Abstract
Background
Attention-deficit/hyperactivity disorder (ADHD) often persists into adulthood, but it remains unclear which childhood factors predict future outcome.
Aim
To identify childhood predictors of ADHD outcome using both dimensional and categorical approaches.
Methods
116 adolescents and young adults with childhood ADHD were followed up on average 6.6 years later. ADHD outcome variables were interview-based parent-reported ADHD symptoms and impairment. Childhood predictors included parent- and teacher-rated ADHD symptoms and co-occurring behaviours; actigraph measures of activity level; socio-economic status (SES); and cognitive measures previously associated with ADHD.
Results
Of the sample, 79% continued to meet clinical criteria of ADHD in adolescence and young adulthood. Higher parent-rated ADHD symptoms and movement intensity in childhood, but not teacher-rated symptoms, predicted ADHD symptoms at follow up. Co-occurring symptoms of oppositional behaviours, anxiety, social and emotional problems were also significant predictors, but these effects disappeared after controlling for ADHD symptoms. IQ and SES were significant predictors of both ADHD symptoms and impairment at follow up, but no other cognitive measures significantly predicted outcome.
Conclusions
SES and IQ emerge as potential moderators for the prognosis of ADHD. Childhood severity of ADHD symptoms, as measured by parent ratings and actigraph movement intensity, also predicts later ADHD outcome. These factors should be considered when identifying ADHD children at most risk of poor long-term outcomes and for the development of interventions to improve prognosis.
Keywords: ADHD, persistence, IQ, SES, actigraph
Introduction
Symptoms of attention deficit hyperactivity disorder (ADHD) decline with age (Biederman et al., 2000, Faraone et al., 2006), yet many individuals with childhood ADHD continue to be affected by the disorder in adolescence and adulthood. Meta-analyses of follow-up studies have suggested that the rates of ADHD persistence vary from 15 to 70% (Faraone et al., 2006, Langley et al., 2010). One of the explanations for the varying levels of persistence reported lies in the criteria used for persistence (Faraone et al., 2006), with some studies including only individuals meeting full diagnostic criteria and other studies including also individuals in partial remission. The high rates of persistence in some studies could also be due to the selection of children with combined-type ADHD, who have high levels of both inattentive and hyperactive symptoms (Lara et al., 2009). Identifying the factors in childhood that lead to persistence of ADHD and associated impairments in adolescence and adulthood is important for early detection and prevention of long term negative outcomes.
Earlier studies found that co-occurring aggression, conduct problems and severity in ADHD symptoms in childhood predicted persistence of ADHD into adolescence and adulthood (Gittelman et al., 1985, Loney et al., 1981, Taylor et al., 1996). A more recent study focusing on a larger cohort of ADHD participants and a wider range of childhood risk factors revealed that other psychiatric comorbidities, oppositional defiant disorder, anxiety disorder and family factors including maternal psychopathology and psychosocial adversity significantly predicted persistence of ADHD in adolescence and adulthood (Biederman et al., 2011). Other longitudinal studies have also suggested socio-economic status (SES) as an important predictor for persistence of hyperactivity symptoms in children (Loney et al., 1981) and outcome severity in early adolescence (Molina et al., 2009). However, this finding has not always been replicated (Biederman et al., 2009, Hart et al., 1995).
Moving beyond behavioural and family factors, the predictive values of neurocognitive functions such as working memory, inhibition and response variability in ADHD persistence have been reported in a few studies, although a limited range of cognitive measures and short follow-up intervals were used. Initial evidence in children suggests that cognitive functions in early childhood may predict future ADHD symptoms or diagnosis a few years later (Brocki et al., 2007, Campbell and von Stauffenberg, 2009, Kalff et al., 2002). General cognitive ability (IQ) in early childhood predicted later ADHD symptoms measured in middle childhood (age 7.5) (Brocki et al., 2007) or in early adolescence (age 14) (Molina et al., 2009), but this was not replicated in another follow-up study in adolescence (ages 12-18), which found childhood IQ and social class to predict conduct disorder outcomes rather than ADHD scores or diagnosis (Langley et al., 2010). Overall the findings across these studies have been mixed, perhaps reflecting differences in study design, variables examined and the definitions of ADHD applied. Furthermore, none of these studies examined whether cognitive impairments in children with ADHD predicted future ADHD outcome in older adolescents and young adults (van Lieshout et al., 2013). Further studies are therefore needed to clarify the predictors of persistence and remission of ADHD.
To address this issue, we have followed a large group of individuals who met DSM-IV combined type criteria during childhood. The sample consisted entirely of children with combined type ADHD, selected as the inclusion criteria to minimise clinical heterogeneity in the sample. At the initial assessment, the children with ADHD demonstrated on average impairments in all the cognitive measures assessed, including performance on reaction time variability (RTV), go/no-go task commission (CE) and omission (OE) errors, choice impulsivity (Kuntsi et al., 2010) and lower IQs (Wood et al., 2011), as well as objectively measured actigraph movement intensity and count (Wood et al., 2009). We now aim to provide a comprehensive investigation of the predictors of adolescent and young adult ADHD outcome, by examining a diverse range of the behavioural, cognitive and family factors that we previously assessed in this sample in childhood. Childhood predictors include parent and teacher ratings on ADHD symptoms and co-occurring symptoms of oppositional behaviours, anxiety, social and emotional problems; actigraph measures of activity level; socio-economic status (SES); and cognitive measures of IQ, digit span, RTV, CE, OE and choice impulsivity. ADHD outcome is examined both as a continuous measure of symptoms and impairment and as a categorical diagnosis of persistence or remittance, to account for the arbitrary nature of definitions of persistence when focusing on diagnostic criteria.
Methods
Sample
Participants who had taken part in our previous research (UK-London sub-sample of the International Multicentre ADHD Genetic (IMAGE) project (Chen et al., 2008, Kuntsi et al., 2010) were invited to take part in this study. All participants were originally recruited from specialist clinics and were of European Caucasian decent, aged 6 to 19 years. The selection criteria for the IMAGE project probands was DSM-IV combined type ADHD. Childhood ADHD was assessed based on the Parental Account of Childhood symptoms (PACS) (Chen et al., 2008, Taylor et al., 1986a, Taylor et al., 1986b), a semi-structured, standardised, investigator interview with high inter-rater reliability (Taylor et al., 1986a). Exclusion criteria applied at the initial childhood assessment included IQ<70, autism, epilepsy, general learning difficulties, brain disorders and any genetic or medical disorder associated with externalising behaviours that might mimic ADHD. The investigation was carried out in accordance with the latest version of the Declaration of Helsinki. The study design was reviewed by an appropriate ethical committee and informed consent of the participants was obtained after the nature of the procedures had been fully explained.
Of the 128 eligible families with clinical, behavioural, actigraph and cognitive data in childhood, 80 (63%) were re-assessed. Of the 48 families not assessed at follow up, 13 (27%) declined previous studies and were not re-contacted; 14 (29%) declined the current study, 11 (23%) had agreed to participate but did not attend the appointments, 7 (15%) were uncontactable at follow up, 2 (4%) moved abroad and 1 (2%) proband was deceased. In addition to the 80 families with complete data in childhood, we also assessed 42 families who had clinical and IQ data in childhood but not cognitive or actigraph data. In total, we therefore assessed 122 families at follow up, 66% with complete data and 34% with only clinical and IQ data at childhood. The average length of follow up was 6.62 (± 0.99) years.
