Abstract
Valeriana glechomifolia, a native species from southern Brazil, presents antidepressant-like activity and diene valepotriates (VAL) contribute to the pharmacological properties of the genus. It is known that depression can develop on an inflammation background in vulnerable patients and antidepressants present anti-inflammatory properties. We investigated the effects of VAL (10 mg/kg, p.o.) on sickness and depressive-like behaviors as well as proinflammatory cytokines (IL-1β and TNF-α) and BDNF expression in the cortex of mice exposed to a 5 min swimming session (as a stressful stimulus) 30 min before the E. coli LPS injection (600 µg/kg, i.p.). The forced swim + LPS induced sickness and depressive-like behaviors, increased the cortical expression of IL-1β and TNF-α, and decreased BDNF expression. VAL was orally administered to mice 1 h before (pretreatment) or 5 h after (posttreatment) E. coli LPS injection. The pretreatment with VAL restored the behavioral alterations and the expression of cortical proinflammatory cytokines in LPS-injected animals but had no effects on BDNF expression, while the posttreatment rescued only behavioral alterations. Our results demonstrate for the first time the positive effects of VAL in an experimental model of depression associated with inflammation, providing new data on the range of action of these molecules.
1. Introduction
Major depressive disorder (MDD) is a recurrent and incapacitating mood disorder being related to high mortality and morbidity [1]. Despite the fact that the accepted “monoamine hypothesis” contributed to the comprehension about the neurobiology of mood disorders, the pathogenesis of MDD has not been completely elucidated yet [2]. Thus, the identification of novel biological targets and pathways that may play a role in MDD pathophysiology is required.
In this regard, several studies have been pointing to the association of the immune system activation with MDD [3, 4]. Of note, depressed patients display elevated plasma levels of proinflammatory cytokines such as interleukin- (IL-) 1β, IL-6, and tumor necrosis factor-alpha (TNF-α) [2, 5–7] as well as increased expression of proinflammatory cytokines in frontal cortex [8]. Also, some studies showed that the association of anti-inflammatories to conventional antidepressants increased the efficacy of these drugs [9–11].
Preclinical studies have been demonstrating that systemic administration of Escherichia coli lipopolysaccharide (LPS) to rodents results in behavioral time-dependent changes related to increased peripheral and central proinflammatory cytokines production [3, 12, 13]. It is known that LPS-injected rodents present behavioral signs of sickness (such as decreased locomotor and exploratory activities) that are followed by depressive-like behavior [3]. The switch from sickness to depression occurs after the activation of indoleamine 2,3-dioxygenase, which culminates in decreased central serotonin levels [14].
Recently, evidences have suggested the involvement of disrupted neuroplasticity in MDD pathophysiology [3, 15]. Furthermore, hippocampal neurogenesis and neurotrophins (especially the brain derived neurotrophic factor, BDNF) expression are thought to be necessary for the behavioral effects of antidepressants [16, 17]. In addition, some authors demonstrated that intraperitoneal administration of LPS to rodents is correlated with decreased BDNF levels in the hippocampus and cortex [13, 18, 19].
Noteworthy, the administration of antidepressants [18, 20] to animals subjected to the above mentioned model of depression prevents the development of sickness and depressive-like behavior. Interestingly, the antidepressant potential of anti-inflammatory drugs has been demonstrated in the forced swimming test in models of depression related to stress in rats [21]. Also, the anti-inflammatory properties of natural products in LPS-injected rodents have been shown by several studies [22–24].
In line with these observations, some studies have been pointing to the anti-inflammatory properties of the Valeriana genus, which is widely known by its sedative and anxiolytic properties [25], representing a new approach to the therapeutic use of the genus. The anti-inflammatory potential of species such as V. wallichii [26], V. amurensis [27], and V. officinalis, which prevents the sickness and depressive-like behavior in rats submitted to a model of depression related to inflammation [28], has been demonstrated. Furthermore, some authors have already shown the antidepressant potential of those species [25, 29, 30].
In this sense, our research group has been investigating the pharmacological properties of V. glechomifolia Meyer, one species native to southern Brazil that is especially enriched in valepotriates [31]. Valepotriates are nonglycosylated carbocyclic iridoids, comprising a family of terpenes [32] that contribute to the pharmacological properties of the genus [33–36]. The antidepressant potential of diene valepotriates was demonstrated by our research group as well as its action on noradrenergic and dopaminergic neurotransmission [37], suggesting a dual-action mechanism distinct of most of the conventional antidepressants.
Considering the above mentioned data, in the present study we investigated the effects of a diene valepotriates fraction (VAL) obtained from V. glechomifolia on sickness and depression-like behavior triggered by intraperitoneal administration of E. coli LPS, in mice previously submitted to a forced swimming session as a stressful stimulus [13]. We also investigated VAL effects on the cortical expression of proinflammatory cytokines (IL-1β and TNF-α) and BDNF. This experimental protocol was chosen on the basis of the concept that internal and external stressors interact, resulting in an illness state that causes an allostatic overload [38, 39].
2. Materials and Methods
2.1. Drugs and Chemicals
For the extract characterization, grade HPLC acetonitrile and methanol were purchased from Merck (Germany). For the behavioral experiments, the following drugs were used: imipramine from Henrifarma (Porto Alegre, Brazil) and lipopolysaccharide (LPS) from Escherichia coli serotype 0111:B4 from Sigma Chemical Co. (St. Louis, MO, USA). All chemicals were obtained in the highest grade.
2.2. Plant Material
Valeriana glechomifolia above-ground and below-ground material was collected during its flowering stage in the region of São José dos Ausentes, state of Rio Grande do Sul, Brazil. The collection was authorized by SISBIO-IBAMA (protocol n° 29495-1). The identification was performed by Dr. M. Sobral (Universidade Federal de São João del-Rei, state of Minas Gerais, Brazil) and a voucher specimen (Sobral, 7733) was deposited in the Herbarium of the Universidade Federal do Rio Grande do Sul (ICN), Brazil.
2.3. VAL Extraction and Characterization by HPLC
To obtain the VAL fraction, 100 g (dry weight) of dried and powdered plant material was submitted to supercritical CO2 (SCCO2) extraction, using a Pilot Equipment as described elsewhere [37, 40, 41]. The conditions of the extraction were 40°C, 90 bar, SCCO2 flow rate through the extraction vessel: 6.67 × 10−4 kg s−1. The SCCO2 extraction recovery was 2.96 g%.
