montagnoli C, Fiorucci S, Casagrande A, Flavell RA, Bistoni F, Romani L. Interleukin 18 restores defective Th1 immunity to Candida albicans in caspase 1-deficient mice. Infect Immun. 2000;68:5126–31. doi: 10.1128/iai.68.9.5126-5131.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van de Veerdonk FL, Joosten LA, Devesa I, Mora-Montes HM, Kanneganti TD, Dinarello CA, van der Meer JW, Gow NA, Kullberg BJ, Netea MG. Bypassing pathogen-induced inflammasome activation for the regulation of interleukin-1beta production by the fungal pathogen Candida albicans. J Infect Dis. 2009;199:1087–96. doi: 10.1086/597274. [DOI] [PubMed] [Google Scholar]
- 40.Colonna M, Samaridis J, Angman L. Molecular characterization of two novel C-type lectin-like receptors, one of which is selectively expressed in human dendritic cells. Eur J Immunol. 2000;30:697–704. doi: 10.1002/1521-4141(200002)30:2<697::AID-IMMU697>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 41.Kerrigan AM, Dennehy KM, Mourão-Sá D, Faro-Trindade I, Willment JA, Taylor PR, Eble JA, Reis e Sousa C, Brown GD. CLEC-2 is a phagocytic activation receptor expressed on murine peripheral blood neutrophils. J Immunol. 2009;182:4150–7. doi: 10.4049/jimmunol.0802808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang T, Li L, Tang J, Li Y, Lin WY, Martin F, Grant D, Solloway M, Parker L, Ye W, Forrest W, Ghilardi N, Oravecz T, Platt KA, Rice DS, Hansen GM, Abuin A, Eberhart DE, Godowski P, Holt KH, Peterson A, Zambrowicz BP, de Sauvage FJ. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol. 2010;28:749–55. doi: 10.1038/nbt.1644. [DOI] [PubMed] [Google Scholar]
- 43.Mourão-Sá D, Robinson MJ, Zelenay S, Sancho D, Chakravarty P, Larsen R, Plantinga M, Van Rooijen N, Soares MP, Lambrecht B, Reis e Sousa C. CLEC-2 signaling via Syk in myeloid cells can regulate inflammatory responses. Eur J Immunol. 2011 doi: 10.1002/eji.201141641. (in press) [DOI] [PubMed] [Google Scholar]
- 44.Fuller GLJ, Williams JAE, Tomlinson MG, Eble JA, Hanna SL, Pöhlmann S, Suzuki-Inoue K, Ozaki Y, Watson SP, Pearce AC. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem. 2007;282:12397–409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, Yamazaki Y, Narimatsu H, Ozaki Y. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993–6001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 46.Chaipan C, Steffen I, Tsegaye TS, Bertram S, Glowacka I, Kato Y, Schmokel J, Munch J, Simmons G, Gerardy-Schahn R, Pohlmann S. Incorporation of podoplanin into HIV released from HEK-293T cells, but not PBMC, is required for efficient binding to the attachment factor CLEC-2. Retrovirology. 7:47. doi: 10.1186/1742-4690-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki-Inoue K, Inoue O, Ding G, Nishimura S, Hokamura K, Eto K, Kashiwagi H, Tomiyama Y, Yatomi Y, Umemura K, Shin Y, Hirashima M, Ozaki Y. Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J Biol Chem. 2010;285:24494–507. doi: 10.1074/jbc.M110.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, Chen CY, Xu B, Lu MM, Zhou D, Sebzda E, Santore MT, Merianos DJ, Stadtfeld M, Flake AW, Graf T, Skoda R, Maltzman JS, Koretzky GA, Kahn ML. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010:1–39. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Séverin S, Pollitt AY, Navarro-Nuñez L, Nash CA, Mourão-Sá D, Eble JA, Senis YA, Watson SP. Syk-dependent phosphorylation of CLEC-2: a novel mechanism of hem-immunoreceptor tyrosine-based activation motif signaling. J Biol Chem. 2011;286:4107–16. doi: 10.1074/jbc.M110.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kulathu Y, Grothe G, Reth M. Autoinhibition and adapter function of Syk. Immunol Rev. 2009;232:286–99. doi: 10.1111/j.1600-065X.2009.00837.x. [DOI] [PubMed] [Google Scholar]
- 51.Pollitt AY, Grygielska B, Leblond B, Désiré L, Eble JA, Watson SP. Phosphorylation of CLEC-2 is dependent on lipid rafts, actin polymerization, secondary mediators, and Rac. Blood. 2010;115:2938–46. doi: 10.1182/blood-2009-12-257212. [DOI] [PubMed] [Google Scholar]
- 52.Hughes CE, Pollitt AY, Mori J, Eble JA, Tomlinson MG, Hartwig JH, O’Callaghan CA, Fütterer K, Watson SP. CLEC-2 activates Syk through dimerization. Blood. 2010;115:2947–55. doi: 10.1182/blood-2009-08-237834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watson AA, Christou CM, James JR, Fenton-May AE, Moncayo GE, Mistry AR, Davis SJ, Gilbert RJC, Chakera A, O’Callaghan CA. The platelet receptor CLEC-2 is active as a dimer. Biochemistry. 2009;48:10988–96. doi: 10.1021/bi901427d. [DOI] [PubMed] [Google Scholar]
- 54.Sancho, Mourão-Sá, Joffre, Schulz, Rogers, Pennington, Carlyle, Reis e Sousa C. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008;118:2098–110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caminschi, Proietto, Ahmet, Kitsoulis, Teh, Lo, Rizzitelli, Wu, Vremec, Dommelen v, Campbell, Maraskovsky, Braley, Davey, Mottram, Velde vd, Jensen, Lew, Wright, Heath, Shortman, Lahoud The dendritic cell subtype restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 2008;112:3264–73. doi: 10.1182/blood-2008-05-155176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen C-JJ, Dunbar PR, Wadley RB, Jeet V, Vulink AJE, Hart DNJ, Radford KJ. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–60. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen J-L, Keller AM, Joffre O, Zelenay S, Nye E, Le Moine A, Faure F, Donckier V, Sancho D, Cerundolo V, Bonnet D, Reis e Sousa C. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8{alpha}+ dendritic cells. J Exp Med. 2010;207:1261–71. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bachem A, Güttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, Henn V, Kloetzel P-M, Gurka S, Kroczek RA. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–81. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre C-A, Ventre E, Vu Manh T-P, Baranek T, Storset AK, Marvel J, Boudinot P, Hosmalin A, Schwartz-Cornil I, Dalod M. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8{alpha}+ dendritic cells. J Exp Med. 2010;207:1283–92. