Abstract
Objectives
Human hematopoietic stem cell (HSC)–containing grafts are most commonly used to treat various blood diseases, including leukemias and autoimmune disorders. CD150 (SLAM) family receptors have recently been shown to be differentially expressed by mouse HSC and progenitor cells. Members of the CD150 family are key regulators of leukocyte activation and differentiation. The goal of the present study is to analyze the expression patterns of the CD150 receptors CD48, CD84, CD150 (SLAM), CD229 (Ly9), and CD244 (2B4) on the different sources of human hematopoietic stem and progenitor cells.
Materials and Methods
Expression of CD150 receptors was analyzed on human mobilized peripheral blood CD133+-isolated cells and CD34+ bone marrow (BM) and umbilical cord blood (CB) cells using multicolor flow cytometry.
Results
CD244 was present on most CD133+Lin−-mobilized cells and CD34+Lin− BM and CB cells, including virtually all CD38−Lin− primitive progenitor cells. CD48 had a restricted expression pattern on CD133+Lin−CD38− cells, while its levels were significantly higher in CD34+Lin− BM and CB cells. In addition, CD84 was present on a significant number of CD133+Lin− cells, but only on a small fraction of CD133+Lin−CD38− peripheral blood mobilized cells. In contrast, CD84 was expressed on practically all CD34+Lin− BM cells. No CD150 expression was observed in mobilized peripheral blood CD133+Lin− or CD34+Lin− BM and CB cells. Furthermore, only a small fraction of CD34+Lin− BM and CB cells expressed CD229.
Conclusions
Our results show that CD150 family molecules are present on human hematopoietic stem and progenitor cells and that their expression patterns differ between humans and mice.
Hematopoietic stem cells (HSCs) are a rare cell type found mainly in the bone marrow (BM), which have the capacity to self-renew and differentiate into all blood cell lineages [1]. HSC-containing grafts are most commonly used to treat leukemias and lymphomas, aplastic anemia, congenital immunodeficiencies, and autoimmune diseases [2]. Autologous HSC transplantations have been widely performed for hematopoietic rescue following high-dose chemo/radiotherapy for treatment of malignancies. Technological advances for HSC enrichment have relied on complex combinations of numerous cell-surface markers and FACS cell-sorting technology [1].
The CD150 (SLAM) is a new family of cell-surface receptors of the immunoglobulin superfamily that regulates several leukocyte functions [3,4]. These molecules, which act as cell–cell interaction and signaling receptors, are differentially expressed on distinct types of leukocytes and lymphocytes [5]. CD150 receptors are selectively expressed among primitive mouse progenitors in the adult BM in such a way that it is possible to highly purify HSCs using a simple combination of monoclonal antibodies (mAbs) against three of these receptors (CD150, CD244, and CD48) [6]. This strategy has recently been extended to isolate HSCs from embryonic and fetal tissues, which has been complicated by developmental variations in the anatomical localization and cell-surface marker profile of these cells [7,8].
Given the success of this strategy in isolating HSCs in mice, as well as the important biological role of these receptors, it is essential to determine whether or not the CD150 family of molecules exhibits similar expression patterns on human hematopoietic progenitors. Thus, here we examined the expression patterns of CD150 markers on human hematopoietic progenitor and stem cells.
Materials and methods
Sample collection and purification
Peripheral blood leukocytes (PBLs) from healthy donors for allogeneic transplantation were used after mobilization with 10 μg/kg subcutaneous granulocyte colony-stimulating factor (G-CSF; Filgrastim; Amgen, Thousand Oaks, CA, USA) every 12 hours for 4 to 7 days. PBLs were collected by leukapheresis and the CD133+ hematopoietic progenitor cells were positively selected using immunomagnetic beads conjugated to a monoclonal mouse anti-human CD133 antibody (clone AC133) (CliniMACS; Miltenyi Biotec, Bergisch Gladbach, Germany). The isolated cell fraction was checked for purity with anti-CD34 and contained >93% of CD34+ cells. Placental umbilical cord blood (CB) units were obtained from the Barcelona Cord Blood Bank (Hospital Duran i Reynals, Barcelona, Spain), stored at 4°C and processed within 24 hours of collection. Donors gave their written informed consent according to the protocol approved by the Ethics Committee of the Hospital Clinic of Barcelona. Cryopreserved human BM Poietics positive immunomagnetic CD34+-isolated cells were purchased from Cambrex (Verviers, Belgium).
