Abstract
A giant abdominal aortic aneurysm (AAA) renders surgical treatment much more difficult by deforming the proximal infrarenal aortic neck (shortened length and disturbed angulation), by altering the iliac arteries (marked tortuosity and aneurysmal dilatation), and by displacing abdominal organs. Because the retroperitoneal rupture of giant AAA makes the mesentery more elongated and deformed, compromising its blood flow and thus increasing the risk of mesenteric ischemia such as colon ischemia. We describe here the surgical repair of a large infrarenal AAA with a ruptured huge left common iliac artery aneurysm of 13.5 cm in diameter, accompanied by colostomy due to colon ischemia which occurred during the operation. We discuss the pathophysiology and preventive strategy of colon ischemia during ruptured giant AAA repair.
Keywords: Abdominal aortic aneurysms, Ruptured aneurysm, Ischemic colitis
INTRODUCTION
As an abdominal aortic aneurysm (AAA) expands, increasing in diameter, the risk of rupture escalates. Reports of giant AAAs, with diameters exceeding 11 cm, seldom appear in the medical literature [1]. An aneurysm of this magnitude complicates open or endovascular surgery considerably by shortening the proximal infrarenal aortic neck and by displacing abdominal organs. Adjacent viscera may be adherent peripherally, with fistula formation [1]. As rupture in a giant AAA makes the mesentery mo re elongated and deformed, its blood flow may be compromised and the risk of mesenteric ischemia such as colon ischemia may increase. In this report, we describe the surgical repair of a large infrarenal AAA with a ruptured huge left common iliac artery (CIA) aneurysm of 13.5 cm in diameter, accompanied by colostomy due to colon ischemia which occurred during the operation, and discuss the pathophysiology and preventive strategy of colon ischemia during ruptured giant AAA repair.
CASE
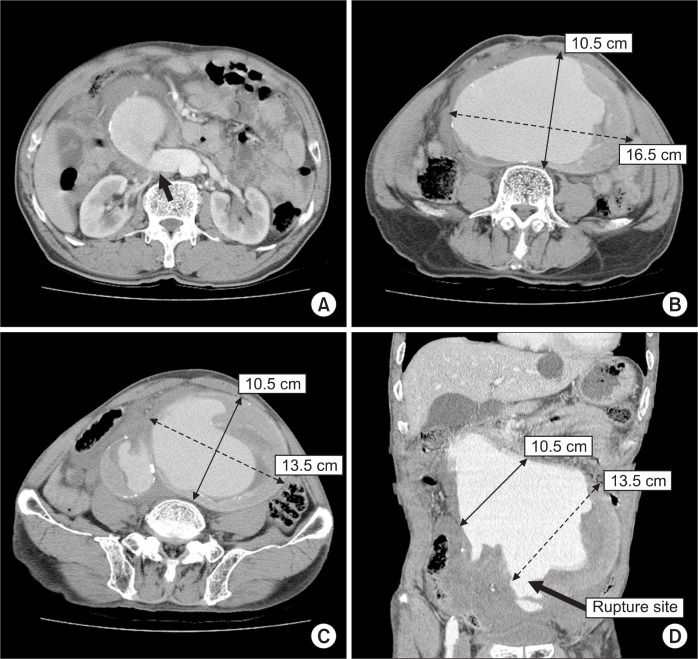
A 75-year-old male patient presented to the emergency department with abdominal pain and pulsating mass of 7-hour duration. His blood pressure was 94/51 mmHg and the pulse rate was 88 beats/min. He had no history of previous abdominal surgery. Computed tomography (CT) revealed a large infrarenal AAA measuring 10.5×16.5 cm maximally at the aortic bifurcation and involving both iliac arteries (Fig. 1). Aneurysms of the common iliac arteries measured 5.5×5.0 cm (right) and 10.5×13.5 cm (left). Rupture occurred at the medial wall of the left CIA aneurysm (Fig. 1D). The proximal infrarenal aortic neck was angled at 120° (Fig. 1A). At the iliac arteries, the angle was nearly 180°. Both internal iliac arteries (IIAs) were patent, but the inferior mesenteric artery (IMA) was deviated to the right side, owing to the massive aneurysm and retroperitoneal hematoma. A splenic artery aneurysm (SAA) of 3 cm diameter was also encountered as an incidental finding. The aberrant angulations of the proximal neck and access vessels, urgent patient’s condition and inadequate facilities for endovascular treatment made open surgery more preferable than endovascular approach.
Fig. 1.
