Abstract
Prenatal diagnosis has been shown to improve preoperative morbidity in newborns with congenital heart defects (CHDs), but there are conflicting data as to the association with mortality. We performed a population-based, retrospective, cohort study of infants with prenatally versus postnatally diagnosed CHDs from 1994 to 2005 as ascertained by the Metropolitan Atlanta Congenital Defects Program. Among infants with isolated CHDs, we estimated 1-year Kaplan-Meier survival probabilities for prenatal versus postnatal diagnosis and estimated Cox proportional hazard ratios adjusted for critical CHD status, gestational age, and maternal race/ethnicity. Of 539,519 live births, 4,348 infants had CHDs (411 prenatally diagnosed). Compared with those with noncritical defects, those with critical defects were more likely to be prenatally diagnosed (58% vs 20%, respectively, p <0.001). Of the 3,146 infants with isolated CHDs, 1-year survival rate was 77% for those prenatally diagnosed (n = 207) versus 96% for those postnatally diagnosed (n = 2,939, p <0.001). Comparing 1-year survival rate among those with noncritical CHDs alone (n = 2,455) showed no difference between prenatal and postnatal diagnoses (96% vs 98%, respectively, p = 0.26), whereas among those with critical CHDs (n = 691), prenatally diagnosed infants had significantly lower survival rate (71% vs 86%, respectively, p <0.001). Among infants with critical CHDs, the adjusted hazard ratio for 1-year mortality rate for those prenatally versus postnatally (reference) diagnosed was 2.51 (95% confidence interval 1.72 to 3.66). In conclusion, prenatal diagnosis is associated with lower 1-year survival rate for infants with isolated critical CHDs but shows no change for those with isolated noncritical CHDs. More severe disease among the critical CHD subtypes diagnosed prenatally might explain these findings.
Conflicting results as to whether prenatal diagnosis leads to decreased preoperative and postoperative mortalities have been reported in studies examining hypoplastic left heart syndrome (HLHS)1–3 and transposition of the great arteries.1,4 A lack of definitive evidence regarding mortality outcomes may be due in part to the difficulties in obtaining adequate patient numbers when examining specific defects at a single center.5 In addition, few studies have examined survival beyond the perioperative period. The objective of our study was to examine the 1-year survival rate of infants with prenatally versus postnatally diagnosed congenital heart defects (CHDs) in a large population-based cohort. We hypothesized that prenatal diagnosis would be associated with improved long-term survival rate.
Methods
Established in 1967, the Centers for Disease Control and Prevention's Metropolitan Atlanta Congenital Defects Program (MACDP) is an active population-based surveillance system for major birth defects among infants, fetuses, and children born to residents of the 5 central counties of metropolitan Atlanta.6 The MACDP operates in collaboration with the Georgia Department of Public Health and has approval of the Centers for Disease Control and Prevention's Institutional Review Board. Trained abstractors visit area birth and pediatric hospitals, maternal-fetal medicine departments, and outpatient perinatal offices to identify affected pregnancies and children in whom a birth defect is diagnosed before 6 years of age. Their medical records are reviewed, and demographic and clinical information collected. Cases in the MACDP are coded using a modified British Pediatric Association code. All cases with CHDs undergo review and classification by clinical experts in pediatric cardiology according to a standard nomenclature adopted from the Society of Thoracic Surgeons and based on current understanding of development morphogenesis.7
For this analysis, prenatal echocardiographic records were obtained from metropolitan Atlanta area pediatric cardiology clinics and were matched to cases in the MACDP. Cases for which no documented prenatal diagnosis existed were assumed to have been diagnosed post-natally. Survival status for live born infants was determined through a review of available clinical records, linkage with death certificates from the Office of Vital Records, Georgia Department of Public Health, or linkage with the National Death Index. Echocardiographic records were available starting from 1994, and National Death Index records available through 2006. With 1-year mortality rate as the primary outcome, the birth cohort was limited to infants born from January 1, 1994, to December 31, 2005.
Potential covariates for the association between timing of diagnosis (prenatal vs postnatal) and 1-year mortality rate included critical CHD (CCHD) status (critical vs noncritical), gestational age at birth (≤36 vs >36 weeks), neighborhood poverty level (<20% of population in census tract living in poverty vs ≥20%), birth weight (<2,500 vs ≥2,500 g), maternal race/ethnicity (white non-Hispanic vs all others), and maternal age.
For this study, we defined CCHDs as 12 defects that are likely to require intervention within the first year of life and are likely to present postnatally with hypoxemia some or most of the time.8 These 12 defects consisted of 7 primary targets of pulse oximetry screening (HLHS, pulmonary atresia, tetralogy of Fallot, transposition of the great arteries, tricuspid atresia, truncus arteriosus, and total anomalous pulmonary venous return) and 5 secondary targets (coarctation of the aorta, double-outlet right ventricle, Ebstein's anomaly, interrupted aortic arch, and single ventricle). As disease severity is not routinely collected by the MACDP, defects such as critical pulmonary stenosis and critical aortic stenosis were not considered as CCHDs.
