Abstract
Corn masa flour, used to make products such as corn tortillas, is a staple food for Hispanic populations residing in the United States, particularly among Mexican Americans and Central Americans. Research has indicated that Hispanic women in the United States continue to be at a higher risk of having a neural tube defect–affected pregnancy than women of other races/ethnicities, even after the introduction of folic acid fortification of cereal grain products labeled as “enriched.” Corn masa flour has, therefore, been suggested as a potential food vehicle for folic acid in the United States. This paper explores the potential impact that folic acid fortification of corn masa flour could have on the Hispanic population in the United States.
Keywords: fortification, corn masa flour, folic acid
Introduction
Neural tube defects are serious birth defects of the brain (anencephaly) and spine (spina bifida) that can occur during the first month of pregnancy.1 Previous research demonstrated that a B vitamin, folic acid, can prevent the majority of neural tube defects if it is consumed before and during early pregnancy2,3 As a result of this research, the U.S. Food and Drug Administration (FDA) mandated in 1998 that cereal grain products labeled as enriched be fortified with folic acid in an effort to reduce a woman's risk of having a pregnancy affected by a neural tube defect.4 Since fortification of enriched cereal grain products was initiated, the prevalence of neural tube defects in the United States has decreased; however, racial/ethnic disparities remain.5 This paper explores the potential impact that folic acid fortification of corn masa flour could have on folic acid intake and rates of neural tube defects, and discusses the current status of federal regulations regarding corn masa flour fortification.
Current folic acid fortification policies
In 1992, the U.S. Public Health Service issued a recommendation that all women capable of becoming pregnant consume at least 400 μg of folic acid daily in order to reduce their risk of having a pregnancy affected by a neural tube defect.6 This recommendation was based on data from randomized controlled trials, intervention studies, and observational studies that indicated that folic acid could reduce the risk of neural tube defects from 35% to 100%, depending on the baseline prevalence of neural tube defects.2,3,7,8 This research and recommendation led to efforts to fortify the U.S. food supply with folic acid. In 1993, an amendment and a proposed addition to existing food regulations regarding folic acid were published in the Federal Register.9 This amendment focused on changing the standards of identity for cereal grain products to require the addition of folic acid at 140 μg/100 g of flour.9 The proposed addition would continue to allow folic acid to be added to ready-to-eat breakfast cereals and dietary supplements as a food additive, but at a lower level (lowered from 400 μg/serving to 100 μg/serving).10 In 1996, the FDA published final rules that modified the standard of identity for cereal grain products labeled as enriched, mandating that they must contain folic acid at 140 μg/100 g of flour and approving folic acid as a food additive for infant formulas, medical foods, and foods for special dietary use, such as meal replacement products, in addition to ready-to-eat cereals.4,11
Manufacturers were given until 1998 to comply with the new federal regulations. As stated previously, the FDA proposed reducing the amount of folic acid allowed per serving of ready-to-eat cereals to 100 μg in conjunction with the required addition of folic acid to enriched cereal grain products;11 however, after receiving concerns about this proposal during the public comment period and because only a small percentage of the market at that time had breakfast cereals with this amount of folic acid, the allowable level remained at 400 μg/serving.11 No further federal regulations relating to folic acid in foods have been approved.
Impact of folic acid fortification
Since folic acid fortification of cereal grain products labeled as enriched, monitoring programs have shown increases in folic acid intake and blood folate concentrations, and a reduction in the prevalence of neural tube defects among the U.S. population. Estimates of usual median daily folic acid intake from cereal grain products labeled as enriched among nonpregnant women aged ≥19 years in the United States were 117 μg from 2003 to 2006.12 This was similar to the amount that fortification had been estimated to provide (approximately 100 μg).13 Blood folate concentrations were also found to increase postfortification. Prefortification (1988–1994) concentrations of serum and red blood cell (RBC) folate increased from 14.0 nmol/L and 686 nmol/L, respectively, to 37.6 nmol/L and 1060 nmol/L, respectively, postfortification (1999–2010) for women aged 15–44 years.14 Prevalence rates of neural tube defects have also decreased by 36% since folic acid fortification, from 10.8/10,000 births in 1995–1996 to 6.9/10,000 births in 2006.5 Overall, there has been a significant positive health impact as a result of folic acid fortification. Some studies have suggested that poor vitamin B12 status could account for many of the neural tube defects that are occurring postfortification,15,16 and additional research into this issue has been recommended. However, the impact of vitamin B12 is beyond the scope of this paper.
