Abstract
Background
Hematopoietic stem cell transplantation (HSCT) involves the infusion of hematopoietic stem cells from a suitable donor to a patient who has undergone chemotherapy. Stem Cell transplantation is used for the treatment for a wide variety of diseases, including leukemia and lymphoma.
Purpose
This study highlights prevention strategies of infectious diseases among HSCT donors and recipients in our institute as guided by International guidelines. We aim to highlight the strategy for extensive screening of HIV, Hepatitis B and C, CMV infection and syphilis cases in all the stem cell units stored in our facility
Methods
We searched the institutional database to identify cases of infectious diseases among HSC transplants. Extensive donor evaluation was conducted through screening and laboratory infectious disease testing for HIV, Hepatitis B and C, CMV infection and syphilis.
Results
Between 1996 and 2014, 263 consecutive adult HSCT were performed. An approximate equal number of autologous and allogeneic HSC collections were undertaken. The median age for autologous donors was 35 years, whereas that of allogeneic donors is 25 years. Of the 263 stem cell donors, we found 18 patients (autologous) and 2 donors (allogeneic) to be infected. We did not find any of the donors infected with HIV by the serology as well as the NAT testing protocol
Conclusion
Donor screening and testing is the most critical parameter in stem cell transplantation in order to ensure the safety of the product to be transplanted. Modifications in the regulations related to donor screening are aimed at providing safe transplantation and negate the risk of accidental infection of the donor.
Keywords: Screening, HSCT, Recipients, Donors, Hepatitis
 Introduction
Introduction
Hematopoietic stem cell transplantation (HSCT) is defined as the transplantation of hematopoietic stem cells originating from bone marrow cord or peripheral blood from a donor to a recipient in case of an allogeneic transplantation or from the same patient in case of an autologous transplantation.1
Stem Cells are harvested in vitro and returned to the individual as part of the transplantation procedure. The goal of HSCT is to achieve engraftment of the administered cells, thus resulting in the recipient’s lymphohematopoietic system derived from the HCT graft. The ultimate goal of many HCT protocols is to achieve optimal graft-versus-tumor (GVT) activity in patients with malignant disease.2
From the time of the first Hematopoietic Stem Cell transplant that was reported by JM Goldman in the late ‘70’s,3 bone marrow transplantation has been used as a treatment of choice in allogeneic transplantation and has shown an improvement in the outcome of a wide array of disorders including leukemia, lymphoma, myelodysplastic syndrome (MDS), myeloproliferative disorders, congenital immunodeficiencies, enzyme deficiencies, bone marrow (BM) failure syndromes and hemoglobinopathies.4 However, significant morbidity and mortality is associated with allogeneic HCT due to regimen-related toxicity (RRT),5 infection,6 and graft versus host disease (GVHD),7 a recommendation regarding transplantation for the individual patient requires careful risk assessment that takes into account disease status,8 comorbidities, previous therapies, other standard therapies available for the underlying disease,9 donor stem cell source,10 and histoincompatibility must be carefully analyzed.11
Recent updates in the regulations have made donor screening for Infectious disease very stringent in order to avoid the chances of an unsuspecting patient to get infected. The idea of the screening parameters is to make the stem cell product safe. Recent introduction of the Nucleic acid testing (NAT) has even brought down the window period to as less as 6 days. In the Prince Sultan Military Medical City (PSMMC), efforts are being made to screen all donors for infectious diseases. Recently, the NAT testing has become mandatory by the American Association of Blood Bank (AABB), knowing that earlier samples were screened by serological parameters only. The infectious disease screening was done as per the AABB standards 5.8.4 & 5.8.5.12 Infectious disease screening has also been necessitated by the US FDA as per their standards 21 CFR 610.40(d).13 All samples that are pending Infectious disease screening results have to be quarantined thus minimizing the chances of cross contamination with the eligible donors. This study highlights prevention strategies due to infectious disease screening among HSCT donors as periodic recommendations and guidelines of the CDC, the Infectious Diseases Society of America, and the American Society of Blood and Marrow Transplantation and the American Association of Blood Banks (AABB). We aim to highlight the effectiveness of disease screening in the elimination of the prevalence of HIV, Hepatitis B and C, CMV infection and syphilis cases among HSCT donors.