The VAL fraction was dissolved in HPLC grade methanol and filtered (0.22 μm pore size, Merck) before the analysis by HPLC according to a method previously described [37, 41, 42], using Shimadzu HPLC system and Waters Nova-Pack C18 column (4 mm, 3.9 × 150 mm i.d. with Waters Nova-Pack C-18 guard column, 60 Å, 3.9 × 20 mm). The isocratic mobile phase consisted of acetonitrile and water (50 : 50 v/v); flow rate of 1 mL/min; UV detection at 254 nm. All diene valepotriates were quantified in terms of mg of valtrate equivalent/g extract. The VAL fraction was suspended in saline with 1% of polysorbate 80 (vehicle) prior to use.
2.4. Animals
Male CF1 mice (25–30 g) were from Fundação Estadual de Produção e Pesquisa em Saúde, Rio Grande do Sul, Brazil. Animals were housed in plastic cages (17 × 28 × 13 cm) at 23° ± 1°C under a 12-hour light/dark cycle, with food and water provided ad libitum. Experiments were approved by Animal Care Local Ethical Committee (CEUA-UFRGS; protocol n° 22648) and were conducted in accordance with Brazilian law [43–45] and European Communities Council Directive of 24 November 1986 (86/609/EEC).
2.5. Experimental Design
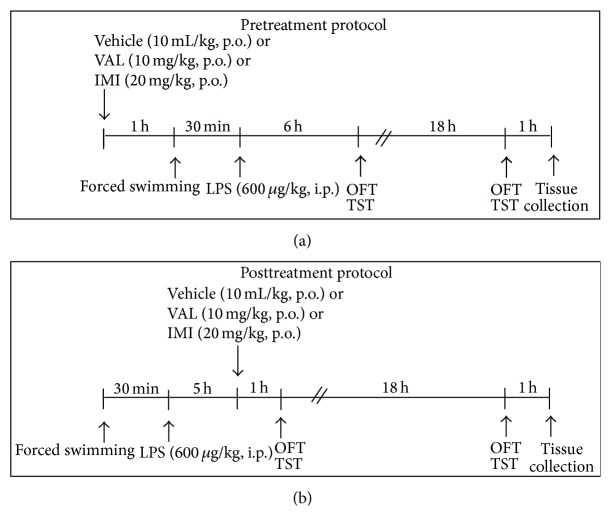
The experimental protocol was carried out according to Viana and coworkers [13] with minor modifications. The animals (n = 8–11 mice/group) were submitted to a prestressful stimulus (6 min forced swimming session, water at 23 ± 1°C) 30 min before receiving LPS from E. coli (600 μg/kg, i.p.) or vehicle (0.9% NaCl solution, 10 mL/kg, i.p.). The animals were submitted to behavioral tests 6 h and 24 h after the LPS injection.
In order to verify the effects of VAL on forced swim + LPS-induced behavioral and neurochemical alterations, independent groups of mice were treated with VAL (10 mg/kg, p.o.) or vehicle (0.9% NaCl solution with 1% of polysorbate 80) 1 h before the forced swimming session (pretreatment protocol, in order to evaluate their preventive potential) or 5 h after LPS injection (posttreatment protocol, in order to evaluate their therapeutic potential). The antidepressant imipramine (IMI) was used as a positive control (20 mg/kg, p.o.). The doses of VAL and IMI were determined on the basis of previous studies from our group [37]. Mice were sacrificed by cervical dislocation 1 h after the final behavioral test for quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis. A naïve group was included for RT-qPCR analysis. A schematic overview of the experimental design is shown in Figure 1.
Figure 1.
Experimental timeline and design. OFT: open field test; TST: tail suspension test.
2.6. Behavioral Paradigms
2.6.1. Open Field Test (OFT)
To assess the effects of the forced swimming session + LPS treatment (as well as the effects of VAL and IMI before and after treatment) on the locomotor activity, mice were evaluated in the OFT, 6 or 24 h after LPS administration. Animals were individually placed in an acrylic box (40 × 30 × 30 cm), with the floor being divided into 24 equal squares. The number of squares crossed with the four paws (crossing) was recorded in a 6 min session.
2.6.2. Tail Suspension Test (TST)
Mice depression-like behavior following the forced swimming session + LPS treatment (as well as the effects of VAL and IMI before and after treatment) was evaluated by using the TST as previously described by Steru and coworkers [46] with minor modifications. Immediately after being submitted to the OFT (6 or 24 h after LPS injection), the animals were suspended by the tail 60 cm above the floor using adhesive tape (1 cm from the tip of the end). The time during which mice remained immobile was recorded (in seconds) during the last 4 min of a total 6 min session [47].
2.7. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR) for Quantification of IL-1β, TNF-α, and BDNF mRNA Expression
Mice cortices were collected and immediately immersed in liquid nitrogen and stored at −80°C until use. Total RNA was extracted using TRIzol Reagent (Invitrogen), according to the manufacturer's instructions. The concentration and purity of the RNA were assessed at 260 nm and 260/280 nm absorbance measurements, respectively. Also, its integrity was evaluated by using agarose gel electrophoresis stained with ethidium bromide. Complementary DNA (cDNA) was synthesized from 2 μg of total RNA using a High Capacity cDNA Transcription kit (Applied Biosystems Inc., Foster City, CA) in a 10 μL reaction. After obtaining the cDNA, samples were stored at −20°C. IL-1β, TNF-α, and BDNF expression was carried out through fluorescence-based real-time PCR, by amplifying 100 ng of cDNA (in duplicates) using TaqMan-based chemistry with specific primers, FAM-labeled probes (Assays-by-Demand, Life Technologies) for mouse IL-1β (#Mm00434228_m1), TNF-α (#Mm00443260_g1), and BDNF (#Mm00432069_m1) and VIC-labeled glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Assays-by-Demand, Life Technologies, #Mm99999915_g1) as the endogenous control for normalization. Amplifications were carried out in a thermal-cycler (StepOne Plus, Applied Biosystems) for 70 cycles; the fluorescence was collected at each amplification cycle and the data were analyzed using the 2−ΔΔCt method for relative quantification. Expression of the target genes was calibrated against conditions found in naïve mice.
2.8. Statistical Analysis
Data from behavioral experiments and RT-qPCR were analyzed by two- or one-way ANOVA, respectively, followed by Student-Newman-Keuls test when appropriate. The statistical procedures were performed using the Sigma Stat software 2.03 (Jandel Scientific Corporation). The level for statistical significance was set as p < 0.05. The results are given as mean ± S.E.M.
