Abstract
Purpose:
Hyperhomocysteinemia has been identified as an independent risk factor in arterial and venous thrombosis. Mutations in genes encoding methylenetetrahydrofolate reductase (MTHFR), involved in the metabolism of homocysteine, may account for reduced enzyme activity and elevated plasma homocysteine levels. In this study, we investigated the interrelation of MTHFR C677T genotype and level of homocysteine in patients with arterial and venous thrombosis.
Materials and Methods:
We retrospectively reviewed the medical records of 146 patients who were diagnosed as having arterial and venous thrombosis. We excluded patients diagnosed with atrial fibrillation. We examined routinely the plasma concentration of total homocysteine level and MTHFR C677T polymorphism for evaluation of thrombotic tendency in all patients. Screening processes of MTHFR C677T polymorphism were performed by real-time polymerase chain reaction.
Results:
Investigated groups consisted of thrombotic arterial occlusion in 48 patients and venous occlusion in 63 patients. The distribution of the three genotypes was as follows: homozygous normal (CC) genotype in 29 (26.1%), heterozygous (CT) genotype in 57 (51.4%), and homozygous mutant (TT) genotype in 25 (22.5%) patients. There were no significant differences among individuals between each genotype group for baseline characteristics. Plasma concentration of homocysteine in patients with the TT genotype was significantly increased compared to the CC genotype (P<0.05).
Conclusion:
We observed a significant interaction between TT genotypes and homocysteine levels in our results. The results might reflect the complex interaction between candidate genes and external factors responsible for thrombosis.
Keywords: Methylenetetrahydrofolate reductase, Mutation, Hyperhomocysteinemia
INTRODUCTION
McCully’s report in 1969 [1] showed widespread arterial thrombosis and atherosclerosis in the autopsy results of children with homocystinuria in which the plasma homocysteine levels were increased; since then, hyperhomocysteinemia has been shown in many studies to be an independent risk factor of several diseases [2–5]. Although severe hyperhomocysteinemia is rare, about 5%-7% of the total population is known to have mild hyperhomocysteinemia [6,7].
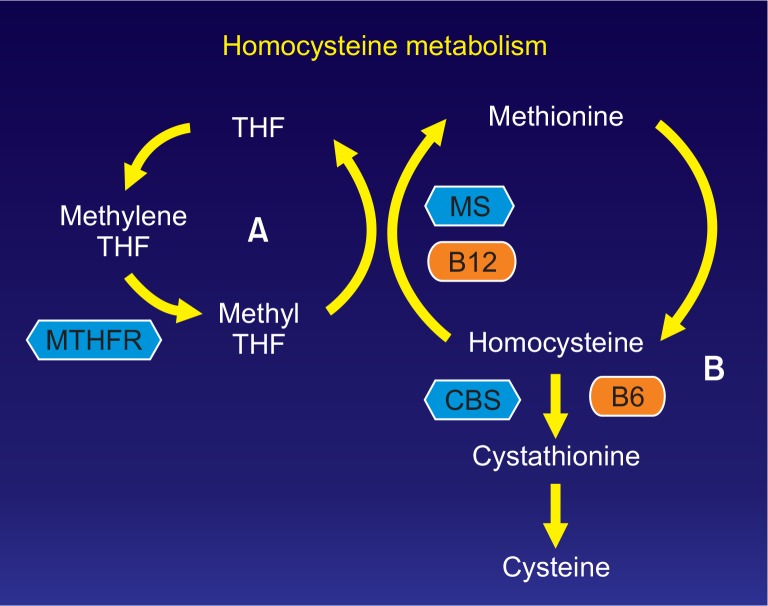
Homocysteine is produced during the metabolism of methionine, which is an essential amino acid, and it is metabolized by one of the two pathways: remethylation or transsulfuration. As 5-methyltetrahydrofolate functions as a methyl donor and vitamin B12 functions as a cofactor in the remethylation process, homocysteine is resynthesized to methionine by methionine synthase. 5-methyltetrahydrofolate is reduced from 5,10-methylenetetrahydrofolate by methylenetetrahydrofolate reductase (MTHFR), and the decreased enzymatic activity that results from the gene mutations is related to the increase in the serum homocysteine concentrations (Fig. 1). The MTHFR-expressing gene is located on chromosome 1p36.3, and the gene mutation occurs when cytosine (C) at the 677th position is replaced by thymidine (T). The MTHFR polymorphisms are divided into a homozygous normal genotype (CC), a heterozygous genotype (CT), and a homozygous mutant genotype (TT), and several studies have reported that the TT genotype is associated with a marked increase in plasma homocysteine concentrations [8–10].