Methods
The screening procedure includes with administration health history questionnaire of the potential stem cell donor in order to ascertain the suitability of the donor. The suitability of the donor therefore depends on the questionnaire which the donor has to fill. The next stage includes Infectious disease screening where it has to be ascertained that the donor is free of infectious diseases such as HIV, Hepatitis, CMV-IgM and syphilis. The details of the assessment of the donor are as follows.
Adult Donor Health History Questionnaire
The questionnaire conforms to the requirements of the Food and Drug Administration (FDA), American Association of Blood Banks (AABB), and the Foundation for the Accreditation of Cellular Therapy (FACT). The questionnaire is written in dual language namely English and Arabic. If the donor is illiterate, assistance is given to the donor to fill the questionnaire. The donor must be asked to fill accurate and correct information especially the medical history section.
The medical history of the prospective HSCT donor should contain information on the history of vaccinations during the 4 week period prior to donation; travel history that includes travel to certain countries that is in the list of countries with prominent endemic diseases that might be transmitted through HSCT (e.g malaria); Chagas’ disease, viral hepatitis and leishmaniasis; It mus also be detrmined if the donor has ever been deferred from plasma or blood donation and the type of deferral; It must be ascertained if there is a history of blood product transfusion, solid organ transplantation in the previous 12 months. There needs to be a determination for the history of risk factors for classic Creutzfelt-Jacob disease and medical history that indicates the donor has clinical evidence of or is at risk for acquiring a bloodborne infection (e.g., HIV-1 or HIV-2, HTLV I or II, Hepatitis B, or Hepatitis C). As there are no significant incidence of Malaria in Saudi Arabia, we have included the screening for malaria as part of the questionnaire to ascertain if the donor has visited malaria infected areas and if necessary the screening for malaria is performed.
Screening of infectious diseases for Stem Cell donors
The screening is undertaken prior to the hematopoietic stem cell collection from the prospective donors. Screening is carried out on donors (allogeneic) and on patients (autologous) as part of the bone marrow transplantation (BMT) workup procedure. HIV, Hepatitis and CMV-IgM screening are performed by the Enzyme Linked Immunosorbent assay (ELISA). Syphilis screening is performed by Venereal Disease Research Laboratory (VDRL) test and confirmed by Treponema pallidum Haemagglutination Assay. In addition to the adherence to the standards for screening and obtaining medical history, all stem cell donors are subjected to a bone marrow transplant workup to ensure that the donor is in good health prior to stem cell donation.
After completion of screening, donors that were clear of infectious diseases were allowed to donate hematopoietic stem cells. Peripheral Blood Stem Cells were collected from the prospective donors after standard apheresis procedures. The total number of donors/patients recruited for stem cell transplantation was 278. A complete Infectious disease screening on all the donors/patients was carried out. This study was approved by departmental research and ethics committee.
Results
We have analyzed a total of 263 patients who have donated stem cells either for autologous or allogeneic usage between 1996 and 2014. The male to female ratio was 1.39 to 1. An approximate equal number of autologous and allogeneic Hematopoietic Stem Cell collections were undertaken. The median age for Autologous donors is 35 years, whereas that of Allogeneic donors is 25 years. The infectious disease of the patients has been enumerated and shown in Table 1.