Gaps in current folic acid fortification policies
Although folic acid fortification has been successful in reducing the number of neural tube defects in the United States and has been identified as one of the top 10 public health achievements in the last decade,5,17 there are still some subpopulations who continue to have higher prevalence rates of neural tube defects and might not be receiving the full benefit of the fortification of cereal grain products labeled as enriched. For example, Mexican American women have lower usual mean total folic acid intake than non-Hispanic white women (244 μg and 332 μg, respectively).18 Serum and RBC folate concentrations increased from pre- to postfortification for both Mexican American and non-Hispanic white women; however, the disparity remained between these groups of women for each of these biomarkers of folate metabolism (median serum folate concentrations: prefortification (1988–1994), 14.0 nmol/L and 17.4 nmol/L, respectively; postfortification (2005–2010), 35.3 nmol/L and 41.9 nmol/L, respectively; median RBC folate concentrations: prefortification (1988–1994), 672 nmol/L and 752 nmol/L, respectively; postfortification (2005–2010), 1020 nmol/L and 1480 nmol/L, respectively).14 Finally, as indicated by data from the 2005–2007 National Birth Defects Prevention Network, Hispanic women were 21% more likely to have a baby affected by a neural tube defect than non-Hispanic white women.5
The number of Hispanics in the United States is increasing19 and the number of Hispanic women is estimated to be over 62 million by the year 2060.20 In 2011, the U.S. Census Bureau estimated that there were almost 11 million Hispanic women aged 15– 44 years in the United States.21 Hispanic women tend to have higher fertility rates and birth rates than non-Hispanic white women.22 However, Hispanic women are not a homogenous group. Research has indicated that differences by acculturation—a term used to describe an individual's integration into the dominant culture23—exist for total folic acid intake,18 folic acid supplement use,18 and neural tube defect prevalence.24 Mexican American women with lower acculturation factors (e.g., reported primarily speaking Spanish, living in the United States for <15 years, or being born in Mexico) have lower total usual folic acid intake than their more acculturated counterparts (i.e., Mexican American women who reported primarily speaking English, or living in the United States for ≥15 years, or being born in the United States).18 In addition, Mexican American women who reported primarily speaking Spanish, were half as likely to report consuming a folic acid–containing supplement as Mexican American women who reported primarily speaking English.18 Finally, Hispanic women, who reported speaking Spanish at home, were almost two times more likely to have a baby born with spina bifida than non-Hispanic white women (OR: 1.87, 95% CI: 1.48, 2.35), while no difference was observed between Hispanic women who reported speaking primarily English at home and white women (OR: 1.17, 95% CI: 0.87, 1.59).24 Given the increasing numbers of Hispanic women in the United States, their increased risk for having a pregnancy affected by a neural tube defect, and their lower folic acid consumption and supplement use, there is a need to address these disparities.
Fortification of corn masa flour with folic acid
These issues led public health experts (e.g., epidemiologists, nutritionists, health education specialists, physicians) to identify potential interventions that could target Hispanic women. One such intervention was the fortification of corn masa flour with folic acid. Corn masa flour is used to make corn tortillas, and Mexican Americans and Central Americans report commonly consuming corn masa flour,25 making it a targeted fortification vehicle.