Procedure
Initial assessment
Families were invited to the research center for assessments, with a parent interview conducted in a separate room from the participants, who completed cognitive assessments with simultaneous actigraph measurement (Kuntsi et al., 2010, Wood et al., 2009). The children completed four tasks in a fixed order: The Wechsler Intelligence Scales for Children (Wechsler, 1991); The Go/No-go task (Borger and van der Meere, 2000, Kuntsi et al., 2005); The Fast task (Borger and van der Meere, 2000, Kuntsi et al., 2005); and the Maudsley index of childhood delay aversion (Kuntsi et al., 2006a, Paloyelis et al., 2009). All tasks are discussed in more detail in Kuntsi et al. (2006a). Children were given short breaks as required and the total length of the test session, including breaks, was approximately 2.5-3 hours. A minimum of a 48-hour medication-free period before testing was required.
Follow-up assessment
Participants were re-contacted by telephone and scheduled for a follow-up clinical interview and a cognitive-EEG assessment with simultaneous actigraph assessment at the same research centre where the initial assessment took place. When sibling pairs were tested, the assessments were carried out in separate rooms. The order of tasks was fixed. For those prescribed stimulants, a 48-hour ADHD medication-free period was required for cognitive and EEG testing. The total length of the test session, including breaks, was approximately 4-4.5 hours. Face-to-face or telephone clinical interviews were administered to the parent of each ADHD proband shortly before or after the participant’s assessment.
Measures
Childhood measures (predictor variables)
ADHD ratings
Inattentive and hyperactive-impulsive symptoms were measured using the Long Version of Conners’ Parent Rating Scale (Conners et al., 1998a) and the Long Version of Conners’ Teacher Rating Scale (Conners et al., 1998b). On both the parent and teacher Conners’ scales, summing the scores on the nine-item hyperactive-impulsive and nine-item inattentive DSM-IV symptoms subscales forms a total DSM-IV ADHD symptoms subscale.
Co-occurring symptoms
Oppositional behaviours, social problems and emotional lability were measured using the subscales of the Long Version of Conners’ Parent Rating Scale (Conners et al., 1998a) and the Long Version of Conners’ Teacher Rating Scale (Conners et al., 1998b). Social communication was measured using the parent-rated Social Communication Questionnaire (Rutter et al., 2003).
Actigraph measures of activity level (Wood et al., 2009)
The actigraph readings used in the current analyses are taken from a laboratory-based test session, when the siblings were apart completing a short-form IQ test and several cognitive-experimental tasks, under the supervision of separate experimenters who administered standardized instructions. The total length of the testing session was approximately 2 hours, excluding a 25-minute unstructured break, given approximately halfway through the session.
Four actigraph measures from each participant were used, which we previously showed to reliably distinguish between ADHD probands and controls (ROC-AUC= 0.53-0.79) (Wood et al., 2009): the cumulative intensity of movements (mean actigraph intensity), number of movements (mean actigraph count), variability (individual’s SD in minute-to-minute readings) of intensity (intra-individual variability of (IIV) actigraph intensity) and the number of movements (IIV actigraph count) from the dominant ankle and the waist.
Socio-economic status (SES)
Socio-economic status was measured based on parental occupational status (employed or unemployed) and types of occupation based on the parent with higher occupational class. The five occupational classes were defined as follows: 1 = unemployed or unclassified or not in search of jobs (e.g. housewife/husband, disabled/on disability allowance) (n=0); 2 = employed laborer (n=6); 3 = employed in service or sales (n=37); 4 = clerical workers (n=18); and 5 employed professionals (n=47).
Wechsler Intelligence Scales for Children, Third Edition (WISC-III) (Wechsler, 1991)
The vocabulary, similarities, picture completion and block design subtests from the WISC-III were used to obtain an estimate of the child’s full-scale IQ (FIQ). Verbal (VIQ) and performance IQ (PIQ) were also calculated separately. The digit span subtest from the WISC-III was administered to obtain digit span forward (verbal short-term memory) and digit span backward (verbal working memory) (Wechsler, 1991).
The Go/No-Go task (Borger and van der Meere, 2000, Kuntsi et al., 2005)
On each trial, one of two possible stimuli appeared for 300ms in the middle of the computer screen. The participant was instructed to respond only to the ‘go’ stimuli and to react as quickly as possible, but to maintain a high level of accuracy. The proportion of ‘go’ stimuli to ‘no-go’ stimuli was 4:1. The participants performed the task under three conditions (slow, fast and incentive), matched for length of time on task. Herein we present data from the slow condition, which had an inter-stimulus interval (ISI) of 8s and consisting of 72 trials, and the fast condition, with an ISI of 1s and consisting of 462 trials. The order of presentation of the slow and fast conditions varied randomly across participants. In this study we focus on standard deviation of RTs (RTV), commission errors (CE) and omission errors (OE) as these variables were most strongly associated with ADHD at initial assessment (Kuntsi et al., 2010).
The Fast Task (Andreou et al., 2007, Kuntsi et al., 2006b)
The baseline condition consists of 72 trials, which followed a standard warned four-choice RT task. Four empty circles (warning signals, arranged horizontally) first appeared for 8s, after which one of them (the target) was colored in. Participants were asked to press the response key that directly corresponded to the position of the target stimulus. Following a response, the stimuli disappeared from the screen and a fixed inter-trial interval of 2.5s followed. Speed and accuracy were emphasized equally in the task instructions. If the child did not respond within 10s, the trial terminated. A comparison condition of 80 trials with a fast event rate (1s) and incentives followed the baseline condition (Andreou et al., 2007). The variable obtained from the task is RTV, herein reported for the baseline condition only.
A preliminary reliability study in the general population revealed moderate-to-good retest reliability for both the Go/No-Go and the Fast Task (Kuntsi et al., 2005). To limit the total number of variables and to create psychometrically robust variables based on previous analyses on the same sample (Kuntsi et al., 2010), the summed unstandardized scores of RTV were obtained across the baseline conditions of the Go/No-Go and the Fast Task. A composite measure of CE and OE were obtained by summing the raw CE scores from both the slow and the fast conditions of the Go/No-Go task.
The Maudsley Index of Childhood Delay Aversion (Kuntsi et al., 2006a, Paloyelis et al., 2009)
Two conditions, each with 20 trials, were administered. In each trial, the child had a choice between a smaller-immediate reward (one point involving a 2-second pre-reward delay) and a larger-delayed reward (two points involving a 30-second pre-reward delay). In the no post-reward delay condition, choosing the small reward led immediately to the next trial, reducing the overall length of the condition. In the post-reward delay condition, choosing the small reward led to a delay period of 30 seconds, and choosing the large reward led to a delay period of 2 seconds before the next trial. The order of the two conditions was randomly chosen for each twin. Choice impulsivity (CI) was calculated here as the number of times the smaller-immediate reward was selected in the no post-reward delay condition, controlling for total number of trials attempted.
Follow-up measures (response variables)
Diagnostic Interview for ADHD in adults (DIVA; (Kooij and Francken, 2007)
This structured interview conducted by trained researchers is based on the DSM-IV criteria for ADHD (9 items for inattentiveness and 9 items for hyperactivity-impulsivity) and provides a list of concrete and realistic examples, for both adult and adolescent behaviour when they are off medication. The DIVA was conducted with both the ADHD proband and his/her parent separately.