3. Results
3.1. VAL Fraction Characterization
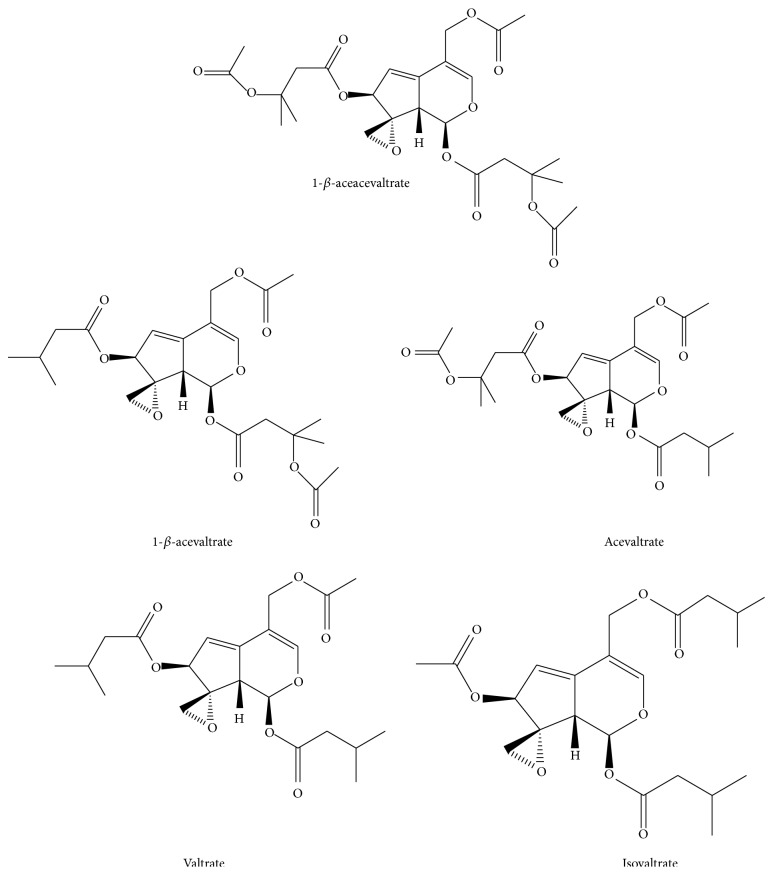
The characterization of the VAL fraction by HPLC revealed that valtrate was the diene valepotriate present in larger quantity (643 ± 56 mg/g), followed by acevaltrate (172 ± 34 mg/g), 1-β-acevaltrate (87 ± 9 mg/g), 1-β-aceacevaltrate (39 ± 5 mg/g), and isovaltrate (37 ± 6 mg/g). Results are expressed in mean ± S.D. HPLC chromatograms of the VAL fraction have already been published [41]. The chemical structures of each diene valepotriate are presented in Figure 2.
Figure 2.
Diene valepotriates found in Valeriana glechomifolia.
3.2. Effects of VAL and IMI on Forced Swim + LPS-Induced Sickness and Depression-Like Behavior
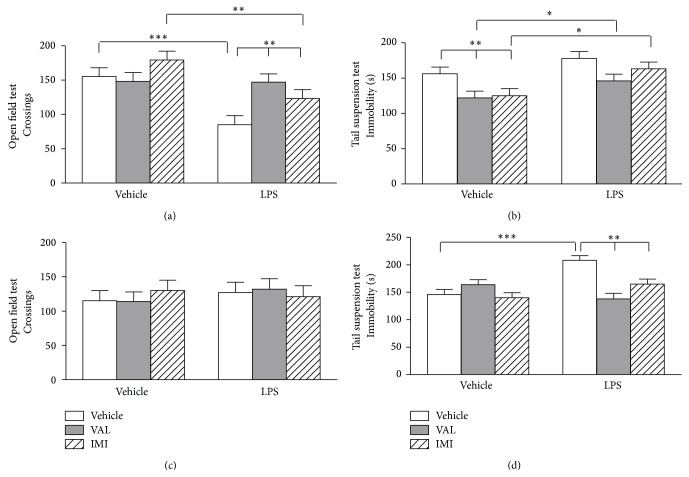
The effects of VAL or IMI pretreatment protocol on forced swim + LPS-induced behavioral alterations are presented in Figure 3. The forced swim + LPS administration elicited a significant (p < 0.001) decrease in mice locomotor activity at 6 h after the injection (Figure 3(a)), which was prevented by mice pretreatment with VAL and IMI [F pretreatment(1,59) = 17.589, p < 0.001; F forced swim+LPS(1,59) = 4.037, p < 0.05; F pretreatment×forced swim+LPS(1,59) = 4.307, p < 0.05]. The oral administration of VAL and IMI significantly (p < 0.01) decreased mice immobility time in the TST 6 h after vehicle injection when compared to the vehicle-vehicle treated group and this effect was not observed when the animals were pretreated with VAL or IMI and received the LPS injection (Figure 3(b)) [F pretreatment(1,59) = 11.556, p < 0.001; F forced swim+LPS(1,59) = 5.922, p < 0.01; F pretreatment×forced swim+LPS(1,59) = 0.398, p = 0.674]. The locomotor activity of the animals returned to basal levels at 24 h (Figure 3(c)) [F pretreatment(1,59) = 0.0001; p = 0.992; F forced swim+LPS(1,59) = 0.122, p = 0.885; F pretreatment×forced swim+LPS(1,59) = 1.431, p = 0.248]. The forced swim + LPS administration significantly (p < 0.001) increased mice immobility time in the TST 24 h after the injection (Figure 3(d)) when compared to vehicle-vehicle treated animals, while VAL and IMI pretreatment prevented the forced swim + LPS-induced immobility injection [F pretreatment(1,59) = 6.601; p < 0.05; F forced swim+LPS(1,59) = 4.721, p < 0.05; F pretreatment×forced swim+LPS(1,59) = 10.863, p < 0.001].
Figure 3.
Effects of diene valepotriates from V. glechomifolia (VAL) pretreatment on sickness and depressive-like behavior induced by a stressful stimulus (6 min forced swimming session) + E. coli LPS injection in mice. The animals were orally treated with VAL (10 mg/kg, p.o.) or imipramine (IMI, used as a positive control, 20 mg/kg, p.o.) 1 hour before being exposed to the forced swim + LPS and were evaluated in the open field and tail suspension tests 6 h (Panels (a) and (b)) and 24 h after (Panels (c) and (d)) the immune challenge. Each column represents the mean ± S.E.M (n = 8–12 mice/group). Two-way ANOVA, post hoc Student-Newman-Keuls test. ∗ p < 0.05; ∗∗ p < 0.01; ∗∗∗ p < 0.001.
