Abstract
Introduction
To assess regional brain injury on magnetic resonance imaging (MRI) after pediatric cardiac arrest (CA) and to associate regional injury with patient outcome and effects of hypothermia therapy for neuroprotection.
Methods
We performed a retrospective chart review with prospective imaging analysis. Children between 1 week and 17 yrs of age who had a brain MRI in the first 2 weeks after CA without other acute brain injury between 2002-2008 were included. Brain MRI (1.5 T General Electric, Milwaukee, WI, USA) images were analyzed by 2 blinded neuroradiologists with adjudication; images were visually graded. Brain lobes, basal ganglia, thalamus, brain stem, and cerebellum were analyzed using T1, T2, and diffusion-weighted images (DWI).
Results
Signal intensity alterations in the basal ganglia on T2 and brain lobes on DWI were associated with unfavorable outcome (all p < .05). Therapeutic hypothermia had no effect on regional brain injury. Repeat brain MRI was infrequently performed but demonstrated evolution of lesions.
Conclusion
Children with lesions in the basal ganglia on conventional MRI and brain lobes on DWI within the first 2 weeks after CA represent a group with increased risk of poor outcome. These findings may be important for developing neuroprotective strategies based on regional brain injury and for evaluating response to therapy in interventional clinical trials.
Keywords: cardiac arrest, neurological outcome, MRI, pediatric, brain injury
INTRODUCTION
Survival after cardiac arrest (CA) in children ranges from 8-45%, with most deaths occurring secondary to neurological failure. More than half of the children who survive CA sustain long-lasting neurological deficits 1-3. Outcomes vary by patient and event characteristics such as age, heart rhythm prior return of spontaneous circulation, etiology, location of CA, and duration of pulselessness 2-4.
Brain magnetic resonance imaging (MRI) is a sensitive tool to identify lesions after hypoxia-ischemia and findings may assist with outcome prognostication, assessment of neuroprotective interventions, and rehabilitation planning 5-8. Post-resuscitative care for pediatric CA is largely supportive 9, 10. Therapeutic hypothermia (32-34 °C) is used commonly as a neuroprotective strategy in adults who remain comatose after ventricular fibrillation and resuscitation and infants with birth asphyxia and is being tested for efficacy in children surviving CA, 85-90% of who have asphyxia or shock as the cause of arrest 2, 3, 9, 11-15. Recent evidence suggests that hypothermia exerts regional protective effects as evidenced on brain MRI in infants with hypoxic-ischemic encephalopathy 16-19.
Regional injury patterns on diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) and the effects of hypothermia on imaging sequences have not been reported in children with CA. This report is an exploratory analysis with the objective to assess patterns of brain injury on conventional MRI and DWI in children surviving CA in the first two weeks after resuscitation, to correlate findings with subject outcome and to describe any effects of therapeutic hypothermia.
METHODS
Design and Setting
This study was approved by the University of Pittsburgh Institutional Review Board. We performed a retrospective chart review of subjects admitted to the pediatric intensive care unit (ICU) at the Children’s Hospital of Pittsburgh between 2002 and 2008.
Inclusion and Exclusion Criteria
We included subjects between 1 week and 17 years of age who survived CA and underwent a brain MRI for clinical care within the first 14 days after resuscitation. Subjects with additional acute brain injury (e.g., brain hemorrhagic or traumatic brain injury) were excluded. Children with congenital heart disease were excluded as they were not in the original study cohort, and up to one-third of these children have pre-existing neurologic lesions 20, 21.
Clinical care
All children were initially intubated and mechanically ventilated and received standard supportive care targeting normotension, normoxia, normocarbia, and treatment of fever. Antiepileptic medications were started only if seizures were suspected clinically or confirmed by electroencephalogram. Pain, sedation, and neuromuscular blockade medications were used at the discretion of the attending ICU physician.
Temperature control
There was no protocol for hypothermia during the study period. The ICU physician decided whether to initiate therapeutic hypothermia, and established the depth, duration, and methods of implementing hypothermia. The primary method of initiating and maintaining hypothermia included a cooling blanket (Cincinnati SubZero Plastipad, Cincinnati, OH) positioned under the patient and controlled by an automated cooling system (Gaymar Medi-Therm III, Orchard Park, NY). Common adjunct cooling methods included surface cooling with ice packs and fans.
Brain MRI
Subjects received a brain MRI if it was clinically indicated by their attending physician. Brain MRI (1.5 T General Electric, Milwaukee, WI, USA) images were assessed by 2 blinded pediatric neuroradiologists with adjudication. Brain regions examined included lobes (frontal, temporal, parietal, insular, and occipital), basal ganglia, thalamus, cerebellum, and brain stem. Generalized edema was defined by the presence of lesions affecting at least four of those regions. MRI sequences performed included T1-weighted- and T2 -weighted images, and DWI/ADC images. T1-weighted and T2-weighted images were visually scored as normal or abnormal for each region. DWI/ADC was not standard of care throughout the entire study period and was therefore not available for each subject. DWI/ADC images were considered abnormal in presence of high signal intensity on DWI and low signal intensity on the corresponding ADC map (cytotoxic edema) or high signal intensity on DWI and high signal intensity of ADC (vasogenic edema)22. Images disclosing high signal intensity on DWI but no significant changes on ADC map were not included in the evaluation because of the possibility of T2-shine-through artifact 23. Subjects with subsequent MRIs were subjectively evaluated by 1 neuroradiologist for the presence of volume loss and changes in lesions compared with the initial MRI.