Barkley’s functional impairment scale (BFIS; (Barkley and Murphy, 2006)
This 10-item scale is used to assess the levels of functional impairments commonly associated with ADHD symptoms in five areas of their everyday life when participants are off medication: family/relationship; work/ education; social interaction; leisure activities and management of daily responsibilities. Each item ranged from 0 (never or rarely) to 3 (very often).
Participants were classified as having persistent ADHD, if they scored a “yes” on ≥ 6 items on the DIVA for either inattention or hyperactivity-impulsivity based on parent report, and scored ≥ 2 on ≥ 2 areas of impairments on the BFIS, rated by their parent at the follow up assessment. Otherwise, they were classified as remitters. ADHD behaviours and impairments were reported and rated based on times when participants were not on medication.
Statistical analyses
We analysed the predictive values of the childhood variables using two analytic steps. First, we ran simple linear regressions to identify the childhood factors (predictor variables) that are associated, at follow-up, with (1) ADHD severity, defined as i) a continuous measure of ADHD symptoms based on the parent DIVA scores, ii) parent-report on Barkley’s functional impairment. Second, we conducted a canonical correlation analysis to determine the multivariate relationship between the childhood factors identified in the regression analysis and ADHD outcome. As a large sample size is required for the canonical correlation analysis, missing data were imputed using Stata version 11.0 (Stata Corporation, College Station, TX). We further examined which childhood factors predicted a clinical status of ADHD persistence and remittance at follow up using logistic regressions.
No group differences were observed between the younger (age <18.78) and older (age >18.78) participants on either ADHD symptoms or impairment at follow-up (Table 1), therefore we analysed our data collapsing the two age groups. We used age-regressed residuals of the cognitive variables in childhood, and of the DIVA symptom scores at follow-up to control for age effects. There was no effect of age on childhood behavioural measures (parent or teacher ratings of ADHD and comorbid symptoms) and clinical impairment at follow-up (t=0.51, p=0.61) and so for these variables we did not covary for age effects. Actigraph scores and DIVA symptoms were skewed and transformed using the optimized minimal skew (lnskew0) command in Stata (Stata Corporation, College Station, TX). Using Stata, we also controlled for the correlation of the sibling pair data by using the ‘robust cluster’ command. To aid interpretation, correlation coefficients (r) are presented as effect sizes for the regression models (Table 4 and 5), where r > 0.1, r > 0.3 and r > 0.5 are considered small, medium and large effects respectively (Cohen, 1988). Cohen’s d effect sizes are presented along with means, SDs and test statistics for the group analyses (Table 6), where 0.2 is considered a small effect, 0.5 considered a medium effect and 0.8 considered a large effect (Cohen, 1992).
Table 1. Clinical characteristics between younger (age <18.78) and older (age >18.78) participants.
| Younger (n=58) | Older (n=58) | χ 2 | p | |
|---|---|---|---|---|
| Persistence /remittance, n (%) | 46 (82%)/ 10 (18%) | 41 (76%)/ 13 (24%) | 0.64 | 0.42 |
| Males (%) | 50 (86%) | 51 (88%) | 0.08 | 0.78 |
| DIVA ADHD symptoms (range 0-18) | 13.56 (3.65) | 12.45 (3.56) | 1.84 | 0.07 |
| Functional impairment (range 0-30) | 14.09 (6.58) | 14.07 (6.77) | −0.01 | 0.99 |
Table 4. Predictive values of childhood measures on interview-based ADHD symptoms in adolescence and adulthood.
| r | t | F | df | p | |
|---|---|---|---|---|---|
| ADHD symptoms | |||||
| Inattention | |||||
| Parent-rated | 0.45 | 5.32 | 28.27 | 1/113 | <0.01 |
| Teacher-rated | 0.01 | 0.13 | 0.02 | 1/107 | 0.90 |
| Hyperactivity-Impulsivity | |||||
| Parent-rated | 0.43 | 4.97 | 24.72 | 1/112 | <0.01 |
| Teacher-rated | 0.08 | 0.85 | 0.73 | 1/107 | 0.40 |
| Activity level | |||||
| Mean intensity | 0.33 | 2.82 | 7.95 | 1/64 | <0.01 |
| Mean count | 0.15 | 1.24 | 1.53 | 1/64 | 0.22 |
| IIV intensity | 0.27 | 2.20 | 4.85 | 1/64 | 0.03 |
| IIV count | 0.03 | 0.24 | 0.06 | 1/64 | 0.81 |
| Co-occurring symptoms | |||||
| Oppositional behaviours | |||||
| Parent-rated | 0.25 | 2.74 | 7.50 | 1/112 | <0.01 |
| Teacher-rated | 0.09 | 0.98 | 0.95 | 1/106 | 0.33 |
| Anxious/shy behaviours | |||||
| Parent-rated | 0.20 | 2.18 | 4.74 | 1/112 | 0.03 |
| Teacher-rated | −0.07 | −0.77 | 0.59 | 1/107 | 0.44 |
| Social problems | |||||
| Parent-rated | 0.27 | 2.99 | 8.94 | 1/113 | <0.01 |
| Teacher-rated | 0.07 | 0.75 | 0.56 | 1/107 | 0.47 |
| Emotional problems | |||||
| Parent-rated | 0.36 | 3.52 | 12.40 | 1/85 | <0.01 |
| Teacher-rated | 0.07 | 0.60 | 0.36 | 1/84 | 0.55 |
| Social communication | 0.23 | 2.53 | 6.38 | 1/113 | 0.01 |
| SES | −0.20 | −2.12 | 4.50 | 1/103 | 0.04 |
| Cognitive performance | |||||
| FIQ | −0.25 | −3.47 | 12.03 | 1/101 | <0.01 |
| VIQ | −0.16 | −2.28 | 5.21 | 1/99 | 0.02 |
| PIQ | −0.29 | −2.70 | 7.30 | 1/99 | <0.01 |
| Digit span forward | −0.13 | −1.22 | 1.49 | 1/86 | 0.23 |
| Digit span backward | −0.06 | −0.55 | 0.31 | 1/86 | 0.58 |
| RTV | 0.03 | 0.22 | 0.05 | 1/53 | 0.83 |
| OE | 0.13 | 1.04 | 1.08 | 1/63 | 0.30 |
| CE | −0.07 | −0.56 | 0.32 | 1/63 | 0.58 |
| Choice impulsivity | 0.15 | 1.25 | 1.56 | 1/65 | 0.22 |
Table 5. Predictive value of childhood measures on parent ratings of functional impairment in adolescence/adulthood.
