Abstract
Aortic aneurysm is a common and life-threatening disease that can cause death from rupture. Current therapeutic options are limited to surgical or endovascular procedures because no pharmacological approaches have been proven to decrease the chance of expansion or rupture. The best approach to the management of aortic aneurysm would be the understanding and prevention of the processes involved in disease occurrence, progression, and rupture. There is a need for animal models that can reproduce the pathophysiological features of human aortic aneurysm, and several such models have been studied. This review will emphasize recent advances in animal models used in the determination of mechanisms and treatments of aortic aneurysms.
Keywords: Aorta, Aneurysm, Animal model, Research
INTRODUCTION
Aortic aneurysm is a degenerative disorder characterized by permanent dilatation of the aortic wall that exceeds the normal diameter by at least 50%. If an aneurysm is left untreated without appropriate management, it progresses to dilate and rupture with high mortality. Recently, with life expectancy increasing, it has become a major cause of death in the elderly population and raises concerns as a socioeconomic issue [1].
Management of abdominal aortic aneurysms still consists of monitoring the disease course without any active treatments or attempts at surgical correction [2]. However, surgical treatments and anesthesia increase morbidity and mortality rates. There have been significant efforts to advance noninvasive endovascular surgery by using stent grafting, but many problems persist, such as endoleaks, stent migration, and limb occlusion [3]. Studies on aortic aneurysms have focused on investigating physical causes and surgical treatments. Early diagnosis of small aortic aneurysms not requiring surgical treatment is more frequent, but problems associated with surgery persist [4].
Ultimately, the best approach to the management of aortic aneurysms is to understand and prevent the processes involved in disease occurrence, progression, and rupture. However, there are no effective non-surgical treatments to prevent progression of early stage disease, or adequate means to monitor disease activity and guide suppressive medical therapies. Information related to this sequence of events is still lacking. A limitation is that human aortic aneurysm tissue for research can only be obtained during surgery. Such tissue is already in late disease stages, and unsuitable for analyzing the occurrence and progression of the disease. Therefore, human studies are restricted to end-stage disease with markedly altered tissue architecture. There is a need for animal models that can reproduce the pathophysiological features of human aortic aneurysm.
In vivo animal models enable researchers to test hypotheses relating to pathogenesis and to investigate potential therapeutic strategies by providing an opportunity to study early stages of disease [5]. Several recently developed animal models of aortic aneurysm have provided novel insights into pathogenesis and pharmacotherapy. We will herein introduce some animal models that have been used, and discuss their relevance to human disease, focusing on contributions to research on aortic aneurysms.
PATHOPHYSIOLOGY OF AORTIC ANEURYSM
A wide variety of pathologic conditions is associated with initiation, maturation, and eventual rupture of aortic aneurysms [6]. These include proteolytic degradation and inflammation of the aortic wall, and increased biomechanical wall stress. Understanding the underlying pathophysiology is critical for basic research on prevention of initial aneurysm formation and limiting growth and expansion.
Loss of two critical structural elements in the aortic wall, elastin and collagen, contributes to degradation, increasing risk of aneurysm formation. Matrix metalloproteinases (MMPs) derived from macrophages and aortic smooth muscle cells are secreted into the extracellular matrix, which activate collagen and elastin degradation [7]. MMP-1 (collagenase), MMP-3 (stromelysin), MMP-2 (gelatinase A), and MMP-9 (gelatinase B) are the principal proteinases in aortic aneurysms that result in collagen and elastin degradation [8–10]. MMP-2 and MMP-9, which are synthesized by local cells in the aortic wall, including infiltrating macrophages and aortic vascular smooth muscle cells, break down collagen [11,12]. Longo et al. [13] reported that a concerted role of MMP-2 and MMP-9 is required for aneurysmal degradation; both could be targeted for the treatment of aortic aneurysms. The contribution of MMPs to aneurysm formation is highlighted by the beneficial effect on aneurysm expansion of medications that reduce MMP levels. The feasibility of pharmacologically suppressing aneurysmal degeneration was demonstrated by using doxycycline, a nonspecific inhibitor of MMP [14].
Chronic transmural inflammation is a prominent histologic feature of aortic aneurysms [15]. Lymphocytes and macrophages are found in the adventitia and media of aortic aneurysms in greater concentration than in the normal aorta [16]. When such cells invade aortic tissue and create an inflammatory environment, the process of elastin and collagen breakdown and aneurysm formation begins. Tissue levels of proinflammatory cytokines, such as tumor necrosis factor-α, interleukin (IL)-1β, IL-8, interferon-γ, and IL-6, are all consistently upregulated in patients with aneurysms [17]. Recent studies showed that limiting inflammation with drugs can reduce aneurysm formation in animal models [18–20].
Hemodynamic stress on the aortic wall is an obvious important factor in aneurysm development and rupture. Compared with the thoracic aorta, the infrarenal abdominal aorta is prone to aneurysmal enlargement due to a combination of differences in structure, composition, and biology of the wall, as well as differences in hemodynamic flow and biomechanical forces [21]. The infrarenal segment is subject to higher levels of oscillating flow and reflected pressure waves than the suprarenal segment, resulting in a higher level of aortic wall tension [22]. Shear stress caused by altered hemodynamics, and wall tension caused by blood pressure acting on the curved aneurysm wall, is a signal for collagen remodeling [23]. Understanding biomechanical factors may help improve assessment of the risk of aneurysm growth and rupture.