Hamner et al. estimated that fortification of corn masa flour could marginally increase usual total folic acid intake for all races/ethnicities but would selectively increase intake for Mexican Americans (Fig. 1).26,27 This modeled increase was observed particularly for Mexican American women with lower acculturation factors, such as those who reported primarily speaking Spanish (Fig. 2).27 Estimates indicated that folic acid intake could increase by a relative percentage of 30.5% for Mexican American women who reported primarily speaking Spanish, while increasing intake for non-Hispanic white women by only a relative percentage of 3.9%.27 Among women who reported only consuming enriched cereal grain products (i.e., did not report consuming any ready-to-eat cereals or folic acid-containing supplements), the estimated impact was even higher for Mexican American women who reported primarily speaking Spanish (relative percentage increase of 42.9%), compared to non-Hispanic white women (relative percentage increase of 7.1%; Fig. 3). Researchers also found that fortification of corn masa flour would increase the percentage of women of childbearing age that meet the recommended intake of 400 μg of folic acid by 6 percentage points for Mexican Americans and 1.1 percentage points for non-Hispanic whites.27
Figure 1.
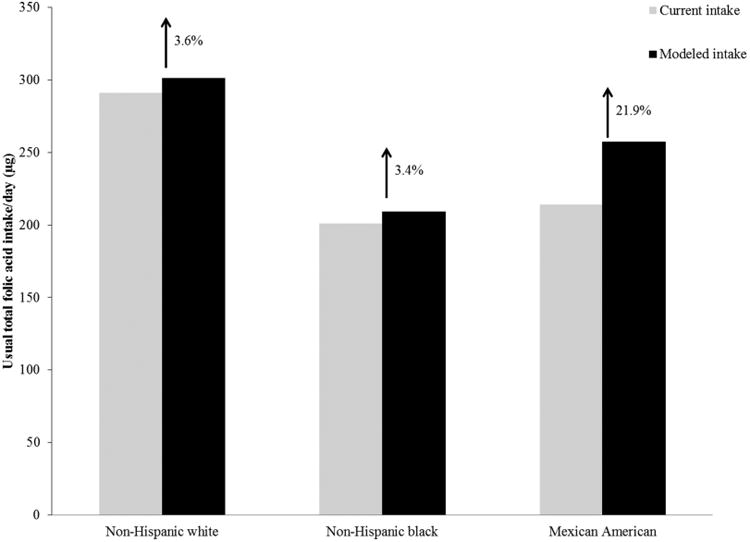
Total usual daily folic acid intake without (current) and with (modeled) folic acid fortification of corn masa flour for the United States population by race/ethnicity, National Health and Nutrition Examination Survey (NHANES) 2001–2008.
Figure 2.
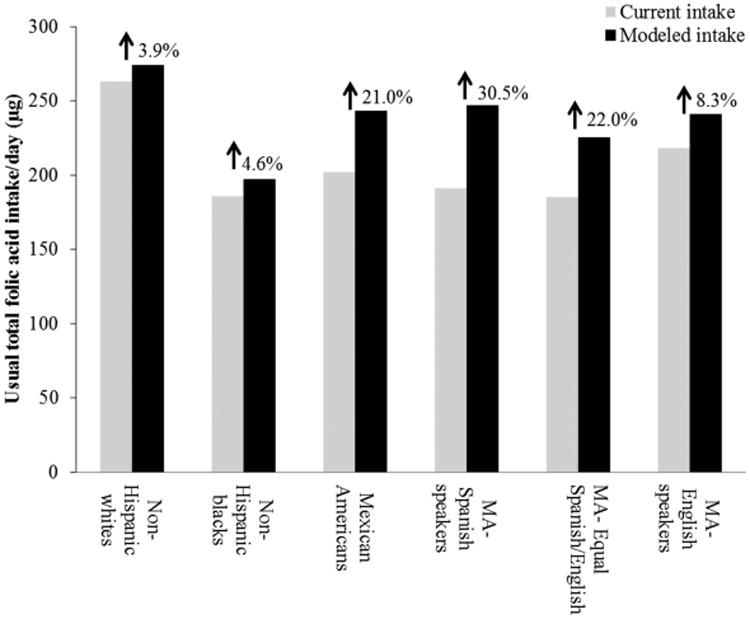
Total usual daily folic acid intake without (current) and with (modeled) folic acid fortification of corn masa flour among women aged 15–44 years by race/ethnicity and acculturation, National Health and Nutrition Examination Survey (NHANES) 2001–2008. MA, Mexican American.