| r | t | F | df | p | |
|---|---|---|---|---|---|
| ADHD symptoms | |||||
| Inattention | |||||
| Parent-rated | 0.37 | 4.28 | 18.29 | 1/113 | <0.01 |
| Teacher-rated | 0.05 | 0.50 | 0.25 | 1/107 | 0.25 |
| Hyperactivity-Impulsivity | |||||
| Parent-rated | 0.33 | 3.75 | 14.03 | 1/112 | <0.01 |
| Teacher-rated | 0.16 | 1.67 | 2.79 | 1/107 | 0.10 |
| Activity level | |||||
| Mean intensity | 0.26 | 2.17 | 4.69 | 1/65 | 0.03 |
| Mean count | 0.22 | 1.78 | 3.15 | 1/65 | 0.08 |
| IIV intensity | 0.21 | 1.77 | 3.14 | 1/65 | 0.08 |
| IIV count | 0.13 | 1.06 | 1.11 | 1/65 | 0.30 |
| Co-occurring symptoms | |||||
| Oppositional behaviours | |||||
| Parent-rated | 0.31 | 3.47 | 12.05 | 1/112 | <0.01 |
| Teacher-rated | 0.05 | 0.50 | 0.25 | 1/106 | 0.25 |
| Anxious/shy behaviours | |||||
| Parent-rated | 0.24 | 2.64 | 6.97 | 1/112 | 0.01 |
| Teacher-rated | 0.00 | 0.05 | 0.00 | 1/107 | 0.96 |
| Social problems | |||||
| Parent-rated | 0.32 | 3.57 | 12.73 | 1/113 | <0.01 |
| Teacher-rated | 0.09 | 0.88 | 0.38 | 1/107 | 0.78 |
| Emotional problems | |||||
| Parent-rated | 0.33 | 3.73 | 13.93 | 1/112 | <0.01 |
| Teacher-rated | 0.07 | 0.76 | 0.58 | 1/107 | 0.45 |
| Social communication | 0.15 | 1.66 | 2.77 | 1/113 | 0.10 |
| SES | −0.22 | −2.32 | 5.38 | 1/103 | 0.02 |
| Cognitive performance | |||||
| FIQ | −2.65 | 7.01 | 1/100 | <0.01 | |
| VIQ | −1.16 | 1.35 | 1/98 | 0.25 | |
| PIQ | - | −4.21 | 17.73 | 1/98 | <0.01 |
| Digit span forward | −0.11 | −0.99 | 0.99 | 1/85 | 0.32 |
| Digit span backward | −0.11 | −0.98 | 0.96 | 1/85 | 0.33 |
| RTV | 0.03 | 0.25 | 0.06 | 1/54 | 0.81 |
| OE | −0.03 | −0.21 | 0.04 | 1/64 | 0.84 |
| CE | −0.07 | −0.58 | 0.34 | 1/64 | 0.57 |
| Choice impulsivity | 0.15 | 1.25 | 1.57 | 1/66 | 0.21 |
Table 6. Predictive value of childhood measures on ADHD status (persistence vs remittance).
| Persistent ADHD (n=87) Mean ± SD | Remittent ADHD (n=23) Mean ± SD | z | p | Cohen’s d | |
|---|---|---|---|---|---|
| ADHD symptoms | |||||
| Inattention | |||||
| Parent-rated | 17.47 ±7.65 | 10.39 ±11.24 | 3.27 | <0.01 | −0.75 |
| Teacher-rated | 7.64 ±11.74 | 7.04 ±10.06 | 0.23 | 0.82 | −0.05 |
| Hyperactivity-Impulsivity | |||||
| Parent-rated | 20.07 ±8.32 | 16.33 ±13.18 | 1.65 | 0.10 | −0.34 |
| Teacher-rated | 9.15 ±14.22 | 8.84 ±10.25 | 0.10 | 0.92 | −0.03 |
| Activity level | |||||
| Mean intensity | 1.47 ±0.46 | 1.30 ±0.42 | 1.40 | 0.16 | −0.39 |
| Mean count | 1.79 ±0.71 | 1.85 ±0.63 | 0.37 | 0.71 | −0.09 |
| IIV intensity | 1.69 ±0.50 | 1.44 ±0.65 | 1.54 | 0.12 | −0.43 |
| IIV count | −1.35 ±0.76 | −1.34 ±0.70 | −0.09 | 0.93 | −0.01 |
| Co-occurring symptoms | |||||
| Oppositional behaviours | |||||
| Parent-rated | 15.11 ±11.44 | 10.08 ±13.94 | 1.79 | 0.07 | −0.39 |
| Teacher-rated | 6.01 ±14.17 | 10.57 ±13.81 | −1.40 | 0.16 | 0.33 |
| Anxious/shy behaviours | |||||
| Parent-rated | 8.14 ±15.10 | 3.91 ±14.47 | 1.24 | 0.22 | −0.29 |
| Teacher-rated | 2.94 ±11.74 | 1.37 ±11.57 | 0.59 | 0.56 | −0.13 |
| Social problems | |||||
| Parent-rated | 13.96 ±14.47 | 5.17 ±15.69 | 2.49 | 0.01 | −0.58 |
| Teacher-rated | 4.88 ±12.64 | 2.29 ±9.80 | 0.94 | 0.35 | −0.23 |
| Emotional problems | |||||
| Parent-rated | 14.54 ±12.00 | 8.38 ±15.10 | 2.05 | 0.04 | −0.45 |
| Teacher-rated | 7.12 ±14.87 | 10.90 ±14.39 | −1.11 | 0.27 | 0.26 |
| Social communication | 3.38 ±6.65 | 2.46 ±7.45 | 0.59 | 0.56 | −0.13 |
| SES | 3.81 ±1.01 | 4.41 ±0.88 | −2.50 | 0.01 | 0.63 |
| Cognitive performance | |||||
| FIQ | 101.41 ±14.04 | 104.41 ±15.62 | −0.99 | 0.32 | 0.20 |
| VIQ | 21.22 ±5.29 | 22 ±5.37 | −0.65 | 0.52 | 0.15 |
| PIQ | 19.87 ±4.62 | 21.30 ±4.70 | −1.32 | 0.19 | 0.31 |
| Digit span forward | 8.23 ±1.93 | 8.62 ±2.29 | −0.40 | 0.69 | 0.18 |
| Digit span backward | 4.71 ±1.80 | 5.62 ±2.03 | −1.57 | 0.12 | 0.47 |
| RTV | 585.37 ±451.49 | 525.74 ±264.67 | −0.08 | 0.94 | −0.16 |
| OE | 23.99 ±20.66 | 24.65 ±21.10 | −0.61 | 0.54 | 0.03 |
| CE | 106.21 ±33.20 | 114.44 ±43.87 | −1.46 | 0.15 | 0.21 |
| Choice impulsivity | 0.30 ±0.33 | 0.17 ±0.27 | 1.10 | 0.27 | −0.43 |
Results
Of the 118 participants with childhood ADHD whom we re-assessed at follow-up, 87 (79%) were classified as ADHD persisters as these individuals continued to meet full DSM-IV ADHD criteria in adolescence/adulthood. No additional cases are classified as affected under revised DSM5 criteria. Among the persistent ADHD group, 60% (n=52) met criteria for the DSM-IV combined subtype, 32% (n=28) met criteria for predominantly inattentive subtype and 8% (n=7) met criteria for predominantly hyperactivity-impulsivity subtype at follow up. Of the 25 remaining participants, 9 did not meet symptom criteria (displayed less than 6 items in either inattention or hyperactivity-impulsivity domains) and were not clinically impaired; and 14 displayed five or more items on either the inattention or hyperactivity-impulsivity symptom domains, but did not show functional impairment (less than two domains). Two individuals, whom met criteria for clinical impairment but not for symptoms, were excluded from analyses. Six individuals had missing data on parent-reported functioning impairment and were excluded from the group analyses, as their diagnostic status could not be determined.