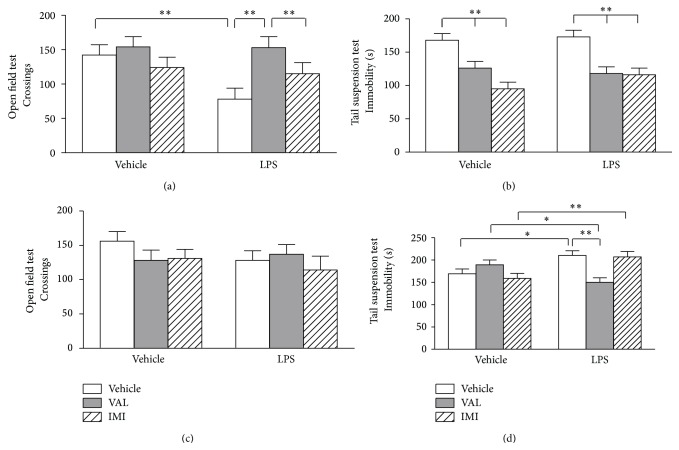
The effects of VAL or IMI posttreatment on forced swim + LPS-induced behavioral alterations are shown in Figure 4. Mice posttreatment with VAL, but not with IMI, prevented the forced swim + LPS-induced decrease in the locomotor activity assessed by the OFT 6 h after LPS injection (Figure 4(a)) [F forced swim+LPS(1,58) = 9.755, p < 0.01; F post treatment(1,58) = 8.3691, p < 0.001; F forced swim+LPS×post treatment(1,59) = 0.918, p = 0.406]. Mice orally posttreated with VAL and IMI presented a significant (p < 0.01) decrease in the immobility time in the TST 6 h after vehicle or LPS injection (Figure 4(b)) [F forced swim+LPS(1,59) = 0.479, p = 0.492; F post treatment(1,58) = 21.114, p < 0.001; F post treatment×forced swim+LPS(1,59) = 0.920, p = 0.406]. The locomotor activity of the animals returned to basal levels 24 h after LPS injection, as can be seen in Figure 4(c) [F forced swim+LPS(1,59) = 0.135, p = 0.715; F post treatment(1,59) = 0.896, p = 0.415; F post treatment×forced swim+LPS(1,59) = 2.036, p = 0.141]. Only VAL posttreatment was able to prevent (p < 0.01) the forced swim + LPS-induced increase in the immobility time in the TST 24 h after LPS injection [F forced swim+LPS(1,59) = 3.405, p = 0.071; F post treatment(1,59) = 1.749, p = 0.184; F post treatment×forced swim+LPS(1,59) = 9.876, p < 0.001].
Figure 4.
Effects of diene valepotriates from V. glechomifolia (VAL) posttreatment on sickness and depressive-like behavior induced by a stressful stimulus (6 min forced swimming session) + E. coli LPS injection in mice. The animals were orally treated with VAL (10 mg/kg, p.o.) or imipramine (IMI, used as a positive control, 20 mg/kg, p.o.) 5 hours after being exposed to the forced swim + LPS and were evaluated in the open field and tail suspension tests 6 h (Panels (a) and (b)) and 24 h after (Panels (c) and (d)) the immune challenge. Each column represents the mean ± S.E.M (n = 8–12 mice/group). Two-way ANOVA, post hoc Student-Newman-Keuls test. ∗ p < 0.05; ∗∗ p < 0.01.
3.3. Effects of VAL and IMI on Forced Swim + LPS-Induced Alterations in the Expression of IL1-β, TNF-α, and BDNF Expression in the Cortex of Mice
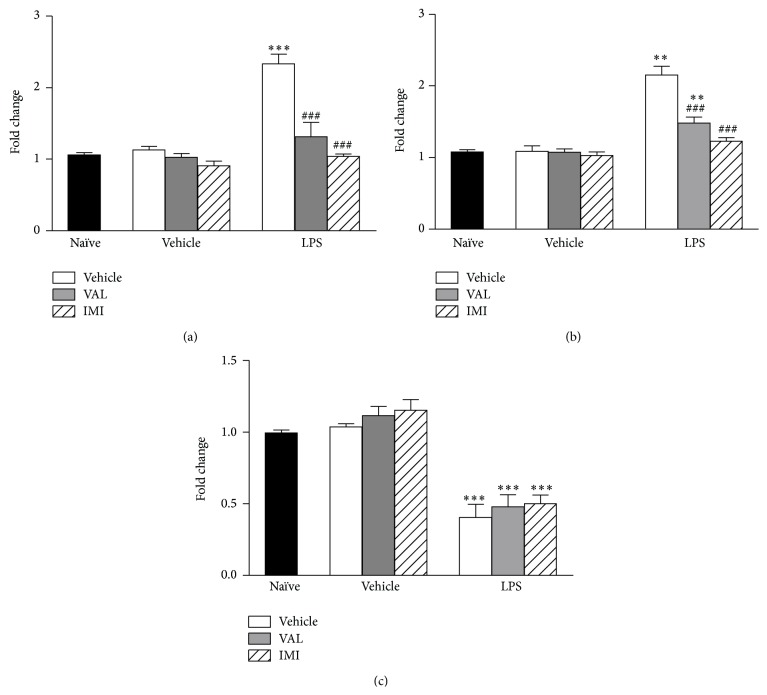
The effects of pretreatment with VAL and IMI on forced swim + LPS-induced changes in the cortical expression of IL1-β, TNF-α, and BDNF are shown in Figure 5. The forced swim + LPS elicited a significant increase in the expression of IL1-β (p < 0.001) (Figure 5(a)) and TNF-α (p < 0.01) (Figure 5(b)) in the cortex of mice, while VAL and IMI pretreatment prevented this effect [IL1-β: F(6,28) = 23.76, p < 0.001; TNF-α: F(6,28) = 32.21, p < 0.001]. Cortical BDNF expression was significantly (p < 0.001) reduced by the forced swim + LPS (Figure 5(c)). Nevertheless, mice pretreatment with VAL and IMI was not effective in preventing this effect [F(6,28) = 25.48, p < 0.001].
Figure 5.
Effects of diene valepotriates from V. glechomifolia (VAL) pretreatment on the alterations in the cortical expression of IL1-β (Panel (a)), TNF-α (Panel (b)), and BDNF (Panel (c)) induced by a stressful stimulus (6 min forced swimming session) + E. coli LPS injection in mice. The animals were orally treated with VAL (10 mg/kg, p.o.) or imipramine (IMI, used as a positive control, 20 mg/kg, p.o.) 1 hour before being exposed to the forced swim + LPS and the tissues were collected 25 h later. Each column represents the mean (n = 4 mice/group). One-way ANOVA, post hoc Student-Newman-Keuls test. ∗∗ p < 0.01; ∗∗∗ p < 0.001 versus Naïve group; ### versus Vehicle+LPS-treated group.