Computed tomographic scan showing ruptured huge infrarenal abdominal aortic aneurysm (AAA), with angulated proximal infrarenal aortic neck but adequate enough for aortic cross-clam ping (black arrow) (A); AAA at aortic bifurcation (B); aneurysmal dilatation of both common iliac arteries (C); coronal view of AAA and rupture site (D).
An emergent open transperitoneal repair was performed, using a Y-shaped Dacron graft (14×7 mm) for aortoiliac re construction. Because the huge AAA allowed for little surgical work space, aortic cross-clamping of the proximal infrarenal aortic neck was performed with difficulty. After aortic cross-clamping, the iliac arteries were dissected and controlled. Aortotomy was made along the anterior wall of the aneurysm. Proximal anastomosis at the infrarenal aorta was performed just below the renal arteries, whereas the distal anastomosis involved both the right iliac bifurcation and the left external iliac artery. The left IIA had aneurysmal change and thus was ligated. Excision of the SAA was also achieved by proximal and distal ligation.
After exclusion of the aortoiliac and SAAs, the sigmoid colon was found to have an impaired motility and discoloration that was suspicious of colon ischemia. IMA re vascularization could not be performed, because the me senteric root of the sigmoid colon had already been ligated, due to right side deviation of the IMA owing to the massive aneurysm and retroperitoneal hematoma. Left IIA revascularization could not be performed, because its distal stump was very deeply located after exclusion of its aneurysm. A proximal transverse loop colostomy was therefore performed, following closure of the retroperitoneal space. Sigmoid colectomy was not done, because the presumed ischemic changes were thought to be reversible and the marginal arteries of the colon had not been sacrificed. The surgery lasted 10 hours overall, during which the patient received 14 units of packed red blood cells. There were no intraoperative episodes of severe hypotension; his systolic blood pressure had been steady at 90 to 100 mmHg, with only intermittent administration of vasopressors.
Pancreatitis did develop after surgery, requiring a brief stay for bowel rest. The patient was thereafter discharged, on postoperative day 17. A follow-up CT scan confirmed that the spleen was preserved; one year later, the colostomy was successfully reversed, with no signs of ischemic colitis. Nonetheless, he presented two years later with infection of the aortic graft. Axillofemoral bypass surgery was performed, rather than direct reconstruction, based on a large accumulation of pus at the previous graft site. Postoperatively (day 18), the patient succumbed to multiorgan failure, despite all surgical efforts and removal of the graft. Methicillin-susceptible Staphylococcus aureus was ultimately identified in graft cultures.
DISCUSSION
An extensive search of the medical literature was conducted via PubMed (keywords: big, giant, huge, large, and mega AAA). Some reports of giant AAAs indicate diameters as large as 15 cm [2,3]. The huge aortoiliac aneurysm cited here is one of the largest AAAs to date. Its association with a SAA is also a rarity.
When an AAA massively expands, surgical treatment (open or endovascular) becomes much more challenging due to physical/spatial limitations. Abdominal viscera are markedly displaced, and the surgical field is typically insufficient. In most instances, the proximal infrarenal aortic neck is shortened, with a significantly deviated angle. Aortic cross-clamping may thus be very difficult. Frequently, adjacent structures are adherent (e.g., bowels, venous structures, and spine) [4], resulting in the development of fistulas and increased chance of rupture. Aneurysmal dilatation and excessive tortuosity of the iliac arteries are commonly found as well. Given these factors, an endovascular approach is usually not feasible, necessitating open surgical repair [1]. However an endovascular approach may become feasible in these cases in the future due to improving endovascular technology and skills. Further difficulties may arise if such a giant AAA ruptures, including shadowy operative view, blunt dissection, coagulopathy and shock that could be due to adjacent hematoma and massive bleeding.
Open repair of a giant AAA is fraught with a number of potential complications, stemming from extreme anatomic distortion. The left colonic mesentery may be injured during approach to the aortic aneurysm as a consequence of right-sided IMA deviation, as in our case. Moreover, it may be difficult to revascularize the aneurysmal IIAs and retain pelvic blood supply, and hemodynamic instability in the case of ruptured aneurysms can cause hypoperfusion, leading to ischemic colitis. Finally, the predictably tight surgical field may force excessive traction upon the aneurysm and/or adjacent organs, so that venous injury, pancreatitis, serosal tears of the bowels, or other such injuries may occur.
In this regard, several technical tips are worthy of mention. An approach aimed more to the right of the aortic aneurysm may strategically prevent injury to the left colonic mesentery. The following procedures may prevent colon ischemia: preservation of IIA (especially left side) and mesenteric collateral vessels (Arc of Riolan and marginal arteries), reimplantation of IMA and avoidance of hypotension after aortic cross-clamping. In addition, it is important to perform aortic cross-clamping rapidly and accurately. Although traction of the aortic aneurysm and/or adjacent organs may widen the surgical field, great traction is mandatory to avoid stated injuries. Vural et al. [5] suggested that open proximal anastomosis under hypothermic circulatory arrest may be helpful to repair giant AAAs.