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Idoyaga J, Lubkin A, Fiorese C, Lahoud MH, Caminschi I, Huang Y, Rodriguez A, Clausen BE, Park CG, Trumpfheller C, Steinman RM. Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proc Natl Acad Sci U S A. 2011;108:2384–9. doi: 10.1073/pnas.1019547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burgdorf S, Kautz A, Böhnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–6. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 62.Joffre OP, Sancho D, Zelenay S, Keller AM, Reis e Sousa C. Efficient and versatile manipulation of the peripheral CD4+ T-cell compartment by antigen targeting to DNGR-1/CLEC9A. Eur. J. Immunol. 2010;40:1255–65. doi: 10.1002/eji.201040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lahoud MH, Ahmet F, Kitsoulis S, Wan SS, Vremec D, Lee CN, Phipson B, Shi W, Smyth GK, Lew AM, Kato Y, Mueller SN, Davey GM, Heath WR, Shortman K, Caminschi I. Targeting Antigen to Mouse Dendritic Cells via Clec9A Induces Potent CD4 T Cell Responses Biased toward a Follicular Helper Phenotype. J Immunol. 2011;187:842–50. doi: 10.4049/jimmunol.1101176. [DOI] [PubMed] [Google Scholar]
- 64.Powlesland AS, Ward EM, Sadhu SK, Guo Y, Taylor ME, Drickamer K. Widely divergent biochemical properties of the complete set of mouse DC-SIGN-related proteins. J Biol Chem. 2006;281:20440–9. doi: 10.1074/jbc.M601925200. [DOI] [PubMed] [Google Scholar]
- 65.Taylor PR, Reid DM, Heinsbroek SEM, Brown GD, Gordon S, Wong SYC. Dectin-2 is predominantly myeloid restricted and exhibits unique activation-dependent expression on maturing inflammatory monocytes elicited in vivo. Eur J Immunol. 2005;35:2163–74. doi: 10.1002/eji.200425785. [DOI] [PubMed] [Google Scholar]
- 66.Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, Verbeek JS, Ruland J, Tybulewicz V, Brown GD, Moita LF, Taylor PR, Reis e Sousa C. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009;206:2037–51. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McGreal EP, Rosas M, Brown GD, Zamze S, Wong SY, Gordon S, Martinez-Pomares L, Taylor PR. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology. 2006;16:422–30. doi: 10.1093/glycob/cwj077. [DOI] [PubMed] [Google Scholar]
- 68.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung S-h, Komatsu R, Miura N, Adachi Y, Ohno N, Shibuya K, Yamamoto N, Kawakami K, Yamasaki S, Saito T, Akira S, Iwakura Y. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–91. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 69.Barrett NA, Maekawa A, Rahman OM, Austen KF, Kanaoka Y. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J Immunol. 2009;182:1119–28. doi: 10.4049/jimmunol.182.2.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ritter M, Gross O, Kays S, Ruland J, Nimmerjahn F, Saijo S, Tschopp J, Layland LE, Prazeres da Costa C. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci U S A. 2010;107:20459–64. doi: 10.1073/pnas.1010337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aragane Y, Maeda A, Schwarz A, Tezuka T, Ariizumi K, Schwarz T. Involvement of dectin-2 in ultraviolet radiation-induced tolerance. J Immunol. 2003;171:3801–7. doi: 10.4049/jimmunol.171.7.3801. [DOI] [PubMed] [Google Scholar]
- 72.Sato K, Yang X-l, Yudate T, Chung J-S, Wu J, Luby-Phelps K, Kimberly RP, Underhill D, Cruz PD, Ariizumi K. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem. 2006;281:38854–66. doi: 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- 73.Bi L, Gojestani S, Wu W, Hsu Y-MS, Zhu J, Ariizumi K, Lin X. CARD9 mediates dectin-2-induced IkappaBalpha kinase ubiquitination leading to activation of NF-kappaB in response to stimulation by the hyphal form of Candida albicans. J Biol Chem. 2010;285:25969–77. doi: 10.1074/jbc.M110.131300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barrett NA, Rahman OM, Fernandez JM, Parsons MW, Xing W, Austen KF, Kanaoka Y. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J Exp Med. 2011 doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wills-Karp M. Allergen-specific pattern recognition receptor pathways. Curr Opin Immunol. 2010;22:777–82. doi: 10.1016/j.coi.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–46. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 77.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Günther G, Johnston I, Lanzavecchia A, Nagasaka T, Okada T, Vermi W, Winkels G, Yamamoto T, Zysk M, Yamaguchi Y, Schmitz J. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–34. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kanazawa N, Tashiro K, Inaba K, Miyachi Y. Dendritic cell immunoactivating receptor, a novel C-type lectin immunoreceptor, acts as an activating receptor through association with Fc receptor gamma chain. J Biol Chem. 2003;278:32645–52. doi: 10.1074/jbc.M304226200. [DOI] [PubMed] [Google Scholar]
- 79.Kaden SA, Kurig S, Vasters K, Hofmann K, Zaenker KS, Schmitz J, Winkels G. Enhanced dendritic cell-induced immune responses mediated by the novel C-type lectin receptor mDCAR1. J Immunol. 2009;183:5069–78. doi: 10.4049/jimmunol.0900908. [DOI] [PubMed] [Google Scholar]
- 80.Martinelli E, Cicala C, Van Ryk D, Goode DJ, Macleod K, Arthos J, Fauci AS. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha} secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2007;104:3396–401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cao W, Zhang L, Rosen DB, Bover L, Watanabe G, Bao M, Lanier LL, Liu Y-J. BDCA2/Fc epsilon RI gamma complex signals through a novel BCR-like pathway in human plasmacytoid dendritic cells. PLoS Biol. 2007;5:e248. doi: 10.1371/journal.pbio.0050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Röck J, Schneider E, Grün JR, Grützkau A, Küppers R, Schmitz J, Winkels G. CD303 (BDCA-2) signals in plasmacytoid dendritic cellsvia a BCR-like signalosome involving Syk, Slp65 and PLCγ2. Eur J Immunol. 2007;37:3564–75. doi: 10.1002/eji.200737711. [DOI] [PubMed] [Google Scholar]