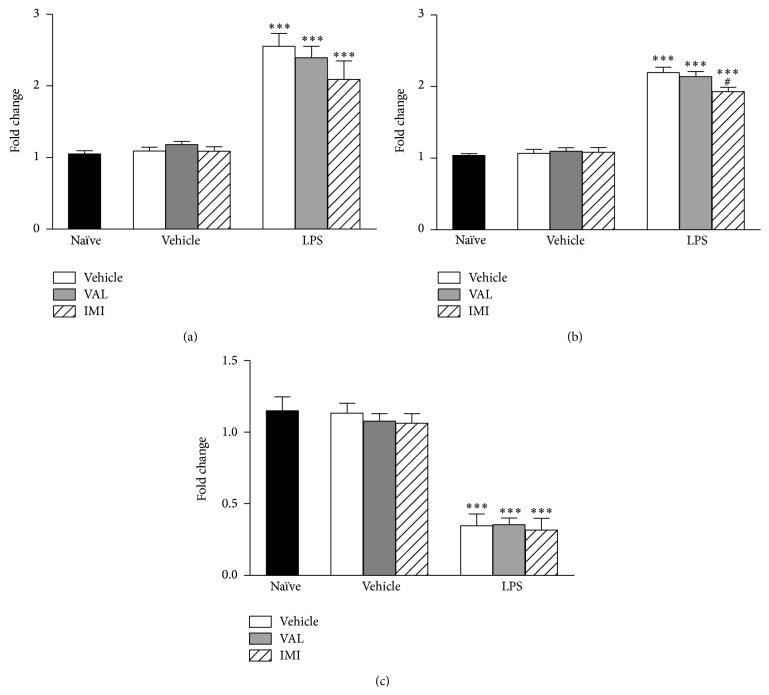
The effects of mice posttreatment with VAL and IMI on forced swim + LPS-induced alterations in the expression of IL1-β, TNF-α, and BDNF in mice cortex are depicted in Figure 6. The forced swim + LPS significantly increased (p < 0.001) the expression of IL1-β (Figure 6(a)) and TNF-α (Figure 6(b)), while a significant (p < 0.001) decrease in BDNF expression was found in the cortex of mice. The posttreatment with VAL or IMI did not prevent these alterations in the expression of proinflammatory cytokines [IL1-β: F(5,28) = 23.89, p < 0.001; TNF-α: F(5, 28) = 83.54, p < 0.001] or BDNF [F(5,58) = 31.84, p < 0.001].
Figure 6.
Effects of diene valepotriates from V. glechomifolia (VAL) posttreatment on the alterations in the cortical expression of IL1-β (Panel (a)), TNF-α (Panel (b)), and BDNF (Panel (c)) induced by a stressful stimulus (6 min forced swimming session) + E. coli LPS injection in mice. The animals were orally treated with VAL (10 mg/kg, p.o.) or imipramine (IMI, used as a positive control, 20 mg/kg, p.o.) 5 hours after being exposed to the forced swim + LPS and the tissues were collected 25 h later. Each column represents the mean (n = 4 mice/group). One-way ANOVA, post hoc Student-Newman-Keuls test. ∗∗∗ p < 0.001 versus Naïve group; # p < 0.05 versus Vehicle + LPS-treated group.
4. Discussion
The present study demonstrated the positive effects of Valeriana glechomifolia diene valepotriates (VAL) in an animal model of depression that correlates the activation of inflammatory pathways with the manifestation of depression-like behavior. Also, the antidepressant-like effect of VAL was accompanied by normalization in the expression of cortical proinflammatory cytokines in E. coli LPS-injected animals previously submitted to a 6 min swimming session as a stressful stimulus. These findings are in accordance with Neamati and coworkers' study [28], who reported that a hydroalcoholic extract of V. officinalis prevented the development of sickness and depression-like behavior in ovalbumin sensitized rats, which is an animal model of depression associated with inflammation. In line with these findings, accumulating evidence points to the antidepressant and anti-inflammatory potential of Valeriana species [25–27, 29, 30, 48].
Sickness behavior is a usual response to infection characterized by endocrine, autonomic, and behavioral changes triggered by the activation of the peripheral innate immune system [3]. In rodents, the sickness behavior can be detected by a reduction in locomotor activity and exploratory behaviors [49]. Herein, we demonstrated that the administration of LPS to mice previously submitted to a 6 min swimming session significantly decreased the locomotor activity 6 h after LPS, indicating the development of sickness behavior. There were no differences between the immobility time of the vehicle-treated and LPS-treated groups in the TST 6 h after the peripheral immune challenge, corroborating with the hypothesis that depression-like behavior develops over a background of sickness and peaks 24 h later [3]. No alterations in mice locomotor activity were observed 24 h after the immune challenge, confirming that the increased immobility time in the TST 24 h after LPS is due to the manifestation of depression-like behavior.
The pre- and posttreatment with VAL resulted in normalization of behavioral alterations elicited by LPS in mice previously submitted to a forced swimming session. On the other hand, mice posttreatment with IMI was not able to restore the forced swim + LPS-triggered effects. In fact, there are several studies in the literature demonstrating the preventive, but not the therapeutic, effects of antidepressants or natural products on LPS-induced behavioral alterations [13, 23, 24, 50]. However, other authors [18] demonstrated that mice posttreatment with IMI restored the depressive-like behavior elicited by the LPS injection. This could be due to the different experimental protocols used, since the animals used in our study were submitted to a forced swimming session before LPS injection (600 μg/kg) and received an oral administration of IMI, whereas Ferreira Mello and coworkers [18] performed the LPS injection only (500 μg/kg) and the administration of IMI by the i.p. route.