Fig. 1.
Homocysteine metabolism. Remethylation cycle (A) and Transsulfuration pathway (B). THF, tetrahydrofolate; MTHFR, methylenetetrahydrofolate reductase; MS, methionine synthase; CBS, cystathionine β synthase; B12, vitamin B12; B6, vitamin B6.
When the plasma homocysteine concentration increases, oxidative stress increases, leading to inflammation of the vascular cells and thrombosis due to endothelial cell dysfunction [11]. However, little is known about the effects of the association between MTHFR gene mutations and hyperhomocysteinemia on thrombosis, and, especially, its role in the prevention and treatment of thrombosis is not yet established. Therefore, in this study, we investigated the clinical significance of the MTHFR gene mutation and serum homocysteine concentrations in patients with arterial and venous thrombosis.
MATERIALS AND METHODS
1. Patients
We conducted a retrospective study on 146 patients who were diagnosed with arterial and venous thrombosis in Chosun University Hospital from September 2008 to October 2013. The investigation was based on medical records, and information, including data on hypertension, diabetes, cardiovascular disease, atrial fibrillation, hyperlipidemia, and smoking history, was collected. Patients with atrial fibrillation (n=19), May-Thurner syndrome (n=14), and other coagulation abnormalities (n=2), which can be potential causes of thrombosis, were excluded from this study. The final study had 111 patients, including 48 patients with arterial thrombosis and 63 patients with venous thrombosis. Patients with arterial thrombosis were a heterogeneous group, composed of a pure thrombosis group (n=28) and an atherothrombosis group (n=20). Patients with evidence of atherosclerosis, such as atherosclerotic plaque, calcification and collateral artery development on CT angiogram were classified as atherothrombosis group. Thrombectomy, thrombolysis, percutaneous transluminal angioplasty or vascular bypass were performed according to the case situation. The study protocol was approved by ethics committee of Chosun University Hospital (No. 2014-09-006-001).
2. Measurement of the plasma homocysteine concentrations
The plasma homocysteine concentrations of all of the patients were measured in the fasting state by using a fluorescence polarization immunoassay, and the normal range of the plasma homocysteine concentration was 5-15 μmol/L [12].
3. MTHFR gene test
The samples were obtained from whole blood in all of the patients, and the MTHFR polymorphism was classified into a homozygous normal genotype (CC), a heterozygous genotype (CT), or a homozygous mutant genotype (TT) with real-time polymerase chain reactions with an AccuPower MTHFR C677T genotyping kit (Bioneer Co., Daejeon, Korea).
4). Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). One-way analysis of variance (ANOVA) was performed to compare the average plasma homocysteine concentrations of each group according to the MTHFR polymorphisms, and the plasma homocysteine values of each group were compared by Tukey’s post-hoc tests. P-values less than 0.05 were considered statistically significant.
RESULTS
Of the 111 patients, 78 (70.3%) were men, and 33 (29.7%) were women, and the average age was 61.8 years (range, 19-91 years). For genotype, 29 (26.1%) belonged to the CC group, 57 (51.4%) belonged to the CT group, and 25 (22.5%) belonged to the TT group. There were no significant differences in the clinical characteristics, including the sex ratio, age, hypertension, diabetes, cardiovascular diseases, hyperlipidemia, and smoking history, among the groups. Of the 111 patients, 59 (53.2%) had hyperhomocysteinemia; among them, 14 (48.3%), 30 (52.6%), and 15 (60.0%) belonged to the CC, CT, and TT groups, respectively (Table 1).