Table 1: Number of autologous and allogeneic donors as well as the percentage of allogeneic donors listed at the Prince Sultan Military Medical City. The number of positive cases in Infectious disease screening are listed. Test for HIV, HBV and HCV are done by serology (designated as S) and by NAT analysis of the DNA/RNA. CMV-IgM is done by serology method. Syphilis is done by VDRL method for detection and later confirmed by TPPA method.
| Stem Cell Collections | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of alloge-neic do-nors | Number of autolo-gous do-nors | Percentage of alloge-neic dona-tion | Positive cases for Infectious disease screening (Total No of positive cases= 20) | ||||||||
| HIV (S) (% ) | HIV-NAT (%) | HBsAg (S) (%) | HBc Ab (S) (%) | HBV-NAT (%) | HCV (S) (%) | HCV-NAT (%) | CMV-IgM (%) | Syphilis (%) | |||
| 128 | 135 | 48.67% | |||||||||
| Autologous Donors | 0 (0%) | 0 (0%) | 3 (1.1%) | 8 (3.04%) | 3 (1.1%) | 1 (0.38%) | 1 (0.38%) | 2 (0.76%) | 0(0%) | ||
| Allogeneic Donors | 0 (0%) | 0 (0%) | 0 (0%) | 2 (0.76%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Total | 0 (0%) | 0 (0%) | 3 (1.1%) | 10 (3.8%) | 3 (1.1%) | 1 (0.38%) | 1(0.38%) | 2 (0.76%) | 0 (0%) | ||
Transplants carried out are broadly classified into two major groups; autologous and allogeneic transplants. Each of these two major groups is further sub classified on the basis of sex and the various age groups of the donor. This is enumerated in Chart 1.
Chart 1:
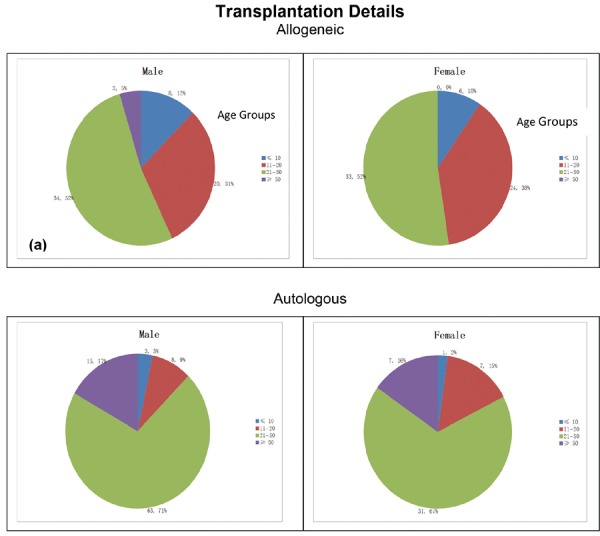
Distribution of Allogeneic and Autologous donors among the various age groups. Chart 1a and 1b shows the distribution of Allogeneic donors among Male and Female subjects respectively. Chart 1c and 1d shows the distribution of Autologous donors among Male and Female subjects resp.
We have tabulated the distribution of the male and female donors among the various age groups from autologous and allogeneic donors which is shown in Table 2.
Table 2: Table showing the distribution of the autologous and allogeneic donors among various age groups.
| Age Groups | Autologous Male | Autologous Female | Allogeneic Male | Allogeneic Female |
|---|---|---|---|---|
| ≤10 years | 3 | 1 | 8 | 6 |
| 11–20 years | 8 | 7 | 20 | 24 |
| 21–50 years | 63 | 31 | 34 | 33 |
| ≥50 years | 15 | 7 | 3 | 0 |
Infectious disease screening was done for all donors irrespective of the nature of transplantation. We have seen the prevalence of infectious diseases in 20 of the 263 donors/patients that have been identified for transplantation. This can be seen in Chart 2. Of the 263 stem cell donors, we found 18 patients (autologous condition) and 2 donors (allogeneic) to be infected
Chart 2:
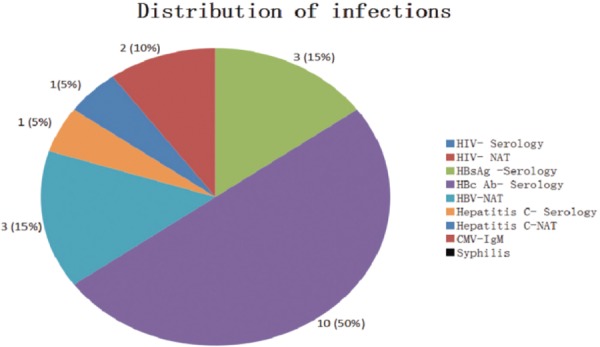
Distribution of the infectious diseases identified on the stem cell units collected for autologous as well as allogeneic transplantation.