Data Collection
Medical record review was used for data collection. Demographical data including age, weight, and sex were recorded. Other data collected included CA and resuscitation details, ICU and hospital length of stay (LOS), temperature management, disposition at hospital discharge, and pre ICU-admission and discharge Glasgow Outcome Score (GOS). The GOS is defined as follows: 1, dead; 2, persistent vegetative state; 3, severe disability; 4, moderate disability; 5, good recovery)24. GOS was scored based on information available in the medical record and favorable outcome was defined as a GOS 4-5.
Data analysis
Data were analyzed for associations between presence of lesions (by region) and 1) favorable or unfavorable outcome at discharge, or 2) receipt of therapeutic hypothermia using Fisher’s exact test. P-values for differences by clinical outcome were 1-sided (since presence of lesions could only plausibly lead to worsening of outcome), whereas p-values for differences by hypothermia treatment were 2-sided. All other statistical tests for binary or categorical variables were also conducted using Fisher’s exact test, whereas differences in continuous variable were assessed using the Wilcoxon rank-sum test. Spearman’s rank was used to correlate the day of MRI after CA with the total number of brain regions affected. P-values < .05 were considered statistically significant. Data were summarized via either medians (inter-quartile range), for continuous variables, or frequencies (%), for binary or categorical data. All analyses were conducted using Stata software, version 10 (College Station, Texas). As part of this exploratory analysis, many different comparisons were tested, and some of the significant results will therefore likely be due to chance alone. Therefore, interpretation of findings focused on the overall pattern of findings, e.g. significance across the same region, and not single findings of p < .05.
RESULTS
Study subjects
Twenty-eight subjects with median age 1.9 years (IQR 0.4-13.0) and 19 (68%) males, were examined (Figure 1). The etiology of CA for most children was asphyxia (82%) and the most common first monitored rhythm was pulseless electrical activity or asystole (77%) (Table 1). Twenty-three (82%) of children had a normal baseline GOS pre-arrest, including all 7 of the children with unfavorable outcome. Twenty-four (86%) subjects survived and 14 (50%) had favorable outcome at hospital discharge.
Figure 1.
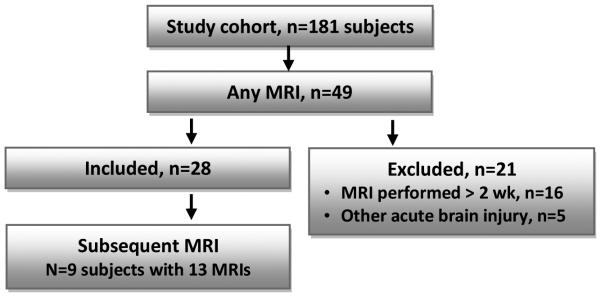
Study flowchart
Table 1.
Subject demographics, details of cardiac arrest and resuscitation, and subject outcome at hospital discharge.
| All subjects (N = 28) |
Favorable outcome (n=14) |
Unfavorable outcome (n=14) |
p-value | |
|---|---|---|---|---|
| Age in years, median (IQR) | 1.9 (.4-13.0) | 2.3 (0.2-6.4) | 1.7 (1.1-15.7) | .48 |
| Sex, male/female (% male) | 19/9 (68) | 11/3 (79) | 8/6 (57) | .42 |
| Chronic disease, n (%) | 13 (46) | 7 (50) | 6 (43) | .50 |
| Etiology of cardiac arrest, n (%) | .33 | |||
| Asphyxia | 23 (82) | 10 (71) | 13 (93) | |
| Cardiac | 5 (18) | 4 (29) | 1 (7) | |
| Location of cardiac arrest, n (%) | .19 | |||
| In-hospital | 11 (39) | 8 (57) | 3 (21) | |
| Out-of-hospital | 17 (61) | 6 (43) | 11 (79) | |
| Witnessed, n (%) | 16 (57) | 9 (64) | 7 (54) | 1.00 |
| Number of epinephrine boluses, median (IQR) | 2 (1-4) | 2 (1-3) | 3 (1-5) | .30 |
| First monitored rhythm, n (%) | .12 | |||
| Pulseless electrical activity | 10 (36) | 8 (57) | 2 (14) | |
| Asystole | 8 (29) | 2 (14) | 6 (43) | |
| Ventricular fibrillation or tachycardia | 4 (14) | 2 (14) | 2 (14) | |
| Sinus tachycardia/normal sinus rhythm | 2 (7) | 1 (7) | 1 (7) | |
| Unknown | 4 (14) | 1 (7) | 3 (21) | |
| Hypothermia for neuroprotection, n (%) | 19 (68) | 9 (64) | 10 (71) | .50 |
| ICU LOS, d (median (IQR)) | 15 (9-29) | 11 (6-19) | 23 (12-33) | .04 |
| Hospital LOS, d (median (IQR)) | 19 (12-33) | 17 (10-21) | 30 (13-41) | .08 |
| Days between cardiac arrest and MRI, d (median (IQR)) | 6 (4-11) | 5 (4-7) | 8 (5-13) | .16 |
| Survived to HD, n (%) | 24 (86) | 14 (100) | 4 (29) | .10 |
| Pre-cardiac arrest, n %) | .33 | |||
| GOS = 5 | 23 (82) | 10 (71) | 13 (93) | |