Table 1.
Demographic and clinical characteristics
| Characteristic | Total (n=111) | CC (n=29, 26.1%) | CT (n=57, 51.4%) | TT (n=25, 22.5%) | P-value |
|---|---|---|---|---|---|
| Male/female | 78/33 (70.3/29.7) | 24/5 (82.8/17.2) | 40/17 (70.2/29.8) | 14/11 (56.0/44.0) | 0.1 |
| Age (y) | 61.85 (19-91) | 58.3 | 61.4 | 66.9 | 0.162 |
| Hypertension | 39 (35.1) | 10 (34.5) | 19 (33.3) | 10 (40.0) | 0.841 |
| Diabetes | 31 (27.9) | 4 (13.8) | 19 (33.3) | 8 (32.0) | 0.141 |
| Coronary artery disease | 4 (3.6) | 1 (3.4) | 2 (3.5) | 1 (4.0) | 0.993 |
| Hyperlipidemia | 5 (4.5) | 2 (6.9) | 2 (3.5) | 1 (4.0) | 0.749 |
| Smoking | 16 (14.4) | 3 (10.3) | 9 (15.8) | 4 (16.0) | 0.654 |
| Hyperhomocysteinemia | 59 (53.2) | 14 (48.3) | 30 (52.6) | 15 (60.0) | 0.686 |
Values are presented as number (%) or age (range).
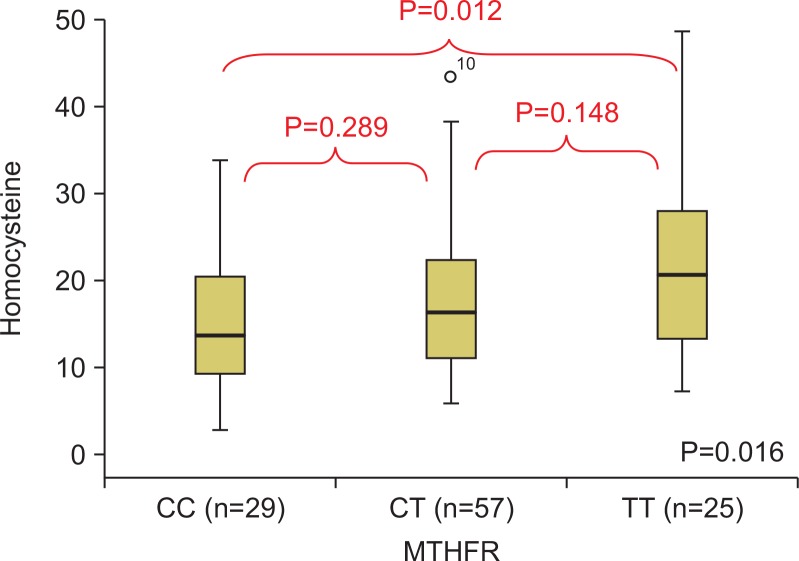
The plasma homocysteine values were significantly different among the different genotype groups (ANOVA, P=0.016). The plasma homocysteine values for each genotype group were compared by Tukey’s post-hoc tests, and the average homocysteine levels of the CC, CT, and TT groups were 14.6 μmol/L, 17.7 μmol/L, and 21.8 μmol/ L, respectively. While there was a statistically significant difference between the CC and TT groups in the plasma homocysteine values (P=0.012), there was no statistically significant difference between the CC and CT groups or between the CT and TT groups (Fig. 2).
Fig. 2.
The comparison of the plasma homocysteine according to methylenetetrahydrofolate reductase (MTHFR) polymorphism.