- 83.Cao W, Liu Y-J. Innate immune functions of plasmacytoid dendritic cells. Curr Opin Immunol. 2007;19:24–30. doi: 10.1016/j.coi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 84.Hara, Ishihara, Takeuchi, Imanishi, Xue, Morris, Inui, Takai, Shibuya A, Saijo, Iwakura, Ohno, Koseki, Yoshida, Penninger, Saito The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors. Nat Immunol. 2007;8:619–29. doi: 10.1038/ni1466. [DOI] [PubMed] [Google Scholar]
- 85.Riboldi E, Daniele R, Cassatella MA, Sozzani S, Bosisio D. Engagement of BDCA-2 blocks TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. Immunobiology. 2009;214:868–76. doi: 10.1016/j.imbio.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 86.Wu P, Wu J, Liu S, Han X, Lu J, Shi Y, Wang J, Lu L, Cao X. TLR9/TLR7-triggered downregulation of BDCA2 expression on human plasmacytoid dendritic cells from healthy individuals and lupus patients. Clin Immunol. 2008;129:40–8. doi: 10.1016/j.clim.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 87.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–88. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 88.Bugarcic A, Hitchens K, Beckhouse AG, Wells CA, Ashman RB, Blanchard H. Human and mouse macrophage-inducible C-type lectin (Mincle) bind Candida albicans. Glycobiology. 2008;18:679–85. doi: 10.1093/glycob/cwn046. [DOI] [PubMed] [Google Scholar]
- 89.Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, Beckhouse AG, Lo Y-L-S, Manzanero S, Cobbold C, Schroder K, Ma B, Orr S, Stewart L, Lebus D, Sobieszczuk P, Hume DA, Stow J, Blanchard H, Ashman RB. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol. 2008;180:7404–13. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]
- 90.Yamasaki S, Matsumoto M, Takeuchi O, Matsuzawa T, Ishikawa E, Sakuma M, Tateno H, Uno J, Hirabayashi J, Mikami Y, Takeda K, Akira S, Saito T. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci USA. 2009;106:1897–902. doi: 10.1073/pnas.0805177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–88. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, Agger EM, Stenger S, Andersen P, Ruland J, Brown GD, Wells C, Lang R. Cutting Edge: Mincle Is Essential for Recognition and Adjuvanticity of the Mycobacterial Cord Factor and its Synthetic Analog Trehalose-Dibehenate. J Immunol. 2010;184:2756–60. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bakker AB, Baker E, Sutherland GR, Phillips JH, Lanier LL. Myeloid DAP12-associating lectin (MDL)-1 is a cell surface receptor involved in the activation of myeloid cells. Proc Natl Acad Sci USA. 1999;96:9792–6. doi: 10.1073/pnas.96.17.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen S-T, Lin Y-L, Huang M-T, Wu M-F, Cheng S-C, Lei H-Y, Lee C-K, Chiou T-W, Wong C-H, Hsieh S-L. CLEC5A is critical for dengue-virus-induced lethal disease. Nature. 2008;453:672–6. doi: 10.1038/nature07013. [DOI] [PubMed] [Google Scholar]
- 95.Inui, Kikuchi Y, Aoki N, Endo S, Maeda T, Sugahara-Tobinai A, Fujimura S, Nakamura A, Kumanogoh A, Colonna M, Takai Signal adaptor DAP10 associates with MDL-1 and triggers osteoclastogenesis in cooperation with DAP12. Proc Natl Acad Sci USA. 2009;106:4816–21. doi: 10.1073/pnas.0900463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Joyce-Shaikh B, Bigler ME, Chao C-C, Murphy EE, Blumenschein WM, Adamopoulos IE, Heyworth PG, Antonenko S, Bowman EP, McClanahan TK, Phillips JH, Cua DJ. Myeloid DAP12-associating lectin (MDL)-1 regulates synovial inflammation and bone erosion associated with autoimmune arthritis. J Exp Med. 2010;207:579–89. doi: 10.1084/jem.20090516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bates EE, Fournier N, Garcia E, Valladeau J, Durand I, Pin JJ, Zurawski SM, Patel S, Abrams JS, Lebecque S, Garrone P, Saeland S. APCs express DCIR, a novel C-type lectin surface receptor containing an immunoreceptor tyrosine-based inhibitory motif. J Immunol. 1999;163:1973–83. [PubMed] [Google Scholar]
- 98.Lee RT, Hsu TL, Huang SK, Hsieh SL, Wong CH, Lee YC. Survey of immune-related, mannose/fucose-binding C-type lectin receptors reveals widely divergent sugar-binding specificities. Glycobiology. 2011;21:512–20. doi: 10.1093/glycob/cwq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klechevsky E, Flamar A-L, Cao Y, Blanck J-P, Liu M, O’Bar A, Agouna-Deciat O, Klucar P, Thompson-Snipes L, Zurawski S, Reiter Y, Palucka AK, Zurawski G, Banchereau J. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010;116:1685–97. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kanazawa N, Okazaki T, Nishimura H, Tashiro K, Inaba K, Miyachi Y. DCIR acts as an inhibitory receptor depending on its immunoreceptor tyrosine-based inhibitory motif. J Invest dermatol. 2002;118:261–6. doi: 10.1046/j.0022-202x.2001.01633.x. [DOI] [PubMed] [Google Scholar]
- 101.Fujikado, Saijo, Yonezawa, Shimamori, Ishii, Sugai, Kotaki, Sudo, Nose, Iwakura Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat Med. 2008;14:176–80. doi: 10.1038/nm1697. [DOI] [PubMed] [Google Scholar]
- 102.Nussenzweig MC, Steinman RM, Witmer MD, Gutchinov B. A monoclonal antibody specific for mouse dendritic cells. Proc Natl Acad Sci USA. 1982;79:161–5. doi: 10.1073/pnas.79.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee H-W, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–11. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 104.Lambert AA, Gilbert C, Richard M, Beaulieu AD, Tremblay MJ. The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways. Blood. 2008;112:1299–307. doi: 10.1182/blood-2008-01-136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Richard M, Thibault N, Veilleux P, Gareau-Pagé G, Beaulieu AD. Granulocyte macrophage-colony stimulating factor reduces the affinity of SHP-2 for the ITIM of CLECSF6 in neutrophils: a new mechanism of action for SHP-2. Mol Immunol. 2006;43:1716–21. doi: 10.1016/j.molimm.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 106.Lambert AA, Barabe F, Gilbert C, Tremblay MJ. DCIR-mediated enhancement of HIV-1 infection requires the ITIM-associated signal transduction pathway. Blood. 2011:1–42. doi: 10.1182/blood-2011-01-331363. [DOI] [PubMed] [Google Scholar]