ANIMAL MODELS OF AORTIC ANEURYSMS
1). Genetically predisposed or engineered animal models
The broad breasted bronze turkey was the first animal model reported to develop spontaneous rupture of dissecting aneurysms [24,25]. Supplementation of feed with various compounds, such as β-aminopropionitrile (BAPN) and diethylstilbestrol, increased risk of rupture [26,27]
The blotchy mouse, with a mutation on the X-chromosome that leads to abnormal copper absorption, developed spontaneous aortic aneurysms [28]. Because copper is a cofactor for lysyl oxidase (Lox), which is important in the crosslinkage of elastin and collagen, these mice have defective tissue. Mice with a genetically engineered deficiency in Lox died in the perinatal period due to ruptured thoracic aortic aneurysms [29]. Electron microscopy showed highly fragmented elastic fibers and discontinuity in the smooth muscle cell layer in the aortic wall. Lox was therefore proposed as an enzyme with an essential role in the resilience and tensile strength of the arterial wall.
High fat diets fed to apolipoprotein E (ApoE) and low-density lipoprotein (LDL) receptor knockout mice promoted the formation of aortic aneurysms [30]. Aortic aneurysms formed under mature atherosclerotic lesions showed medial elastolysis, dilatation of the lumen, and the presence of necrotic cores with a predominant lipid component. Because most reports on these mice focused on the development of atherosclerosis, there are limited data on aortic aneurysm development. For this reason, subcutaneous infusion of angiotensin II in hyperlipidemic strains has been widely employed, generating data of mechanistic and pharmacologic relevance [31–33].
Aneurysm development in both the thoracic and abdominal aorta has been noted in mice with MMP-3 or tissue inhibitor of MMP-1 deficiency [34,35]. The lack of specificity for the region may reflect a generalized destruction of extracellular matrix. However, some of these mice are not suitable as models because they died from early-stage ruptured aneurysms, or aneurysms developed at atypical locations.
2). Elastase infusion models
Temporary infusion of porcine pancreatic elastase into an isolated segment of infrarenal aorta produced aneurysmal degeneration in rats [36]. This rat model has been modified to produce aortic aneurysms in mice [37]. The rationale for development of aortic dilatation was based on an influx of inflammatory cells, increased production of MMPs, and destruction of medial elastin [38]. These findings supported the hypothesis that elastolytic activity within the media plays a key role in aneurysm formation.
The procedure of elastase infusion involves the introduction of a catheter via the femoral artery into the infrarenal aorta, and isolation of a segment of the abdominal aorta. The aorta is clamped at the level of the left renal vein and ligated around the catheter 1cm distally. This isolated segment is perfused with 2 ml of elastase for 2 hours. The elastase results in extensive destruction of the elastin network with immediate aortic dilatation followed by aneurysm formation. After 1–2 weeks, gradual expansion in the corresponding area can be confirmed, and tissues that are histologically similar to human aortic aneurysms can be obtained.
In the elastase infusion model, administration of indo-methacin [38] and doxycycline [39] significantly attenuated aneurysm formation, presumably because of inhibition of MMPs. Recently, it was reported that muscle-derived stem cells attenuated the rate of aneurysm formation in elastase-induced aortic aneurysms in rats [40].
The advantages of this model include circumferential aneurysmal degeneration with precise localization and structural stabilization, without proceeding to rupture. However, there are discrepancies in the rate of aneurysm formation related to differences in technique and elastase preparation [41,42]. In addition, the fatality rate is increased because this model requires direct manipulation of the aorta, with the possibility of aortic injury. Injection over a long time period resulted in extensive aortic elastolysis, and was also associated with higher rates of complications, such as lower limb ischemia [43].
3). Calcium chloride models
Extraluminal application of calcium chloride (CaCl2) to the aorta induces aneurysm formation. A pathogenic association between calcium deposition and aneurysm formation was first suggested in a study using periarterial application of CaCl2 to rabbit common carotid artery [44]. Calcium is deposited due to its high affinity for elastin, and the calcium-elastin complex destroys the elastin network and weakens the vascular walls to cause an aortic aneurysm; inflammatory responses accelerate due to this complex. This method was then applied to rabbit aortas through placement of a gauze pad soaked in a CaCl2 solution [45]. A mouse model was also successfully produced using the same technique [46].
The abluminal incubation of CaCl2 led to structural disruption of the media elastic network and inflammatory responses, as seen in human aortic aneurysms [47]. Moreover, a number of mechanisms, such as calcification, oxidative stress, neovascularization, and vascular smooth muscle cell apoptosis, may be relevant to the pathogenesis of human aortic aneurysms. Calcification of medial elastin itself has been associated with elastin degradation, vascular smooth muscle cell apoptosis, and inflammation. However, the CaCl2 models had some different features, with an inability to observe thrombus, atherosclerosis, and ruptures, which are seen in human aortic aneurysms.
CaCl2 models can be applied to wild-type mice, making assessment of transgenic rodent models more straightforward and rapid; pathology usually developed in the infrarenal abdominal aorta, the most common location of human aortic aneurysms [47].