Monoclonal antibodies
The following biotinylated anti-human mAbs were produced in our laboratory: CD84 (CD84.1.21), CD150 (SLAM.4), CD229 (HLy9.1.84) and CD244 (2B4.69) [9–12]. Biotinylated anti-human CD48 (99A) was provided by R. Vilella (Hospital Clinic, Barcelona, Spain) and CD150 mAbs (clones A12, 1B12, 10F5, 11G2, and 5G4) by Cox Terhorst (Harvard Medical School, Cambridge, MA, USA). Phycoerythrin (PE)-conjugated anti-human CD34, CD38, and allophycocyanin (APC)-conjugated anti-human CD38 antibodies were purchased from Becton-Dickinson Pharmingen (San Diego, CA, USA) and APC-conjugated anti-human CD34 and CD45 antibodies from Immunotools (Friesoythe, Germany). The following lineage (Lin)-specific fluorescein isothiocyanate (FITC)–conjugated mAbs were used: CD3, CD14, CD16, CD19, CD56 (Becton-Dickinson), CD21, CD31, and CD41 (ImmunoTools). Directly conjugated mouse IgG1 and IgG2a (ImmunoTools) were used for isotype controls.
Immunofluorescence analysis
Single-cell suspensions were washed in phosphate-buffered saline plus 2% heat-inactivated fetal calf serum (Biological Industries, Israel) and 0.01% sodium azide. They were then incubated with biotinylated and directly labeled mAbs for 45 minutes at 4°C. After washing, cells were stained with Streptavidin-PE-Cy5 (Becton-Dickinson) for an additional 15 minutes at 4°C. Red cells were lysed using FACS Lysing Solution (Becton-Dickinson). In all experiments, cells were also stained with isotype-matched monoclonal antibodies and Streptavidin-PE-Cy5. The samples were acquired on a FACSCalibur flow cytometer (Becton-Dickinson). Gates were set on CD133+Lin− or CD34+Lin− populations after using forward and side-scatter parameters to exclude dead cells and debris. A minimum of 50,000 events from each tube was analyzed using CellQuest software. Fluorescence compensation was performed with FITC-, PE-, and APC anti-human CD34 as well as biotinylated anti-human CD244 (2B4.69). Fluorescence for both indirect staining and isotype-matched negative controls was set at a mean fluorescence intensity of 5.
Results and Discussion
The CD150 molecule is expressed on mouse HSCs, but not on multipotent or restricted hematopoietic progenitors [6]. In contrast, CD244 is expressed by multipotent and by some restricted progenitors, while CD48 expression is restricted to B-lineage and myeloerythroid progenitors [6]. Because mobilized peripheral, umbilical CB, and BM cells are currently used as sources of human HSCs, we studied the expression of CD150 family molecules on these cells using flow cytometry in order to assess whether their expression patterns are conserved across species.
In contrast to the restricted expression of CD244 in mouse BM cells [6], this receptor was expressed at high levels on most of the progenitor cells (Figs. 1 and 2). Moreover, 92.9% of the more primitive CD133+Lin− CD38− progenitor cells, which contain most of the repopulating HSCs [13], expressed CD244 (Table 1). Moreover, almost all CD34+Lin−CD38− CB and BM cells also expressed CD244 (Tables 2 and 3). These data indicate that in humans CD244 is a pan-hematopoietic progenitor/stem cell marker. This finding is surprising because until now this molecule was thought to be restricted to more mature or lineage-committed cell types such as human natural killer cells, a subset of cytotoxic T lymphocytes, basophils, and eosinophils [5,14]. Consistent with the expression pattern found in this study, it has been reported that only a small fraction of thymocytes express CD244, with positive cells corresponding to immature CD4−CD8− double-negative thymocytes [5].
Figure 1.