The incidence of colon ischemia is 0.6%–3% in nonruptured AAA repairs and 7%–27% in ruptured AAA repairs [6], and is 22% for aortofemoral grafts, 4% for tube grafts and 2.7% for aortoiliac grafts [7]. It can be classified according to severity; patchy mucosal necrosis (grade I), mucosal and muscularis involvement (grade II) and transmural necrosis, gangrene, and perforation (grade III) [8]. Risk factors include hypotension, IMA patency, collateral supply from the superior mesenteric artery and IIA, use of vasopressors and intraoperative transfusions [9]. Djavani Gidlund et al. [10] suggested that extraluminal tonometry may serve as a screening test for colon ischemia.
The source of the lethal aortic graft infection suffered by this patient was indeterminable. Specifically, the prior colostomy could not be directly implicated in the graft infection since the methicillin-susceptible Staphylococcus aureus identified in the graft cultures do not normally inhabit in the gut.
A giant AAA shortens the proximal infrarenal aortic neck and distorts its angulation, incites tortuosity and dilatation of the iliac arteries, and displaces abdominal organs. Open surgical repair is thus preferable over endovascular treatment. In doing so, cautious dissection is vital to prevent complications, such as colonic ischemia, pancreatitis, serosal tears of the bowels and other traumatic organ injuries.
Footnotes
Conflict of interest: None.
REFERENCES
- 1.Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Bocci L, et al. Images in cardiovascular medicine: giant abdominal aortic aneurysm. Circulation. 2010;122:e392–e393. doi: 10.1161/CIRCULATIONAHA.110.946442. [DOI] [PubMed] [Google Scholar]
- 2.Ferrero E, Gaggiano A, Berardi G, Ferri M, Piazza S, Viazzo A, et al. Giant infrarenal aortic aneurysm: a huge size of 15 cm on diameter. Minerva Chir. 2009;64:321–322. [PubMed] [Google Scholar]
- 3.Ebaugh JL, Raffetto JD. Images in clinical medicine. Giant abdominal aortic aneurysm. N Engl J Med. 2010;362:66. doi: 10.1056/NEJMicm0808225. [DOI] [PubMed] [Google Scholar]
- 4.Mii S, Mori A, Yamaoka T, Sakata H. Penetration by a huge abdominal aortic aneurysm into the lumbar vertebrae: report of a case. Surg Today. 1999;29:1299–1300. doi: 10.1007/BF02482229. [DOI] [PubMed] [Google Scholar]
- 5.Vural H, Türk T, Göncü T, Yalçinkaya S, Yavuz S, Ozyazicioğlu A. Giant size abdominal aortic aneurysm repair using open proximal anastomosis under hypothermic circulatory arrest: a report of two cases. J Vasc Surg. 2007;46:363–365. doi: 10.1016/j.jvs.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Levison JA, Halpern VJ, Kline RG, Faust GR, Cohen JR. Perioperative predictors of colonic ischemia after ruptured abdominal aortic aneurysm. J Vasc Surg. 1999;29:40–45. doi: 10.1016/S0741-5214(99)70348-4. [DOI] [PubMed] [Google Scholar]
- 7.Becquemin JP, Majewski M, Fermani N, Marzelle J, Desgrandes P, Allaire E, et al. Colon ischemia following abdominal aortic aneurysm repair in the era of endovascular abdominal aortic repair. J Vasc Surg. 2008;47:258–263. doi: 10.1016/j.jvs.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Champagne BJ, Darling RC, 3rd, Daneshmand M, Kreienberg PB, Lee EC, Mehta M, et al. Outcome of aggressive surveillance colonoscopy in ruptured abdominal aortic aneurysm. J Vasc Surg. 2004;39:792–796. doi: 10.1016/j.jvs.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Biros E, Staffa R. Incidence and risk factors of ischemic colitis after AAA repair in our cohort of patients from 2005 through 2009. Rozhl Chir. 2011;90:682–687. [PubMed] [Google Scholar]
- 10.Djavani Gidlund K, Wanhainen A, Björck M. A comparative study of extra- and intraluminal sigmoid colonic tonometry to detect colonic hypoperfusion after operation for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2011;42:302–308. doi: 10.1016/j.ejvs.2011.05.002. [DOI] [PubMed] [Google Scholar]