The final sample consisted of 116 individuals (10 sibling pairs and 96 singletons). The mean age was 11.79 years (S.D. = 2.93, range 6-17) at the baseline assessment and 18.44 (S.D. = 2.98, range 11-26) at follow up. There were no significant differences between those lost to follow up and those who participated in the follow up on baseline age, gender, IQ or ADHD symptoms (Table 2), but those who were lost to follow up had significantly lower SES (χ2 = 10.02; p=0.04). At follow up, the ADHD-persistent, ADHD-remittent and control groups did not differ in age (F=2.05, p=0.20), but there were significantly more males in the remitted group than the other two groups (χ2 =7.65, p=0.02) (Table 3). As the age range at follow up is wide, spanning from late childhood and early adulthood, we additionally split the group by median age (18.78 years) and examined the effect of age on ADHD symptoms and clinical impairment (Table 3). Almost half (47%) of the participants were under medication treatment for ADHD at the time of the follow-up assessment. Those who were on medication had significantly higher ADHD symptoms (F=11.34, p<0.01) and exhibited more functional impairment (F=5.22, p<0.01) than those who were not on medication at follow up. However, medication status did not predict a categorical status of ADHD persistence vs remittance (Table 3).
Table 2. Baseline sample characteristics between participants (individuals who were successfully reassessed) and non-participants (individuals lost to follow-up).
| Participants (n=116) | Non-participants (n=50) | t / χ | p | |
|---|---|---|---|---|
| Mean age (SD) | 11.79 (2.93) | 11.86 (2.61) | 0.11 | 0.92 |
| Male, n (%) | 101 (87%) | 46 (92%) | 0.77 | 0.38 |
| Mean parent-rated ADHD symptoms (SD) | 81.26 (8.93) | 81.28 (9.27) | 0.04 | 0.97 |
| Mean IQ (SD) | 102.77 (14.09) | 99.31 (17.99) | −0.83 | 0.41 |
Table 3. Sample characteristics between ADHD persisters and remitters at follow-up.
| ADHD remitters (n=23) | ADHD persisters (n=87) | t / χ | p | |
|---|---|---|---|---|
| Mean age (SD) | 18.89 (3.06) | 18.27 (3.03) | 0.87 | 0.39 |
| Male, n (%) | 23 (100%) | 72 (83%) | 4.59 | 0.03 |
| Mean ADHD symptoms (SD) | 9.71 (4.16) | 14.14 (2.83) | 4.76 | <0.01 |
| Functional impairment (SD) | 5.57 (3.64) | 16.44 (5.32) | 6.73 | <0.01 |
| On medication (%) | 49 | 65 | 1.95 | 0.16 |
Predictors of ADHD symptoms and impairments
Linear regression was first conducted to determine which childhood variables were associated with the total DIVA ADHD symptom scores (Table 4) and ratings of functional impairment (Table 5). Parent-rated childhood inattention and hyperactivity-impulsivity symptoms, as well as co-occurring symptoms including oppositional behavior, anxiety, emotional lability and social problems, were predictive of higher ADHD symptoms and impairment at follow up. Teacher-rated ADHD symptoms or co-occurring symptoms in childhood did not significantly predict parent interview-based ADHD symptoms at follow up. The actigraph measure of mean intensity of movement level in childhood significantly predicted both ADHD symptoms and impairment at follow up, while variability of movement intensity only significantly predicted ADHD symptoms but not impairment, and neither mean nor variability of movement count were significant predictors. Higher IQ scores (FIQ, VIQ and PIQ) and higher SES in childhood were each associated with lower ADHD symptoms (Table 4), as well as with fewer reports of clinical impairments (Table 5), in adolescence/adulthood. None of the other cognitive variables in childhood significantly predicted either ADHD symptoms or clinical impairments at follow up (Tables 4 and 5).
Including parent-rated childhood ADHD symptoms (inattention and hyperactivity-impulsivity total scores) as a covariate in a multiple linear regression eradicated the predictive values of all co-occurring clinical symptoms, but not those of SES or FIQ measures (Table S1). The predictive value of actigraph mean activity reduced to a trend level (p=0.07). Based on these results, we selected four childhood predictor measures (Parent-rated ADHD symptoms, actigraph mean intensity, FIQ and SES) for the second stage of the analysis, to explore their relationship with ADHD outcome (DIVA symptoms and impairment). A canonical correlation analysis was performed to determine (i) the relationship between the combined effects of the four identified childhood predictors of interest and ADHD outcome, and (ii) the relative contribution of each of these factors on this association.
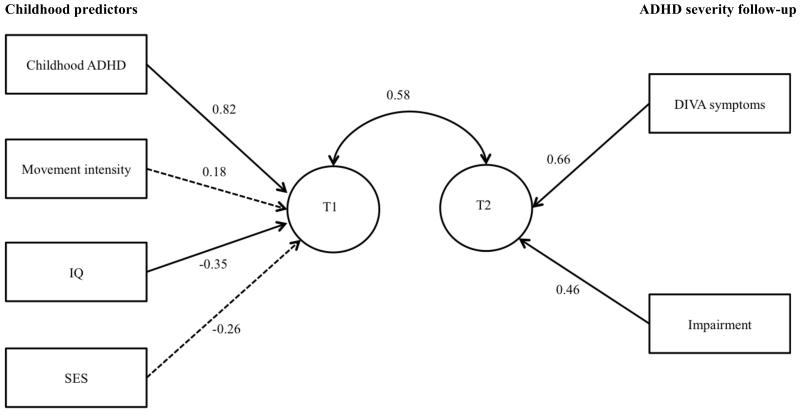
The two canonical correlations were 0.58 and 0.13, respectively. Only the first canonical correlation was interpreted, as it was significant (Wilks’ Λ= 0.66, F= 5.25, p<0.01). The canonical correlation of 0.58 indicates that the combined effect of the four selected childhood predictor (T1 in Fig. 1) explained 34% (0.582 * 100) of the variance in outcome variate (T2 in Fig. 1) ADHD symptoms and impairment. Parent-rated childhood ADHD symptoms and IQ made significant contributions to the predictor variate (T1) (t = 5.50, p < 0.01; t = −2.29, p = 0.02, respectively) while SES and mean actigraph intensity did not (t = −1.59, p = 0.12; t = 1.17, p= 0.24, respectively). DIVA ADHD symptoms and clinical impairment both contributed significantly to the outcome variate (t = 3.72, p < 0.01; t = 2.60, p = 0.01, respectively).
Figure 1. Standardised coefficients estimating the relative contribution of each variable on the canonical variates (T1/T2), where T1 reflects the linear combination of the childhood measures and T2 reflects the linear combination of the outcome measures. The relationship between the two canonical variates (T1 and T2) is represented by the canonical correlation.
Significant paths (p<0.05) are indicated as solid lines and non-significant paths (p≥0.05) are indicated as dotted lines.
Predictors for categorical diagnosis of ADHD persistence
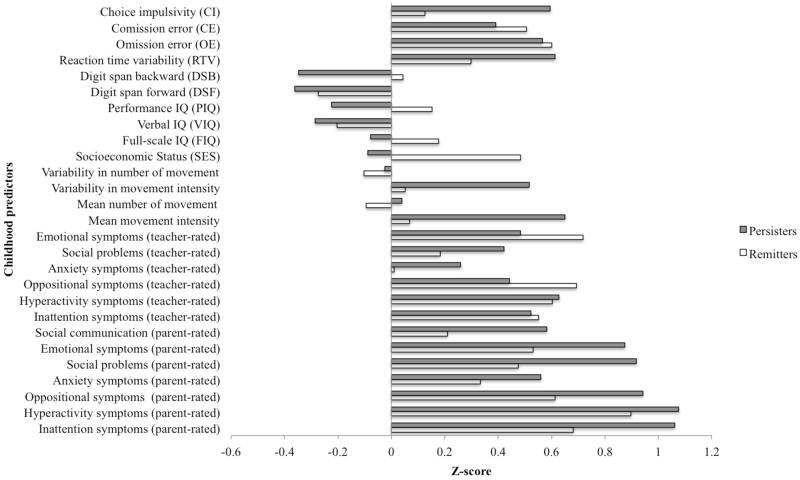
Logistic regression was conducted to examine, which variables in childhood predicted a clinical ADHD diagnosis in adolescence/adulthood (Table 6). Inattention symptoms, social and emotional problems in childhood significantly differentiated between ADHD persisters and remitters at follow up. However, after controlling for childhood ADHD symptoms, social and emotional problems were no longer significant predictors. SES was significantly higher in remitters than in persisters, even when childhood IQ was controlled for (z = −2.47, p = 0.01). None of the cognitive variables measured in childhood, including IQ, significantly predicted ADHD diagnostic status in adolescence/adulthood. To illustrate the differences between persisters and remitters on these childhood variables, mean standardized scores (z-scores) are presented for each variable (Figure 2).