4). Angiotensin II model
The elastin model, in which pancreatic elastase is injected into the aorta, and the CaCl2 model, in which CaCl2 is applied directly around the aorta, can induce aortic aneurysms with degenerative changes of the aortic wall, in the absence of intravascular hemodynamic force. However, these have disadvantages associated with the need to avulse the artery directly and manipulate it; in addition, aneurysms can be induced only in the infrarenal abdominal aorta, and human aneurysm features, such as thrombus, arterial stenosis, and ruptures, cannot be observed.
The angiotensin II model, which is the most widely known, induces aortic aneurysms in LDL receptor –/– or ApoE−/− mice by injecting angiotensin II with an osmotic pump for 4–6 weeks [31,48]. Angiotensin II contributes to the formation of aneurysms through various effects on the vasculature, in addition to its hypertensive effect. Angiotensin II induces changes similar to aortic aneurysms in humans, such as degeneration of the medial aortic wall, inflammatory responses, and thrombosis [49]. Aneurysm formation in this model tends to occur in the suprarenal aorta, in contrast to humans, where aneurysms develop in the infrarenal aorta.
Although the majority of angiotensin II infusion models have used hyperlipidemic mice, recently it was reported that aortic aneurysms develop during angiotensin II infusion into wild type C57BL/6 mice, which is the background strain of the hyperlipidemic mice [50]. However, the incidence was much lower in C57BL/6 mice compared with the hyperlipidemic mice. Therefore, although hyperlipidemia is not essential for development of aneurysms, its presence augments the incidence of aneurysm formation.
Recently, aortic aneurysm models have been induced by injecting angiotensin II together with BAPN, which breaks down elastin crosslinks, directly induces degenerative changes in the aortic wall, directly stimulates endothelial cells, and causes the expression of several chemokines and adhesion molecules [51,52]. The combination infusion of angiotensin II and BAPN, which causes the degeneration of elastic lamina, induced both thoracic and abdominal aortic aneurysms in wild-type mice. Although aneurysms can be induced successfully in both the thoracic and abdominal regions, the results differ morphologically and histologically between the regions, and the frequency of ruptures or avulsion is relatively high, suggesting the need for further studies.
The pharmacological manipulation of aneurysm development in this model has been investigated. Hydralazine lowered systolic blood pressure, but had no effect on aneurysm formation in angiotensin II infused ApoE−/− mice, showing that aneurysm formation was independent of blood pressure [53]. On the other hand, doxycycline [54], vitamin E [55], simvastatin [56], and rosiglitazone [57] reduced the incidence and extent of aneurysm formation in angiotensin II treated hyperlipidemic mice.
5). Deoxycorticosterone acetate and the high salt model
Clinically, there is evidence that systemic hypertension is closely associated with aortic aneurysm formation [58,59]. In an experiment on hypertension using mice, deoxycorticosterone acetate (DOCA), a precursor of aldosterone, was correlated with the occurrence of aortic aneurysms, with some animal specimens showing aortic ruptures. When DOCA was administered with a high salt content, an aortic aneurysm was induced, with features of human aneurysm, such as changes in elastin, infiltration of inflammatory cells, and degeneration of smooth muscle cells [51].
However, when angiotensin II or DOCA were used alone in wild-type mice, reports on incidence rates varied. In cases with high incidence rates, BAPN, an inhibitor of Lox (which induces the formation of elastin-collagen cross-links in the medial aortic wall), was co-administered [60]. Consequently, currently known animal models are not yet well developed, with regard to either the mechanisms underlying the occurrence of aortic aneurysms, or similarities to human aneurysms. In particular, studies on co-administration of angiotensin II and DOCA have not been reported.
OUR EXPERIENCE ON ANIMAL MODELS OF ANEURYSM RESEARCH
For more productive research, knowing the proper incidence of aneurysm formation in animal model is an essential factor. The incidence of animal models for aortic aneurysm has been reported at different rates according to its causative substances (Table 1) [36,40,51,53,60–64].
Table 1.
The incidence of aortic aneurysm formation
| Reference | Substance | Animal | Incidence of aneurysm formation (n/total, %) |
|---|---|---|---|
| Anidjar et al. (1990) [36] | Elastase | Wistar rat | 10/10 (100) |
| Park et al. (2013) [40] | Elastase | SD rat | 5/6 (83) |
| Bi et al. (2013) [61] | Elastase | New Zeland white rabbit | 6/6 (100) |
| Tanaka et al. (2009) [62] | Elastase | SD rat | 2/12 (16.7) |
| CaCl2 | 0/9 (0) | ||
| Elastase+CaCl2 | 13/14 (92.7) | ||
| Isenburg et al. (2007) [63] | CaCl2 | SD rat | 8/12 (66.7) |
| Cassis et al. (2009) [53] | Angiotensin II | ApoE−/− mouse | 13/26 (50) |
| Cao et al. (2010) [64] | Angiotensin II | ApoE−/− mouse | 41/65 (63) |
| Kanematsu et al. (2010) [51] | Angiotensin II+BAPN | C57BL/6J mouse | 22/45 (48.9) |
| DOCA+BAPN | 7/10 (70) | ||
| Liu et al. (2013) [60] | DOCA+salt | C57BL/6J mouse | 28/45 (62) |
SD rat, Sprague-Dawley rat; BAPN, β-aminopropionitrile; DOCA, deoxycorticosterone acetate.