- 107.Meyer-Wentrup, Benitez-Ribas, Tacken, Punt, Figdor, Vries d, Adema Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-{alpha} production. Blood. 2008 doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- 108.Meyer-Wentrup F, Cambi A, Joosten B, Looman MW, de Vries IJM, Figdor CG, Adema GJ. DCIR is endocytosed into human dendritic cells and inhibits TLR8-mediated cytokine production. J Leukocyte Biol. 2009;85:518–25. doi: 10.1189/jlb.0608352. [DOI] [PubMed] [Google Scholar]
- 109.Marshall ASJ, Willment JA, Lin H-H, Williams DL, Gordon S, Brown GD. Identification and characterization of a novel human myeloid inhibitory C-type lectin-like receptor (MICL) that is predominantly expressed on granulocytes and monocytes. J Biol Chem. 2004;279:14792–802. doi: 10.1074/jbc.M313127200. [DOI] [PubMed] [Google Scholar]
- 110.Han Y, Zhang M, Li N, Chen T, Zhang Y, Wan T, Cao X. KLRL1, a novel killer cell lectinlike receptor, inhibits natural killer cell cytotoxicity. Blood. 2004;104:2858–66. doi: 10.1182/blood-2004-03-0878. [DOI] [PubMed] [Google Scholar]
- 111.Bakker ABH, van den Oudenrijn S, Bakker AQ, Feller N, van Meijer M, Bia JA, Jongeneelen MAC, Visser TJ, Bijl N, Geuijen CAW, Marissen WE, Radosevic K, Throsby M, Schuurhuis GJ, Ossenkoppele GJ, de Kruif J, Goudsmit J, Kruisbeek AM. C-type lectin-like molecule-1: a novel myeloid cell surface marker associated with acute myeloid leukemia. Cancer Res. 2004;64:8443–50. doi: 10.1158/0008-5472.CAN-04-1659. [DOI] [PubMed] [Google Scholar]
- 112.Chen C-H, Floyd H, Olson NE, Magaletti D, Li C, Draves K, Clark EA. Dendritic-cell-associated C-type lectin 2 (DCAL-2) alters dendritic-cell maturation and cytokine production. Blood. 2006;107:1459–67. doi: 10.1182/blood-2005-08-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lahoud MH, Proietto AI, Ahmet F, Kitsoulis S, Eidsmo L, Wu L, Sathe P, Pietersz S, Chang H-W, Walker ID, Maraskovsky E, Braley H, Lew AM, Wright MD, Heath WR, Shortman K, Caminschi I. The C-Type Lectin Clec12A Present on Mouse and Human Dendritic Cells Can Serve as a Target for Antigen Delivery and Enhancement of Antibody Responses. J Immunol. 2009;182:7587–94. doi: 10.4049/jimmunol.0900464. [DOI] [PubMed] [Google Scholar]
- 114.Pyz E, Huysamen C, Marshall ASJ, Gordon S, Taylor PR, Brown GD. Characterisation of murine MICL (CLEC12A) and evidence for an endogenous ligand. Eur. J. Immunol. 2008;38:1157–63. doi: 10.1002/eji.200738057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoffmann, Schellack, Textor, Konold, Schmitz, Cerwenka, Pflanz, Watzl Identification of CLEC12B, an Inhibitory Receptor on Myeloid Cells. J Biol Chem. 2007;282:22370–5. doi: 10.1074/jbc.M704250200. [DOI] [PubMed] [Google Scholar]
- 116.Toyama-Sorimachi N, Tsujimura Y, Maruya M, Onoda A, Kubota T, Koyasu S, Inaba K, Karasuyama H. Ly49Q, a member of the Ly49 family that is selectively expressed on myeloid lineage cells and involved in regulation of cytoskeletal architecture. Proc Natl Acad Sci USA. 2004;101:1016–21. doi: 10.1073/pnas.0305400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kamogawa-Schifter Y, Ohkawa J, Namiki S, Arai N, Arai K-I, Liu Y. Ly49Q defines 2 pDC subsets in mice. Blood. 2005;105:2787–92. doi: 10.1182/blood-2004-09-3388. [DOI] [PubMed] [Google Scholar]
- 118.Toyama-Sorimachi N, Omatsu Y, Onoda A, Tsujimura Y, Iyoda T, Kikuchi-Maki A, Sorimachi H, Dohi T, Taki S, Inaba K, Karasuyama H. Inhibitory NK receptor Ly49Q is expressed on subsets of dendritic cells in a cellular maturation- and cytokine stimulation-dependent manner. J Immunol. 2005;174:4621–9. doi: 10.4049/jimmunol.174.8.4621. [DOI] [PubMed] [Google Scholar]
- 119.Scarpellino L, Oeschger F, Guillaume P, Coudert JD, Lévy F, Leclercq G, Held W. Interactions of Ly49 family receptors with MHC class I ligands in trans and cis. J Immunol. 2007;178:1277–84. doi: 10.4049/jimmunol.178.3.1277. [DOI] [PubMed] [Google Scholar]
- 120.Yoshizaki M, Tazawa A, Kasumi E, Sasawatari S, Itoh K, Dohi T, Sasazuki T, Inaba K, Makrigiannis AP, Toyama-Sorimachi N. Spatiotemporal regulation of intracellular trafficking of Toll-like receptor 9 by an inhibitory receptor, Ly49Q. Blood. 2009;114:1518–27. doi: 10.1182/blood-2008-12-192344. [DOI] [PubMed] [Google Scholar]
- 121.Tai L-H, Goulet M-L, Belanger S, Toyama-Sorimachi N, Fodil-Cornu N, Vidal SM, Troke AD, Mcvicar DW, Makrigiannis AP. Positive regulation of plasmacytoid dendritic cell function via Ly49Q recognition of class I MHC. J Exp Med. 2008;205:3187–99. doi: 10.1084/jem.20080718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sasawatari S, Yoshizaki M, Taya C, Tazawa A, Furuyama-Tanaka K, Yonekawa H, Dohi T, Makrigiannis AP, Sasazuki T, Inaba K, Toyama-Sorimachi N. The Ly49Q receptor plays a crucial role in neutrophil polarization and migration by regulating raft trafficking. Immunity. 2010;32:200–13. doi: 10.1016/j.immuni.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 123.Kerrigan AM, Brown GD. C-type lectins and phagocytosis. Immunobiology. 2009;214:562–75. doi: 10.1016/j.imbio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Largent BL, Walton KM, Hoppe CA, Lee YC, Schnaar RL. Carbohydrate-specific adhesion of alveolar macrophages to mannose-derivatized surfaces. J Biol Chem. 1984;259:1764–9. [PubMed] [Google Scholar]
- 125.Burgdorf S, Lukacs-Kornek V, Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol. 2006;176:6770–6. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 126.Zehner M, Chasan AI, Schuette V, Embgenbroich M, Quast T, Kolanus W, Burgdorf S. Mannose receptor polyubiquitination regulates endosomal recruitment of p97 and cytosolic antigen translocation for cross-presentation. Proc Natl Acad Sci U S A. 2011;108:9933–8. doi: 10.1073/pnas.1102397108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schweizer A, Stahl PD, Rohrer J. A di-aromatic motif in the cytosolic tail of the mannose receptor mediates endosomal sorting. J Biol Chem. 2000;275:29694–700. doi: 10.1074/jbc.M000571200. [DOI] [PubMed] [Google Scholar]
