Abstract
Background
Trophic factors (TFs) play important role during development and adult tissue maintenance. In neurodegenerative diseases (ND) TF supplementation provides protection. Stromal cells (HUMS) derived from the human umbilical cord matrix provide neuroprotection in the ND models of mice.
Purpose
Though TF mediated protection is known, the exact mechanism of protection is not clear. So, here the essential TFs (secreted by HUMS cells) and the pathway of induction of neurite extension, differentiation and networking is addressed.
Methods
The HUMS cells from the human umbilical cord matrix were derived and the mouse spinal cord motor neuron cell line, NSC-34 was extensively used. Flow cytometry, immunohistochemistry, RT- PCR, western blot, ELISA and antibody/inhibitor treatment were carried out to figure out the TF pathway.
Results
The HUMS cells secrete six neurotrophic factors (sTFs), namely, NT-3, NGF, BDNF, VEGF, IGF-1 and GDNF (TFs). These TFs are sufficient to induce differentiation, neurite extension and neural networking in a motor neuron cell line, NSC34. All the 5 TFs need to be neutralized simultaneously with their antibodies to abrogate neurite extension. These motor neurons express the concomitant receptors, which are either receptor tyrosine kinase (TrK) coupled or to the receptor followed by the TrKs, for the above trophic factors (except for BDNF). The tyrosine kinase inhibitor, K252a, drastically reduces neurite extension. In NSC34, the TFs are coupled to the PI3K–Akt–pathway and the RAS-MAP kinase signaling through phosphorylation of ERK1 and ERK2. PI3K inhibitor, Ly 294002, abolishes neural differentiation and neurite extension. Thus, differentiation, neurite extension and networking could be achieved through the PI3K pathway. Intriguingly, the cAMP second messenger system coupling was not required. H89, PKA-inhibitor caused extensive cell death. But, had no effect in the presence of HUMS-secreted-TFs(HSTFs) suggesting a pathway switch for cell survival itself.
Conclusion
HUMS cells and their secreted factors could be of great use in regenerative medicine (RM). The activators of PI3K pathway, the major route of these HUMS-TFs action could be explored in RM and in the neurobiology of neural differentiation and extension.
Keywords: Human umbilical cord, Motor neurons, Neurotrophic factors, Differentiation, PI3K, MSC, Tyrosine kinase
 Introduction
Introduction
The secreted trophic factor (TF) signaling is crucial for growth, development, differentiation, formation and maintenance of various organs and complex systems.1 The TFs could be of various types like mitogenic – growth factors, cytokines and neurotrophins. The neurotrophins are crucial for neuronal growth, differentiation and plasticity. Some of the known neurotrophic factors2 are: the neurotrophin family consisting of nerve growth factor (NGF), brain derived neurotrophic growth factor (BDNF) and NT-3 which act through the tyrosine kinase (TrK) receptors, insulin like growth factor-1 (IGF-1), glial derived neurotrophic factor (GDNF) and vascular endothelial growth factor (VEGF) which have a TrK domain in the receptors or coupled to a TrK. GDNF receptor couples to tyrosine kinase receptor RET. They act through complex signaling network. Interestingly, their downstream intracellular- signalling converges.
One commonality between many neurodegenerative diseases like Alzheimer’s disease,3 Amyotrophic lateral sclerosis,4 Huntington disease5 and Parkinson’s disease is reduced/impaired trophic support. In some diseases like ALS, transgenic or lentivirus mediated supplementation of trophic factors delays the disease progression and increases survival.4–6 But, this cannot be applied to human patients. Hence, an alternative approach is needed.
Protection against various neurodegenerative diseases (ND) and stroke requires regeneration of the nervous system (Central and Peripheral) - neurons, including axon and dendrite growth and networking to restore normalcy. Generation of various tissue types from the embryonic7 (Thomson et al., 1998) or adult tissues to stem cells8–10 has caused both hype and hope for potential cell replacement therapy for these diseases11,12 mainly as a source of trophic factor support in various diseases.8,13–15 The healthy stem cell based approach is essential to derive the differentiated cells,16,17 trophic support8,13,15 and immune modulation. As human ES cells are ridden with ethical controversy and teratoma formation, other potential human tissue based approach is essential. Therefore, various adult tissues are being explored to obtain stem cells. One of the most effective cells is mesenchymal stem cells (MSC) derived from bone marrow.18 But, they have limitations like: less number of cells, invasive procurement and long time lag for the cells to proliferate and more importantly less cell homing and survival in vivo.19 Moreover, in some diseases like Amyotrophic lateral sclerosis, the autologous stem cells are defective.20 Hence, we explore here the non controversial, abundantly available tissue, (with inbuilt immunosuppression capacity) the human umbilical cord, to obtain human umbilical cord matrix stromal (HUMS) cells that could be provided in a scaffold or multiple times as a universal MSC source.
Mesenchymal stromal cells are shown to provide protection in stroke through secreted trophic factor, VEGF.11 In neurodegenerative diseases, the exact mechanism of the TF mediated protection is not well understood. One of the anticipated protection mechanisms is by trophic factors which induce neural differentiation, neurite outgrowth and networking. For this, they activate multiple signaling pathways21–23 downstream of their receptor tyrosine kinases or the receptors coupled to tyrosine kinase. They are: PI3K-Akt; Mitogen activated kinase pathway through Ras-ERK1-Elk1 or instead of ELK1 the cAMP response element binding protein CREB; Phospholipase Cg which activates the DAG, Ca2+ and phosphoinositide pathway; the adaptor protein (SH2-containing Protein Tyrosine Phosphatase-2) and Suc-Associated Neurotrophic Factor-Induced Tyrosine Phosphorylated Target-SNT signaling pathways, to name a few.