We performed a pilot study to overcome the limitations of the previously presented elastase infusion and CaCl2 models, and to increase the incidence of aortic aneurysms in hyperlipidemic mice through co-administration of angiotensin II and DOCA.
Seven-week-old ApoE−/− hyperlipidemic mice (n=10) were purchased from Japan SLC Inc. (Shizuoka, Japan) and subjected to inhalation anesthesia using isoflurane, following an adaptation period of one week. The mice were divided into four groups: control group (n=1), angiotensin II group (n=3), DOCA group (n=3), and angiotensin II+DOCA group (n=3). Angiotensin II was administered for the first 4 weeks through a subcutaneously implanted osmotic-pump (Alzet model 2004, 28-day release; Durect Corporation, Cupertino, CA, USA), and DOCA pellets (50 mg, 21-day release; Innovative Research of America, Sarasota, FL, USA) were also subcutaneously implanted in the dorsal region (Fig. 1). Four weeks later, at the study endpoint, mice were anesthetized for tissue harvesting, and aortas were isolated for pathological and immunological analysis.
Fig. 1.
Photography of mouse showing subcutaneously implanted osmotic pump filled with angiotensin II and deoxycorticosterone acetate pellet.
In all hyperlipidemic mice, atherosclerotic lesions were seen in abdominal aorta and thoracic aorta. Among them, angiotensin II+DOCA mice showed most prominent atherosclerotic changes on the aorta (Fig. 2) and one of them showed an aneurysmal change on the ascending aorta (Fig. 3).
Fig. 2.
Atherosclerotic lesion on thoracic (A) and abdominal (B) aorta in angiotensin II+deoxycorticosterone acetate mouse.
Fig. 3.
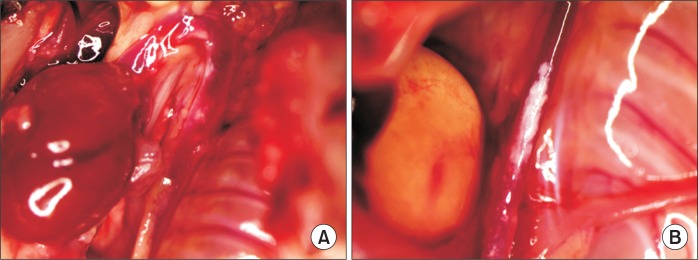
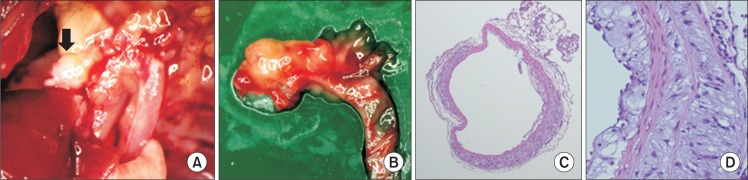
Aneurysm formation on ascending aorta in angiotensin II+deoxycorticosterone acetate mouse. (A, B) Dissected aortic arch showing aneurysmal change (black arrow) on ascending aorta. (C, D) H&E staining of ascending aorta (×5, ×20).
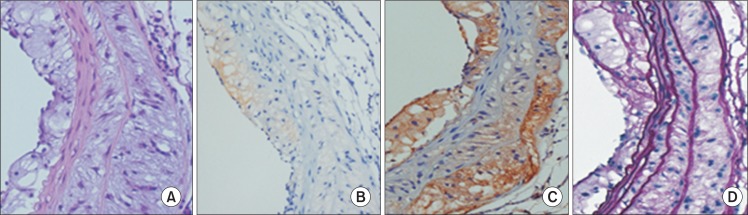
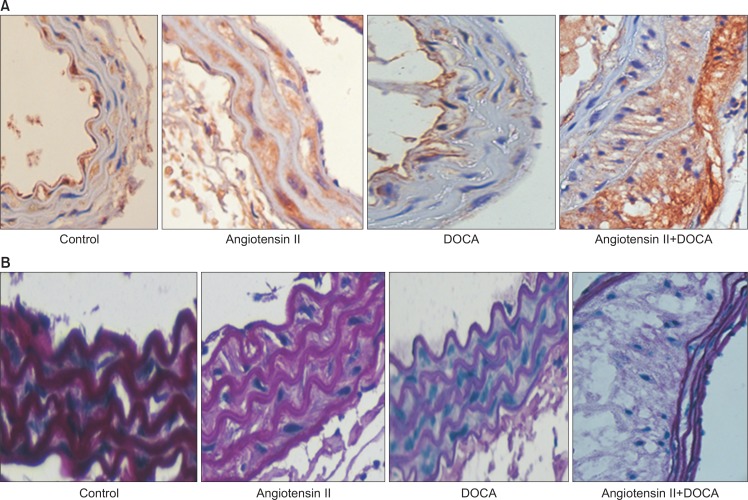
Through this preliminary experiment, we confirmed that administration of angiotensin II and DOCA augmented atherosclerotic lesions on the aorta and that aneurysms occurred in the ascending aorta when angiotensin II and DOCA were co-administered to hyperlipidemic mice. This was confirmed by histological testing of the expanded regions of the aorta, showing degeneration of the medial wall, accumulation of extracellular matrix, and infiltration of inflammatory cells (Fig. 3). Moreover, the results of immunohistochemical staining showed expression of MMP-2 and MMP-9, and Elastica von Gieson (EVG) staining results showed changes in the elastic lamina (Fig. 4).