- 128.Kruskal BA, Sastry K, Warner AB, Mathieu CE, Ezekowitz RA. Phagocytic chimeric receptors require both transmembrane and cytoplasmic domains from the mannose receptor. J Exp Med. 1992;176:1673–80. doi: 10.1084/jem.176.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang J, Zhu J, Bu X, Cushion M, Kinane TB, Avraham H, Koziel H. Cdc42 and RhoB activation are required for mannose receptor-mediated phagocytosis by human alveolar macrophages. Mol Biol Cell. 2005;16:824–34. doi: 10.1091/mbc.E04-06-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Le Cabec V, Emorine LJ, Toesca I, Cougoule C, Maridonneau-Parini I. The human macrophage mannose receptor is not a professional phagocytic receptor. J Leukoc Biol. 2005;77:934–43. doi: 10.1189/jlb.1204705. [DOI] [PubMed] [Google Scholar]
- 131.Cambi A, Netea MG, Mora-Montes HM, Gow NAR, Hato SV, Lowman DW, Kullberg B-J, Torensma R, Williams DL, Figdor CG. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem. 2008;283:20590–9. doi: 10.1074/jbc.M709334200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, Monti P, Piemonti L, Biondi A, Mantovani A, Introna M, Allavena P. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–60. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- 133.Chavele K-M, Martinez-Pomares L, Domin J, Pemberton S, Haslam SM, Dell A, Cook HT, Pusey CD, Gordon S, Salama AD. Mannose receptor interacts with Fc receptors and is critical for the development of crescentic glomerulonephritis in mice. J Clin Invest. 2010;120:1469–78. doi: 10.1172/JCI41560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang J, Tachado SD, Patel N, Zhu J, Imrich A, Manfruelli P, Cushion M, Kinane TB, Koziel H. Negative regulatory role of mannose receptors on human alveolar macrophage proinflammatory cytokine release in vitro. J Leukocyte Biol. 2005;78:665–74. doi: 10.1189/jlb.1204699. [DOI] [PubMed] [Google Scholar]
- 135.Rajaram MVS, Brooks MN, Morris JD, Torrelles JB, Azad AK, Schlesinger LS. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor gamma linking mannose receptor recognition to regulation of immune responses. J Immunol. 2010;185:929–42. doi: 10.4049/jimmunol.1000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sweet L, Singh PP, Azad AK, Rajaram MV, Schlesinger LS, Schorey JS. Mannose receptor-dependent delay in phagosome maturation by Mycobacterium avium glycopeptidolipids. Infect Immun. 2010;78:518–26. doi: 10.1128/IAI.00257-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Royer P-J, Emara M, Yang C, Al-Ghouleh A, Tighe P, Jones N, Sewell HF, Shakib F, Martinez-Pomares L, Ghaemmaghami AM. The mannose receptor mediates the uptake of diverse native allergens by dendritic cells and determines allergen-induced T cell polarization through modulation of IDO activity. J Immunol. 2010;185:1522–31. doi: 10.4049/jimmunol.1000774. [DOI] [PubMed] [Google Scholar]
- 138.Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–5. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 139.Mahnke K, Guo M, Lee S, Sepulveda H, Swain SL, Nussenzweig M, Steinman RM. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol. 2000;151:673–84. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tacken, Vries d, Torensma, Figdor Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007 doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 141.Shrimpton RE, Butler M, Morel A-S, Eren E, Hue SS, Ritter MA. CD205 (DEC-205): A recognition receptor for apoptotic and necrotic self. Mol Immunol. 2009;46:1229–39. doi: 10.1016/j.molimm.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nickel T, Schmauss D, Hanssen H, Sicic Z, Krebs B, Jankl S, Summo C, Fraunberger P, Walli AK, Pfeiler S, Weis M. oxLDL uptake by dendritic cells induces upregulation of scavenger-receptors, maturation and differentiation. Atherosclerosis. 2009;205:442–50. doi: 10.1016/j.atherosclerosis.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 143.Zhang S-s, Park CG, Zhang P, Bartra SS, Plano GV, Klena JD, Skurnik M, Hinnebusch BJ, Chen T. Plasminogen activator Pla of Yersinia pestis utilizes murine DEC-205 (CD205) as a receptor to promote dissemination. J Biol Chem. 2008;283:31511–21. doi: 10.1074/jbc.M804646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–85. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 145.Lai WK, Sun PJ, Zhang J, Jennings A, Lalor PF, Hubscher S, McKeating JA, Adams DH. Expression of DC-SIGN and DC-SIGNR on human sinusoidal endothelium: a role for capturing hepatitis C virus particles. Am J Pathol. 2006;169:200–8. doi: 10.2353/ajpath.2006.051191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Engering A, Geijtenbeek TBH, van Vliet SJ, Wijers M, van Liempt E, Demaurex N, Lanzavecchia A, Fransen J, Figdor CG, Piguet V, van Kooyk Y. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168:2118–26. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- 147.Geijtenbeek TBH, van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CMJE, Appelmelk B, van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang P, Snyder S, Feng P, Azadi P, Zhang S, Bulgheresi S, Sanderson KE, He J, Klena J, Chen T. Role of N-acetylglucosamine within core lipopolysaccharide of several species of gram-negative bacteria in targeting the DC-SIGN (CD209) J Immunol. 2006;177:4002–11. doi: 10.4049/jimmunol.177.6.4002. [DOI] [PubMed] [Google Scholar]
- 149.Hodges A, Sharrocks K, Edelmann M, Baban D, Moris A, Schwartz O, Drakesmith H, Davies K, Kessler B, McMichael A, Simmons A. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat Immunol. 2007;8:569–77. doi: 10.1038/ni1470. [DOI] [PubMed] [Google Scholar]
- 150.Kwon DS, Gregorio G, Bitton N, Hendrickson WA, Littman DR. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity. 2002;16:135–44. doi: 10.1016/s1074-7613(02)00259-5. [DOI] [PubMed] [Google Scholar]
- 151.Caparrós E, Munoz P, Sierra-Filardi E, Serrano-Gómez D, Puig-Kröger A, Rodríguez-Fernández JL, Mellado M, Sancho J, Zubiaur M, Corbí AL. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood. 2006;107:3950–8. doi: 10.1182/blood-2005-03-1252. [DOI] [PubMed] [Google Scholar]