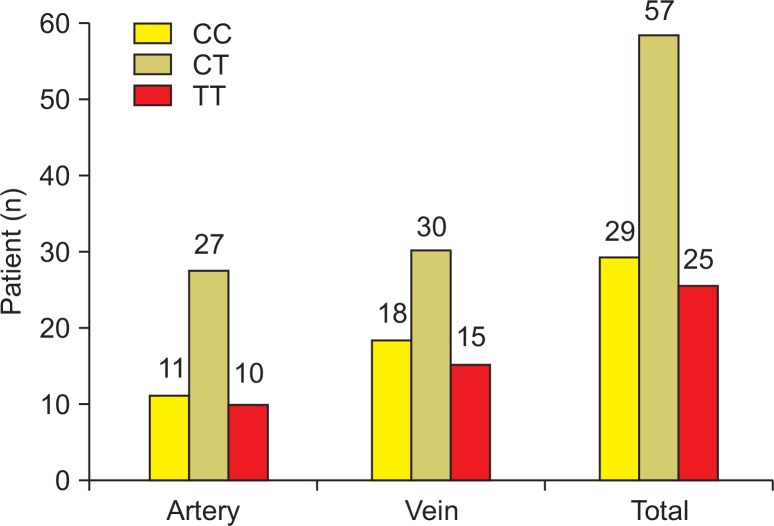
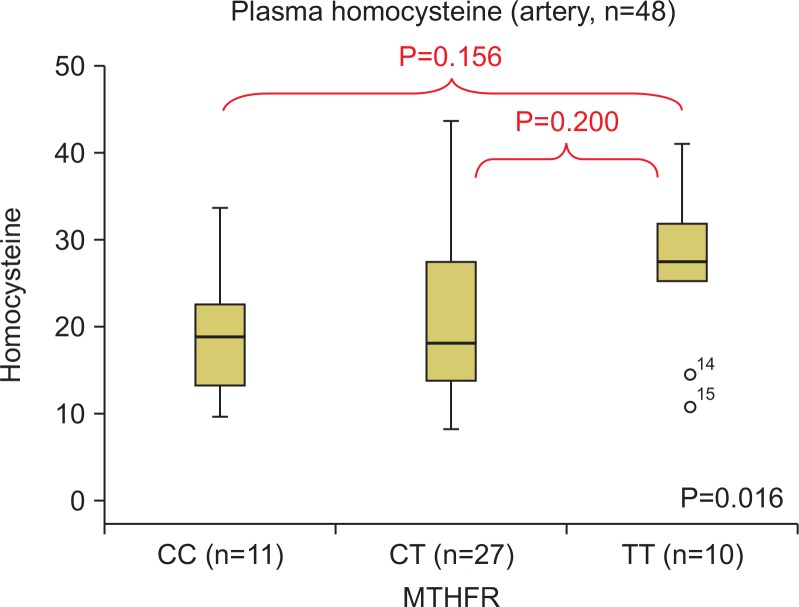
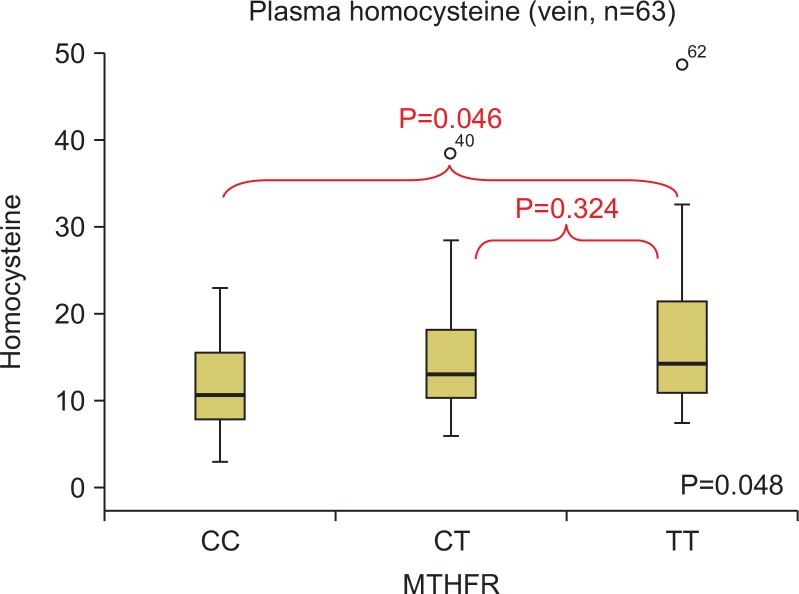
After dividing the groups according to their arterial and venous thrombosis lesions, the homocysteine concentrations per genotype were compared. Out of the 48 patients with arterial thrombosis, eight had a lesion in the iliac artery, 22 had a lesion in the femoral artery, 11 had a lesion in the popliteal artery, one had a lesion in the below-knee artery, and three had a lesion in the upper-extremity artery (subclavian and brachial artery). There were 11, 27, and 10 patients in the CC, CT, and TT groups, respectively (Fig. 3). The plasma homocysteine values between the genotype groups were compared by a Tukey’s post-hoc test, and there were no statistically significant differences (Fig. 4). Out of the 63 patients with venous thrombosis, there were 57 patients with deep vein thrombosis, five patients with superior mesenteric vein thrombosis, and one patient with portal vein thrombosis. There were 18, 30, and 15 patients in the CC, CT, and TT groups, respectively (Fig. 3). The plasma homocysteine values between the genotype groups were compared by a Tukey’s post-hoc test. As was the case for arterial thrombosis, there were no statistically significant differences between the groups (Fig. 5).