Fig. 4.
(A) H&E staining and immunohistochemical staining for (B) matrix metalloproteinase (MMP)-2, (C) MMP-9, and (D) Elastica von Gieson on aneurysmal portion in angiotensin II+deoxycorticosterone acetate mouse (A–D: ×20).
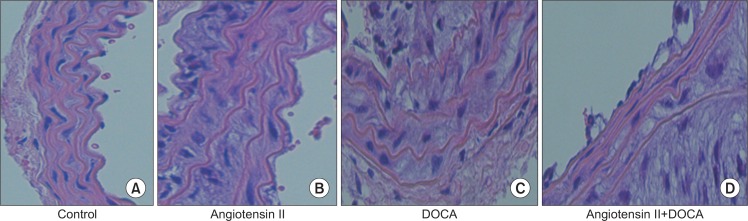
In mice given either angiotensin II or DOCA alone, no visible morphological changes in the aortic aneurysm were observed. However, on histological examination, medial degeneration was much more defined in these mice than in the control group, with the co-administration group showing the most severe degeneration (Fig. 5). MMP-9 expression was distinct in the angiotensin II and co-administration groups (Fig. 6A). EVG staining results showed that, in comparison with the control group, the angiotensin II and DOCA groups showed medial degeneration and thickening; the co-administration group showed medial degeneration and accumulation of extracellular matrix (Fig. 6B).
Fig. 5.
H&E staining of thoracic aorta in (A) control, (B) angiotensin II, (C) deoxycorticosterone acetate (DOCA) and (D) angiotensin II+DOCA mouse (A–D: ×40).
Fig. 6.
Immunohistochemical staining of thoracic aorta for matrix metalloproteinase-9 (A) and Elastica von Gieson (B) in each group (×40). DOCA, deoxycorticosterone acetate.
Although it is difficult to draw conclusions because of the small number of specimens, changes occurring in the course of aortic aneurysm formation were observed in both the angiotensin II and DOCA groups. When animal models were created by administering a mixture of drugs, synergism between the two was believed to improve the incidence rate and reproduce the characteristics of the aortic aneurysm induced by the two drugs.
CONCLUSION
Animal models have been important in increasing our understanding of the pathophysiology of aortic aneurysms, and continue to be valuable and indispensable tools for basic research on pharmacotherapy. Each animal model has features that are remarkably similar to clinical disease in humans, but no one model faithfully recreates the human condition in its entirety. Understanding of specific features and limitations inherent in each model is necessary for interpreting the significance and potential generalizability of results obtained from any one model. The advances in these animal models will provide an understanding of signaling systems at the cellular and molecular levels that are associated with the occurrence of human aortic aneurysms, along with predictions for the risk of rupture.
Acknowledgments
This study was supported by research fund from Chosun University, 2013.
Footnotes
Conflict of interest: None.
REFERENCES
- 1.Moxon JV, Parr A, Emeto TI, Walker P, Norman PE, Golledge J. Diagnosis and monitoring of abdominal aortic aneurysm: current status and future prospects. Curr Probl Cardiol. 2010;35:512–548. doi: 10.1016/j.cpcardiol.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell JT, Greenhalgh RM. Clinical practice. Small abdominal aortic aneurysms. N Engl J Med. 2003;348:1895–1901. doi: 10.1056/NEJMcp012641. [DOI] [PubMed] [Google Scholar]
- 3.Jones WB, Taylor SM, Kalbaugh CA, Joels CS, Blackhurst DW, Langan EM, 3rd, et al. Lost to follow-up: a potential under-appreciated limitation of endovascular aneurysm repair. J Vasc Surg. 2007;46:434–440. doi: 10.1016/j.jvs.2007.05.002. discussion 440–441. [DOI] [PubMed] [Google Scholar]
- 4.Rughani G, Robertson L, Clarke M. Medical treatment for small abdominal aortic aneurysms. Cochrane Database Syst Rev . 2012;9:CD009536. doi: 10.1002/14651858.CD009536.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaragoza C, Gomez-Guerrero C, Martin-Ventura JL, Blanco-Colio L, Lavin B, Mallavia B, et al. Animal models of cardiovascular diseases. J Biomed Biotechnol. 2011;2011:497841. doi: 10.1155/2011/497841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wassef M, Baxter BT, Chisholm RL, Dalman RL, Fillinger MF, Heinecke J, et al. Pathogenesis of abdominal aortic aneurysms: a multidisciplinary research program supported by the National Heart, Lung, and Blood Institute. J Vasc Surg. 2001;34:730–738. doi: 10.1067/mva.2001.116966. [DOI] [PubMed] [Google Scholar]
- 7.Vine N, Powell JT. Metalloproteinases in degenerative aortic disease. Clin Sci (Lond) 1991;81:233–239. doi: 10.1042/cs0810233. [DOI] [PubMed] [Google Scholar]