Figure 2. Mean standardised scores on childhood predictors for future ADHD outcome (persistent vs remittent).
Discussion
In this follow-up investigation of 116 participants with childhood DSM-IV combined type ADHD, 79% continued to meet clinical criteria of ADHD in adolescence and young adulthood. Childhood variables of actigraph movement intensity, full-scale IQ, performance IQ and SES predicted greater ADHD symptoms and impairment in adolescence and early adulthood. Verbal IQ predicted greater ADHD symptoms, but not clinical impairment. Apart from IQ measures, none of the cognitive measures assessed in childhood predicted future ADHD symptoms or impairment, despite our test battery measuring cognitive impairments that showed strong phenotypic and familial association with ADHD in the same sample in cross-sectional analyses at time 1 (in childhood) (Kuntsi et al., 2010). For the categorical diagnostic outcome measure, only low SES in childhood significantly predicted the follow-up group status of persistent ADHD. The remitted ADHD group was small (n=23), however, reflecting the high degree of ADHD persistence observed in this sample. These findings were consistent across both younger and older participants.
Our findings raise the possibility of IQ and SES as potential moderators of ADHD outcome, as these variables reflect baseline characteristics that predict change in ADHD symptoms over development. While some previous studies did not find ADHD persisters to differ from remitters on SES (Biederman et al., 2011, Biederman et al., 2010, Halperin et al., 2008, Hart et al., 1995), others have either reported higher SES in ADHD remitters than persisters (Bedard et al., 2010, Halperin et al., 2008) or have shown a positive association between socio-economic advantage and treatment response (Loney et al., 1981, Molina et al., 2009). Consistent with these findings, we show that lower SES based on parental occupation alone has predictive value in ADHD outcome in adolescence and early adulthood. However, the canonical correlation analysis indicated that IQ made a larger contribution to the relationship between childhood predictors and ADHD outcome, relative to SES.
The association between childhood IQ and ADHD severity at follow up is consistent with previous findings that found IQ to moderate treatment outcomes in ADHD (Handen et al., 1997, Owens et al., 2003). We extended previous findings to show that both verbal and performance domains of IQ predicted future ADHD symptoms, but only performance IQ significantly predicted clinical impairment. Overall, the findings raise the possibility that individuals with higher IQ, particularly in the performance domain, may develop better coping strategies to deal with their ADHD symptoms or be more responsive to treatment. IQ did not significantly differentiate between diagnostic status of ADHD-persistent and remittent, which likely reflects the insufficient sample size to detect mean group differences using a categorical approach. Future replications with larger samples and older age ranges – with increased numbers of individuals crossing the threshold for remittance – will be important. Clinicians should note that, although high IQ may help ADHD patients cope with their disorder, studies of high IQ ADHD youths and adults show that they are nonetheless at risk for multiple psychiatric and functional impairments (Antshel et al., 2010, Antshel et al., 2007).
The severity of childhood ADHD symptoms, as reported by parents, was a strong predictor for ADHD outcome at follow up. The stability of ADHD symptoms was also evident from objective measures of actigraph measures of activity level, which are not subject to rater bias effects. Co-occurring symptoms, such as social and emotional functioning or oppositional behaviours rated by parents, also predicted more severe symptoms and impairment at follow up. However, the predictive value of these co-occurring symptoms became trivial once childhood ADHD symptoms were controlled for, suggesting that the co-occurring problems are related to the severity of ADHD symptoms. Teacher ratings of childhood ADHD symptoms and co-occurring symptoms did not predict parent interview-based ADHD symptoms or diagnosis at follow up. This is in line with the only moderate correlations (r=0.30) observed cross-sectionally between parent and teacher ratings of ADHD symptoms (Newcorn et al., 1994, Wolraich et al., 2004). The validity of teacher reports in older children or adolescents may also be compromised (Merwood et al., 2013, Sibley et al., 2012).
Some methodological limitations should be considered. The age range of the participants was wide, reflecting a limitation inherent in recruiting a large clinical sample. We controlled for the age effects by including age as a covariate for all cognitive and clinical variables, as well as conducting additional analyses with a median split in age for younger vs older participants. The SES measure used in this study did not take into account parental education or income. Future studies should replicate these findings with a more comprehensive measure of SES. While our study adds to previous research on predictors of ADHD persistence by including multiple domains of impairments that are most sensitive to ADHD, the exploratory approach to considering the multiple dependent measures emphasizes the need for future replication of the findings. We did not study the effects of treatment access, type and duration on ADHD outcome in this study, which should be investigated in future studies. The cognitive and actigraph measures had a lower number of participants compared to the behavioural and IQ measures, which may have affected the power to detect group differences. However, the sample size for these measures was moderate and the effect sizes were considered in conjunction with statistical significance. Further application and development of more complex models will also be required to test the moderating effect of IQ and SES directly in a developmental framework. Finally, the exclusive reliance on parent report as outcome measure in adolescence and young adulthood is also a potential limitation. However, research to date suggests the use of parent ratings of ADHD symptoms as the most reliable source of information compared to self-ratings, in both children and adults (Chang et al., 2013, Epstein et al., 2000, Merwood et al., 2013). Furthermore, we wished to use the same rater for both baseline and outcomes measures as otherwise any observed changes might related to change in the rater (parent to self-report) rather than reflecting the developmental change. Further investigations are needed to explore in more detail the differences and similarities across parent and self-report of ADHD symptoms and impairments in both clinical and research settings.
It should be noted that in the most recent version of the Diagnostic and Statistical Manual for ADHD (DSM-V), the diagnostic threshold for adolescents and adults with ADHD (age over 17 years) has been lowered, with a requirement of only five instead of six symptoms on either the inattention or hyperactivity-impulsivity domains to meet clinical criteria for ADHD. In the present study, we used the previous DSM version (DSM-IV) to be consistent with the childhood diagnostic criteria.
Taken together, whereas none of the cognitive measures except IQ was associated with ADHD outcome in the current sample, we demonstrate the predictive value of childhood measures of lower IQ and SES, as well as severity of ADHD symptoms as measured by parent ratings and actigraph movement intensity, on later ADHD outcome. In accordance with existing evidence from treatment studies, SES and IQ, in particular PIQ, therefore emerge as potential moderators for the prognosis of ADHD. The separation between the developmental roles of IQ vs other cognitive impairments in ADHD mirrors the etiological separation we have previously reported between them (Wood et al., 2010, Wood et al., 2011). By identifying childhood predictors of later outcome – and separating these from factors that do not predict – we can improve the early identification of individuals at greatest risk.