- 152.Gringhuis SI, Den Dunnen J, Litjens M, Van Der Vlist M, Geijtenbeek TBH. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol. 2009;10:1081–8. doi: 10.1038/ni.1778. [DOI] [PubMed] [Google Scholar]
- 153.Gringhuis, Dunnen d, Litjens, Hof vH, Kooyk v, Geijtenbeek C-Type Lectin DC-SIGN Modulates Toll-like Receptor Signaling via Raf-1 Kinase-Dependent Acetylation of Transcription Factor NF-kappaB. Immunity. 2007;26:605–16. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 154.Hovius JWR, de Jong MAWP, Den Dunnen J, Litjens M, Fikrig E, Van Der Poll T, Gringhuis SI, Geijtenbeek TBH. Salp15 binding to DC-SIGN inhibits cytokine expression by impairing both nucleosome remodeling and mRNA stabilization. PLoS Pathog. 2008;4:e31. doi: 10.1371/journal.ppat.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kang Y-S, Yamazaki S, Iyoda T, Pack M, Bruening SA, Kim JY, Takahara K, Inaba K, Steinman RM, Park CG. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int Immunol. 2003;15:177–86. doi: 10.1093/intimm/dxg019. [DOI] [PubMed] [Google Scholar]
- 156.Taylor PR, Brown GD, Herre J, Williams DL, Willment JA, Gordon S. The role of SIGNR1 and the beta-glucan receptor (dectin-1) in the nonopsonic recognition of yeast by specific macrophages. J Immunol. 2004;172:1157–62. doi: 10.4049/jimmunol.172.2.1157. [DOI] [PubMed] [Google Scholar]
- 157.Zhou Y, Kawasaki H, Hsu S-C, Lee RT, Yao X, Plunkett B, Fu J, Yang K, Lee YC, Huang S-K. Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1. Nat Med. 2010;16:1128–33. doi: 10.1038/nm.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cheong C, Matos I, Choi J-H, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, Koh H, Rodriguez A, Idoyaga J, Pack M, Velinzon K, Park CG, Steinman RM. Microbial Stimulation Fully Differentiates Monocytes to DC-SIGN/CD209(+) Dendritic Cells for Immune T Cell Areas. Cell. 2010;143:416–29. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nagaoka K, Takahara K, Tanaka K, Yoshida H, Steinman RM, Saitoh S, Akashi-Takamura S, Miyake K, Kang YS, Park CG, Inaba K. Association of SIGNR1 with TLR4-MD-2 enhances signal transduction by recognition of LPS in gram-negative bacteria. Int Immunol. 2005;17:827–36. doi: 10.1093/intimm/dxh264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Takahara K, Tokieda S, Nagaoka K, Takeda T, Kimura Y, Inaba K. C-type lectin SIGNR1 enhances cellular oxidative burst response against C. albicans in cooperation with Dectin-1. Eur. J. Immunol. 2011;41:1435–44. doi: 10.1002/eji.200940188. [DOI] [PubMed] [Google Scholar]
- 161.Kato C, Kojima N. SIGNR1 ligation on murine peritoneal macrophages induces IL-12 production through NFkappaB activation. Glycoconj J. 2010;27:525–31. doi: 10.1007/s10719-010-9298-x. [DOI] [PubMed] [Google Scholar]
- 162.Wieland CW, Koppel EA, den Dunnen J, Florquin S, McKenzie AN, van Kooyk Y, van der Poll T, Geijtenbeek TB. Mice lacking SIGNR1 have stronger T helper 1 responses to Mycobacterium tuberculosis. Microbes Infect. 2007;9:134–41. doi: 10.1016/j.micinf.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 163.Srivastava V, Manchanda M, Gupta S, Singla R, Behera D, Das G, Natarajan K. Toll-like receptor 2 and DC-SIGNR1 differentially regulate suppressors of cytokine signaling 1 in dendritic cells during Mycobacterium tuberculosis infection. J Biol Chem. 2009;284:25532–41. doi: 10.1074/jbc.M109.006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, Duvert-Frances V, Vincent C, Schmitt D, Davoust J, Caux C, Lebecque S, Saeland S. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 165.Idoyaga J, Suda N, Suda K, Park C, Steinman R. Antibody to Langerin/CD207 localizes large numbers of CD8{alpha}+ dendritic cells to the marginal zone of mouse spleen. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0812247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.de Jong MA, Vriend LE, Theelen B, Taylor ME, Fluitsma D, Boekhout T, Geijtenbeek TB. C-type lectin Langerin is a beta-glucan receptor on human Langerhans cells that recognizes opportunistic and pathogenic fungi. Mol Immunol. 2010;47:1216–25. doi: 10.1016/j.molimm.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MAWP, de Gruijl T, Piguet V, van Kooyk Y, Geijtenbeek TBH. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13:367–71. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 168.Hunger RE, Sieling PA, Ochoa MT, Sugaya M, Burdick AE, Rea TH, Brennan PJ, Belisle JT, Blauvelt A, Porcelli SA, Modlin RL. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701–8. doi: 10.1172/JCI19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Higashi N, Fujioka K, Denda-Nagai K, Hashimoto S-I, Nagai S, Sato T, Fujita Y, Morikawa A, Tsuiji M, Miyata-Takeuchi M, Sano Y, Suzuki N, Yamamoto K, Matsushima K, Irimura T. The macrophage C-type lectin specific for galactose/N-acetylgalactosamine is an endocytic receptor expressed on monocyte-derived immature dendritic cells. J Biol Chem. 2002;277:20686–93. doi: 10.1074/jbc.M202104200. [DOI] [PubMed] [Google Scholar]
- 170.Raes G. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol. 2004;77:321–7. doi: 10.1189/jlb.0304212. [DOI] [PubMed] [Google Scholar]
- 171.Singh SK, Streng-Ouwehand I, Litjens M, Weelij DR, García-Vallejo JJ, van Vliet SJ, Saeland E, van Kooyk Y. Characterization of murine MGL1 and MGL2 C-type lectins: distinct glycan specificities and tumor binding properties. Mol Immunol. 2009;46:1240–9. doi: 10.1016/j.molimm.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 172.van Vliet SJ, Aarnoudse CA, Broks-van den Berg VCM, Boks M, Geijtenbeek TBH, van Kooyk Y. MGL-mediated internalization and antigen presentation by dendritic cells: a role for tyrosine-5. Eur. J. Immunol. 2007;37:2075–81. doi: 10.1002/eji.200636838. [DOI] [PubMed] [Google Scholar]