| GOS = 4 | 4 (14) | 3 (21) | 1 (7) | |
| GOS = 3 | 1 (7) | 1 (7) | 0 (0) | |
| Hospital discharge, n (%) | <.001 | |||
| GOS = 5 | 8 (29) | 8 (38) | 0 (0) | |
| GOS = 4 | 5 (18) | 5 (36) | 0 (0) | |
| GOS = 3 | 8 (29) | 1 (7) | 7 (50) | |
| GOS = 2 | 3 (11) | 0 (0) | 3 (21) | |
| GOS = 1 | 4 (14) | 0 (0) | 4 (29) | |
ICU, intensive care unit; LOS, length of stay; GOS, Glasgow outcome score; HD, hospital discharge
Nineteen (68%) subjects were treated with therapeutic hypothermia (target temperature 33.7 ± 0.9°C (mean ± standard deviation) for 36.0 ± 20.6 h), all of whom had a brain MRI obtained following rewarming. All five of the subjects with cardiac etiology and 14 of 23 subjects with asphyxia were treated with hypothermia. The number of subjects with favorable outcome was the same regardless of hypothermia status (p = 1.00).
Overall outcomes
Brain MRI was obtained at a median of 6 days (IQR 4-11) after resuscitation, and was similar between outcome groups and hypothermia status. Of the 16 (57%) children without lesions on MRI, all survived to hospital discharge, and 10 had no change in their GOS at hospital discharge. The 12 children with brain MRI lesions were more likely to have increased hospital LOS (p = .05), decreased survival to hospital discharge (p < .05), and more frequent neurological disability at hospital discharge (p = .02) versus children without MRI lesions. There was no difference in the number of days between CA and brain MRI when analyzing by presence of any brain lesion on T2-weighted images (p = .93).
Regional MRI lesions on T1-weighted and T2-weighted images
Brain MRI results are shown by favorable and unfavorable outcome at hospital discharge groups in Table 2. Regional brain lesions were most numerous on T2-weighted images and were located in the brain lobes in 10 subjects (parietal n = 9, frontal n = 8, occipital n = 8, temporal n = 5, and insular n = 3) and basal ganglia in 10 subjects (lenticular n = 9, caudate n = 7). None of the subjects with a single brain lobe lesion died. There was an association between having multiple brain lobes affected and worse outcome (p < .01). Lesions in the occipital brain lobe and lenticular nucleus on T1-weighted imaging and the lenticular and caudate nuclei on T2-weighted images were each associated with unfavorable outcome (all p < .05). Only 1 of 4 of the subjects with cardiac etiology had lesions on T2-weighted imaging, making it impossible to determine differences in injury patterns versus subjects with asphyxia. Figure 2 illustrates typical MRI lesion pattern seen after asphyxial CA in a patient with unfavorable outcome.
Table 2.
MRI lesion location by imaging sequence and favorable or unfavorable outcome at hospital discharge.
| Region, n (%) | T1 | T2 | DWI/ADC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fav ( n=14) |
Unfav
(n=14) |
P-value | Fav (n=13) |
Unfav
(n=14) |
P-value | Fav (n=10) |
Unfav
(n=14) |
P-value | |
| Brain lobes | 1 (7) | 5 (36) | .08 | 3 (23) | 7 (50) | .15 | 1 (10) | 8 (57) | .02 a |
| Temporal | 0 (0) | 2 (15) | .22 | 1 (8) | 4 (31) | .16 | 1 (10) | 4 (31) | .25 |
| Frontal | 0 (0) | 3 (23) | .10 | 2 (15) | 6 (46) | .10 | 0 (0) | 7 (54) | <.01a |
| Occipital | 0 (0) | 4 (29) | .05 a | 2 (15) | 6 (46) | .10 | 1 (10) | 8 (62) | .02 a |
| Parietal | 0 (0) | 3 (21) | .11 | 3 (23) | 6 (46) | .21 | 1 (11) | 7 (54) | .05 a |
| Insular | 0 (0) | 0 (0) | 1.00 | 0 (0) | 3 (23) | .11 | 0 (0) | 1 (8) | .57 |
| Basal ganglia | 0 (0) | 4 (43) | .05 a | 1 (8) | 9 (65) | <.01a | 0 (0) | 4 (31) | .08 |
| Lenticular | 0 (0) | 4 (31) | .04 a | 1 (8) | 8 (62) | <.01 a | 1 (6) | 3 (43) | .07 |
| Caudate | 0 (0) | 1 (8) | .48 | 0 (0) | 7 (54) | <.01 a | 0 (0) | 2 (15) | .31 |
| Thalamus | 0 (0) | 1 (7) | .50 | 1 (8) | 4 (29) | .19 | 0 (0) | 1 (7) | .58 |
| Cerebellum | 0 (0) | 1 (7) | .50 | 0 (0) | 1 (7) | .52 | 0 (0) | 2 (14) | .33 |
| Brainstem | 0 (0) | 1 (7) | .50 | 0 (0) | 4 (29) | .06 | 0 (0) | 1 (7) | .58 |
| Generalized edema | 0 (0) | 2 (14) | .24 | 0 (0) | 2 (14) | .26 | 0 (0) | 2 (14) | .33 |
p < .05 for unfavorable versus favorable outcome at hospital discharge
Fav, favorable; Unfav, unfavorable; DWI, diffusion-weighted imaging; ADC, apparent diffusion coefficient