Discussion
HSCTis defined as the transfer of hematopoietic stem cells from a suitable donor to an immunocompatable recipient. HSCT may be the transfer of stem cells from one individual to another (allogeneic HCT) or the return of previously harvested cells to the same individual (autologous HCT). HSCT is currently done without any manipulation of the cells but there is a future prospect for stem cell manipulation prior to transplantation. Main challenges in HCT include infectious disease screening to ensure safe stem cell transplantation. Apart from the HSCs that are required to be transplanted, considerable amount of patient plasma is also transplanted along with the cells and there is a considerable amount of risk to the patient from Infectious diseases being transmitted. The regulations are being modified periodically, so that new transmittable infections are included in endemic areas and chances of accidental transmission of infection is completely eliminated.14
Isolation and transplantation regime of Hematopoietic Stem Cells
The isolation and transplantation regime of Hematopoietic Stem Cells has been published by us recently in the Journal of Transplantation.15 We have devised a new and a novel strategy to isolate stem cells from suitable donors and enumerate the number of stem cells that will be isolated from each collection. We have correlated the number of CD 34+ cells that are enumerated from the peripheral blood with the Final number of Stem Cells that are isolated per kg body weight of the patient.
We collect a minimum of 2.5–5 × 106 CD 34+ cells from each donor/patient per kg body weight. Each collection is performed when the CD 34+ level reaches a minimum of 10 cells/μl. Multiple collections (generally 2-3) are performed from a single donor till the minimum amount is attained. Cryopreserved stem cells are transplanted post chemotherapy.
A strict regime in Infectious disease screening for allogeneic donors and all donors was maintained and found to be free from HIV, Hepatitis, syphilis and cytomegalovirus (CMV) prior to being accepted as a potential donor. Autologous patients are also screened for infectious diseases so as to segregate any infected sample from the rest in the cryopreservation tank.
Infectious disease testing for HIV and Hepatitis B and C was done by analyzing the antibody titer by ELISA method but later advancements in the analysis led to more advanced methods of detection. The Nucleic acid testing (NAT) HIV and Hepatitis screening, thus bringing down the window period of detection to 9-10 days. This became part of the AABB guidelines only a couple of years ago. In the earlier guidelines HIV and Hepatitis testing was done only by ELISA but it became mandatory in 2010 as per the FDA and AABB guidelines that all donors must be screened by NAT testing in addition to the serological testing. NAT testing was mandatory for HIV and HCV where it became important to detect small quantities of RNA from the serum.14,16
Eligible donors for allogeneic donation are searched within the family for a suitable HLA match with that of the patient. At this stage every donor or patient is screened in the hospital as part of the BMT workup. The infectious disease screening is done as per the AABB Standards (5.8.5) involving Infectious disease screening. The tests are done for HIV, HBsAg, HBc, HCV and syphilis.