Fig. 3.
Distribution of methylenetetrahydrofolate reductase polymorphism.
Fig. 4.
The comparison of the plasma homocysteine according to methylenetetrahydrofolate reductase (MTHFR) polymorphism in arterial thrombosis.
Fig. 5.
The comparison of the plasma homocysteine according to methylenetetrahydrofolate reductase (MTHFR) polymorphism in venous thrombosis.
During the follow-up monitoring period, six patients had a relapse, and all were patients with arterial thrombosis. Three had the CT genotype, and the other three had the TT genotype (Table 2).
Table 2.
Clinical characteristics of recurrence
| Patient | Lesion | MTHFR | Homocysteine |
|---|---|---|---|
| 1 | Artery | TT | High (35.56) |
| 2 | Artery | CT | High (27.71) |
| 3 | Artery | TT | High (31.90) |
| 4 | Artery | CT | High (16.74) |
| 5 | Artery | TT | High (26.54) |
| 6 | Artery | CT | High (23.68) |
MTHFR, methylenetetrahydrofolate reductase.
DISCUSSION
Although the mechanisms by which hyperhomocysteinemia causes atherosclerosis and thrombosis are not clear, endothelial dysfunction, damage to endothelial cells, and inflammation in the blood vessels caused by reactive oxygen species, including superoxide, are thought to be responsible for these diseases [11]. A number of factors cause hyperhomocysteinemia, including malnutrition, chronic renal failure, hypothyroidism, cancer, drugs, and gene mutations. Deficiencies in vitamin B12, vitamin B6, and folic acid, which are necessary for homocysteine metabolism, can cause hyperhomocysteinemia [13,14]. In addition, increased levels of creatinine in patients with chronic renal failure can cause increase in the plasma homocysteine concentrations. Although it is not clear whether this mechanism originates from a metabolic disorder or an excretion disorder, it can be speculated that the deterioration of atherosclerosis in patients with chronic renal failure is associated with hyperhomocysteinemia caused by renal insufficiency [15,16]. Hypothyroidism; pernicious anemia; cancers in the breast, ovary, or pancreas; acute lymphoblastic leukemia; drugs such as methotrexate, phenytoin, or theophylline; or smoking can cause hyperhomocysteinemia [7,17].