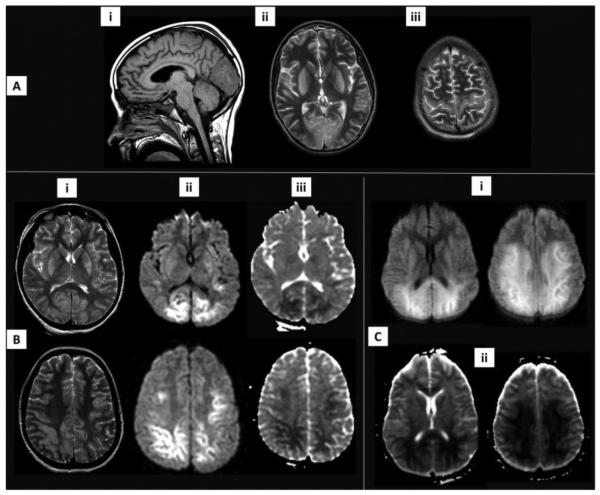
Figure 2A-C.
Brain MRI sequences. A) a 15 yo male with asphyxia from anaphylaxis, found asystolic and required CPR for 36 min. T1 sagittal image (i) shows low signal intensity in the occipital lobe. T2-weighted images demonstrates high signal intensity alterations in the basal ganglia, occipital lobe (ii), and motor strip (iii). B) 1 ½ yo male who was found asystolic after drowning required ~30 min of CPR; increased signal is seen in the basal ganglia and brain lobes on T2-weighted images (i). Restricted diffusion is noted on DWI (ii) and ADC (iii) images in the deep layers of the occipital, parietal and frontal lobes. C) a 17 yo male with ventricular fibrillation after electrocution. Extensive cytotoxic edema is seen on DWI (i) and ADC (ii) images involving the frontal-parietal white matter and the deep layers of the cortex. Subjects A and C were treated with hypothermia for neuroprotection; subjects A and B had profound neurologic dysfunction and had life support withdrawn and subject C had severe neurologic disability on hospital discharge to rehabilitation.
Regional MRI lesions on DWI/ADC
Lesions on DWI/ADC in the brain lobes were associated with unfavorable outcome (p = .02), and were most commonly located in the occipital (n = 9), parietal (n = 8), and frontal (n = 7) lobes. Lesions on DWI/ADC sequences in general represented cytotoxic edema in both the brain lobes (n = 8 subjects) and basal ganglia (n = 4 subjects). One subject had evidence of both cytotoxic and vasogenic edema on DWI/ADC sequence in all regions examined.
There was no correlation between day of MRI after CA and the number of lesions on either T2-weighted imaging (p = .29) or DWI (p = .68). Visually, however, T2-weighted images appear to have a bell-shaped curve, peaking between days 3-7 while DWI/ADC changes appeared sooner after CA than on conventional imaging.
Subjects receiving therapeutic hypothermia had the same frequency of brain lesions from all regions analyzed as normothermic subjects on conventional and diffusion imaging (Table 4). There was no effect of hypothermia on the frequency of generalized edema.
Table 4.
Follow-up brain MRI scans (n=8 subjects, n=12 scans). Changes are from T2-weighted images unless noted otherwise.
| Subject | Days from Initial MRI |
Days from Cardiac Arrest |
Changes from Initial Brain MRI | Volume loss |
|---|---|---|---|---|
| 1 | 14 | 21 | Unchanged T2 brain lobe lesions | Mild, global (unchanged) |
| 2 | 13 | 16 | Increased cortical and basal ganglia hyperintensity | Mild, global (unchanged) |
| 20 | 23 | Unchanged | Mild, global (unchanged) | |
| 36 | 39 | Increased basal ganglia hyperintensity | Mild, global (unchanged) | |
| 90 | 93 | Unchanged | Mild, global (unchanged) | |
| 3 | 14 | 16 | New brain lobe increased T2 signal | None |
| 4 | 252 | 264 | Normal scan unchanged | Normal (improved from mild) |
| 5 | 3 | 12 | New left parietal lobe and thalamic lesions; basal ganglia lesions unchanged; improved PLIC lesion | Mild, global (unchanged) |
| 6 | 14 | 18 | New brain lobe lesions; diffusion lesions resolved | None |
| 7 | 7 | 14 | Unchanged brain lobe and basal ganglia lesions; DWI/ADC changes nearly resolved | None |
| 8 | 3 | 8 | Unchanged brain lobe lesions | Mild, global (unchanged) |
| 19 | 24 | Unchanged brain lobe lesions | Mild, global (unchanged) | |
PLIC, posterior limb of the internal capsule; DWI, diffusion-weighted imaging; ADC, apparent diffusion coefficient
Follow-up imaging studies
Twelve follow-up brain MRIs were available from 8 subjects, performed 3-252 days from the initial scan (Table 4). New brain lobe or basal ganglia lesions were found in 3 subjects and lesions were unchanged in another 3 subjects on follow-up MRI. Resolution of DWI/ADC lesions occurred in 2 subjects – one in a subject with new T2 lesions on day 14 after CA and another in a subject with persistent T2 lesions on day 7 after CA. Global volume loss was present 2 subjects with chronic disease and in 2 subjects that were previously well. One child had focal volume loss in the occipital lobes on follow-up MRI that correlated with early changes in DWI/ADC but was absent on T2-weighted images on the initial scan.