Between 1996 till date, peripheral blood stem cell units were harvested from 263 donors/patients. There is an almost equal number of autologous and allogeneic units that have been collected of which around 49% are allogeneic donors. We analyzed the data from the infectious disease screening and ascertained that 7.6% of units stored were from donors who were tested positive during the infectious disease screening. We analyzed the infected donors in each of the infectious diseases such as HIV, Hepatitis, CMV-IgM and Syphilis. We did not find any of the donors infected with HIV as per the NAT testing protocol. We next tested the number of donors that showed a positive result for Hepatitis B surface antigen (HBsAg). As per FACT and AABB guidelines (12), donors that are seropositive for HBsAg are permanently deferred to donate. 3 autologous donors (1.1%) who were found to be positive for HBsAg. Autologous donors are acceptable provided the stem cell units are segregated from other tested samples. We analyzed more common antibody detection for Hepatitis (HBc or Hepatitis B core antibody). This antibody can result at the onset of symptoms in acute hepatitis B that persist for life. Anti-HBc indicates previous or ongoing infection with hepatitis B virus. We have observed that the donors who were HBc antibody positive were negative for HBsAg or Hepatitis test done by NAT assay. This also indicates that all the patients had no current Hepatitis incidence. This indicates that either the infection is resolved or it is a false positive result for Hepatitis B. We do not have the data for anti-HBs (Hepatitis B surface antibodies) in order to indicate whether the presence of HBc antibodies is due to any recent vaccination. We next analyzed the prevalence of Hepatitis C antibody in the donors recruited for stem cell transplant. There was a single autologous case (0.38%) where there was a positive indication for Hepatitis C virus. This was confirmed by the NAT testing protocol. We found 2 autologous cases (0.76%) of CMV-IgM. The Hepatitis C and CMV-IgM samples were also quarantined from the other stem cells stored and used for autologous administration only.
The age that is most common for Stem Cell donation was 21–50 years among all donors with the median age for autologous donors being 36 years and the median age for allogeneic donors was 35 years.
Cases of autologous utility of stem cells included patients who suffered from lymphoma or multiple myeloma. Allogeneic usage of stem cells involved donors who are close relatives such as siblings for a probability of a good HLA match. The patients included those of leukemia which necessitates stem cells to be transplanted from an HLA matched donor. The donor-patient match is maintained at 100% to eliminate the possibility of GvHD after the stem cell transplant.
As shown in Chart 2, there were just 20 cases of Infectious diseases that were reported from 263 donors/recipients. Analysis of Table 1 showed that there were only 2 cases (10%) of allogenenic donors that showed a seropositive result to infectious disease screening. These 2 cases indicated a positive result for HBcAb (Hepatitis B core antibody). Confirmation of tests for HBsAg (Hepatitis B surface antigen) and Hepatitis B-NAT testing showed a negative result. This indicates that there was a previous incidence of Hepatitis B but not currently prevalent in the donor at the moment. We have not yet performed any tests on the Hepatitis B surface antibody (anti-HBs) nor have we performed tests for Anti-Hepatitis B e antibody (Anti-HBe).
As reported earlier,17 the national average of HIV cases in Saudi Arabia is ≤ 4 cases per 100,000 subjects. There is a high prevalence of Hepatitis cases in Saudi Arabia with around 9,000 new cases being detected every year (18) with Hepatitis B virus forming majority of the cases (53%) followed by Hepatitis C virus (40%). However, Syphilis cases amounts to only 0.03-0.85% in the population in Saudi Arabia (19).
As per the regulations of AABB, all stem cell donors that have been cleared as Seronegative for HIV and Hepatitis C have to be cleared by the mandatory Nucleic acid test (NAT). NAT testing for Hepatitis B DNA was discontinued in December 2010 (16); we nevertheless conduct HIV as well as Hepatitis test by ELISA as well as the Nucleic Acid Test (NAT).