In our experiments, the antidepressant-like effects of VAL and IMI were accompanied by a decrease in the expression of IL-1β and TNF-α in mice cortex, which is a cerebral area related to the pathophysiology of depression [51, 52]. Consistently, the pivotal proinflammatory cytokines involved in sickness and depression-related behaviors following infection are IL-1β and TNF-α [3]. Thus, the effects of VAL and IMI on these cytokines may explain the positive results of VAL and IMI pretreatment in the behavioral tests. Of note, Jacobo-Herrera and coworkers [53] demonstrated that sesquiterpenes from V. officinalis showed inhibitory activity against the nuclear factor NF-kB in in vitro studies. These findings are particularly relevant, since this nuclear factor has been considered to play a role in the proinflammatory signaling pathway, mainly in the expression of proinflammatory genes including cytokines, such as IL-1β and TNF-α [54] and is activated upon the interaction of Toll-like receptors with the LPS [55]. Considering that the valepotriates comprise a large family of terpenes [32], we may suggest that these compounds decrease the production of proinflammatory cytokines by inhibiting the NF-kB activation and modulating the activation of cytokine signaling, which results in the decrease of cortical cytokines expression and, consequently, normalization of behavioral alterations. In line with our findings, some ex vivo studies demonstrated that antidepressants decrease the levels of proinflammatory cytokines in the serum and brain of LPS-injected rodents [18, 20]. These data are supported by in vitro studies demonstrating the anti-inflammatory potential of antidepressants by using central and peripheral derived cells [56–58]. Also, the inhibition of microglia activation by terpenes has been demonstrated in an in vitro study [59] as well as the anti-inflammatory potential of these molecules in vivo [60–62]. To the best of our knowledge, there are no previous studies regarding the anti-inflammatory properties of diene valepotriates.
Interestingly, mice posttreatment with VAL, but not with IMI, prevented the forced swim + LPS-induced behavioral alterations and had no effects on IL-1β and TNF-α expression in the cortex, while VAL pretreatment decreased the cortical expression of IL-1β and TNF-α. These findings may suggest that the effects of VAL posttreatment on behavioral alterations could be due to its action on dopaminergic and noradrenergic neurotransmissions. Our research group showed that the acute antidepressant-like effect of VAL was prevented by mice pretreatment with yohimbine (α2 adrenoceptor antagonist), SCH 23390 (D1 dopamine receptor antagonist), and sulpiride (D2 dopamine receptor antagonist) [37]. The findings of the present study may also suggest that VAL prevents the activation of IL-1β and TNF-α signaling cascades, but it is not able to block these cascades once they were activated. Another possibility is that other brain regions, than cortex, might be involved in the anti-inflammatory effects of VAL posttreatment.
Our results demonstrate that the stressful stimulus followed by the peripheral immune challenge decreases the cortical expression of BDNF mRNA at 24 h after LPS and these findings are in agreement with several studies that used animal models of depression associated to inflammation [13, 18–20]. However, mice before and after treatment with VAL or IMI were not effective in preventing these alterations. In fact, several authors have reported that the acute administration of conventional antidepressants, including IMI, is not able to increase the expression of BDNF [63–67]. Moreover, our results suggest that VAL could exert its effects in the cortex acting mainly by inhibiting an inflammatory pathway and not by interfering with BDNF.
5. Conclusion
Altogether, the results so far reinforce the antidepressant-like potential of V. glechomifolia diene valepotriates and demonstrate the positive effects of these compounds in an animal model that associates the activation of inflammatory pathways to depression etiology. The behavioral effects of diene valepotriates administration to the animals were accompanied by the normalization of proinflammatory cytokines expression, which brings a new focus on the range of action of these molecules.
Acknowledgments
The authors wish to acknowledge CNPq (Grant 480647/2011-9) and CAPES (PRODOC Edital 2010) Brazilian agencies as well as Programa de Pós Graduação em Ciências Farmacêuticas (PPGCF-UFRGS) for the financial support. The authors are thankful to Dr. Eduardo Cassel, Dr. Rubem Vargas, and Dr. Gilsane Lino von Poser, for assistance with the extraction and characterization of the fraction.
Abbreviations
- ANOVA:
Analysis of variance
- BDNF:
Brain derived neurotrophic factor
- HPLC:
High performance liquid chromatography
- IL:
Interleukin
- IMI:
Imipramine
- LPS:
Lipopolysaccharide
- MDD:
Major depressive disorder
- OFT:
Open field test
- RT-qPCR:
Quantitative reverse transcription polymerase chain reaction
- S.E.M:
Standard error of the mean
- SCCO2:
Supercritical CO2
- SISBIO-IBAMA:
Sistema de Autorização e Informação em Biodiversidade do Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis
- TNF-α:
Tumor necrosis factor alpha
- TST:
Tail suspension test
- VAL:
Diene valepotriates fraction.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Kessler R. C., Berglund P., Demler O., et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Journal of the American Medical Association. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Hannestad J., Dellagioia N., Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36(12):2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dantzer R., O'Connor J. C., Freund G. G., Johnson R. W., Kelley K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maes M., Mihaylova I., Kubera M., Uytterhoeven M., Vrydags N., Bosmans E. Increased plasma peroxides and serum oxidized low density lipoprotein antibodies in major depression: markers that further explain the higher incidence of neurodegeneration and coronary artery disease. Journal of Affective Disorders. 2010;125(1–3):287–294. doi: 10.1016/j.jad.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Maes M. The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuroendocrinology Letters. 2008;29(3):287–291. [PubMed] [Google Scholar]
- 6.Dowlati Y., Herrmann N., Swardfager W., et al. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 7.Raison C. L., Miller A. H. Is depression an inflammatory disorder? Current Psychiatry Reports. 2011;13(6):467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shelton R. C., Claiborne J., Sidoryk-Wegrzynowicz M., et al. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Molecular Psychiatry. 2011;16(7):751–762. doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendlewicz J., Kriwin P., Oswald P., Souery D., Alboni S., Brunello N. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. International Clinical Psychopharmacology. 2006;21(4):227–231. doi: 10.1097/00004850-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Akhondzadeh S., Jafari S., Raisi F., et al. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depression and Anxiety. 2009;26(7):607–611. doi: 10.1002/da.20589. [DOI] [PubMed] [Google Scholar]