DISCUSSION
In this case series of children with brain MRI within 14 days of CA, we found that lesions in the basal ganglia on conventional MRI and across multiple brain lobes on DWI were associated with unfavorable outcome at hospital discharge. The brain lobes and basal ganglia are regions that are especially vulnerable to hypoxic-ischemic injury and have been associated with poor outcome other studies after hypoxic-ischemic injury reviewed later 25-30. These brain regions have unique susceptibilities to excitotoxicity and free radical stress, postulated to be related to differences in receptor expression and type, stage of brain development, and other variances of cellular metabolism 31-34.
Although this is the first report of DWI to predict outcome in pediatric CA, Dubowitz et al (n = 22 subjects) previously found that in children with poor outcome after CA due to drowning had more lesions in the cortex, basal ganglia, and generalized edema on conventional imaging, and abnormal N-acetylaspartate and lactate peaks on brain MR spectroscopy versus children with good outcome 8. They also found that lesions present on MRI on day 3 or4 post-CA correlated best with patient outcome versus MRI on days 1 or 2 post-CA. Christophe et al (n = 40 subjects) tested a scoring system based on the presence of watershed and basal ganglia lesions on conventional MRI in children with hypoxia or CA. Although many children had serial MRIs, the initial MRI had the best sensitivity (96%) and specificity (50%) to predict outcome on follow-up examination by a neurologist 7. Specificity was optimal if the MRI was performed on days 1-3, and sensitivity was best after day 3.
In our subjects, DWI/ADC was most accurate in identifying cortical lesions associated with unfavorable outcome while T2-weighted imaging was most accurate in detecting basal ganglia lesions. DWI/ADC images can detect brain injury from hypoxia-ischemia early (hours) after the insult. DWI images 1-2 days after birth asphyxia was found to have superior sensitivity versus conventional imaging in locating brain lesions but occasionally missed lesions present later (3-5 days) after birth asphyxia. One to two weeks after the acute brain injury, some DWI lesions recede and therefore can be missed if MRI is performed too late35. The clinical significance of transient versus long-lasting DWI lesions is unknown but should be evaluated.
Cytotoxic edema on DWI/ ADC was primarily responsible for diffusion changes in our subjects, with only one subject having evidence of both cytotoxic and vasogenic edema. In both cytotoxic and vasogenic edema, the DWI/ADC signal is abnormal in the affected region. However, the ADC signal is decreased and less anisotropic in cytotoxic edema while in vasogenic edema the ADC signal is increased with large anisotropy 22. Increase in cell volume relative to the extracellular space is thought to be due to dysfunctional transmembrane Na+/K+ pumps in cytotoxic edema. Vasogenic edema is secondary to disruption of the blood-brain barrier, in which the volume of the extracellular water compartment is increased relative to the intracellular component. Cytotoxic edema may portend a worse outcome because it is less amenable to treatment with hyperosmotic therapies and the global nature of brain injury after CA.
There are many challenges in the interpretation of a brain MRI in children. Developmental changes, including increases in brain size, functional connectivity, and myelination, and decreased brain water content result in prolonged T2 relaxation constants, increased diffusivity and isotropy in children compared with adults, all which change rapidly in the first several years of life 36-38. In addition, the immature brain is more vulnerable to insults versus more mature brains, with the immature brain being more predisposed to delayed (apoptotic) cell death in addition to the primary cell death due to the original primary brain insult 39-42 . Timing of the MRI after initial injury is critical to capturing information as lesions on conventional and DWI/ADC will evolve over time. Risks are associated with the transport of a critically ill patient, consequently a patient’s clinical status often influences the timing of imaging acquisition 43. Finally, secondary brain insults from hypotension, hypoxia, seizures, and fever can have negative neurological effects but are difficult to quantify apart from the original hypoxic-ischemic insult.
Physicians in our ICU frequently used therapeutic hypothermia in children surviving CA without an explicit protocol during the study period 20. Neonatal studies have found a mixture of brain lesion patterns after birth asphyxia comparing therapeutic hypothermia versus normothermia for neuroprotection16, 17,18. Whether hypothermia has similar effects in children with CA remains to be seen.