It is important that all potential donors be screened for an evaluation for their general state of health and whether they pose any risk in the transmission of infectious diseases to the recipient. The evaluation of donors are achieved through screening and laboratory testing as described earlier.20
Assessment of Donor safety
Disorders pertaining with HCT are categorized according to its prevalence and severity. Different areas have different assessment criteria. National laws of a particular country take precedence over international laws. Thus, concerns pertaining to the likelihood of the disorder within the potential donor population is highly dependent upon the donor’s geographic location. Severity is related to the consequences of the disorder and ease of managing these consequences. We have included pathogenic conditions of highly pertinent disorders21–23which include HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), human T-cell leukemia viruses type I and II (HTLV-I and HTLV-II), and West Nile virus.24–26
The donor screening regime includes physical examination of the donor, history of previous donation and review of medical records. This screening must be done preceding donation. 2 ml of the donor plasma was stored in Stem Cell Collection due to the possibility of the development of new risk assessment. This is a critical aspect because discovery of new risk findings necessitates that the potential donor might require further evaluation or deferral.27
Potential Donors first undergo an HLA matching analysis. The HLA match is done between all the siblings of the donor to find a perfect 6 out of 6 match. Only a perfect match is allowed to donate as part of the directed donation regime. The selected donor’s medical history is compiled which includes a complete BMT work up such as Infectious disease screening, complete hematology analysis, blood grouping analysis, biochemistry analysis, microbiology analysis and X ray investigation. Physical examination of the donor is also undertaken with the aim to detect stigmata associated with transfusion transmissible disease. Only those donors that have undergone all the required tests and have no significant findings that can defer Stem Cell donation are allowed to donate. The documentation for the Donor selection is completed. The incidence of low or almost negligible prevalence of Infectious disease is due to the stringent criteria adopted prior to collection of the stem cell sample. We, however do not perform any infectious disease screening of the patient post transplantation.
Hematopoietic stem cell graft safety
It is important that the current standards in the prevention of extrinsic bacterial and fungal contamination are followed. Personnel involved in the collection, transportation, processing, storage and finally transplantation require to follow a strict protocol to avoid accidental contamination of the stem cell product. It has been noted in an earlier literature28 that cryogenic temperature can harvest Hepatitis B virus-infected peripheral blood progenitor cell product. Thus, it is imperative to ensure absolute Hematopoietic Stem Cell Graft safety for a successful transplantation outcome. Transportation services that involve long distance transportation need to follow the IATA guidelines. These are detailed in the Food and Drug Administration (FDA) “Good Tissue Practice regulations,29 in the regulation of the European Commission30 and in the FACT NetCord Standards for Cord Blood Collection, Processing, Testing, Banking, Selection and Release.31 Regulations are being updated to ensure a risk free transplantation to the patients.
Future strategies to improve Infectious disease screening
It is our constant endeavor to improve the quality of stem cell product for safe transplantation in addition to the effectiveness of the Transplant graft. Human T Lymphocyte Virus (HTLV) has been included in the infectious disease screening parameters by AABB. We have also recently included this parameter as part of the infectious disease screening regime. Local and International regulations might request for particular testing depending on the severity and intensity of the disease as defined by the World Health Organization (WHO). The severity and intensity of the disease may to localized to a particular region or may be country specific. Recently discovered Coronavirus that primarily infect the upper respiratory and gastrointestinal tract which cause the Severe Acute Respiratory Syndrome (SARS) has been detected in many parts of the world. The WHO has confirmed the prevalence of this disease in the Middle East with a significant number of deaths taking place due to SARS. Thus, introducing screening parameters for the Coronavirus is a future prospect.
Conclusions
Donor screening is one of the most critical parameter in stem cell transplantation. All regulations and standards stress on donor screening in order to provide safe transplantation and negate the risk of accidental infection to the recepient. Blood banking has very stringent criteria for donation. Similarly, stem cell banking has taken the cue from the blood banking directive to provide safe transplanted products to the patient. In our facility, the BMT workup including infectious disease screening is done 7 days prior to collection of the Stem Cell unit. We perform the NAT testing for HIV, HCV, and HBV on the donor samples so retesting of the donor sample is not required. All samples for PBSC are stored transiently until they are transplanted back to the patient. Thus, it is imperative to perform the serology as well as the NAT testing on the initial sample before Stem Cell collection.