Here, we address i) derivation of HUMS cells from the human umbilical cord ii) the secreted trophic factors of HUMS cells and iii) induction of differentiation, neurite extension and networking iv) the signaling through PI3K- MAPK pathway for (iii) in the motor neuron cell line, NSC34 by the TFs of HUMS cells.
Methods
In vitro culture of HUMS cells: Propagation and proliferation
The standard ethical guidelines were followed. First, the human umbilical cords were obtained just before disposal from full-term births from local obstetricians after informed consent. Then, the umbilical cord was washed several times with PBS to flush out the blood followed by removal of the umbilical blood vessels. The remaining tissue was cut into small pieces. The explants (~ 2- to 5-cm lengths) were placed in 60 mm culture dishes and cultured in DMEM medium with high glucose (Hyclone) supplemented with 10% fetal bovine serum (FBS) (Invitrogen), penicillin G (100 units/ml), streptomycin (100 µg/ml), and amphotericin B (25 mg/ml).24 The cells were incubated at 37°C in the incubator with 5% CO2 and 95% humidity. The plates were left undisturbed for 5 to 7 days and thereafter medium was changed every 3rd day. The cells begin to appear in 8 to 10 days of culture. When cells reached 70% to 80% confluency, the cells were detached with 0.25% trypsin-EDTA (Hyclone) and passaged subsequently.
Immunocytochemistry
The cells were taken and fixed with acetone (for c-KIT) at -20oC or 4% paraformaldehyde (for OCT-4) at room temperature for 15 min. The immunocytochemistry was carried out as described in Rajan et al.25 Primary antibodies concentrations used were 1:10 for c-KIT and OCT-4 (Cell Signalling). Secondary antibody (Jackson Immuno Research Laboratories) was used at 1:500 dilution. All cultures were counterstained with 1mg/ml Hoechst 33342 (Sigma-Aldrich) to visualize the nucleus in individual cells.
FACS and ELISA
Around 70% confluent HUMS cells were harvested and stained with the fluorophore- tagged antibodies against CD markers following the provided protocols (Serotec). The quantitation of the TFs was carried out using the standard ELISA kits (R & D Systems).
RT-PCR
Total RNA was extracted from umbilical cord MS cell cultures (P5-8) or the NSC34 motor neurons (27) using TRI reagent (Sigma). RNA was then reverse transcribed (RT) with MMLV reverse transcriptase using random hexamers in the presence of RNAse inhibitor. Primers used are given in (Table 1). PCR was carried out using Veriti Thermal Cycler (Applied Biosystems). The amplicons were then separated by agarose gel electrophoresis (1–1.8%).
Table 1: Primers for RT-PCR.
| Primer Name | Sequence | PCR Prdt (bp) | Primer Name | Sequence | PCR Prdt (bp) |
|---|---|---|---|---|---|
| Oct4-FP | gacaacaatgaaaatcttca | 218 | TrkA-FP | aaccatcgtgaagagtggcct | 525 |
| Oct4- RP | ttctggcgccggttacagaa | TrkA-RP | attctctgcccagcacgtca | ||
| Nanog-FP | caaaggcaaacaacccactt | 140 | TrkB-FP | taacagcgttgacccggaga | 362 |
| Nanog-RP | tctgctggaggctgaggtat | TrkB-RP | acaattgggtatctgcaggt | ||
| sox2-FP | tgaaccagcgcatggacagtta | 410 | TrkC-FP | agcaagactgagatcaattg | 502 |
| sox2-RP | gctgggacatgtgaagtctg | TrkC-RP | atcacactgactgatgttcatg | ||
| Alk.Phs-FP | Atatgtggctctgtccaagaca | 350 | GRa1-FP | gatcagtgcctgaaggaaca | 450 |
| Alk.Phs-RP | aatgtccatgttggagatgagct | GRa1-RP | tgcagacttcattggacatg | ||
| GDNF-FP | atcagttcgatgatgtcatggat | 330 | GRa2-FP | attgtatgactgccgctgca | 740 |
| GDNF-RP | gccttctatttctggataagt | GRa2-RP | cagggcagctggtgattgt | ||
| BDNF-FP | tgacatcattggctgacact | 285 | GRa3-FP | tgactacgagttggatgtct | 550 |
| BDNF-RP | ttacccactcactaatactgtca | GRa3-RP | tgttgaccttgctgatgaagt | ||
| VEGF-FP | aagttcatggatgtctatcag | 198 | VEGFR2-FP | acctcacctgtttcctgtat | 500 |
| VEGF-RP | cataatctgcatggtgatgt | VEGFR2-RP | agcacctctctcgtgattt | ||
| NGFb-FP | aagctgcagacactcaggat | 394 | IGF1R-FP | agagattgcagatggcatg | 600 |
| NGFb-RP | cgtatctatccggataaacc | IGF1R-RP | gacgctctccatgttctca | ||
| CNTF-FP | aagattcgttcagacctgact | 311 | |||
| CNTF-RP | agtatcattaactcctctat | ||||
| IGF-1 FP | tcttgaaggtgaagatgcac | 277 | |||
| IGF1-RP | ggtgcgcaatacatctcca | ||||
| NT-3-FP | aagctgatccaggcagatat | 261 | |||
| NT-3 -RP | gtaatcctccatgagatacaa |
HUMS cells Conditioned Medium (CM)
HUMS cell cultures at 70% confluence were maintained in DMEM medium with high glucose (Hyclone) supplemented with 10% fetal bovine serum (FBS), penicillin G (100 units/ml), streptomycin (100 µg/ml), and amphotericin B (25mg/ml) at 37°C for 48 hours. The medium of the HUMS cells referred to as conditioned medium (CM) from these cultures was collected, filtered through 0.2mm filter (Millipore) and used for neurite outgrowth assay.