- 8.Herron GS, Unemori E, Wong M, Rapp JH, Hibbs MH, Stoney RJ. Connective tissue proteinases and inhibitors in abdominal aortic aneurysms. Involvement of the vasa vasorum in the pathogenesis of aortic aneurysms. Arterioscler Thromb. 1991;11:1667–1677. doi: 10.1161/01.ATV.11.6.1667. [DOI] [PubMed] [Google Scholar]
- 9.Newman KM, Ogata Y, Malon AM, Irizarry E, Gandhi RH, Nagase H, et al. Identification of matrix metalloproteinases 3 (stromelysin-1) and 9 (gelatinase B) in abdominal aortic aneurysm. Arterioscler Thromb. 1994;14:1315–1320. doi: 10.1161/01.ATV.14.8.1315. [DOI] [PubMed] [Google Scholar]
- 10.Newman KM, Malon AM, Shin RD, Scholes JV, Ramey WG, Tilson MD. Matrix metalloproteinases in abdominal aortic aneurysm: characterization, purification, and their possible sources. Connect Tissue Res. 1994;30:265–276. doi: 10.3109/03008209409015042. [DOI] [PubMed] [Google Scholar]
- 11.Newman KM, Jean-Claude J, Li H, Scholes JV, Ogata Y, Nagase H, et al. Cellular localization of matrix metalloproteinases in the abdominal aortic aneurysm wall. J Vasc Surg. 1994;20:814–820. doi: 10.1016/S0741-5214(94)70169-5. [DOI] [PubMed] [Google Scholar]
- 12.Patel MI, Melrose J, Ghosh P, Apple-berg M. Increased synthesis of matrix metalloproteinases by aortic smooth muscle cells is implicated in the etiopathogenesis of abdominal aortic aneurysms. J Vasc Surg. 1996;24:82–92. doi: 10.1016/S0741-5214(96)70148-9. [DOI] [PubMed] [Google Scholar]
- 13.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI0215334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson RW, Liao S, Curci JA. Therapeutic potential of tetracycline derivatives to suppress the growth of abdominal aortic aneurysms. Adv Dent Res. 1998;12:159–165. doi: 10.1177/08959374980120011301. [DOI] [PubMed] [Google Scholar]
- 15.McMillan WD, Pearce WH. Inflammation and cytokine signaling in aneurysms. Ann Vasc Surg. 1997;11:540–545. doi: 10.1007/s100169900088. [DOI] [PubMed] [Google Scholar]
- 16.Satta J, Laurila A, Pääkkö P, Haukipuro K, Sormunen R, Parkkila S, et al. Chronic inflammation and elastin degradation in abdominal aortic aneurysm disease: an immunohistochemical and electron microscopic study. Eur J Vasc Endovasc Surg. 1998;15:313–319. doi: 10.1016/S1078-5884(98)80034-8. [DOI] [PubMed] [Google Scholar]
- 17.Golledge AL, Walker P, Norman PE, Golledge J. A systematic review of studies examining inflammation associated cytokines in human abdominal aortic aneurysm samples. Dis Markers. 2009;26:181–188. doi: 10.1155/2009/352319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultz G, Tedesco MM, Sho E, Nishimura T, Sharif S, Du X, et al. Enhanced abdominal aortic aneurysm formation in thrombin-activatable procarboxypeptidase B-deficient mice. Arterioscler Thromb Vasc Biol. 2010;30:1363–1370. doi: 10.1161/ATVBAHA.109.202259. [DOI] [PubMed] [Google Scholar]
- 19.Leeper NJ, Tedesco MM, Kojima Y, Schultz GM, Kundu RK, Ashley EA, et al. Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation. Am J Physiol Heart Circ Physiol. 2009;296:H1329–H1335. doi: 10.1152/ajpheart.01341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannawa KK, Cho BS, Sinha I, Roelofs KJ, Myers DD, Wakefield TJ, et al. Attenuation of experimental aortic aneurysm formation in P-selectin knockout mice. Ann N Y Acad Sci. 2006;1085:353–359. doi: 10.1196/annals.1383.014. [DOI] [PubMed] [Google Scholar]
- 21.Vorp DA, Trachtenberg JD, Webster MW. Arterial hemodynamics and wall mechanics. Semin Vasc Surg. 1998;11:169–180. [PubMed] [Google Scholar]
- 22.Moore JE, Jr, Ku DN, Zarins CK, Glagov S. Pulsatile flow visualization in the abdominal aorta under differing physiologic conditions: implications for increased susceptibility to atherosclerosis. J Biomech Eng. 1992;114:391–397. doi: 10.1115/1.2891400. [DOI] [PubMed] [Google Scholar]
- 23.Finol EA, Amon CH. Flow-induced wall shear stress in abdominal aortic aneurysms: Part I--steady flow hemodynamics. Comput Methods Biomech Biomed Engin. 2002;5:309–318. doi: 10.1080/1025584021000009742. [DOI] [PubMed] [Google Scholar]
- 24.Gresham GA, Howard AN. Aortic rupture in the turkey. J Atheroscler Res. 1961;1:75–80. doi: 10.1016/S0368-1319(61)80056-2. [DOI] [PubMed] [Google Scholar]
- 25.Neumann F, Ungar H. Spontaneous aortic rupture in turkeys and the vascularization of the aortic wall. Can Vet J. 1973;14:136–138. [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson CF, Boucek RJ. The B-amino-propionitrile-fed turkey: a model for detecting potential drug action on arterial tissue. Cardiovasc Res. 1983;17:26–32. doi: 10.1093/cvr/17.1.26. [DOI] [PubMed] [Google Scholar]