Figure 3.
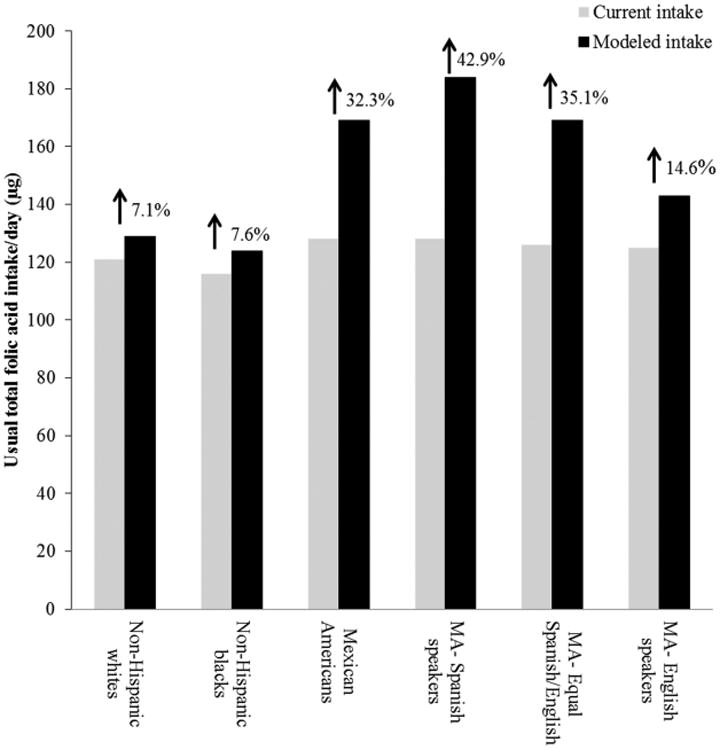
Total usual daily folic acid intake without (current) and with (modeled) folic acid fortification of corn masa flour by race/ethnicity and acculturation among women aged 15–44 years, who report consuming only enriched cereal grain products, National Health and Nutrition Examination Survey (NHANES) 2001–2008. MA, Mexican American.
Using data from multiple sources, researchers estimated the impact that fortification of corn masa flour might have on the number of neural tube defects. They estimated that 40 cases/year of neural tube defects could be prevented among Hispanic women in the United States with fortification of corn masa flour with folic acid, although there were some limitations with this estimate and many assumptions were made;28 this estimate could be as low as zero or as high as 120.28 Even though fortification of corn masa flour with folic acid would selectively increase folic acid intake among Mexican Americans, researchers found that it did not significantly change the percentage of the U.S. population exceeding the tolerable upper intake level of 1000 μg of folic acid,29 regardless of race/ethnicity or age, including among lower acculturated Mexican Americans, for whom intake would increase the most.30
Taking into account the increasing number of Hispanics in the United States and their increased risk for having a pregnancy affected by a neural tube defect, the potential impact that fortification of corn masa flour with folic acid could have is significant. Current U.S. federal regulations do not allow for the addition of folic acid to corn masa flour and would require a change in current standards of identity of corn masa flour or the approval of folic acid as a food additive for corn masa flour. In 2012, a petition to allow folic acid to be added to corn masa flour as a food additive was submitted to the FDA by industry and nonprofit organizations.31 This petition is under review by the FDA and a final ruling could take several years.