Deficiencies in MTHFR, which is involved in the remethylation step in which methionine is resynthesized into homocysteine, causes severe hyperhomocysteinemia [18] and can result in severe clinical outcomes, such as coronary artery disease, deep vein thrombosis, peripheral arterial occlusive disease, and cerebral infarction [2–5]. Among the common MTHFR mutations, the thermolabile variant C677T of MTHFR is a point mutation of the 667th C in the MTHFR coding sequence to T, which results in a change in the aminoacid sequence from alanine to valine, leading to a decrease in enzymatic activity [19,20]. While this gene mutation was found in 5%-15% of the cases abroad [21], there have been no large-scale studies on the MTHFR gene mutation frequency in normal subjects or disease-specific patient groups in Korea [22,23]. In a study on 243 patients with thrombosis in the middle Black Sea area (Tokat) of Turkey, the distribution ratio of the MTHFR gene genotype was CC:CT:TT=40.0%:47.3%:12.7% [24]. On the other hand, in a study on 106 people in China, the distribution ratio was CC:CT:TT=63.8%:25.7%:10.5% [25] and in our study, the distribution ratio was CC:CT:TT= 26.1%:51.4%:22.5%. As such, the distribution ratio of the MTHFR gene genotype is different for each study and population. According to a few studies besides the one by Jacques et al. [8], the homozygous TT genotype among the MTHFR mutations has reportedly caused a marked increase in plasma homocysteine concentrations [8,9]. In addition, when the homozygous TT genotype is accompanied by a folate deficiency, hyperhomocysteinemia reportedly becomes much more severe [10]. In our study, there was a statistically significant difference in the homocysteine concentrations between the TT and CT genotypes, which was consistent with the findings of the study by Jacques et al. [8].
There is controversy on whether the MTHFR gene mutation itself causes thrombosis. Even though there is clear evidence that the MTHFR gene mutation can cause hyperhomocysteinemia and hyperhomocysteinemia causes atherosclerosis and thrombosis, the MTHFR gene mutation cannot be said to be a direct cause of thrombosis [10]. According to Abbate et al. [26], the TT genotype of the MTHFR gene mutation is reportedly not a risk factor of thrombosis or restenosis in patients with coronary artery disease. In addition, according to a study by Bezemer et al. [27] on 4,375 patients with deep vein thrombosis and pulmonary thromboembolism and 4,856 normal controls, the MTHFR gene mutation was not a risk factor for venous thrombosis. Although the MTHFR gene mutation has not been proven to have a direct association with the risk for arterial and venous thrombosis, we speculate that it plays an important indirect role by influencing the plasma homocysteine concentrations. According to Morita et al. [28], hyperhomocysteinemia in patients with ischemic heart disease is related to prognosis and is a risk factor for restenosis after percutaneous transluminal coronary angioplasty. Botto et al. [29] have claimed that the TT genotype of the MTHFR gene mutation is a risk factor that is related to prognosis after coronary revascularization. Furthermore, Kibbe et al. [30] have reported that the TT genotype of the MTHFR gene mutation is a risk factor for low-graft patency rates and graft thrombosis after peripheral bypass surgery in patients with peripheral arterial occlusive disease. Based on the results of these studies, although the MTHFR gene mutation is not a direct risk factor for atherosclerosis and thrombosis, it does have clinical significance with respect to prognosis. In this study, six out of 116 patients had a relapse, three of whom had the CT genotype while the other three had the TT genotype of the MTHFR mutation. All of them were accompanied by hyperhomocysteinemia, and the average homocysteine concentration was 22.7 μmol/L and 31.3 μmol/L for the CT and TT genotypes, respectively. Because there were no statistically significant differences in the results of this study, the conclusion that the TT genotype is a risk relapse factor could not be drawn. To obtain more reliable results, a large-scale study and a prospective study are necessary in the future.
The limitations of this study were that it was not a control group study or a prospective study, and it did not consider nutritional factors such as vitamin B6 and vitamin B12.
CONCLUSION
Although the TT genotype in the MTHFR gene mutation was more closely associated with hyperhomocysteinemia compared to the other genotypes, it was not shown to be a prognostic factor for disease factors, such as relapse. However, we suspect that the TT genotype indirectly influences atherosclerosis and thrombosis based on its relationship with hyperhomocysteinemia and that it is associated with prognosis. To prove this, more efforts are required in the future in large-scale studies and prospective studies, and the clinical usefulness of the MTHFR gene test should be supported in order to recommend the follow-up observation of patients with the TT genotype and active treatment of hyperhomocysteinemia.
Acknowledgments
This study was supported by a research fund from Chosun University, 2014.
Footnotes
Conflict of interest: None.