- 27.Simpson CF. Relation of hemodynamics to the incidence of diethylstilbestrol-induced aortic ruptures in hypertensive and hypotensive lines of turkeys. Atherosclerosis. 1978;30:249–254. doi: 10.1016/0021-9150(78)90117-X. [DOI] [PubMed] [Google Scholar]
- 28.Andrews EJ, White WJ, Bullock LP. Spontaneous aortic aneurysms in blotchy mice. Am J Pathol. 1975;78:199–210. [PMC free article] [PubMed] [Google Scholar]
- 29.Mäki JM, Räsänen J, Tikkanen H, Sormunen R, Mäkikallio K, Kivirikko KI, et al. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. doi: 10.1161/01.CIR.0000038109.84500.1E. [DOI] [PubMed] [Google Scholar]
- 30.Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res. 1995;36:2320–2328. [PubMed] [Google Scholar]
- 31.Daugherty A, Manning MW, Cassis LA. Antagonism of AT2 receptors augments angiotensin II-induced abdominal aortic aneurysms and atherosclerosis. Br J Pharmacol. 2001;134:865–870. doi: 10.1038/sj.bjp.0704331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rush C, Nyara M, Moxon JV, Trollope A, Cullen B, Golledge J. Whole genome expression analysis within the angiotensin II-apolipoprotein E deficient mouse model of abdominal aortic aneurysm. BMC Genomics. 2009;10:298. doi: 10.1186/1471-2164-10-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue N, Muramatsu M, Jin D, Takai S, Hayashi T, Katayama H, et al. Involvement of vascular angiotensin II-forming enzymes in the progression of aortic abdominal aneurysms in angiotensin II-infused ApoE-deficient mice. J Atheroscler Thromb. 2009;16:164–171. doi: 10.5551/jat.E611. [DOI] [PubMed] [Google Scholar]
- 34.Silence J, Lupu F, Collen D, Lijnen HR. Persistence of atherosclerotic plaque but reduced aneurysm formation in mice with stromelysin-1 (MMP-3) gene inactivation. Arterioscler Thromb Vasc Biol. 2001;21:1440–1445. doi: 10.1161/hq0901.097004. [DOI] [PubMed] [Google Scholar]
- 35.Silence J, Collen D, Lijnen HR. Reduced atherosclerotic plaque but enhanced aneurysm formation in mice with inactivation of the tissue inhibitor of metalloproteinase-1 (TIMP-1) gene. Circ Res. 2002;90:897–903. doi: 10.1161/01.RES.0000016501.56641.83. [DOI] [PubMed] [Google Scholar]
- 36.Anidjar S, Salzmann JL, Gentric D, Lagneau P, Camilleri JP, Michel JB. Elastase-induced experimental aneurysms in rats. Circulation. 1990;82:973–981. doi: 10.1161/01.CIR.82.3.973. [DOI] [PubMed] [Google Scholar]
- 37.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmes DR, Petrinec D, Wester W, Thompson RW, Reilly JM. Indo-methacin prevents elastase-induced abdominal aortic aneurysms in the rat. J Surg Res. 1996;63:305–309. doi: 10.1006/jsre.1996.0265. [DOI] [PubMed] [Google Scholar]
- 39.Petrinec D, Liao S, Holmes DR, Reilly JM, Parks WC, Thompson RW. Doxycycline inhibition of aneurysmal degeneration in an elastase-induced rat model of abdominal aortic aneurysm: preservation of aortic elastin associated with suppressed production of 92 kD gelatinase. J Vasc Surg. 1996;23:336–346. doi: 10.1016/S0741-5214(96)70279-3. [DOI] [PubMed] [Google Scholar]
- 40.Park HS, Choi GH, Hahn S, Yoo YS, Lee JY, Lee T. Potential role of vascular smooth muscle cell-like progenitor cell therapy in the suppression of experimental abdominal aortic aneurysms. Biochem Biophys Res Commun. 2013;431:326–331. doi: 10.1016/j.bbrc.2012.12.099. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi T, Yokokawa M, Suzuki M, Higashide S, Katoh Y, Sugiyama S, et al. The time course of elastin fiber degeneration in a rat aneurysm model. Surg Today. 2000;30:727–731. doi: 10.1007/s005950070085. [DOI] [PubMed] [Google Scholar]
- 42.Dobrin PB. Animal models of aneurysms. Ann Vasc Surg. 1999;13:641–648. doi: 10.1007/s100169900315. [DOI] [PubMed] [Google Scholar]
- 43.Carsten CG, 3rd, Calton WC, Johanning JM, Armstrong PJ, Franklin DP, Carey DJ, et al. Elastase is not sufficient to induce experimental abdominal aortic aneurysms. J Vasc Surg. 2001;33:1255–1262. doi: 10.1067/mva.2001.112706. [DOI] [PubMed] [Google Scholar]
- 44.Gertz SD, Kurgan A, Eisenberg D. Aneurysm of the rabbit common carotid artery induced by periarterial application of calcium chloride in vivo. J Clin Invest. 1988;81:649–656. doi: 10.1172/JCI113368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freestone T, Turner RJ, Higman DJ, Lever MJ, Powell JT. Influence of hypercholesterolemia and adventitial inflammation on the development of aortic aneurysm in rabbits. Arterioscler Thromb Vasc Biol. 1997;17:10–17. doi: 10.1161/01.ATV.17.1.10. [DOI] [PubMed] [Google Scholar]