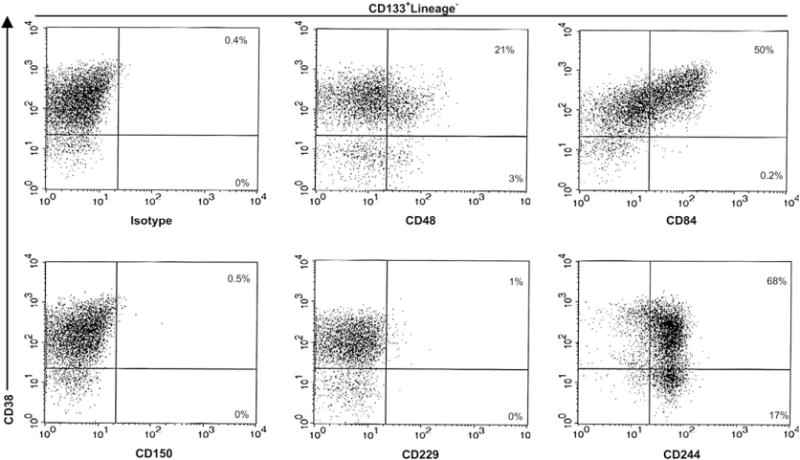
CD150 family markers expression on human mobilized peripheral blood hematopoietic stem and progenitor cells. Mobilized peripheral blood CD133+-isolated cells of healthy donors were stained with anti-human biotin-conjugated antibodies against the CD150 family members (CD48, CD84, CD150, CD229, and CD244), anti–CD38-phycoerythrin and with a cocktail of fluorescein isothiocyanate lineage-specific monoclonal antibodies. The percentages of CD38+ and CD38− subsets within each two-parameter histogram are shown and are representative of four independent experiments.
Figure 2.
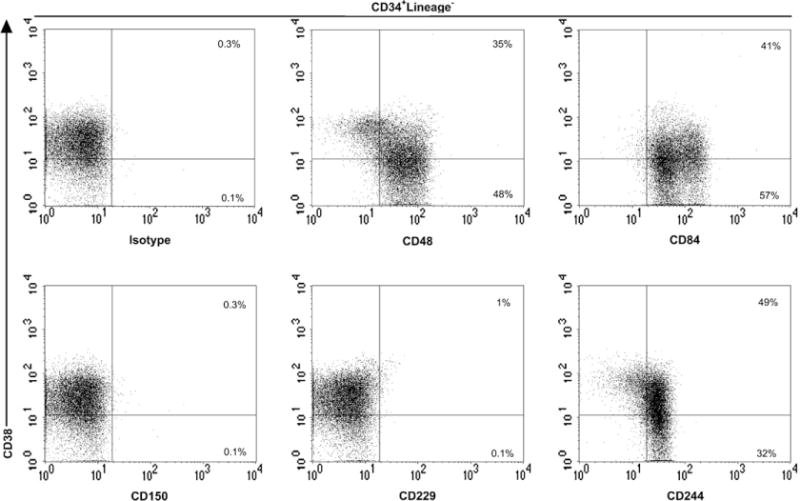
CD150 family markers expression on human bone marrow (BM) hematopoietic stem and progenitor cells. BM CD34+-isolated cells of healthy donors were stained with anti-human biotin-conjugated antibodies against the CD150 family members (CD48, CD84, CD150, CD229, and CD244), anti–CD38 and with a cocktail of fluorescein isothiocyanate lineage-specific monoclonal antibodies. The percentages of CD38+ and CD38− subsets within each two-parameter histogram are shown and are representative of four independent experiments.
Table 1.
Expression of CD150 members on mobilized peripheral blood CD133+-isolated cells was analyzed using flow cytometry
| CTL
|
CD48
|
CD84
|
CD150
|
CD229
|
CD244
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | MFI | % | MFI | % | MFI | % | MFI | % | MFI | % | MFI | |
| CD133+Lin− | 0.09 ± 0.05 | 5.57 ± 1.11 | 21.62 ± 13.92 | 24.17 ± 8.75 | 38.74 ± 2.59 | 43.27 ± 5.46 | 0.31 ± 0.49 | 6.68 ± 3.37 | 0.56 ± 0.30 | 6.57 ± 0.75 | 85.51 ± 6.07 | 70.67 ± 14.50 |
| CD133+Lin−CD38− | 0.21 ± 0.06 | 4.33 ± 0.72 | 34.16 ± 10.25 | 20.83 ± 12.67 | 16.35 ± 20.06 | 11.76 ± 4.92 | 1.17 ± 1.76 | 4.46 ± 0.60 | 0.48 ± 0.42 | 3.50 ± 1.77 | 92.92 ± 6.04 | 80.00 ± 23.81 |
CTL = control.
Values are mean percent of positive cells and mean fluorescence intensity (MFI) ± standard deviation of four independent donors.
Table 2.