Supplementary Material
Highlights.
Of those with childhood ADHD, 79% continued to meet DSM criteria for ADHD at follow-up.
Childhood parent-rated ADHD symptoms and movement intensity predicted future outcome.
SES and IQ are potential moderators for the prognosis of ADHD.
None of the childhood cognitive measures except IQ predicted later ADHD severity.
Acknowledgement
We thank all who make this research possible: our participants and their families; Jessica Deadman, Hannah Collyer and Sarah-Jane Gregori.
Role of Funding Source
This project was supported by generous grants from Action Medical Research and the Peter Sowerby Charitable Foundation (grant reference GN1777). Initial sample recruitment of the ADHD sample was supported by NIMH Grant R01MH062873 to SV Faraone; the recruitment of the control sample and initial cognitive assessments of ADHD and control groups were supported by UK Medical Research Council grant G0300189 to J Kuntsi.
Footnotes
Conflict of Interest
Prof. Asherson, on behalf of King’s College London (nonpersonal pecuniary funds), has served a consultant for Janssen-Cilag, Eli Lilly and Co., Shire, Novartis, and Continuum. He has received educational or research grants from and has spoken at sponsored talks from Shire, Vifor, Janssen-Cilag, Eli Lilly and Co., and Qbech. Prof. Faraone receives royalties from books published by Guilford Press (Straight Talk about Your Child’s Mental Health) and Oxford University Press (Schizophrenia: The Facts). All other authors report no financial interests or potential conflict of interest.
References
- Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A, Meidad S, Muller UC, Uebel H, Banaschewski T, Manor I, Oades R, Roeyers H, Rothenberger A, Sham P, Steinhausen HC, Asherson P, Kuntsi J. Reaction time performance in ADHD: improvement under fast-incentive condition and familial effects. Psychol Med. 2007;37:1703–15. doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antshel KM, Faraone SV, Maglione K, Doyle AE, Fried R, Seidman LJ, Biederman J. Executive functioning in high-IQ adults with ADHD. Psychol Med. 2010;40:1909–18. doi: 10.1017/S0033291709992273. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Faraone SV, Stallone K, Nave A, Kaufmann FA, Doyle A, Fried R, Seidman L, Biederman J. Is attention deficit hyperactivity disorder a valid diagnosis in the presence of high IQ? Results from the MGH Longitudinal Family Studies of ADHD. J Child Psychol Psychiatry. 2007;48:687–94. doi: 10.1111/j.1469-7610.2007.01735.x. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy K. Attention Deficit Hyperactivity Disorder: A Clinical Workbook. 3rd Edition Guildford Press; New York: 2006. [Google Scholar]
- Bedard AC, Trampush JW, Newcorn JH, Halperin JM. Perceptual and motor inhibition in adolescents/young adults with childhood-diagnosed ADHD. Neuropsychology. 2010;24:424–34. doi: 10.1037/a0018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157:816–8. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Ball SW, Fried R, Doyle AE, Cohen D, Henderson C, Faraone SV. Are cognitive deficits in attention deficit/hyperactivity disorder related to the course of the disorder? A prospective controlled follow-up study of grown up boys with persistent and remitting course. Psychiatry Res. 2009;170:177–82. doi: 10.1016/j.psychres.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Clarke A, Lomedico A, Faraone SV. Predictors of persistent ADHD: an 11-year follow-up study. J Psychiatr Res. 2011;45:150–5. doi: 10.1016/j.jpsychires.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Evans M, Small J, Faraone SV. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177:299–304. doi: 10.1016/j.psychres.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borger N, van der Meere J. Motor control and state regulation in children with ADHD: a cardiac response study. Biol Psychol. 2000;51:247–67. doi: 10.1016/s0301-0511(99)00040-x. [DOI] [PubMed] [Google Scholar]
- Brocki KC, Nyberg L, Thorell LB, Bohlin G. Early concurrent and longitudinal symptoms of ADHD and ODD: relations to different types of inhibitory control and working memory. J Child Psychol Psychiatry. 2007;48:1033–41. doi: 10.1111/j.1469-7610.2007.01811.x. [DOI] [PubMed] [Google Scholar]
- Campbell SB, von Stauffenberg C. Delay and inhibition as early predictors of ADHD symptoms in third grade. J Abnorm Child Psychol. 2009;37:1–15. doi: 10.1007/s10802-008-9270-4. [DOI] [PubMed] [Google Scholar]
- Chang Z, Lichtenstein P, Asherson PJ, Larsson H. Developmental twin study of attention problems: high heritabilities throughout development. JAMA Psychiatry. 2013;70:311–8. doi: 10.1001/jamapsychiatry.2013.287. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhou K, Sham P, Franke B, Kuntsi J, Campbell D, Fleischman K, Knight J, Andreou P, Arnold R, Altink M, Boer F, Boholst MJ, Buschgens C, Butler L, Christiansen H, Fliers E, Howe-Forbes R, Gabriels I, Heise A, Korn-Lubetzki I, Marco R, Medad S, Minderaa R, Muller UC, Mulligan A, Psychogiou L, Rommelse N, Sethna V, Uebel H, McGuffin P, Plomin R, Banaschewski T, Buitelaar J, Ebstein R, Eisenberg J, Gill M, Manor I, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Sonuga-Barke E, Steinhausen HC, Taylor E, Thompson M, Faraone SV, Asherson P. DSM-IV combined type ADHD shows familial association with sibling trait scores: a sampling strategy for QTL linkage. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1450–60. doi: 10.1002/ajmg.b.30672. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavoural Sciences. second ed. Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998a;26:257–68. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998b;26:279–91. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Conners CK, Erhardt D, Arnold LE, Hechtman L, Hinshaw SP, Hoza B, Newcorn JH, Swanson JM, Vitiello B. Familial aggregation of ADHD characteristics. J Abnorm Child Psychol. 2000;28:585–94. doi: 10.1023/a:1005187216138. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36:159–65. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Gittelman R, Mannuzza S, Shenker R, Bonagura N. Hyperactive boys almost grown up. I. Psychiatric status. Arch Gen Psychiatry. 1985;42:937–47. doi: 10.1001/archpsyc.1985.01790330017002. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Trampush JW, Miller CJ, Marks DJ, Newcorn JH. Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. J Child Psychol Psychiatry. 2008;49:958–66. doi: 10.1111/j.1469-7610.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handen BL, Janosky J, McAuliffe S. Long-term follow-up of children with mental retardation/borderline intellectual functioning and ADHD. J Abnorm Child Psychol. 1997;25:287–95. doi: 10.1023/a:1025760302598. [DOI] [PubMed] [Google Scholar]
- Hart EL, Lahey BB, Loeber R, Applegate B, Frick PJ. Developmental change in attention-deficit hyperactivity disorder in boys: a four-year longitudinal study. J Abnorm Child Psychol. 1995;23:729–49. doi: 10.1007/BF01447474. [DOI] [PubMed] [Google Scholar]