- 173.Denda-Nagai K, Aida S, Saba K, Suzuki K, Moriyama S, Oo-Puthinan S, Tsuiji M, Morikawa A, Kumamoto Y, Sugiura D, Kudo A, Akimoto Y, Kawakami H, Bovin NV, Irimura T. Distribution and function of macrophage galactose-type C-type lectin 2 (MGL2/CD301b): efficient uptake and presentation of glycosylated antigens by dendritic cells. J Biol Chem. 2010;285:19193–204. doi: 10.1074/jbc.M110.113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Singh SK, Streng-Ouwehand I, Litjens M, Kalay H, Saeland E, van Kooyk Y. Tumour-associated glycan modifications of antigen enhance MGL2 dependent uptake and MHC class I restricted CD8 T cell responses. Int J Cancer. 2011;128:1371–83. doi: 10.1002/ijc.25458. [DOI] [PubMed] [Google Scholar]
- 175.Saba K, Denda-Nagai K, Irimura T. A C-type lectin MGL1/CD301a plays an anti-inflammatory role in murine experimental colitis. Am J Pathol. 2009;174:144–52. doi: 10.2353/ajpath.2009.080235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yuita H, Tsuiji M, Tajika Y, Matsumoto Y, Hirano K, Suzuki N, Irimura T. Retardation of removal of radiation-induced apoptotic cells in developing neural tubes in macrophage galactose-type C-type lectin-1-deficient mouse embryos. Glycobiology. 2005;15:1368–75. doi: 10.1093/glycob/cwj028. [DOI] [PubMed] [Google Scholar]
- 177.Sobanov Y, Bernreiter A, Derdak S, Mechtcheriakova D, Schweighofer B, Düchler M, Kalthoff F, Hofer E. A novel cluster of lectin-like receptor genes expressed in monocytic, dendritic and endothelial cells maps close to the NK receptor genes in the human NK gene complex. Eur J Immunol. 2001;31:3493–503. doi: 10.1002/1521-4141(200112)31:12<3493::aid-immu3493>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 178.Ryan EJ, Marshall AJ, Magaletti D, Floyd H, Draves KE, Olson NE, Clark EA. Dendritic cell-associated lectin-1: a novel dendritic cell-associated, C-type lectin-like molecule enhances T cell secretion of IL-4. J Immunol. 2002;169:5638–48. doi: 10.4049/jimmunol.169.10.5638. [DOI] [PubMed] [Google Scholar]
- 179.Ryan EJ, Magaletti D, Draves KE, Clark EA. Ligation of dendritic cell-associated lectin-1 induces partial maturation of human monocyte derived dendritic cells. Hum Immunol. 2009;70:1–5. doi: 10.1016/j.humimm.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Balch SG, McKnight AJ, Seldin MF, Gordon S. Cloning of a novel C-type lectin expressed by murine macrophages. J Biol Chem. 1998;273:18656–64. doi: 10.1074/jbc.273.29.18656. [DOI] [PubMed] [Google Scholar]
- 181.Arce I, Martínez-Muñoz L, Roda-Navarro P, Fernández-Ruiz E. The human C-type lectin CLECSF8 is a novel monocyte/macrophage endocytic receptor. Eur. J. Immunol. 2004;34:210–20. doi: 10.1002/eji.200324230. [DOI] [PubMed] [Google Scholar]
- 182.Mehta JL, Li D. Identification, regulation and function of a novel lectin-like oxidized low-density lipoprotein receptor. J Am Coll Cardiol. 2002;39:1429–35. doi: 10.1016/s0735-1097(02)01803-x. [DOI] [PubMed] [Google Scholar]
- 183.Oka K, Sawamura T, Kikuta K, Itokawa S, Kume N, Kita T, Masaki T. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci USA. 1998;95:9535–40. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xie J, Zhu H, Guo L, Ruan Y, Wang L, Sun L, Zhou L, Wu W, Yun X, Shen A, Gu J. Lectin-like oxidized low-density lipoprotein receptor-1 delivers heat shock protein 60-fused antigen into the MHC class I presentation pathway. J Immunol. 2010;185:2306–13. doi: 10.4049/jimmunol.0903214. [DOI] [PubMed] [Google Scholar]
- 185.Vohra RS, Walker JH, Howell GJ, Homer-Vanniasinkam S, Ponnambalam S. The LOX-1 scavenger receptor cytoplasmic domain contains a transplantable endocytic motif. Biochem Biophys Res Commun. 2009;383:269–74. doi: 10.1016/j.bbrc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 186.Sakurai K, Cominacini L, Garbin U, Fratta Pasini A, Sasaki N, Takuwa Y, Masaki T, Sawamura T. Induction of endothelin-1 production in endothelial cells via co-operative action between CD40 and lectin-like oxidized LDL receptor (LOX-1) J Cardiovasc Pharmacol. 2004;44(Suppl 1):S173–80. doi: 10.1097/01.fjc.0000166243.43616.8b. [DOI] [PubMed] [Google Scholar]
- 187.Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, Kawakami-Honda N, Goetsch L, Sawamura T, Bonnefoy J, Jeannin P. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–62. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 188.Parlato S, Romagnoli G, Spadaro F, Canini I, Sirabella P, Borghi P, Ramoni C, Filesi I, Biocca S, Gabriele L, Belardelli F. LOX-1 as a natural IFN-alpha-mediated signal for apoptotic cell uptake and antigen presentation in dendritic cells. Blood. 2010;115:1554–63. doi: 10.1182/blood-2009-07-234468. [DOI] [PubMed] [Google Scholar]
- 189.Liu W, Tang L, Zhang G, Wei H, Cui Y, Guo L, Gou Z, Chen X, Jiang D, Zhu Y, Kang G, He F. Characterization of a novel C-type lectin-like gene, LSECtin: demonstration of carbohydrate binding and expression in sinusoidal endothelial cells of liver and lymph node. J Biol Chem. 2004;279:18748–58. doi: 10.1074/jbc.M311227200. [DOI] [PubMed] [Google Scholar]
- 190.Dominguez-Soto A, Aragoneses-Fenoll L, Martin-Gayo E, Martinez-Prats L, Colmenares M, Naranjo-Gomez M, Borras FE, Munoz P, Zubiaur M, Toribio ML, Delgado R, Corbi AL. The DC-SIGN-related lectin LSECtin mediates antigen capture and pathogen binding by human myeloid cells. Blood. 2007;109:5337–45. doi: 10.1182/blood-2006-09-048058. [DOI] [PubMed] [Google Scholar]
- 191.Tang L, Yang J, Liu W, Tang X, Chen J, Zhao D, Wang M, Xu F, Lu Y, Liu B, Sun Q, Zhang L, He F. Liver sinusoidal endothelial cell lectin, LSECtin, negatively regulates hepatic T-cell immune response. Gastroenterology. 2009;137:1498–508. e1–5. doi: 10.1053/j.gastro.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Powlesland AS, Fisch T, Taylor ME, Smith DF, Tissot B, Dell A, Pohlmann S, Drickamer K. A novel mechanism for LSECtin binding to Ebola virus surface glycoprotein through truncated glycans. J Biol Chem. 2008;283:593–602. doi: 10.1074/jbc.M706292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pipirou Z, Powlesland AS, Steffen I, Pohlmann S, Taylor ME, Drickamer K. Mouse LSECtin as a model for a human Ebola virus receptor. Glycobiology. 2010;21:806–12. doi: 10.1093/glycob/cwr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tang L, Yang J, Tang X, Ying W, Qian X, He F. The DC-SIGN family member LSECtin is a novel ligand of CD44 on activated T cells. Eur J Immunol. 2010;40:1185–91. doi: 10.1002/eji.200939936. [DOI] [PubMed] [Google Scholar]