Expression of CD150 members on CD34+ cord blood cells was analyzed using flow cytometry
| CTL
|
CD48
|
CD84
|
CD150
|
CD229
|
CD244
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | MFI | % | MFI | % | MFI | % | MFI | % | MFI | % | MFI | |
| CD34+Lin− | 0.29 ± 0.25 | 3.81 ± 0.54 | 66.40 ± 9.30 | 38.90 ± 8.32 | 54.76 ± 10.01 | 75.41 ± 11.86 | 1.12 ± 1.49 | 4.01 ± 0.65 | 11.38 ± 6.51 | 7.91 ± 3.36 | 90.96 ± 11.8 | 75.41 ± 11.86 |
| CD34+Lin−CD38− | 0.27 ± 0.33 | 4.45 ± 0.99 | 77.50 ± 15.74 | 46.07 ± 26.66 | 47.49 ± 9.40 | 15.52 ± 4.90 | 1.44 ± 1.09 | 4.65 ± 0.78 | 4.54 ± 2.63 | 5.90 ± 2.21 | 86.87 ± 9.21 | 66.91 ± 14.28 |
CTL = control.
Values are mean percent of positive cells and mean fluorescence intensity (MFI) ± standard deviation of four independent donors.
Table 3.
Expression of CD150 members on CD34+ bone marrow cells was analyzed using flow cytometry
| CTL
|
CD48
|
CD84
|
CD150
|
CD229
|
CD244
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | MFI | % | MFI | % | MFI | % | MFI | % | MFI | % | MFI | |
| CD34+Lin− | 0.52 ± 0.14 | 5.35 ± 0.91 | 68.34 ± 11.98 | 40.65 ± 16.42 | 95.06 ± 2.53 | 58.64 ± 14.52 | 0.66 ± 0.43 | 5.39 ± 1.15 | 1.69 ± 0.59 | 5.03 ± 0.25 | 71.53 ± 4.37 | 23.86 ± 4.41 |
| CD34+Lin−CD38− | 0.50 ± 0.12 | 6.11 ± 0.87 | 90.06 ± 9.89 | 56.69 ± 18.08 | 96.38 ± 2.39 | 62.66 ± 8.61 | 0.66 ± 0.49 | 6.12 ± 1.12 | 0.35 ± 0.03 | 5.44 ± 0.33 | 91.72 ± 3.44 | 33.19 ± 9.71 |
CTL = control.
Values are mean percent of positive cells and mean fluorescence intensity (MFI) ± standard deviation of four independent donors.
Here we found that CD48, the natural ligand of CD244 [15], showed a more restricted expression pattern. Only 21.6% of CD133+Lin− cells expressed CD48, although a significant number of the more immature CD133+Lin−CD38− cells expressed CD48 (34.2%) (Table 1). This difference between CD38+ and CD38− populations was also observed in CB and BM counterpart cells (Table 2 and Table 3). However, significantly higher CD48 expression levels were found in the following cells: CD34+Lin−CD38− (77.5%) compared with CD34+Lin− (66.4%) umbilical CB cells, and CD34+Lin−CD38− (90%) compared with CD34+Lin− (68.3%) BM cells (Table 2 and Table 3). These results contrast with the observation that mouse BM HSCs and multipotent progenitors are all CD48− [6]. Interestingly, CD34+CD38− CB cells have a higher cloning efficiency and generate more progenitors than their BM counterparts [16]. CD48 is expressed not only on some progenitors and leukocytes, but also by other nonhematopoietic cells such as endothelial cells [17]. In humans, the interaction between CD244+ HSCs, or between these and other CD48+ cells, may determine HSC localization and differentiation.
CD84 was present on a subpopulation of mobilized peripheral blood cells, expressing very low levels on CD133+Lin−CD38− (Table 1). The expression of human CD84 we observed was identical to that reported in another study, in which the authors proposed that CD84 serves as a marker for committed hematopoietic progenitor cells [18]. However, significantly higher levels of this marker were observed on CB and BM counterparts, particularly in immature CD34+CD38− cells (Table 2 and Table 3). As reported previously, our data indicate that there are some phenotypic differences between hematopoietic progenitors from peripheral blood-mobilized, CB, and BM cells [19]. Interestingly, we have recently observed that virtually all Sca-1+c-kit+Lin− mouse BM cells express CD84 (unpublished data).