Authorship Contribution
Omar Alsuhaibani: Verification of Data and part of the group to conceptualize the project, Winston C Pereira: The analysis of the data, design of the project and project write up for subsequent approval, Mohammed Tareeqanwar: Contributed in conceptualizing the project, data analysis, project write up and subsequent constructive suggestions, Nora El Khizzi: Contributed in giving the inputs from Microbiological results in infectious disease testing, Saadia Bakheswain: Analyzing and verifying data of Microbiological results in infectious disease testing, Ghaleb Elyamany: Initiating the project, design of the project, project write up and final approval
Footnotes
This article complies with International Committee of Medical Journal editor’s uniform requirements for manuscript.
Competing interests: No competing interests have been declared by any of the authors.
References
- 1.Dykewicz CA. Centers for Disease Control and Prevention (U.S.); Infectious Diseases Society of America; American Society of Blood and Marrow TransplantationSummary of the Guidelines for Preventing Opportunistic Infections among Hematopoietic Stem Cell Transplant Recipients. Clin Infect Dis. 2001 Jul;15; 33(2):139–144. doi: 10.1086/321805. [DOI] [PubMed] [Google Scholar]
- 2.Welniak LA Blazar BR, Murphy WJ et al. Immunobiology of allo- geneic hematopoietic stem cell transplantation. Annu RevImmunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 3.Goldman JM, Th’ng KH, Park DS et al. Collection, cryopreservation and subsequent viability of haemapoietic stem cells intended for treatment of chronic granulocytic leukaemia in blast-cell transformation. British Journal of Haematology. 1978;40(2):185–195. doi: 10.1111/j.1365-2141.1978.tb03656.x. [DOI] [PubMed] [Google Scholar]
- 4.Giralt S. Allogeneic hematopoietic progenitor cell transplanta-tion for the treatment of chronic myelogenous leukemia in the era of tyrosine kinase inhibitors: lessons learned to date. Clin Lymphoma Myeloma. 2007;7(Suppl 3):S102–S104. doi: 10.3816/clm.2007.s.009. [DOI] [PubMed] [Google Scholar]
- 5.Aschan J. Risk assessment in haematopoietic stem cell trans-plantation: conditioning. Best Pract Res Clin Haematol. 2007;20:295, 310. doi: 10.1016/j.beha.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Leather HL, Wingard JR et al. Infections following hematopoietic stem cell transplantation. Infect Dis Clin North Am. 2001;15:483–520. doi: 10.1016/s0891-5520(05)70157-4. [DOI] [PubMed] [Google Scholar]
- 7.Fraser CJ, Scott Baker K. The management and outcome of chronic graft-versus-host disease. Br J Haematol. 2007;138:131–145. doi: 10.1111/j.1365-2141.2007.06652.x. [DOI] [PubMed] [Google Scholar]
- 8.Chaidos A, Kanfer E, Apperley JF. Risk assessment in haemo-topoietic stem cell transplantation: disease and disease stage. Best Pract Res Clin Haematol. 2007;20:125–154. doi: 10.1016/j.beha.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Baccarani M, Saglio G, Goldman J et al. Evolving concepts in the management of chronic myeloid leukemia: recommenda-tions from an expert panel on behalf of the European Leukemia Net. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 10.Urbano-Ispizua A. Risk assessment in haematopoietic stem cell transplantation: stem cell source. Best Pract Res Clin Haematol. 2007;20:265–280. doi: 10.1016/j.beha.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Petersdorf EW. Risk assessment in haematopoietic stem cell transplantation: histocompatibility. Best Pract Res Clin Haematol. 2007;20:155–170. doi: 10.1016/j.beha.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Standards in Blood Bank and Transfusion services, American Association of Blood Banks (AABB), Chapter 5, Process Control, 2011 May;:pg 10–19. [Google Scholar]