Similar to other groups, we found that brain lesions evolved over time in subjects with subsequent imaging, although our available sample size was small. Global volume loss was found in 5 children on the initial scan, and surprisingly 3 children had no known prior medical disease. Evaluation for global volume loss was subjective due to the various ages of the subjects and small sample size and deserves further study both after acute brain injury and perhaps to study the effects of the ICU without acute brain injury. The etiology of the global volume loss after acute brain injury is unclear but includes early and ongoing cell death, undiagnosed medical disease, retrograde neuronal cell death, and finally, hydrocephalus 44-47. Innovative imaging strategies including prospective regional volumetric measurements, longitudinal measurement of diffusion tensor and cerebral blood flow and metabolism in concert with longer term patient outcome would add to our understanding of connectivity disturbances and ischemic thresholds after CA, potentially leading to novel therapeutic interventions. Until then, conventional and DWI are sequences that do not require complex and time-consuming analyses and are readily available at tertiary care facilities caring for these patients.
Study limitations
Clinical data collection was limited by available documentation. MRI was performed when clinically indicated, biasing the study population. Decision to use and methods of therapeutic hypothermia were not standardized during the study period. Secondary brain insults such as fever were not evaluated in this study but may have influenced imaging findings and subject outcomes. GOS was not blinded and assigned retrospectively, and as an indicator of gross function, often performs poorly in infants, and does not provide information on detailed neuropsychological function. Long term outcomes were not assessed in this study.
CONCLUSIONS
Children with lesions in the basal ganglia on conventional MRI and brain lobes on DWI within the first 2 weeks after CA represent a group with increased risk of poor outcome. These preliminary findings may be important for targeting neuroprotective strategies and for stratification of subjects in interventional clinical trials. The combination of conventional MRI sequences and DWI/ADC images may be helpful to serially evaluate brain lesions.
Table 3.
MRI lesions in subjects by those treated with hypothermia therapy or normothermia
| Region, n (%) | T1 | T2 | DWI/ADC | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
NT
(n=9) |
HT
(n=18) |
P-value |
NT
(n=9) |
HT
(n=18) |
P-value |
NT
(n=7) |
HT
(n=17) |
P-value | |
| Brain lobes | 2 (22) | 4 (21) | 1.00 | 5 (56) | 5 (28) | .22 | 3 (43) | 6 (35) | 1.00 |
| Temporal | 1 (11) | 1 (6) | 1.00 | 2 (22) | 3 (18) | 1.00 | 1 (14) | 4 (25) | 1.00 |
| Frontal | 2 (22) | 1 (6) | .25 | 4 (44) | 4 (24) | .38 | 3 (43) | 4 (25) | .63 |
| Occipital | 1 (11) | 3 (16) | 1.000 | 3 (33) | 5 (29) | 1.00 | 3 (43) | 6 (38) | 1.00 |
| Parietal | 1 (11) | 2 (11) | 1.00 | 4 (44) | 5 (29) | .67 | 2 (29) | 6 (40) | 1.00 |
| Insular | 0 (0) | 0 (0) | 1.00 | 1 (11) | 2 (12) | 1.00 | 1 (14) | 0 (0) | .30 |
| Basal ganglia | 2 (22) | 2 (11) | .57 | 2 (22) | 8 (44) | .41 | 1 (14) | 3 (18) | 1.00 |
| Lenticular | 2 (22) | 2 (11) | .58 | 2 (22) | 7 (41) | .42 | 1 (14) | 3 (19) | 1.00 |
| Caudate | 1 (11) | 0 (0) | .33 | 2 (22) | 5 (29) | 1.00 | 1 (14) | 1 (6) | .53 |
| Thalamus | 1 (11) | 0 (0) | .32 | 1 (11) | 4 (22) | .64 | 1 (14) | 0 (0) | .29 |
| Cerebellum | 1 (11) | 0 (0) | .32 | 1 (11) | 0 (0) | .33 | 2 (29) | 0 (0) | .08 |
| Brainstem | 1 (11) | 0 (0) | .32 | 2 (22) | 2 (11) | .58 | 1 (14) | 0 (0) | .29 |
| Generalized edema | 1 (11) | 1 (5) | 1.00 | 1 (11) | 1 (6) | 1.00 | 1 (14) | 1 (6) | .51 |
HT, hypothermia; NT, normothermia
Acknowledgments
Sources of Funding: Fink: NINDS 1K23NS065132-01; NICHD 5K12HD047349-02; Fink, Landsittel: 1U54RR023506-04, University of Pittsburgh Clinical and Translational Science Institute; Clark: R01-HD045968.
The study’s sources of funding had no involvement in the study design, collection, analysis, and interpretation of data, writing of the manuscript or in the decision to submit the manuscript for publication.