- 195.Gramberg T, Hofmann H, Moller P, Lalor PF, Marzi A, Geier M, Krumbiegel M, Winkler T, Kirchhoff F, Adams DH, Becker S, Munch J, Pohlmann S. LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus. Virology. 2005;340:224–36. doi: 10.1016/j.virol.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nish S, Medzhitov R. Host defense pathways: role of redundancy and compensation in infectious disease phenotypes. Immunity. 2011;34:629–36. doi: 10.1016/j.immuni.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Puel A, Picard C, Cypowyj S, Lilic D, Abel L, Casanova JL. Inborn errors of mucocutaneous immunity to Candida albicans in humans: a role for IL-17 cytokines? Curr Opin Immunol. 2010;22:467–74. doi: 10.1016/j.coi.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, Jamal S, Manguiat A, Rezaei N, Amirzargar AA, Plebani A, Hannesschlager N, Gross O, Ruland J, Grimbacher B. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–35. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, Jacobs L, Jansen T, Verheijen K, Masthoff L, Morré SA, Vriend G, Williams DL, Perfect JR, Joosten LAB, Wijmenga C, Van der Meer JWM, Adema GJ, Kullberg BJ, Brown GD, Netea MG. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–7. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chai LY, de Boer MG, van der Velden WJ, Plantinga TS, van Spriel AB, Jacobs C, Halkes CJ, Vonk AG, Blijlevens NM, van Dissel JT, Donnelly PJ, Kullberg BJ, Maertens J, Netea MG. The Y238X stop codon polymorphism in the human beta-glucan receptor dectin-1 and susceptibility to invasive aspergillosis. J Infect Dis. 2011;203:736–43. doi: 10.1093/infdis/jiq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cunha C, Di Ianni M, Bozza S, Giovannini G, Zagarella S, Zelante T, D’Angelo C, Pierini A, Pitzurra L, Falzetti F, Carotti A, Perruccio K, Latge JP, Rodrigues F, Velardi A, Aversa F, Romani L, Carvalho A. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood. 2010;116:5394–402. doi: 10.1182/blood-2010-04-279307. [DOI] [PubMed] [Google Scholar]
- 202.Plantinga TS, van der Velden WJ, Ferwerda B, van Spriel AB, Adema G, Feuth T, Donnelly JP, Brown GD, Kullberg BJ, Blijlevens NM, Netea MG. Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49:724–32. doi: 10.1086/604714. [DOI] [PubMed] [Google Scholar]
- 203.Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, DesJardin LE, Schlesinger LS. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–99. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tailleux L, Schwartz O, Herrmann J-L, Pivert E, Jackson M, Amara A, Legres L, Dreher D, Nicod LP, Gluckman JC, Lagrange PH, Gicquel B, Neyrolles O. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med. 2003;197:121–7. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yadav M, Schorey JS. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–75. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rothfuchs AG, Bafica A, Feng CG, Egen JG, Williams DL, Brown GD, Sher A. Dectin-1 interaction with Mycobacterium tuberculosis leads to enhanced IL-12p40 production by splenic dendritic cells. J Immunol. 2007;179:3463–71. doi: 10.4049/jimmunol.179.6.3463. [DOI] [PubMed] [Google Scholar]
- 207.Dorhoi A, Desel C, Yeremeev V, Pradl L, Brinkmann V, Mollenkopf H-J, Hanke K, Gross O, Ruland J, Kaufmann SHE. The adaptor molecule CARD9 is essential for tuberculosis control. J Exp Med. 2010;207:777–92. doi: 10.1084/jem.20090067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Marakalala MJ, Guler R, Matika L, Murray G, Jacobs M, Brombacher F, Rothfuchs AG, Sher A, Brown GD. The Syk/CARD9-coupled receptor Dectin-1 is not required for host resistance to Mycobacterium tuberculosis in mice. Microbes Infect. 2011;13:198–201. doi: 10.1016/j.micinf.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Geijtenbeek TBH, van Vliet SJ, Engering A, ’t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 210.Lee SJ, Evers S, Roeder D, Parlow AF, Risteli J, Risteli L, Lee YC, Feizi T, Langen H, Nussenzweig MC. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science. 2002;295:1898–901. doi: 10.1126/science.1069540. [DOI] [PubMed] [Google Scholar]
- 211.van Gisbergen KPJM, Aarnoudse CA, Meijer GA, Geijtenbeek TBH, van Kooyk Y. Dendritic cells recognize tumor-specific glycosylation of carcinoembryonic antigen on colorectal cancer cells through dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin. Cancer Res. 2005;65:5935–44. doi: 10.1158/0008-5472.CAN-04-4140. [DOI] [PubMed] [Google Scholar]
- 212.Aarnoudse CA, Garcia Vallejo JJ, Saeland E, van Kooyk Y. Recognition of tumor glycans by antigen-presenting cells. Curr Opin Immunol. 2006;18:105–11. doi: 10.1016/j.coi.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 213.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–94. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhang P, Schwartz O, Pantelic M, Li G, Knazze Q, Nobile C, Radovich M, He J, Hong SC, Klena J, Chen T. DC-SIGN (CD209) recognition of Neisseria gonorrhoeae is circumvented by lipooligosaccharide variation. J Leukoc Biol. 2006;79:731–8. doi: 10.1189/jlb.0405184. [DOI] [PubMed] [Google Scholar]
- 215.Geijtenbeek TBH, Groot PC, Nolte MA, van Vliet SJ, Gangaram-Panday ST, van Duijnhoven GCF, Kraal G, van Oosterhout AJM, van Kooyk Y. Marginal zone macrophages express a murine homologue of DC-SIGN that captures blood-borne antigens in vivo. Blood. 2002;100:2908–16. doi: 10.1182/blood-2002-04-1044. [DOI] [PubMed] [Google Scholar]
- 216.van Vliet SJ, Saeland E, van Kooyk Y. Sweet preferences of MGL: carbohydrate specificity and function. Trends Immunol. 2008;29:83–90. doi: 10.1016/j.it.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 217.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S-I, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–24. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]