No significant expression of CD150 was observed in mobilized peripheral blood, CB or BM cells (Tables 1, 2, and 3). The absence of expression on these cells was not dependent on the epitope recognized by the CD150 mAb used in this study, because five other CD150 mAbs were tested and all yielded identical results (Table 4). This finding does not exclude the possibility that CD150 is expressed on a very small subset of CD133−CD34− HSCs [20]. Analysis of CD150 on cytokine-mobilized PBLs before CD133 magnetic selection showed that 1.64% of CD34−CD45+Lin− cells expressed CD150 (data not shown). Whether this small subset of CD150+CD34−CD45+Lin− cells represents HSCs with the capacity for long-term multilineage reconstitution remains to be determined by performing in vivo transplantation assays.
Table 4.
Reactivity of CD150 monoclonal antibodies and isotype control with human mobilized peripheral blood CD133+-isolated cells
| Clone | CD150 expression
|
|
|---|---|---|
| % | MFI | |
| CTL | 0.11 ± 0.0 | 4.21 ± 0.5 |
| SLAM.4 | 0.36 ± 0.2 | 4.98 ± 0.5 |
| A12 | 0.16 ± 0.1 | 4.73 ± 0.4 |
| 1B12 | 0.24 ± 0.2 | 5.02 ± 0.3 |
| 10F5 | 0.29 ± 0.1 | 5.11 ± 0.6 |
| 11G2 | 0.90 ± 1.0 | 6.01 ± 2.2 |
| 5G4 | 0.38 ± 0.4 | 5.58 ± 1.6 |
CTL = control.
Values are mean percentage of positive cells and mean fluorescence intensity (MFI) ± standard deviation of three independent donors.
Mouse CD229 has recently been detected on virtually all Sca-1+c-kit+Lin− mouse HSCs [21]. In contrast, no significant levels of this molecule could be detected on mobilized CD133+ and BM cells (Tables 1 and 3). Only a small fraction of CD34+Lin− (11.3%) and CD34+CD38− (4.5%) CB cells were detected (Table 2).
Although a direct comparison of the phenotype and functional properties between mouse and human progenitor and HSCs cannot be clearly established, our results show that the expression of the CD150 family of cell-surface receptors differs between these two species (Fig. 3). This is not an uncommon observation. Two receptors closely related to the CD150 family, CD2 and CD58, are good examples illustrating the differences between mice and humans. While human CD2 expression is restricted to T cells, mouse CD2 is highly expressed on both T and B cells [17]. Moreover, CD58 is not expressed on mouse cells [17]. The physiological role of CD150 receptors in HSCs remains to be determined. Mice deficient in CD150 family members lack gross alterations of hematopoiesis, although functional redundancy between the receptors of this family may explain this observation.
Figure 3.
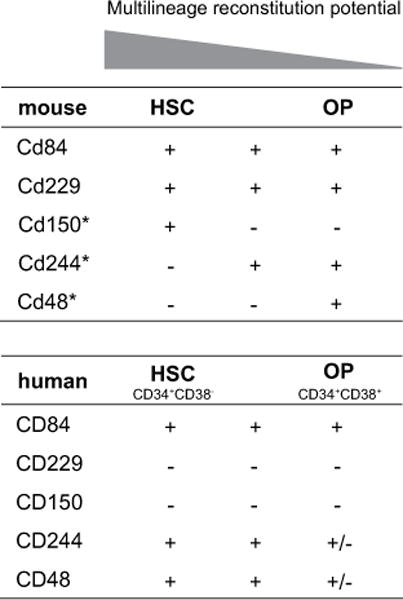
Comparison of CD150 family markers expression on bone marrow (BM) hematopoietic stem cells (HSCs) and oligo-potent progenitors (OP) between mice and humans. Results on human BM chart represent the percentage of positive cells: + (>90%), ± (75–60%) and − (<2%). *Data from Kiel et al. [6].
In conclusion, our data show that CD150 family molecules are present on human HSCs and progenitor cells and that their expression differs between mice and humans. Future functional studies may help to characterize the hematopoietic potential of the populations identified using CD150-family receptors.
Acknowledgments
This publication was made possible by a Senior Research Award from Chron’s and Colitis Foundation of America (CCFA) and supported by a grant from the Ministerio Educación y Ciencia (SAF 2006-00490). We are grateful to the Barcelona Cord Blood Bank (Duran i Reynals Hospital, l’Hospitalet de Llobregat, Spain) for providing us with umbilical cord blood units.
Footnotes
The authors reported no potential conflicts of interest.