Neurite outgrowth assay
NSC34, a mouse spinal cord motor neuron cell line26 was plated at a density 1 × 103 cells/ml in 35mm culture plate(s). After 24 hours, the medium was replaced with CM. Control NSC34 cells were maintained in normal growth medium (DMEM supplemented with 10% FBS) under the same conditions. Neurite outgrowth was measured after 48 and 96 hours.
Quantification of the differentiated and undifferentiated NSC34 cells
Identification of the differentiated & undifferentiated NSC34 cells was done on the basis of their characteristic size and distinctive morphology. The undifferentiated cells are smaller in size and generally circular in shape while the differentiated cells are bigger with neuronal morphology and having extensive neurites. Approximately 100–150 cells/field were quantified in each set of experiments and the fraction of differentiated cells was determined after 48 hours and 96 hours.
Measurement of neurite outgrowth
NSC34 cells were viewed using phase contrast microscopy (OlympusI×71). Images were acquired using CCD camera (Jenoptik) and analysed using ImageProPlus software. Images were taken of 5 non-overlapping visual fields (using 10 × objective) for each culture condition and in 3 independent experiments. Neurite lengths of every NSC34 cell (~100–150 cells/field) within each field of view were measured by tracing the lengths of the neurites using the measurement tool of ImageProPlus software.
Trophic factor neutralization/inhibitors treatment of the NSC34 cells
Trophic factor neutralization with antibodies
For this, antibodies against human GDNF, BDNF, NT-3, NGF, VEGF and IGF-1 (SantaCruz) were used. Around 1 mg/ml antibody was added to the CM and incubated with shaking for 4 hrs at room temperature.27
Inhibitors
The inhibitor treatment was carried out following the standard protocols with minor modifications. For the inhibitor studies, initially a dose response was carried out to determine the optimal concentration and applied for further assays. All the inhibitors were purchased from Sigma Aldrich and DMSO was used as the solvent. These were diluted with the culture medium to specific concentrations just before use. In all instances, the vehicle control was the same volume of DMSO. For the tyrosine kinase inhibitor –K252a28 – 20 nM; and PI3K inhibitor, Ly29400229 – 50 mM; adenylate cyclase inhibitor, SQ22536 - 500 mM, cAMP antagonist Rolipram-cAMP – 500 mM and the Protein kinase A inhibitor, H89 – 20 mM30 were used.
The NSC34 cells were plated at a cell density of 5 X102 /well in a 12 well plate and grown with 500 ml of either regular medium or CM for 24 hrs. Then the antibodies neutralized CM/vehicle –DMSO/inhibitor was added and maintained at 37oC in the CO2 incubator for 48 hrs. The motor neuron (NSC34) cells were scored for inhibition of neurite extension. The cells were viewed and photographed in an Olympus IX71 inverted microscope.
Cell Viability
Cell viability was determined by the standard MTT assay. Briefly, the cells were washed with PBS twice, followed by the addition of 1ml of 5mg/ml MTT in PBS and incubated at 37oC for 3 hrs. This solution was removed and 1 ml of formazan solubilizing solution was added, kept for 15 min., solubilised and O.D. was measured at 570 nm.
Western Blotting
Western blotting was carried out as described in Rajan et al.25
Statistical Analysis
Mean, standard deviation and P values were calculated using the Statistical Analysis software Sigma Plot 10.0. In all the graphs the error bars represent standard deviation. The P values were determined using t-test.
Results
Derivation and propagation of HUMS cells
When the human umbilical cord Wharton jelly devoid of the blood and blood vessels were placed in the regular culture medium in vitro, colonies started growing from them (Fig. 1A-a). From these, individual cells could be obtained (Fig. 1A-b). These cells, when passaged, became the source of the HUMS cells. The HUMS cells could be maintained in in vitro cultures for more than 4 months. These were mixed population of cells. The HUMS cells were positive for several pluripotency markers like OCT-4 (Fig. 1B- and b) & c-KIT (Fig.1 B- c & d), nanog, sox -2 and alkaline phosphatase (Fig. 1C). These cells were CD44+ CD73+ CD90 + CD105 + CD34+ and HLA-DR- (Fig. 1 D).
Fig. 1:
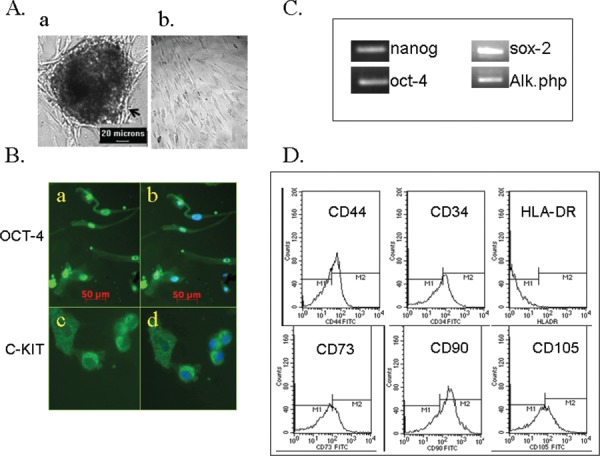
Derivation and characterization of HUMS cells.