- 46.Chiou AC, Chiu B, Pearce WH. Murine aortic aneurysm produced by periarterial application of calcium chloride. J Surg Res. 2001;99:371–376. doi: 10.1006/jsre.2001.6207. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Krishna S, Golledge J. The calcium chloride-induced rodent model of abdominal aortic aneurysm. Atherosclerosis. 2013;226:29–39. doi: 10.1016/j.atherosclerosis.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daugherty A, Cassis L. Angiotensin II and abdominal aortic aneurysms. Curr Hypertens Rep. 2004;6:442–446. doi: 10.1007/s11906-004-0038-0. [DOI] [PubMed] [Google Scholar]
- 50.Deng GG, Martin-McNulty B, Sukovich DA, Freay A, Halks-Miller M, Thinnes T, et al. Urokinase-type plasminogen activator plays a critical role in angiotensin II-induced abdominal aortic aneurysm. Circ Res. 2003;92:510–517. doi: 10.1161/01.RES.0000061571.49375.E1. [DOI] [PubMed] [Google Scholar]
- 51.Kanematsu Y, Kanematsu M, Kurihara C, Tsou TL, Nuki Y, Liang EI, et al. Pharmacologically induced thoracic and abdominal aortic aneurysms in mice. Hypertension. 2010;55:1267–1274. doi: 10.1161/HYPERTENSIONAHA.109.140558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kurobe H, Hirata Y, Matsuoka Y, Sugasawa N, Higashida M, Nakayama T, et al. Protective effects of selective mineralocorticoid receptor antagonist against aortic aneurysm progression in a novel murine model. J Surg Res. 2013;185:455–462. doi: 10.1016/j.jss.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, et al. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1660–H1665. doi: 10.1152/ajpheart.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2003;23:483–488. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- 55.Gavrila D, Li WG, McCormick ML, Thomas M, Daugherty A, Cassis LA, et al. Vitamin E inhibits abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:1671–1677. doi: 10.1161/01.ATV.0000172631.50972.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Naggar JC, Welzig CM, Beasley D, Moulton KS, Park HJ, et al. Simvastatin inhibits angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-knockout mice: possible role of ERK. Arterioscler Thromb Vasc Biol. 2009;29:1764–1771. doi: 10.1161/ATVBAHA.109.192609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones A, Deb R, Torsney E, Howe F, Dunkley M, Gnaneswaran Y, et al. Rosiglitazone reduces the development and rupture of experimental aortic aneurysms. Circulation. 2009;119:3125–3132. doi: 10.1161/CIRCULATIONAHA.109.852467. [DOI] [PubMed] [Google Scholar]
- 58.Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 1997;126:441–449. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
- 59.Kuzmik GA, Sang AX, Elefteriades JA. Natural history of thoracic aortic aneurysms. J Vasc Surg. 2012;56:565–571. doi: 10.1016/j.jvs.2012.04.053. [DOI] [PubMed] [Google Scholar]
- 60.Liu S, Xie Z, Daugherty A, Cassis LA, Pearson KJ, Gong MC, et al. Mine-ralocorticoid receptor agonists induce mouse aortic aneurysm formation and rupture in the presence of high salt. Arterioscler Thromb Vasc Biol. 2013;33:1568–1579. doi: 10.1161/ATVBAHA.112.300820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bi Y, Zhong H, Xu K, Ni Y, Qi X, Zhang Z, et al. Performance of a modified rabbit model of abdominal aortic aneurysm induced by topical application of porcine elastase: 5-month follow-up study. Eur J Vasc Endovasc Surg. 2013;45:145–152. doi: 10.1016/j.ejvs.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka A, Hasegawa T, Chen Z, Okita Y, Okada K. A novel rat model of abdominal aortic aneurysm using a combination of intraluminal elastase infusion and extraluminal calcium chloride exposure. J Vasc Surg. 2009;50:1423–1432. doi: 10.1016/j.jvs.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 63.Isenburg JC, Simionescu DT, Starcher BC, Vyavahare NR. Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation. 2007;115:1729–1737. doi: 10.1161/CIRCULATIONAHA.106.672873. [DOI] [PubMed] [Google Scholar]
- 64.Cao RY, Amand T, Ford MD, Piomelli U, Funk CD. The murine angiotensin ii-induced abdominal aortic aneurysm model: rupture risk and inflammatory progression patterns. Front Pharmacol. 2010;1:9. doi: 10.3389/fphar.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]