REFERENCES
- 1.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- 2.Stampfer MJ, Malinow MR, Willett WC, Newcomer LM, Upson B, Ullmann D, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA. 1992;268:877–881. doi: 10.1001/jama.1992.03490070059042. [DOI] [PubMed] [Google Scholar]
- 3.den Heijer M, Koster T, Blom HJ, Bos GM, Briet E, Reitsma PH, et al. Hyper-homocysteinemia as a risk factor for deep-vein thrombosis. N Engl J Med. 1996;334:759–762. doi: 10.1056/NEJM199603213341203. [DOI] [PubMed] [Google Scholar]
- 4.Malinow MR, Kang SS, Taylor LM, Wong PW, Coull B, Inahara T, et al. Prevalence of hyperhomocyst(e)inemia in patients with peripheral arterial occlusive disease. Circulation. 1989;79:1180–1188. doi: 10.1161/01.CIR.79.6.1180. [DOI] [PubMed] [Google Scholar]
- 5.Perry IJ, Refsum H, Morris RW, Ebrahim SB, Ueland PM, Shaper AG. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet. 1995;346:1395–1398. doi: 10.1016/S0140-6736(95)92407-8. [DOI] [PubMed] [Google Scholar]
- 6.Ueland PM, Refsum H. Plasma homo-cysteine, a risk factor for vascular disease: plasma levels in health, disease, and drug therapy. J Lab Clin Med. 1989;114:473–501. [PubMed] [Google Scholar]
- 7.McCully KS. Homocysteine and vascular disease. Nat Med. 1996;2:386–389. doi: 10.1038/nm0496-386. [DOI] [PubMed] [Google Scholar]
- 8.Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rosenberg IH, et al. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996;93:7–9. doi: 10.1161/01.CIR.93.1.7. [DOI] [PubMed] [Google Scholar]
- 9.Kluijtmans LA, Kastelein JJ, Linde-mans J, Boers GH, Heil SG, Bruschke AV, et al. Thermolabile methylenetetrahydrofolate reductase in coronary artery disease. Circulation. 1997;96:2573–2577. doi: 10.1161/01.CIR.96.8.2573. [DOI] [PubMed] [Google Scholar]
- 10.Deloughery TG, Evans A, Sadeghi A, McWilliams J, Henner WD, Taylor LM, Jr, et al. Common mutation in methylenetetrahydrofolate reductase. Correlation with homocysteine metabolism and late-onset vascular disease. Circulation. 1996;94:3074–3078. doi: 10.1161/01.CIR.94.12.3074. [DOI] [PubMed] [Google Scholar]
- 11.Lentz SR. Mechanisms of homocysteine-induced atherothrombosis. J Thromb Haemost. 2005;3:1646–1654. doi: 10.1111/j.1538-7836.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- 12.Ueland PM, Refsum H, Stabler SP, Malinow MR, Andersson A, Allen RH. Total homocysteine in plasma or serum: methods and clinical applications. Clin Chem. 1993;39:1764–1779. [PubMed] [Google Scholar]
- 13.Kang SS, Wong PW, Norusis M. Homocysteinemia due to folate deficiency. Metabolism. 1987;36:458–462. doi: 10.1016/0026-0495(87)90043-6. [DOI] [PubMed] [Google Scholar]
- 14.Stabler SP, Marcell PD, Podell ER, Allen RH, Savage DG, Lindenbaum J. Elevation of total homocysteine in the serum of patients with cobalamin or folate deficiency detected by capillary gas chromatography-mass spectrometry. J Clin Invest. 1988;81:466–474. doi: 10.1172/JCI113343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilcken DE, Gupta VJ. Sulphr containing amino acids in chronic renal failure with particular reference to homocystine and cysteine-homo-cysteine mixed disulphide. Eur J Clin Invest. 1979;9:301–307. doi: 10.1111/j.1365-2362.1979.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 16.Chauveau P, Chadefaux B, Coudé M, Aupetit J, Hannedouche T, Kamoun P, et al. Hyperhomocysteinemia, a risk factor for atherosclerosis in chronic uremic patients. Kidney Int Suppl. 1993;41:S72–S77. [PubMed] [Google Scholar]