- 11.Abbasi S.-H., Hosseini F., Modabbernia A., Ashrafi M., Akhondzadeh S. Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: randomized double-blind placebo-controlled study. Journal of Affective Disorders. 2012;141(2-3):308–314. doi: 10.1016/j.jad.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Frenois F., Moreau M., O'Connor J., et al. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32(5):516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viana A. F., Maciel I. S., Dornelles F. N., et al. Kinin B1 receptors mediate depression-like behavior response in stressed mice treated with systemic E. coli lipopolysaccharide. Journal of Neuroinflammation. 2010;7, article no. 98 doi: 10.1186/1742-2094-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller N., Myint A.-M., Schwarz M. J. Inflammatory biomarkers and depression. Neurotoxicity Research. 2011;19(2):308–318. doi: 10.1007/s12640-010-9210-2. [DOI] [PubMed] [Google Scholar]
- 15.Ota K. T., Duman R. S. Environmental and pharmacological modulations of cellular plasticity: role in the pathophysiology and treatment of depression. Neurobiology of Disease. 2013;57:28–37. doi: 10.1016/j.nbd.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saarelainen T., Hendolin P., Lucas G., et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. The Journal of Neuroscience. 2003;23(1):349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adachi M., Barrot M., Autry A. E., Theobald D., Monteggia L. M. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biological Psychiatry. 2008;63(7):642–649. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira Mello B. S., Monte A. S., McIntyre R. S., et al. Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. Journal of Psychiatric Research. 2013;47(10):1521–1529. doi: 10.1016/j.jpsychires.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Guo J., Lin P., Zhao X., et al. Etazolate abrogates the lipopolysaccharide (LPS)-induced downregulation of the cAMP/pCREB/BDNF signaling, neuroinflammatory response and depressive-like behavior in mice. Neuroscience. 2014;263:1–14. doi: 10.1016/j.neuroscience.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Ohgi Y., Futamura T., Kikuchi T., Hashimoto K. Effects of antidepressants on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacology Biochemistry and Behavior. 2013;103(4):853–859. doi: 10.1016/j.pbb.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Santiago R. M., Barbiero J., Martynhak B. J., et al. Antidepressant-like effect of celecoxib piroxicam in rat models of depression. Journal of Neural Transmission. 2014;121(6):671–682. doi: 10.1007/s00702-014-1159-5. [DOI] [PubMed] [Google Scholar]
- 22.Nöldner M., Schötz K. Inhibition of lipopolysaccharid-induced sickness behavior by a dry extract from the roots of Pelargonium sidoides (EPs 7630) in mice. Phytomedicine. 2007;14(1):27–31. doi: 10.1016/j.phymed.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Veloso C. C., Bitencourt A. D., Cabral L. D. M., et al. Pyrostegia venusta attenuate the sickness behavior induced by lipopolysaccharide in mice. Journal of Ethnopharmacology. 2010;132(1):355–358. doi: 10.1016/j.jep.2010.07.053. [DOI] [PubMed] [Google Scholar]
- 24.Park S.-M., Choi M.-S., Sohn N.-W., Shin J.-W. Ginsenoside Rg3 attenuates microglia activation following systemic lipopolysaccharide treatment in mice. Biological & Pharmaceutical Bulletin. 2012;35(9):1546–1552. doi: 10.1248/bpb.b12-00393. [DOI] [PubMed] [Google Scholar]
- 25.Hattesohl M., Feistel B., Sievers H., Lehnfeld R., Hegger M., Winterhoff H. Extracts of Valeriana officinalis L. s.l. show anxiolytic and antidepressant effects but neither sedative nor myorelaxant properties. Phytomedicine. 2008;15(1-2):2–15. doi: 10.1016/j.phymed.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 26.Khuda F., Iqbal Z., Khan A., Nasir F., Shah Y. Anti-inflammatory activity of the topical preparation of Valeriana wallichii and Achyranthes aspera leaves. Pakistan Journal of Pharmaceutical Sciences. 2013;26(3):451–454. [PubMed] [Google Scholar]
- 27.Zhang Z.-L., Zuo Y.-M., Wang Q.-H., Xiao H.-B., Kuang H.-X. Effects of Valeriana amurensis on the expressions of iNOS, COX-2 and IkappaCB-alpha in Alzheimer's disease model rat's brain. Zhong Yao Cai. 2010;33(4):581–583. [PubMed] [Google Scholar]
- 28.Neamati A., Chaman F., Hosseini M., Boskabady M. H. The effects of Valeriana officinalis L. hydro-alcoholic extract on depression like behavior in ovalbumin sensitized rats. Journal of Pharmacy and Bioallied Sciences. 2014;6(2):97–103. doi: 10.4103/0975-7406.129174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subhan F., Karim N., Gilani A. H., Sewell R. D. E. Terpenoid content of Valeriana wallichii extracts and antidepressant-like response profiles. Phytotherapy Research. 2010;24(5):686–691. doi: 10.1002/ptr.2980. [DOI] [PubMed] [Google Scholar]
- 30.Sah S. P., Mathela C. S., Chopra K. Antidepressant effect of Valeriana wallichii patchouli alcohol chemotype in mice: behavioural and biochemical evidence. Journal of Ethnopharmacology. 2011;135(1):197–200. doi: 10.1016/j.jep.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Salles L. D. A., L. Silva A., Rech S. B., Zanatta N., Von Poser G. L. Constituents of Valeriana glechomifolia Meyer. Biochemical Systematics and Ecology. 2000;28(9):907–910. doi: 10.1016/s0305-1978(99)00124-6. [DOI] [PubMed] [Google Scholar]
- 32.Geu-Flores F., Sherden N. H., Courdavault V., et al. An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis. Nature. 2012;491(7427):138–142. doi: 10.1038/nature11692. [DOI] [PubMed] [Google Scholar]
- 33.Andreatini R., Leite J. R. Effect of valepotriates on the behavior of rats in the elevated plus-maze during diazepam withdrawal. European Journal of Pharmacology. 1994;260(2-3):233–235. doi: 10.1016/0014-2999(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 34.Backlund A., Moritz T. Phylogenetic implications of an expanded valepotriate distribution in the Valerianaceae. Biochemical Systematics and Ecology. 1998;26(3):309–335. doi: 10.1016/s0305-1978(97)00121-x. [DOI] [Google Scholar]
- 35.Andreatini R., Sartori V. A., Seabra M. L. V., Leite J. R. Effect of valepotriates (valerian extract) in generalized anxiety disorder: a randomized placebo-controlled pilot study. Phytotherapy Research. 2002;16(7):650–654. doi: 10.1002/ptr.1027. [DOI] [PubMed] [Google Scholar]
- 36.Maurmann N., Reolon G. K., Rech S. B., Fett-Neto A. G., Roesler R. A valepotriate fraction of valeriana glechomifolia shows sedative and anxiolytic properties and impairs recognition but not aversive memory in mice. Evidence-Based Complementary and Alternative Medicine. 2011;2011:7. doi: 10.1093/ecam/nep232.720853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller L. G., Salles L. A., Stein A. C., et al. Antidepressant-like effect of Valeriana glechomifolia Meyer (Valerianaceae) in mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;36(1):101–109. doi: 10.1016/j.pnpbp.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Progress in Neuropsychopharmacology and Biological Psychiatry. 1995;19(1):11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- 39.Robinson M. J., Edwards S. E., Iyengar S., Bymaster F., Clark M., Katon W. Depression and pain. Frontiers in Bioscience. 2009;14(13):5031–5051. doi: 10.2741/3585. [DOI] [PubMed] [Google Scholar]
- 40.Cassel E., Vargas R. M. F., Brun G. W., et al. Supercritical fluid extraction of alkaloids from Ilex paraguariensis St. Hil. Journal of Food Engineering. 2010;100(4):656–661. doi: 10.1016/j.jfoodeng.2010.05.015. [DOI] [Google Scholar]
- 41.Müller L. G., de Andrade Salles L., Sakamoto S., et al. Effect of storage time and conditions on the diene valepotriates content of the extract of Valeriana glechomifolia obtained by supercritical carbon dioxide. Phytochemical Analysis. 2012;23(3):222–227. doi: 10.1002/pca.1346. [DOI] [PubMed] [Google Scholar]
- 42.Silva A. L., Rech S. B., Von Poser G. L. Quantitative determination of valepotriates from Valeriana native to South Brazil. Planta Medica. 2002;68(6):570–572. doi: 10.1055/s-2002-32544. [DOI] [PubMed] [Google Scholar]
- 43. Ministério Público, Brasília, Brazil, Lei no. 11.794, de 8 de outubro de 2008, Publicada no DOU 9.10.2008.