Conclusion
Folic acid fortification in the United States has had a significant impact on multiple measures, including folic acid intake, blood folate concentrations, and the prevalence of neural tube defects.5,12,14 However, some subpopulations, including the Hispanic population, exhibit higher rates of neural tube defects and lower total folic acid intake.5,18,32 Folic acid fortification of corn masa flour, a staple food product within the Hispanic community, has been estimated to potentially reduce the disparity in folic acid intake.26,27 This policy-level intervention would require a change in federal regulations but could reduce the number of neural tube defects among Hispanic women28 and increase total folic acid intake among an at-risk population.26,27
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This article was presented at the World Health Organization consultation “Technical Considerations for Maize Flour and Corn Meal Fortification in Public Health” in collaboration with the Sackler Institute for Nutrition Science at the New York Academy of Sciences and the Flour Fortification Initiative (FFI), convened on 8 and 9 April 2013, at the New York Academy of Sciences in New York, USA. This article is being published individually but will be consolidated with other articles as a special issue of Annals of the New York Academy of Sciences. The coordinators of this issue were Drs. Maria Nieves Garcia-Casal, Mireille McLean, Helena Pachon, and Juan Pablo Peña-Rosas. The special issue is the responsibility of the editorial staff of Annals of the New York Academy of Sciences, who delegated to the coordinators preliminary supervision of both technical conformity to the publishing requirements of Annals of the New York Academy of Sciences and general oversight of the scientific merit of each article. The workshop was supported by the Sackler Institute for Nutrition Science at the New York Academy of Sciences and the FFI. The authors alone are responsible for the views expressed in this article; they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated or the decisions, policies, or views of the World Health Organization. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors, publisher, or editorial staff of Annals of the New York Academy of Sciences.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Botto LD, et al. Neural-tube defects. N Engl J Med. 1999;341:1509–1519. doi: 10.1056/NEJM199911113412006. [DOI] [PubMed] [Google Scholar]
- 2.MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- 3.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid, final rule. Fed Reg. 1996;61:8781–8797. [Google Scholar]
- 5.CDC. CDC Grand Rounds: additional opportunities to prevent neural tube defects with folic acid fortification. MMWR Morb Mortal Wkly Rep. 2010;59:980–984. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Morb Mortal Wkly Rep. 1992;41:1–7. [Google Scholar]
- 7.Shaw GM, et al. Periconceptional vitamin use, dietary folate, and the occurrence of nueral tube defects. Epidemiology. 1995;6:219–226. doi: 10.1097/00001648-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Mulinare J, et al. Periconceptional use of multivita-mins and the occurrence of neural tube defects. JAMA. 1988;260:3141–3145. [PubMed] [Google Scholar]
- 9.Food and Drug Admininstration. Food standards: amendment of the standards of identity for enriched grain products to require addition of folic acid. Fed Reg. 1993;58:53305–53317. [Google Scholar]
- 10.Food and Drug Admininstration. Food additives permitted for direct addition to food for human consumption; folic acid (folacin) Fed Reg. 1993;58:53312–53317. [PubMed] [Google Scholar]
- 11.Food and Drug Administration. Food additives permitted for direct addition to food for human consumption; folic acid (folacin), final rule. Fed Reg. 1996;61:8797–8807. [PubMed] [Google Scholar]
- 12.Yang QH, et al. Folic acid source, usual intake, and folate and vitamin B12 status in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2006. Am J Clin Nutr. 2010;91:64–72. doi: 10.3945/ajcn.2009.28401. [DOI] [PubMed] [Google Scholar]
- 13.Food and Drug Administration. Food labeling: health claims and labeling statements: folate and neural tube defects. Fed Reg. 1993;58:53254–53295. [Google Scholar]