A. a. The HUMS cells are forming from the colony. b. Individual HUMS cells in vitro. Scale bar- 20 mm.
B. & C. HUMS cells are positive for pluripotency markers B. a. OCT-4 –green; Scale bar- 50 mm.
C. C-KIT- green; b & d. Merged with the nuclear DNA staining (blue). C. RT-PCR for pluirpotentcy markers – nanog, sox-2, alkaline phosphatase and oct-4.
D. The HUMS cells are CD44+CD73+ CD90+CD105+ and HLA-DR–.
Neurotrophic factors of the HUMS cells and their signaling pathway for differentiation, neurite extension and networking in the motor neuron cell line
The MSCs are known to home in the injured site and provide protection through paracrine factors and immune modulation. Hence, we measured the neurotrophic factors secreted by the HUMS cells. First, we carried out the expression analysis (Fig. 2A), quantitated the content and determined their functionality (Fig. 2B and C).
Fig. 2:
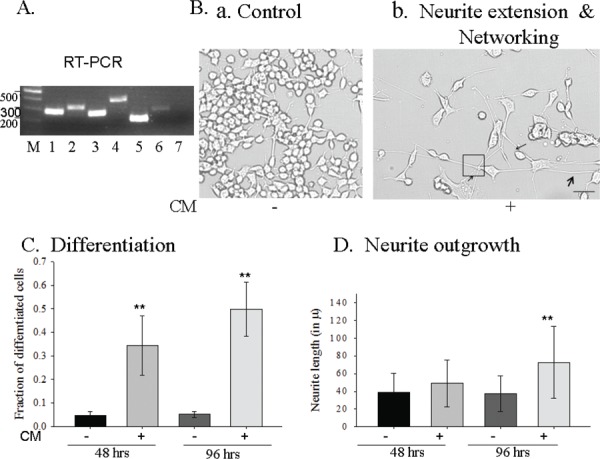
HUMS cells expressed- trophic factors and their functionality.
Trophic factor expression- RT-PCR : 1- BDNF, 2- GDNF, 3- NT-3, 4- NGF, 5-VEGF, 6- IGF-1 and 7- CNTF. B-D. Functionality of HUMS cells secreted trophic factors in the CM.
B. Motor neuronal cells, NSC34, are: a. rarely differentiated; b. Neuronal differentiation, extensive long neurite outgrowth and networking upon CM treatment; Arrows- neurite extension; Square –several neurites networking. Scale bar = 100 mm.
C. ~3 fold and ~5 fold neuronal differentiation upon 48 hrs and 96 hrs CM treatment respectively.
D. CM induces extensive long neurite outgrowth. Statistical significance- ** -P&0.001. Error bars represent standard deviation.
Expression analysis
The HUMSs were expressing an array of trophic factors. They were expressing the neurotrophin family of trophic factors, NGF, BDNF and NT-3 (Fig. 2A) as determined by the mRNA expression RT-PCR. Interestingly, all the three neurotrophic factors are expressed in the HUMS cells. We further analysed the expression of other trophic factors which act as neurotrophic factors for neurons, namely, GDNF, IGF-1, VEGF and CNTF. Of these, except CNTF the other three TFs were expressed (Fig. 2A- RT-PCR). Thus, the HUMS cells were expressing several trophic factors. Of these, the neurotrophin family TFs, BDNF, GDNF and NT-3 are expressed at high levels (Table 2). The rest of the three trophic factor levels were below the detection of spectrophotometric ELISA method. Then we addressed whether these trophic factors are functional.
Table 2: Trophic factors quantitation.
| Trophic Factor | Quantity (ng/ml) |
|---|---|
| BDNF | 2.4 ± 0.059 |
| GDNF | 2.4 ± 0.56 |
| NT-3 | 1.2 ± 0.035 |
Functionality of the neurotrophic factors secreted by the HUMS cells
HUMS cells conditioned medium (CM) induce differentiation in the motor neuron cell line, NSC34.
The well established mouse spinal cord motor neuron cell line, NSC34 mostly remain undifferentiated under normal conditions. The undifferentiated cells are smaller, generally grow in clusters or aggregates and have rounded morphology (Fig. 2B-a). After treatment with CM, there was significantly higher number of differentiated cells (Fig. 2B-b and 2C), which increased with time (Fig. 2C). Differentiated NSC34 cells exhibited typical neuronal morphology (Fig. 2B-b), were bigger in size and had long characteristic neurites. A proportional increase in the number of differentiated cells (Fig. 2C) clearly seen after 48 hours and 96 hours of CM treatment further validates the functionality of trophic factors present in the conditioned medium.