- 44.Ministério da Ciência. Diretrizes da prática de eutanásia do CONCEA. Portaria no. 596, de 25 de junho de 2013, Brasília, Brazil, 2013.
- 45.Ministério da Ciência. Diretriz brasileira para o cuidado e a utilização de animais para fins científicos e didáticos—DBCA. Portaria no. 465, de 23 de maio de 2013, Brasília, Brazil, 2013.
- 46.Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85(3):367–370. doi: 10.1007/bf00428203. [DOI] [PubMed] [Google Scholar]
- 47.Kwon S., Lee B., Kim M., Lee H., Park H.-J., Hahm D.-H. Antidepressant-like effect of the methanolic extract from Bupleurum falcatum in the tail suspension test. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34(2):265–270. doi: 10.1016/j.pnpbp.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 48.Neill M., Dixon P. S. Effects of a preincisional 14-day course of valerian on natural killer cell activity in Sprague-Dawley male rats undergoing abdominal surgery. Holistic Nursing Practice. 2007;21(4):187–193. doi: 10.1097/01.hnp.0000280930.75883.e7. [DOI] [PubMed] [Google Scholar]
- 49.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain, Behavior, and Immunity. 2001;15(1):7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 50.Lee J.-S., Song J.-H., Sohn N.-W., Shin J.-W. Inhibitory effects of ginsenoside Rb1 on neuroinflammation following systemic lipopolysaccharide treatment in mice. Phytotherapy Research. 2013;27(9):1270–1276. doi: 10.1002/ptr.4852. [DOI] [PubMed] [Google Scholar]
- 51.Nestler E. J., Carlezon W. A., Jr. The mesolimbic dopamine reward circuit in depression. Biological Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 52.Krishnan V., Nestler E. J. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobo-Herrera N. J., Vartiainen N., Bremner P., Gibbons S., Koistinaho J., Heinrich M. NF-κB modulators from Valeriana officinalis . Phytotherapy Research. 2006;20(10):917–919. doi: 10.1002/ptr.1972. [DOI] [PubMed] [Google Scholar]
- 54.Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harbor Perspectives in Biology. 2009;1(6) doi: 10.1101/cshperspect.a001651.a001651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Souza J. A. C., Rossa C., Jr., Garlet G. P., Nogueira A. V. B., Cirelli J. A. Modulation of host cell signaling pathways as a therapeutic approach in periodontal disease. Journal of Applied Oral Science. 2012;20(2):128–138. doi: 10.1590/s1678-77572012000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diamond M., Kelly J. P., Connor T. J. Antidepressants suppress production of the Th1 cytokine interferon-γ, independent of monoamine transporter blockade. European Neuropsychopharmacology. 2006;16(7):481–490. doi: 10.1016/j.euroneuro.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 57.Horikawa H., Kato T. A., Mizoguchi Y., et al. Inhibitory effects of SSRIs on IFN-γ induced microglial activation through the regulation of intracellular calcium. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34(7):1306–1316. doi: 10.1016/j.pnpbp.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 58.Liu D., Wang Z., Liu S., Wang F., Zhao S., Hao A. Anti-inflammatory effects of fluoxetine in lipopolysaccharide(LPS)-stimulated microglial cells. Neuropharmacology. 2011;61(4):592–599. doi: 10.1016/j.neuropharm.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 59.Chen H., Xie C., Wang H., et al. Sesquiterpenes inhibiting the microglial activation from laurus nobilis. Journal of Agricultural and Food Chemistry. 2014;62(20):4784–4788. doi: 10.1021/jf501515v. [DOI] [PubMed] [Google Scholar]
- 60.Jardón-Delgado A., Magos-Guerrero G. A., Martínez-Vázquez M. Isolation of a new anti-inflammatory 20, 21, 22, 23, 24, 25, 26, 27-octanorcucurbitacin-typetriterpene from Ibervillea sonorae . Natural Product Communications. 2014;9(1):15–16. [PubMed] [Google Scholar]
- 61.Juergens U. R. Anti-inflammatory properties of the monoterpene 1.8-cineole: current evidence for co-medication in inflammatory airway diseases. Drug Research. 2014;64(12):638–646. doi: 10.1055/s-0034-1372609. [DOI] [PubMed] [Google Scholar]
- 62.Tang S.-A., Zhu H., Qin N., et al. Anti-inflammatory terpenes from flowers of Inula japonica . Planta Medica. 2014;80(7):583–589. doi: 10.1055/s-0034-1368353. [DOI] [PubMed] [Google Scholar]
- 63.Coppell A. L., Pei Q., Zetterström T. S. C. Bi-phasic change in BDNF gene expression following antidepressant drug treatment. Neuropharmacology. 2003;44(7):903–910. doi: 10.1016/s0028-3908(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 64.Molteni R., Calabrese F., Bedogni F., et al. Chronic treatment with fluoxetine up-regulates cellular BDNF mRNA expression in rat dopaminergic regions. International Journal of Neuropsychopharmacology. 2006;9(3):307–317. doi: 10.1017/S1461145705005766. [DOI] [PubMed] [Google Scholar]
- 65.Duman R. S., Monteggia L. M. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 66.Castrén E., Võikar V., Rantamäki T. Role of neurotrophic factors in depression. Current Opinion in Pharmacology. 2007;7(1):18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 67.Martinowich K., Manji H., Lu B. New insights into BDNF function in depression and anxiety. Nature Neuroscience. 2007;10(9):1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]