- 13.Code of Federal Regulations (CFR) Title 21, Vol 7, Chapter 1- Food and Drug Administration, Dept of Health and Human Services, Subchapter F- Biologics, part 610-General Biological Products Standards, subpart E-Testing Requirements for Communicable Disease agents. [Google Scholar]
- 14.FDA Guidance for Industry, May 20, 2010, “Nucleic Acid Testing (NAT) for Human Immunodeficiency Virus Type (HIV-1) AND Hepatitis C Virus (HCV): Testing, Product Disposition, and Donor Deferral and Reentry. [Google Scholar]
- 15.Winston CP, Omar A, Ghaleb E et al. A novel approach for the enumeration of Peripheral Blood Stem Cells suitable for Transplantation. J Transplant. 2014;2014:1–7. doi: 10.1155/2014/473503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Susan A Galel et al. Infectious Disease Screening, Chapter 8, AABB Technical Manual. 2011;239:270. [Google Scholar]
- 17.Mazroa MAAI, Kabbash IA, Felemban SM et al. HIV case notification rates in the kingdom of Saudi Arabia over the past decade (2000–2009). PLoS One. 2012;7(9): doi: 10.1371/journal.pone.0045919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Memish ZA, Al Knawy B, EL- Saed A et al. Incidence trends of viral Hepatitis A. B and C seropositivity over eight years of surveillance in Saudi Arabia. Int J Infect Dis. 2010;14(2):115–120. doi: 10.1016/j.ijid.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Al-Sibiani SA. 4. Vol. 15. JKAU: Med.Sci; 2008. Prenatal Screening Syphilis: Is Universal Screening necessary in Saudi Arabia? pp. 41–48. [Google Scholar]
- 20.Confer D, Gress R, Tomblyn M et al. Hematopoietic Graft safety. Bone Marrow Transplant. 2009 Oct;44(8):463–465. doi: 10.1038/bmt.2009.256. [DOI] [PubMed] [Google Scholar]
- 21.AABBStandards for cellular therapy product services. Bethesda, MD: AABB. 2007 [Google Scholar]
- 22.FACT-JACIE.International standards for cellular therapy product collection, processing, and administration. Omaha: Foundation for the Accreditation of Cellular Therapy. 2006 [Google Scholar]
- 23.19. Minneapolis: National Marrow Donor Program; 2004. National Marrow Donor Program.National Marrow Donor Program Standards. [Google Scholar]
- 24.Centers for Disease Control and Prevention. West Nile virus transmission through blood transfusion–South Dakota,MMWR - Morbidity & Mortality Weekly Report. 2006;2007;56:76–79. [PubMed] [Google Scholar]
- 25.Centers for Disease Control and PreventionWest Nile virus update–United States, MMWR - Morbidity & Mortality Weekly Report. 2007 2007 Jan-Nov;56:1191–1192. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention.Investigation of blood transfusion recipients with West Nile virus infections. MMWR - Morbidity & Mortality Weekly Repor. 2002;51:823. [PubMed] [Google Scholar]
- 27.Washington, DC: U.S. Department of Health and Human Services; 2007. Guidance for Industry: Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps). [Google Scholar]
- 28.Husebekk A Skaug, K Kolstad A et al. Hepatitis B virus-infected peripheral blood progenitor cell harvests in liquid nitrogen freezer containing non-infectious products. Transfusion. 2004;44:942–943. doi: 10.1111/j.1537-2995.2004.00379.x. [DOI] [PubMed] [Google Scholar]
- 29.Washington, DC: GPO; 2004. 21 CFR, Part 1271, Subpart D—Good Tissue Practice. [Google Scholar]
- 30.European Commission. Commission Directive 2004/23/EC.Official J Eur Union. 2004;L 102:48–58. [Google Scholar]
- 31.Omaha. NE: Foundation for the Accreditation of Cellular Therapy; 2006. International Standards for Cord Blood Collection, Processing, Testing, Banking, Selection, and Release. [Google Scholar]