HUMS cells CM induce neurite extension and networking of the motor neurons
Generally, neurotrophic factors induce differentiation and neurite outgrowth in the neuronal cells. The secreted-TFs from the HUMS cells, present in the CM robustly induced differentiation, neurite extension (Fig. 2B-b, arrows) and more importantly networking (shown in the black square Fig 2B-b) in the mouse spinal cord motor neuron cell line, NSC34 (Fig 2B-b, D). The neurites’ extension increased with longer incubation with the CM. Around 0.6 mm long neurite extension could be achieved when the NSC34 motor neurons were treated with CM for 6 days. Thus, proving that the TFs expressed in the HUMS cells are functional and they can induce differentiation and neurite outgrowth (arrows) (Fig. 2B-D). Moreover, only when we inhibited all the 5 TFs with their respective antibodies simultaneously, the neurite extension was abolished (Fig. 3B) to the control level indicating several backup mechanisms for neurite extension in the motor neurons.
Fig. 3:
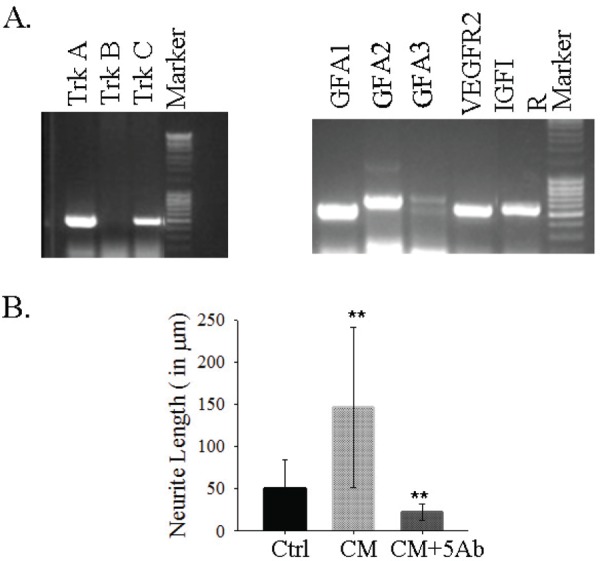
Neurotrophic factors requirement for neurite extension.
A. TF receptors expression in the motor neuron cell line.
B. Five trophic factors expressed/secreted by HUMS cells are required for neurite extension. CM induces extensive long neurite outgrowth in the motor neuron cell line, NSC34, which is inhibited by the simultaneous neutralization with all the 5 Trophic Factors’ antibodies excluding BDNF. Statistically significant. **- P<0.001.
Trophic factor mediated neurite extension is dependent on tyrosine kinase
In order to determine that the trophic factors and their signaling contributed to neurite extension, we determined the expression of receptors for the TFs that were found to be secreted by HUMS cells. Except for the BDNF receptor, Trk B, the motor neuron cell line expressed the receptors for NGF- TrkA; NT-3- TrkC; GDNF- GDNFRa1, a2 and a3; VEGF receptor–Flk-1 or VEGFR2; IGF1 receptor -IGF1R as determined by RT-PCR (Fig. 3A). Further, to reinforce that the TFs are indeed functional and acting through their tyrosine kinase pathway, we treated the motor neuron cells with the TrK inhibitor, K252a. K252a had the effect in a narrow window of 20 nM. At 2 nM the motor neurons cells were healthy with long processes (data not shown) while 20 nM shortened the processes drastically. The higher concentration of 200 nM became toxic to the cells with a flattened and bloated appearance with thin neurites in few cells. The reduction in neurites with K252a provides the evidence that TFs are acting through the TrKs.
The TFs act through the PI3K and MAPK pathway
Though the TFs use multiple pathways to bring about neuronal differentiation and neurite outgrowth, the pathway utilized will depend on the cell type. One of the important pathways is through PI3K-Akt. When the motor neurons cells in the CM was treated with the PI3K inhibitor Ly294002, the neurite extension and differentiation was completely abolished at the routinely used concentration of 50 mM (Fig. 4B-c). Hence, PI3K –Akt pathway is the major player in the motor neuron differentiation and neurite extension. Additionally, the motor neurons utilize the MAPK pathway of RAS- ErK1/2 as ErK1/2 is strongly phosphorylated (~30% increase) upon CM treatment (Fig. 5 A & B). Thus, these two pathways seem to be the major pathways in the motor neurons for differentiation and neurite extension.
Fig. 4:
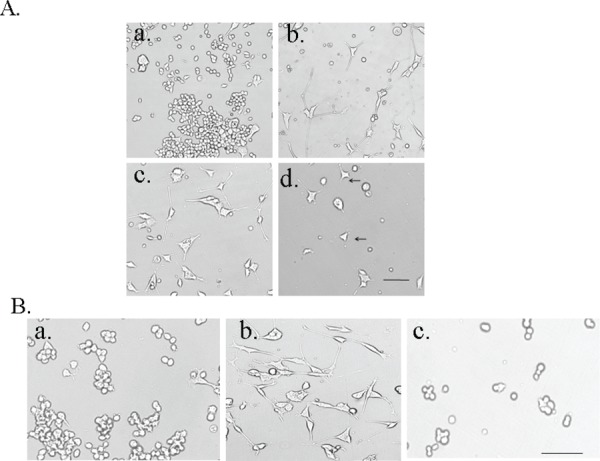
Mechanism of induction of neurite extension.
A. TF receptors tyrosine kinases are needed for neurite extension, Inhibition with TrK inhibitor K252a, drastically reduces neurite extension. a. Control +DMSO; b) CM; c) CM+DMSO; d) CM+K252a. Scale bar = 100 mm.
B. The TFs activate the PI3K- Akt pathway in the motor neurons for neurite extension. a. control; b. CM; c. CM + Ly294002 – PI3K inhibitor blocks differentiation and neurite extension. Scale bar = 100 mm.