REFERENCES
- 1.Young KD, Seidel JS. Pediatric cardiopulmonary resuscitation: a collective review. Ann Emerg Med. 1999;33:195–205. doi: 10.1016/s0196-0644(99)70394-x. [DOI] [PubMed] [Google Scholar]
- 2.Young KD, Gausche-Hill M, McClung CD, Lewis RJ. A prospective, population-based study of the epidemiology and outcome of out-of-hospital pediatric cardiopulmonary arrest. Pediatrics. 2004;114:157–64. doi: 10.1542/peds.114.1.157. [DOI] [PubMed] [Google Scholar]
- 3.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295:50–7. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 4.Meaney PA, Nadkarni VM, Cook EF, et al. Higher survival rates among younger patients after pediatric intensive care unit cardiac arrests. Pediatrics. 2006;118:2424–33. doi: 10.1542/peds.2006-1724. [DOI] [PubMed] [Google Scholar]
- 5.Rutherford M, Pennock J, Schwieso J, Cowan F, Dubowitz L. Hypoxic-ischaemic encephalopathy: early and late magnetic resonance imaging findings in relation to outcome. Arch Dis Child Fetal Neonatal Ed. 1996;75:F145–51. doi: 10.1136/fn.75.3.f145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chau V, Poskitt KJ, Sargent MA, et al. Comparison of computer tomography and magnetic resonance imaging scans on the third day of life in term newborns with neonatal encephalopathy. Pediatrics. 2009;123:319–26. doi: 10.1542/peds.2008-0283. [DOI] [PubMed] [Google Scholar]
- 7.Christophe C, Fonteyne C, Ziereisen F, et al. Value of MR imaging of the brain in children with hypoxic coma. AJNR Am J Neuroradiol. 2002;23:716–23. [PMC free article] [PubMed] [Google Scholar]
- 8.Dubowitz DJ, Bluml S, Arcinue E, Dietrich RB. MR of hypoxic encephalopathy in children after near drowning: correlation with quantitative proton MR spectroscopy and clinical outcome. AJNR Am J Neuroradiol. 1998;19:1617–27. [PMC free article] [PubMed] [Google Scholar]
- 9.2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: pediatric advanced life support. Pediatrics. 2006;117:e1005–28. doi: 10.1542/peds.2006-0346. [DOI] [PubMed] [Google Scholar]
- 10.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–83. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 11.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 12.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 13.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 14.Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 15.Moler FW, Meert K, Donaldson AE, et al. In-hospital versus out-of-hospital pediatric cardiac arrest: a multicenter cohort study. Crit Care Med. 2009;37:2259–67. doi: 10.1097/CCM.0b013e3181a00a6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inder TE, Hunt RW, Morley CJ, et al. Randomized trial of systemic hypothermia selectively protects the cortex on MRI in term hypoxic-ischemic encephalopathy. J Pediatr. 2004;145:835–7. doi: 10.1016/j.jpeds.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 17.Rutherford MA, Azzopardi D, Whitelaw A, et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics. 2005;116:1001–6. doi: 10.1542/peds.2005-0328. [DOI] [PubMed] [Google Scholar]
- 18.Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9:39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thoresen M, Hellstrom-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics. 2010;126:e131–9. doi: 10.1542/peds.2009-2938. [DOI] [PubMed] [Google Scholar]
- 20.Fink EL, Clark RS, Kochanek PM, Bell MJ, Watson RS. A tertiary care center's experience with therapeutic hypothermia after pediatric cardiac arrest. Pediatr Crit Care Med. 2010;11:66–74. doi: 10.1097/PCC.0b013e3181c58237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McQuillen PS, Barkovich AJ, Hamrick SE, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–41. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 22.Ebisu T, Naruse S, Horikawa Y, et al. Discrimination between different types of white matter edema with diffusion-weighted MR imaging. J Magn Reson Imaging. 1993;3:863–8. doi: 10.1002/jmri.1880030612. [DOI] [PubMed] [Google Scholar]
- 23.Le Bihan D, Poupon C, Amadon A, Lethimonnier F. Artifacts and pitfalls in diffusion MRI. J Magn Reson Imaging. 2006;24:478–88. doi: 10.1002/jmri.20683. [DOI] [PubMed] [Google Scholar]
- 24.Jennet B, Teasdale G, Braakman R, Minderhoud J, Knill-Jones R. Predicting outcome in individual patients after severe head injury. Lancet. 1976:1031–4. doi: 10.1016/s0140-6736(76)92215-7. [DOI] [PubMed] [Google Scholar]
- 25.Mattson MP, Guthrie PB, Kater SB. Intrinsic factors in the selective vulnerability of hippocampal pyramidal neurons. Prog Clin Biol Res. 1989;317:333–51. [PubMed] [Google Scholar]