- Kalff AC, Hendriksen JG, Kroes M, Vles JS, Steyaert J, Feron FJ, van Zeben TM, Jolles J. Neurocognitive performance of 5- and 6-year-old children who met criteria for attention deficit/hyperactivity disorder at 18 months follow-up: results from a prospective population study. J Abnorm Child Psychol. 2002;30:589–98. doi: 10.1023/a:1020859629994. [DOI] [PubMed] [Google Scholar]
- Kooij JJS, Francken MH. Diagnostic Interview for ADHD (DIVA) in adults. 2007. [Google Scholar]
- Kuntsi J, Andreou P, Ma J, Borger NA, van der Meere JJ. Testing assumptions for endophenotype studies in ADHD: reliability and validity of tasks in a general population sample. BMC Psychiatry. 2005;5:40. doi: 10.1186/1471-244X-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Neale BM, Chen W, Faraone SV, Asherson P. The IMAGE project: methodological issues for the molecular genetic analysis of ADHD. Behav Brain Funct. 2006a;2:27. doi: 10.1186/1744-9081-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Rogers H, Swinard G, Borger N, van der Meere J, Rijsdijk F, Asherson P. Reaction time, inhibition, working memory and ‘delay aversion’ performance: genetic influences and their interpretation. Psychol Med. 2006b;36:1613–24. doi: 10.1017/S0033291706008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Wood AC, Rijsdijk F, Johnson KA, Andreou P, Albrecht B, Arias-Vasquez A, Buitelaar JK, McLoughlin G, Rommelse NN, Sergeant JA, Sonuga-Barke EJ, Uebel H, van der Meere JJ, Banaschewski T, Gill M, Manor I, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Steinhausen HC, Faraone SV, Asherson P. Separation of cognitive impairments in attention-deficit/hyperactivity disorder into 2 familial factors. Arch Gen Psychiatry. 2010;67:1159–67. doi: 10.1001/archgenpsychiatry.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley K, Fowler T, Ford T, Thapar AK, van den Bree M, Harold G, Owen MJ, O’Donovan MC, Thapar A. Adolescent clinical outcomes for young people with attention-deficit hyperactivity disorder. Br J Psychiatry. 2010;196:235–40. doi: 10.1192/bjp.bp.109.066274. [DOI] [PubMed] [Google Scholar]
- Lara C, Fayyad J, de Graaf R, Kessler RC, Aguilar-Gaxiola S, Angermeyer M, Demytteneare K, de Girolamo G, Haro JM, Jin R, Karam EG, Lepine JP, Mora ME, Ormel J, Posada-Villa J, Sampson N. Childhood predictors of adult attention-deficit/hyperactivity disorder: results from the World Health Organization World Mental Health Survey Initiative. Biol Psychiatry. 2009;65:46–54. doi: 10.1016/j.biopsych.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney J, Kramer J, Millich RS. The hyperactive child grows up: predictors of symptoms, delinquency and achievement at follow-up. In: Gadow KS, Loney J, editors. Psychosocial aspects of drug treatment for hyperactivity. CO: Westview Press; Boulder: 1981. [Google Scholar]
- Merwood A, Greven CU, Price TS, Rijsdijk F, Kuntsi J, McLoughlin G, Larsson H, Asherson PJ. Different heritabilities but shared etiological influences for parent, teacher and self-ratings of ADHD symptoms: an adolescent twin study. Psychol Med. 2013:1–12. doi: 10.1017/S0033291712002978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, Epstein JN, Hoza B, Hechtman L, Abikoff HB, Elliott GR, Greenhill LL, Newcorn JH, Wells KC, Wigal T, Gibbons RD, Hur K, Houck PR. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcorn JH, Halperin JM, Schwartz S, Pascualvaca D, Wolf L, Schmeidler J, Sharma V. Parent and teacher ratings of attention-deficit hyperactivity disorder symptoms: implications for case identification. J Dev Behav Pediatr. 1994;15:86–91. [PubMed] [Google Scholar]
- Owens EB, Hinshaw SP, Kraemer HC, Arnold LE, Abikoff HB, Cantwell DP, Conners CK, Elliott G, Greenhill LL, Hechtman L, Hoza B, Jensen PS, March JS, Newcorn JH, Pelham WE, Severe JB, Swanson JM, Vitiello B, Wells KC, Wigal T. Which treatment for whom for ADHD? Moderators of treatment response in the MTA. J Consult Clin Psychol. 2003;71:540–52. doi: 10.1037/0022-006x.71.3.540. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, Kuntsi J. Are ADHD symptoms associated with delay aversion or choice impulsivity? A general population study. J Am Acad Child Adolesc Psychiatry. 2009;48:837–46. doi: 10.1097/CHI.0b013e3181ab8c97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire. Western Psycholoigcal Services; Los Angeles, CA: 2003. [Google Scholar]
- Sibley MH, Pelham WE, Molina BS, Gnagy EM, Waxmonsky JG, Waschbusch DA, Derefinko KJ, Wymbs BT, Garefino AC, Babinski DE, Kuriyan AB. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol. 2012;80:1052–61. doi: 10.1037/a0029098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E, Chadwick O, Heptinstall E, Danckaerts M. Hyperactivity and conduct problems as risk factors for adolescent development. J Am Acad Child Adolesc Psychiatry. 1996;35:1213–26. doi: 10.1097/00004583-199609000-00019. [DOI] [PubMed] [Google Scholar]
- Taylor E, Everitt B, Thorley G, Schachar R, Rutter M, Wieselberg M. Conduct disorder and hyperactivity: II. A cluster analytic approach to the identification of a behavioural syndrome. Br J Psychiatry. 1986a;149:768–77. doi: 10.1192/bjp.149.6.768. [DOI] [PubMed] [Google Scholar]
- Taylor E, Schachar R, Thorley G, Wieselberg M. Conduct disorder and hyperactivity: I. Separation of hyperactivity and antisocial conduct in British child psychiatric patients. Br J Psychiatry. 1986b;149:760–7. doi: 10.1192/bjp.149.6.760. [DOI] [PubMed] [Google Scholar]
- van Lieshout M, Luman M, Buitelaar J, Rommelse NN, Oosterlaan J. Does neurocognitive functioning predict future or persistence of ADHD? A systematic review. Clin Psychol Rev. 2013;33:539–560. doi: 10.1016/j.cpr.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechler Intelligence Scale for Children. 3rd ed. The Psychological Corporation; London: 1991. [Google Scholar]
- Wolraich ML, Lambert EW, Bickman L, Simmons T, Doffing MA, Worley KA. Assessing the impact of parent and teacher agreement on diagnosing attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2004;25:41–7. doi: 10.1097/00004703-200402000-00007. [DOI] [PubMed] [Google Scholar]
- Wood AC, Asherson P, Rijsdijk F, Kuntsi J. Is overactivity a core feature in ADHD? Familial and receiver operating characteristic curve analysis of mechanically assessed activity level. J Am Acad Child Adolesc Psychiatry. 2009;48:1023–1030. doi: 10.1097/CHI.0b013e3181b54612. [DOI] [PubMed] [Google Scholar]
- Wood AC, Asherson P, van der Meere JJ, Kuntsi J. Separation of genetic influences on attention deficit hyperactivity disorder symptoms and reaction time performance from those on IQ. Psychol Med. 2010;40:1027–37. doi: 10.1017/S003329170999119X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AC, Rijsdijk F, Johnson KA, Andreou P, Albrecht B, Arias-Vasquez A, Buitelaar JK, McLoughlin G, Rommelse NN, Sergeant JA, Sonuga-Barke EJ, Uebel H, van der Meere JJ, Banaschewski T, Gill M, Manor I, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Steinhausen HC, Faraone SV, Asherson P, Kuntsi J. The relationship between ADHD and key cognitive phenotypes is not mediated by shared familial effects with IQ. Psychol Med. 2011;41:861–71. doi: 10.1017/S003329171000108X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.