Fig. 5:
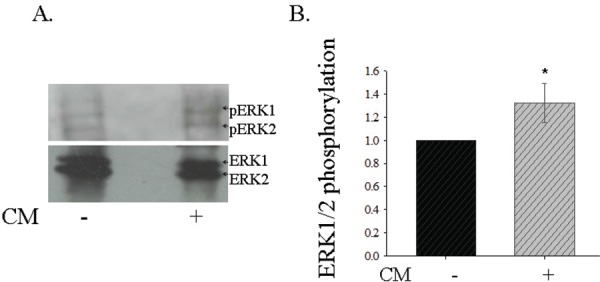
Identification of the signal cascade coupling for motor neuron differentiation.
Phosphorylation of the MAP kinases ERK1 and ERK2 is increased upon CM treatment of motor neurons. A. Ctrl- Control –without CM treatment.
Treated - CM treatment B. Fold increase in ERK1/2 phosphorylation upon CM treatment. P<0.01.
The TFs act independent of the cAMP pathway
To further understand the signaling cascades activated by the TFs in the motor neurons, we treated the motor neurons with and without CM with cAMP pathway activators forskolin (adenylate cyclase activator), Dibutyryl cAMP (dbcAMP – analog of cAMP). Both these treatments did not induce any differentiation of neurite extension in the motor neurons without CM. No increase in the differentiation, neurite extension and networking was noticed with these cAMP activators in the presence of the cAMP activators. But the motor neurons do require optimal cAMP signaling as these cells were dead in the presence of higher concentration of forskolin or H89 the Protein kinase A inhibitor. Similarly, treatment with the inhibitors of the cAMP pathway, SQ22536 and Rp-cAMP did not have any effect on the motor neurons with and without CM (Fig. 6). Thus, the motor neurons do not require the cAMP cascade for differentiation and networking in the presence of the CM.
Fig. 6:
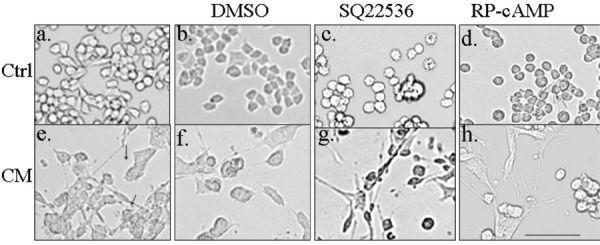
TFs mediated differentiation, neurite extension and networking is independent of the cAMP pathway.
a -d: Ctrl – Control; e-h – CM - Conditioned medium treated; c & g: SQ22536; d& h: Rp-cAMP. Inhibition of adenylate cyclase (SQ22536) or treatment with cAMP antagonist (Rp-cAMP) did not affect neurite extension by CM. Scale bar = 100 mm.
CM switches the Motor neuron cell line survival pathway
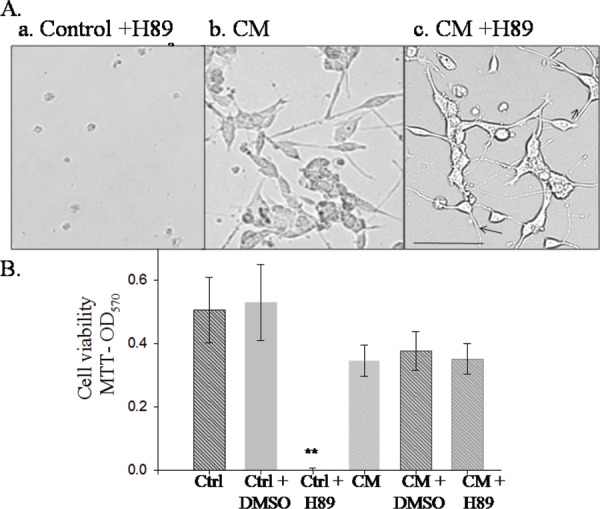
Despite generally known as the specific cAMP pathway Protein kinase A inhibitor, H89, completely inhibits MSK1, ROCKII and RSK or S6K in in vitro kinase assay. In order to inhibit PKA, we treated the motor neuron cells with H89. This led to almost complete death while motor neurons treated with CM did not show cell death (Fig. 7A and B). Intriguingly a pathway switch is happening for cell survival itself, when the TFs in the CM are inducing differentiation and neurite extension in the motor neurons. Moreover, H89 induces more extensive networking in the CM treated motor neurons suggesting that a combination of HUMS. cells and H89 could be tried for the protection in the ALS model mice/rats.
Fig. 7:
A & B. CM switches the pathway for motor neuron cell survival.
a. Control; b. CM +DMSO; c. CM + H89.
H89 treatment causes cell death in the control but not in the CM treated motor neurons. Scale bar = 100 mm.
Discussion
Stem cells have given hope for the treatment of chronic and fatal diseases which currently have no treatment. The potential source of stem cells vary from embryonic stem cells, autologous bone marrow derived mesenchymal stem cells and induced pluirpotent stem cells by reprogramming of autologous tissues.10 But, the autologous approach will not be applicable in situations where the derived MSCs too are defective. For example, in ALS, MSCs from either patients or ALS model are defective.20 Hence, an allologous source is crucial to treat such diseases. Towards this, here, we have derived Matrix stromal cells from the human umbilical cord (non-controversial, highly abundant with inbuilt immunosuppression) (Fig. 1) and characterized them for the expression of pluripotency markers like c-Kit,32 Oct-4, nanog,33 sox-2 and alkaline phosphatase (Fig. 1D) and their capacity to induce neurite outgrowth (Fig. 2), networking and the pathway (Fig 3–7) through which this process is brought about.