- 26.Fink EL, Alexander H, Marco CD, et al. An experimental model of pediatric asphyxial cardiopulmonary arrest in rats. Pediatr Crit Care Med. 2004;5:139–44. doi: 10.1097/01.pcc.0000112376.29903.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu CL, Siesjo BK, Hu BR. Pathogenesis of hippocampal neuronal death after hypoxia-ischemia changes during brain development. Neuroscience. 2004;127:113–23. doi: 10.1016/j.neuroscience.2004.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niwa T, Aida N, Shishikura A, Fujita K, Inoue T. Susceptibility-weighted imaging findings of cortical laminar necrosis in pediatric patients. AJNR Am J Neuroradiol. 2008;29:1795–8. doi: 10.3174/ajnr.A1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinoshita T, Ogawa T, Yoshida Y, Tamura H, Kado H, Okudera T. Curvilinear T1 hyperintense lesions representing cortical necrosis after cerebral infarction. Neuroradiology. 2005;47:647–51. doi: 10.1007/s00234-005-1398-0. [DOI] [PubMed] [Google Scholar]
- 30.Martin LJ, Brambrink A, Koehler RC, Traystman RJ. Primary sensory and forebrain motor systems in the newborn brain are preferentially damaged by hypoxia-ischemia. Journal of Comparative Neurology. 1997;377:262–85. doi: 10.1002/(sici)1096-9861(19970113)377:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Forder JP, Tymianski M. Postsynaptic mechanisms of excitotoxicity: Involvement of postsynaptic density proteins, radicals, and oxidant molecules. Neuroscience. 2009;158:293–300. doi: 10.1016/j.neuroscience.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Ye H, Jalini S, Zhang L, Charlton M, Carlen PL. Early ischemia enhances action potential-dependent, spontaneous glutamatergic responses in CA1 neurons. J Cereb Blood Flow Metab. 30:555–65. doi: 10.1038/jcbfm.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen V, McQuillen PS. AMPA and metabotropic excitoxicity explain subplate neuron vulnerability. Neurobiol Dis. 2010;37:195–207. doi: 10.1016/j.nbd.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guerguerian AM, Brambrink AM, Traystman RJ, Huganir RL, Martin LJ. Altered expression and phosphorylation of N-methyl-D-aspartate receptors in piglet striatum after hypoxia-ischemia. Brain Res Mol Brain Res. 2002;104:66–80. doi: 10.1016/s0169-328x(02)00285-1. [DOI] [PubMed] [Google Scholar]
- 35.Arbelaez A, Castillo M, Mukherji SK. Diffusion-weighted MR imaging of global cerebral anoxia. AJNR Am J Neuroradiol. 1999;20:999–1007. [PMC free article] [PubMed] [Google Scholar]
- 36.Leppert IR, Almli CR, McKinstry RC, et al. T(2) relaxometry of normal pediatric brain development. J Magn Reson Imaging. 2009;29:258–67. doi: 10.1002/jmri.21646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–87. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 38.Engelbrecht V, Scherer A, Rassek M, Witsack HJ, Modder U. Diffusion-weighted MR imaging in the brain in children: findings in the normal brain and in the brain with white matter diseases. Radiology. 2002;222:410–8. doi: 10.1148/radiol.2222010492. [DOI] [PubMed] [Google Scholar]
- 39.Vannucci RC, Towfighi J, Vannucci SJ. Secondary energy failure after cerebral hypoxia-ischemia in the immature rat. Journal of Cerebral Blood Flow & Metabolism. 2004;24:1090–7. doi: 10.1097/01.WCB.0000133250.03953.63. [DOI] [PubMed] [Google Scholar]
- 40.Nakajima W, Ishida A, Lange MS, et al. Apoptosis has a prolonged role in the neurodegeneration after hypoxic ischemia in the newborn rat. J Neurosci. 2000;20:7994–8004. doi: 10.1523/JNEUROSCI.20-21-07994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Northington FJ, Ferriero DM, Graham EM, Traystman RJ, Martin LJ. Early Neurodegeneration after Hypoxia-Ischemia in Neonatal Rat Is Necrosis while Delayed Neuronal Death Is Apoptosis. Neurobiol Dis. 2001;8:207–19. doi: 10.1006/nbdi.2000.0371. [DOI] [PubMed] [Google Scholar]
- 42.Zhu C, Wang X, Huang Z, et al. Apoptosis-inducing factor is a major contributor to neuronal loss induced by neonatal cerebral hypoxia-ischemia. Cell Death Differ. 2007;14:775–84. doi: 10.1038/sj.cdd.4402053. [DOI] [PubMed] [Google Scholar]
- 43.Andrews PJ, Piper IR, Dearden NM, Miller JD. Secondary insults during intrahospital transport of head-injured patients. Lancet. 1990;335:327–30. doi: 10.1016/0140-6736(90)90614-b. [DOI] [PubMed] [Google Scholar]
- 44.Maneru C, Junque C, Salgado-Pineda P, et al. Corpus callosum atrophy in adolescents with antecedents of moderate perinatal asphyxia. Brain Inj. 2003;17:1003–9. doi: 10.1080/0269905031000110454. [DOI] [PubMed] [Google Scholar]
- 45.Hayasaki K, Marmarou A, Barzo P, Fatouros P, Corwin F. Detection of brain atrophy following traumatic brain injury using gravimetric techniques. Acta Neurochir Suppl. 1997;70:75–7. doi: 10.1007/978-3-7091-6837-0_23. [DOI] [PubMed] [Google Scholar]
- 46.Siren AL, Radyushkin K, Boretius S, et al. Global brain atrophy after unilateral parietal lesion and its prevention by erythropoietin. Brain. 2006;129:480–9. doi: 10.1093/brain/awh703. [DOI] [PubMed] [Google Scholar]
- 47.Tasker RC. Changes in white matter late after severe traumatic brain injury in childhood. Dev Neurosci. 2006;28:302–8. doi: 10.1159/000094156. [DOI] [PubMed] [Google Scholar]