- 14.Pfeiffer C, et al. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortifi-cation using assay-adjusted data from the NHANES 1988– 2010. J Nutr. 2012;142:886–893. doi: 10.3945/jn.111.156919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray JG, et al. Vitamin B12 and the risk of neural tube defects in a folic-acid-fortified population. Epidemiology. 2007;18:362–366. doi: 10.1097/01.ede.0000257063.77411.e9. [DOI] [PubMed] [Google Scholar]
- 16.Thompson MD, Cole DE, Ray JG. Vitamin B12 and neural tube defects: the Canadian experience. Am J Clin Nutr. 2009;89(Suppl.):697S–701S. doi: 10.3945/ajcn.2008.26947B. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Ten great public health achievements—United States, 2001—2010. MMWR Morb Mortal Wkly Rep. 2011;60:619–623. [PubMed] [Google Scholar]
- 18.Hamner HC, Cogswell ME, Johnson MA. Acculturation factors are associated with folate intakes among Mexican American women. J Nutr. 2011;141:1889–1897. doi: 10.3945/jn.111.143412. [DOI] [PubMed] [Google Scholar]
- 19.Ennis SR, Rios-Vargas M, Albert NG. Census Briefs. United States Census Bureau; Washington, D.C: 2011. The Hispanic population: 2010. http://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf. [Google Scholar]
- 20.U.S. Census Bureau, Population Division. Table 4. Projections of the Population by Sex, Race, and Hispanic Origin for the United States: 2015 to 2060 (NP2012-T4) 2012 http://www.census.gov/population/projections/data/national/2012/summarytables.html.
- 21.U.S. Census Bureau, Current Population Survey. Table 1. Population by sex, age, Hispanic origin, and race: 2011. Annual Social and Economic Supplement. 2011 http://www.census.gov/population/hispanic/data/2011.html.
- 22.Martin JA, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1–72. [PubMed] [Google Scholar]
- 23.Cabassa LJ. Measuring acculturation: where we are and where we need to go. Hisp J Behav Sci. 2003;25:127–146. [Google Scholar]
- 24.Canfield MA, et al. Anencephaly and spina bifida among Hispanics: maternal, sociodemographic, and acculturation factors in the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2009;85:637–646. doi: 10.1002/bdra.20582. [DOI] [PubMed] [Google Scholar]
- 25.Bressani R, Rooney LW, Quintero X, Serna Saldivar SO. Fortification of corn masa flour with iron and/or other nutrients: a literature and industry experience review. Washington, D.C.: SUSTAIN; 1997. pp. 1–177. [Google Scholar]
- 26.Hamner HC, et al. Predicted contribution of folic acid fortification of corn masa flour to the usual folic acid intake for the US population: National Health and Nutrition Examination Survey, 2001–2004. Am J Clin Nutr. 2009;89:305–315. doi: 10.3945/ajcn.2008.26331. [DOI] [PubMed] [Google Scholar]
- 27.Hamner HC, et al. Modelling fortification of corn masa flour with folic acid and the potential impact on Mexican American women with lower acculturation. Public Health Nutr. 2013;16:912–921. doi: 10.1017/S1368980012004582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tinker SC, et al. Folic acid fortification of corn masa flour and neural tube defect prevention. Birth Defects Res A Clin Mol Teratol. 2013;97:649–657. doi: 10.1002/bdra.23158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- 30.Hamner HC, et al. Modeling fortification of corn masa flour with folic acid: the potential impact on exceeding the tolerable upper intake level for folic acid, NHANES 2001–2008. Food Nutr Res. 2013;57:19146. doi: 10.3402/fnr.v57i0.19146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.U.S. Food and Drug Admininstration. Gruma Corporation, Spina Bifida Association, March of Dimes Foundation, American Academy of Pediatrics, Royal DSM N.V., and National Council of La Raza; Filing of Food Additive Petition FAP 2A4796. Fed Reg. 2012;77:35317. [Google Scholar]
- 32.Yang Q, et al. Race/ethnicity differences in folic acid intake among women of childbearing age in the United States after folic acid fortification: findings from the National Health and Nutrition Examination Survey, 2001–2002. Am J Clin Nutr. 2007;85:1409–1416. doi: 10.1093/ajcn/85.5.1409. [DOI] [PubMed] [Google Scholar]