Earlier reports have shown that human umbilical cord could be a good source of HUMS cells.24 The HUMS cells are CD44+ CD73+ CD90 + CD105 + CD34+ and HLA-DR- (Fig. 1C).31 Here, we show that the HUMS cells secret six trophic factors NT-3, NGF, BDNF, GDNF, VEGF and IGF-1 but not CNTF. Of these, the neurotrophin family TFs, BDNF, GDNF and NT-3 are expressed at high levels of 2.414 ± 59 ng/ml, 2.4 ng/ml and 1.2 ng/ml, respectively. More importantly, the TF containing CM induced extensive neurite extension and networking in the mouse spinal cord motor neuron cell line, NSC34.26 Unlike the DRG neurons, where NGF and BDNF blocking could suffice,34 the motor neuron cell line needed all five trophic factors to be neutralized to abrogate differentiation and neurite extension. While the motor neuron cell line expressed the receptors for all the trophic factors, the common TF, BDNF receptor TrKB was conspicuously absent (Fig. 4A) in NSC34. This opens a new window for treating/activation of neurite extension and neurogenesis independent of BDNF in neurodegenerative diseases as well as in the complex process of learning and memory.
Trophic factors are known to act through multiple signaling cascades which are essentially determined by the cell type. In the CM- treated motor neurons neurite extension, the involvement of Trk receptors are validated through their inhibition with (20 nM) K252a (Fig. 5A). Further, in order to delineate the specific signaling cascade, we treated these NSC34 cells with and without CM with PI3K inhibitor. The TF signaling was predominantly brought about through PI3K-Akt pathway as evidenced by the inhibition of neurite extension by the PI3K inhibitor, LY294002 (Fig. 4B).28 Further the MAPK pathway of Ras - ERK1/ERK2 is utilized as evidenced by the increased phosphorylation of ERK1/2 (Fig.5). The major corroborating evidence is neural regrowth after spinal cord injury through PI3K pathway.35 Hence, PI3K-Akt pathway activation could be explored in the context of development and in regeneration.
Generally, motor neurons are known to utilize cAMP signaling for axon regeneration by overcoming the inhibition of reticulon receptor NOGO.30 Strangely, though the NSC34 motor neuron cell line expresses choline acetyl transferase for synthesizing acetylcholine and generate action potential upon depolarization, they did not utilize the cAMP pathway for neurite extension (Fig. 6). While overstimulation with the cAMP pathway activator forskolin25 or the Protein Kinase A inhibitor, H89, caused cell death, no neurite extension was noticed upon cAMP pathway stimulation as determined by us (data not shown) and reported.36 Another important observation is the switching of pathway for CM – induced differentiation, neurite extension and networking in the NSC34 motor neurons as evidenced by H89 treatment. Normally, H89 induced cell death of the NSC34 cells (Fig. 7). CM treatment could rescue the NSC34 motor neurons from the cell death. H89, though commonly used as a Protein Kinase An inhibitor, could inhibit S6Kinase, MSK1 and ROCKII with equal potency. In addition, several more kinases are partially inhibited by H89.36 As neither the adenylate cyclase inhibitor, SQ22536, nor the cAMP antagonist, Rp-cAMP brings about the same effect as H89; cAMP activated protein kinase A pathway is not involved. Further characterization is needed to identify the specific pathway involved in the H89 induced cell death. Deciphering this pathway could potentially unravel impairments in the neurodegenerative diseases which lead to neuronal degeneration.
More importantly, the motor neurons (NSC34) responded to the trophic factors secreted by the HUMS cells and showed extensive neurite extension and robust networking. This strongly reinforces the motor neuron disease mouse model studies where several trophic factors have been shown to provide protection against motor neuron disease.4,37 Thus, the HUMS cells and their secreted factors is a viable approach to induce neurite extension and networking and could be applied to regenerative medicine, more specifically, neurodegenerative diseases. A better means to provide the HUMS cells and maintain them viable over a longer duration in vivo will help to harness the tremendous potential of the HUMS cells.
Authorship Contribution
Ajeet Kumar: (majority), Himanshu K Mishra, Priyanka Dwivedi: Carried out the experiments. Jamuna R Subramaniam: Designed, carried out (some experiments), supervised, and wrote the manuscript.
Acknowledgement
The authors would like to thank the Obstetricians. They thank the Flow Cytometry facility of Central Drug Research Institute, Lucknow. The authors acknowledge the financial support by the Department of Biotechnology, Government of India.
Footnotes
This article complies with International Committee of Medical Journal editor’s uniform requirements for manuscript.