References
- 1.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287:1442–1446. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- 3.Engel P, Eck MJ, Terhorst C. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nat Rev Immunol. 2003;3:813–821. doi: 10.1038/nri1202. [DOI] [PubMed] [Google Scholar]
- 4.Veillette A. Immune regulation by SLAM family receptors and SAP-related adaptors. Nat Rev Immunol. 2006;6:56–66. doi: 10.1038/nri1761. [DOI] [PubMed] [Google Scholar]
- 5.Romero X, Benitez D, March S, Vilella R, Miralpeix M, Engel P. Differential expression of SAP and EAT-2-binding leukocyte cell-surface molecules CD84, CD150 (SLAM), CD229 (Ly9) and CD244 (2B4) Tissue Antigens. 2004;64:132–144. doi: 10.1111/j.1399-0039.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 6.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim I, He S, Yilmaz OH, Kiel MJ, Morrison SJ. Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood. 2006;108:737–744. doi: 10.1182/blood-2005-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin M, Romero X, de la Fuente MA, et al. CD84 functions as a homophilic adhesion molecule and enhances IFN-gamma secretion: adhesion is mediated by Ig-like domain 1. J Immunol. 2001;167:3668–3676. doi: 10.4049/jimmunol.167.7.3668. [DOI] [PubMed] [Google Scholar]
- 10.de la Fuente MA, Tovar V, Villamor N, et al. Molecular characterization and expression of a novel human leukocyte cell-surface marker homologous to mouse Ly-9. Blood. 2001;97:3513–3520. doi: 10.1182/blood.v97.11.3513. [DOI] [PubMed] [Google Scholar]
- 11.Romero X, Zapater N, Calvo M, et al. CD229 (Ly9) lymphocyte cell surface receptor interacts homophilically through its N-terminal domain and relocalizes to the immunological synapse. J Immunol. 2005;174:7033–7042. doi: 10.4049/jimmunol.174.11.7033. [DOI] [PubMed] [Google Scholar]
- 12.Saborit-Villarroya I, Del Valle JM, Romero X, et al. The adaptor protein 3BP2 binds human CD244 and links this receptor to Vav signaling, ERK activation, and NK cell killing. J Immunol. 2005;175:4226–4235. doi: 10.4049/jimmunol.175.7.4226. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immunedeficient mice. Proc Natl Acad Sci U S A. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNerney ME, Kumar V. The CD2 family of natural killer cell receptors. Curr Top Microbiol Immunol. 2006;298:91–120. doi: 10.1007/3-540-27743-9_5. [DOI] [PubMed] [Google Scholar]
- 15.Brown MH, Boles K, van der Merwe PA, Kumar V, Mathew PA, Barclay AN. 2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J Exp Med. 1998;188:2083–2090. doi: 10.1084/jem.188.11.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao QL, Shah AJ, Thiemann FT, Smogorzewska EM, Crooks GM. A functional comparison of CD34+ CD38− cells in cord blood and bone marrow. Blood. 1995;86:3745–3753. [PubMed] [Google Scholar]
- 17.Tangye SG, Phillips JH, Lanier LL. The CD2-subset of the Ig superfamily of cell surface molecules: receptor-ligand pairs expressed by NK cells and other immune cells. Semin Immunol. 2000;12:149–157. doi: 10.1006/smim.2000.0217. [DOI] [PubMed] [Google Scholar]
- 18.Zaiss M, Hirtreiter C, Rehli M, et al. CD84 expression on human hematopoietic progenitor cells. Exp Hematol. 2003;31:798–805. doi: 10.1016/s0301-472x(03)00187-5. [DOI] [PubMed] [Google Scholar]
- 19.De Bruyn C, Delforge A, Lagneaux L, Bron D. Characterization of CD34+ subsets derived from bone marrow, umbilical cord blood and mobilized peripheral blood after stem cell factor and interleukin 3 stimulation. Bone Marrow Transplant. 2000;25:377–383. doi: 10.1038/sj.bmt.1702145. [DOI] [PubMed] [Google Scholar]
- 20.Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998;4:1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- 21.Sintes J, Vidal-Laliena M, Romero X, Tovar V, Engel P. Characterization of mouse CD229 (Ly9), a leukocyte cell surface molecule of the CD150 (SLAM) family. Tissue Antigens. 2007;70:355–362. doi: 10.1111/j.1399-0039.2007.00909.x. [DOI] [PubMed] [Google Scholar]