- 17.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338:1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 18.Mudd SH, Uhlendorf BW, Freeman JM, Finkelstein JD, Shih VE. Homo-cystinuria associated with decreased methylenetetrahydrofolate reductase activity. Biochem Biophys Res Commun. 1972;46:905–912. doi: 10.1016/S0006-291X(72)80227-4. [DOI] [PubMed] [Google Scholar]
- 19.Kang SS, Zhou J, Wong PW, Kowalisyn J, Strokosch G. Intermediate homo-cysteinemia: a thermolabile variant of methylenetetrahydrofolate reductase. Am J Hum Genet. 1988;43:414–421. [PMC free article] [PubMed] [Google Scholar]
- 20.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 21.Arruda VR, von Zuben PM, Chiaparini LC, Annichino-Bizzacchi JM, Costa FF. The mutation Ala677 → Val in the methylene tetrahydrofolate reductase gene: a risk factor for arterial disease and venous thrombosis. Thromb Hae-most. 1997;77:818–821. [PubMed] [Google Scholar]
- 22.Lee HA, Yang DH, Hong SY, Choi JS, Ha KS. Influence of 5,10-methylenetetrahydrofolate reductase (MTHFR) polymorphism toplasma homocysteine concentration in ESRD patients on maintenance hemodialysis. Korean J Med. 1998;55:1065–1069. [Google Scholar]
- 23.Moon K W, Chung WS, Youn HJ, Baek SH, Yoo KD, Oh YS, et al. The frequency distribution of methylenetetrahydrofolate reductase (MTHFR) polymorphism and association between the genotypes and total homo-cysteine level in patients with coronary artery disease. Korean Circ J. 1999;29:781–787. doi: 10.4070/kcj.1999.29.8.781. [DOI] [Google Scholar]
- 24.Şahin Ş, Benli İ, Aydoğan L. Distribution of prothrombin G20210A, factor V Leiden, and MTHFR C677T mutations in the middle Black Sea area (Tokat) of Turkey. Turk J Med Sci. 2012;42:1093–1097. [Google Scholar]
- 25.Ho CH, Kuo BI, Kong CW, Chau WK, Hsu HC, Gau JP, et al. Influence of methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism, B vitamins and other factors on plasma homocysteine and risk of thromboembolic disease in Chinese. J Chin Med Assoc. 2005;68:560–565. doi: 10.1016/S1726-4901(09)70094-2. [DOI] [PubMed] [Google Scholar]
- 26.Abbate R, Sardi I, Pepe G, Marcucci R, Brunelli T, Prisco D, et al. The high prevalence of thermolabile 5–10 methylenetetrahydrofolate reductase (MTHFR) in Italians is not associated to an increased risk for coronary artery disease (CAD) Thromb Haemost. 1998;79:727–730. [PubMed] [Google Scholar]
- 27.Bezemer ID, Doggen CJ, Vos HL, Rosendaal FR. No association between the common MTHFR 677C → T polymorphism and venous thrombosis: results from the MEGA study. Arch Intern Med. 2007;167:497–501. doi: 10.1001/archinte.167.5.497. [DOI] [PubMed] [Google Scholar]
- 28.Morita H, Kurihara H, Kuwaki T, Hamada C, Kitaoka M, Suzuki S, et al. Homocysteine as a risk factor for restenosis after coronary angioplasty. Thromb Haemost. 2000;84:27–31. [PubMed] [Google Scholar]
- 29.Botto N, Andreassi MG, Rizza A, Berti S, Bevilacqua S, Federici C, et al. C677T polymorphism of the methyl-enetetrahydrofolate reductase gene is a risk factor of adverse events after coronary revascularization. Int J Cardiol. 2004;96:341–345. doi: 10.1016/j.ijcard.2003.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Kibbe MR, Hassett AL, McSherry F, Conner P, Bontempo FA, Williford W, et al. Can screening for genetic markers improve peripheral artery bypass patency? J Vasc Surg. 2002;36:1198–1206. doi: 10.1067/mva.2002.128937. [DOI] [PubMed] [Google Scholar]