Source of Funding: DBT Competing interest: None
References
- 1.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD, (eds) in. 3rd ed, Garland Publishing Inc; 2001. Molecular Biology of the Cell. [Google Scholar]
- 2.Huang EJ, Reichardt LF. Neurotrophins roles in neuronal development and function. Neurotrophins roles in neuronal development and function. Annual Review of Neuroscience. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J Neurochem. 2005;93(6):1412–21. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- 4.Azouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KE, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- 5.Strand AD, Banquet ZC, Argali AK et al. Expression profiling of Huntington’s disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. J Neurosci. 2007;27:11758–68. doi: 10.1523/JNEUROSCI.2461-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Brakefield, D, Pan Y et al. Muscle-derived but not centrally derived transgene GDNF is neuroprotective in G93A-SOD1 mouse model of ALS. Exp Neurol. 2007;203:457–71. doi: 10.1016/j.expneurol.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Thomson JA, Itskovitz-Eldor J, Shapiro SS et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Tanabe K, Ohnuki M et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 1997;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Vodyanik MA, Smuga-Otto K et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 10.Okita K, Ichisaka T. Yamanaka S Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 11.Horie N, Pereira MP, Niizuma K et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29(2):274–85. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uccelli A, Laroni A. Freedman MS Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10:649–56. doi: 10.1016/S1474-4422(11)70121-1. [DOI] [PubMed] [Google Scholar]
- 13.Ebert AD, Beres AJ, Barber AE et al. Human neural progenitor cells over-expressing IGF-1 protect dopamine neurons and restore function in a rat model of Parkinson’s disease. Exp Neurol. 2008;209(1):213–23. doi: 10.1016/j.expneurol.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Nagahara AH, Merrill DA, Coppola G et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nature Medicine. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr D, Llado J, Shamblott M et al. Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury. J Neurosci. 2003;23:5131–5140. doi: 10.1523/JNEUROSCI.23-12-05131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wichterle H, Lieberam I, Porter JA et al. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110(3):385–97. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 17.Silani V, Corbo M. Cell replacement therapy with stem cells in neurodegenerative diseases. aourceCurrent NeuroVas Res. 2004;1:283–289. doi: 10.2174/1567202043362243. [DOI] [PubMed] [Google Scholar]
- 18.Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 19.Copeland IB. Mesenchymal stromal cells for cardiovascular disease. J. of Cardiovascular disease Res. 2011;2:3–13. doi: 10.4103/0975-3583.78581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boucherie C, Caumont AS, Maloteaux JM et al. In vitro evidence for impaired neuroprotective capacities of adult mesenchymal stem cells derived from a rat model of familial amyotrophic lateral sclerosis [hSOD1(G93A)]. Exp Neurol. 2008;212:557–61. doi: 10.1016/j.expneurol.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 21.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection and neural repair. Annu. Rev. Neurosci. 2001;24:1217–281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 22.Cheung E, Slack RS. Emerging role for ERK as a key regulator of neuronal apoptosis. PE45. Sci STKE. 2004 doi: 10.1126/stke.2512004pe45. [DOI] [PubMed] [Google Scholar]
- 23.Schramm A, Schulte JH. Astrahantseff K et al. Biological effects of TrkA and TrkB receptor signaling in neuroblastoma. Cancer Lett. 2005;228:143–53. doi: 10.1016/j.canlet.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell KE, Weiss ML, Mitchell BM et al. Matrix cells from wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 25.Rajan TS, Tripathi PP, Arya U et al. ALS associated mutant SOD1 impairs the motor neurons and astrocytes and wild type astrocyte secreted-factors reverse the impaired motor neurons. Annals of neurosciences. 2011;18(2):48–55. doi: 10.5214/ans.0972.7531.1118205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cashman NR, Durham HD, Blusztajn JK et al. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn. 1992;194(3):209–21. doi: 10.1002/aja.1001940306. [DOI] [PubMed] [Google Scholar]
- 27.Vijayalakshmi K, Alladi PA, Sathyaprabha TN et al. Cerebrospinal fluid from sporadic amyotrophic lateral sclerosis patients induces degeneration of a cultured motor neuron cell line. Brain Res. 2009;1263:122–33. doi: 10.1016/j.brainres.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi S, Isa K, Hayashi K et al. K252a, a potent inhibitor of protein kinases, inhibits the migration of cerebellar granule cells in vitro. Developmental Brain Research. 1995;90:122–128. doi: 10.1016/0165-3806(96)83492-4. [DOI] [PubMed] [Google Scholar]
- 29.Schwartzbauer G, Robbins J. The tumor suppressor gene PTEN can regulate cardiac hypertrophy and survival. J. Biol. Chem. 2001;276:35786–357937. doi: 10.1074/jbc.M102479200. [DOI] [PubMed] [Google Scholar]
- 30.Aglah C, Gordon T, Posse de Chaves EI. cAMP promotes neurite outgrowth and extension through protein kinase A but independently of Erk activation in cultured rat motoneurons. Neuropharmacology. 2008;55:8–17. doi: 10.1016/j.neuropharm.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Koh SH, Kim KS, Choi MR et al. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 2008;1229:233–248. doi: 10.1016/j.brainres.2008.06.087. [DOI] [PubMed] [Google Scholar]
- 32.Kim JB, Sebastiano V, Wu G et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 1998;136(3):411–9. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Mitsui K, Tokuzawa Y, Itoh H et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. 2003;Cell 113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 34.Mahay D, Terenghi G, Shawcross SG. Schwann cell mediated trophic effects by differentiated mesenchymal stem cells. Experimental Cell Res. 2008;314:2692–2701. doi: 10.1016/j.yexcr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Zhao T, Qi Y, Li Y et al. PI3 Kinase regulation of neural regeneration and muscle hypertrophy after spinal cord injury. DOI 10.1007/s11033-011-112. Mol Biol Rep. 2011 doi: 10.1007/s11033-011-1127-1. [DOI] [PubMed] [Google Scholar]
- 36.Davies SP, Reddy H, Caivano M et al. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaspar BK, Lladó L, Sherkat N et al. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]