Chi-square analyses were performed to compare baseline characteristics of each covariate between the prenatally and postnatally diagnosed cohorts. Survival probabilities were estimated using Kaplan-Meier methods, and the log-rank test was used to determine significance (p <0.05). All infants in the MACDP identified with a CHD were included in the baseline statistical summary, but only infants with isolated CHDs (those without chromosomal abnormalities or noncardiac defects) were included in the Kaplan-Meier survival curves or proportional hazards analyses. Covariates were also analyzed using univariate logistic regression modeling, with death at 1 year as the outcome. Covariates that were significantly different between prenatal and post-natal cohorts and were also significantly associated with 1-year mortality rate (p <0.05) were identified as potential confounders and included in Cox proportional hazards models to obtain adjusted hazard ratios for mortality. Finally, a separate Kaplan-Meier curve was constructed to compare 1-year survival rate based on timing of diagnosis for those with isolated CCHDs: prenatal diagnosis versus early postnatal diagnosis (<1 day of age) versus late post-natal diagnosis (>1 day of age). All analyses were performed in SAS, version 9.3 (Cary, North Carolina).
Results
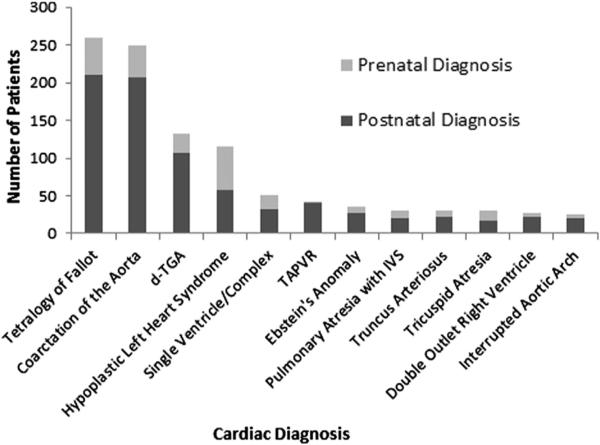
Of the 539,519 live births in the 5-county metropolitan Atlanta area from 1994 to 2005, 4,348 (0.8%) were identified as having CHDs. Overall, 411 (9.5%) were diagnosed prenatally, with this rate increasing from 6.6% (3.5% for non-CCHDs and 15% for CCHDs) in 1994 to 1999 to 11.4% (6.3% for non-CCHDs and 30% for CCHDs) in 2000 to 2005. Prenatal detection rates for CCHDs are illustrated in Figure 1, ranging from 2% for total anomalous pulmonary venous return to 50% for HLHS.
Figure 1.
Detection of CCHDs by prenatal echocardiography in Atlanta, Georgia, 1994 to 2005. IVS = intact ventricular septum; TAPVR = total anomalous pulmonary venous return; TGA = transposition of the great arteries.
Comparing infants with prenatally versus postnatally diagnosed CHDs, significant differences were seen with respect to the proportion of infants with CCHDs, associated defects, gestational age, and maternal race/ethnicity. No significant differences were seen with respect to neighborhood poverty level, low birth weight, or maternal age. All covariates were significantly associated with 1-year mortality rate by logistic regression. Among all infants with CHDs, those diagnosed prenatally had a significantly greater unadjusted 1-year mortality rate (33%) compared with those diagnosed postnatally (8.8%; Table 1).
Table 1.
Baseline characteristics for prenatally versus postnatally diagnosed congenital heart defects (CHDs) in metropolitan Atlanta, Georgia: 1994 to 2005
| Variable | Prenatally Diagnosed, n = 411 (%)* | Postnatally Diagnosed, n = 3,937 (%)* | p Value |
|---|---|---|---|
| CCHD† | 238 (58) | 769 (20) | <0.001 |
| Associated defects | |||
| None (isolated) | 207 (50) | 2,939 (75) | <0.001 |
| Multiple CHD | 70 (17) | 453 (12) | 0.001 |
| Laterality defects | 33 (8) | 47 (1) | <0.001 |
| Chromosomal abnormality | 101 (25) | 498 (13) | <0.001 |
| Gestational age (weeks) | |||
| <36 | 107 (27) | 989 (27) | <0.001 |
| 37–38 | 146 (37) | 955 (26) | |
| 39–40 | 126 (32) | 1,404 (39) | |
| >40 | 12 (3) | 280 (8) | |
| Neighborhood poverty level‡ | |||
| 0–4.9% | 127 (32) | 1,287 (34) | 0.66 |
| 5.0–9.9% | 113 (28) | 1,056 (28) | |
| 10.0–19.9% | 100 (25) | 964 (26) | |
| ≥20% | 56 (14) | 459 (12) | |
| Low birth weight, <2,500 g | 120 (29) | 988 (25) | 0.07 |
| Race/ethnicity | |||
| White, non-Hispanic | 177 (43) | 1,757 (45) | 0.002 |
| Black, non-Hispanic | 170 (41) | 1,360 (35) | |
| Hispanic | 38 (9) | 609 (15) | |
| Others | 26 (6) | 211 (5) | |
| Maternal age (yrs) | |||
| <20 | 37 (9) | 325 (8) | 0.14 |
| 20–24 | 76 (18) | 727 (18) | |
| 25–29 | 82 (20) | 984 (25) | |
| ≥30 | 216 (53) | 1,901 (48) | |
| 1-yr mortality rate | 137 (33) | 345 (9) | <0.001 |
Not all subcategories sum to total n because of missing values.
Defined as 7 primary CHD targets for pulse oximetry screening (HLHS, truncus arteriosus, tricuspid atresia, total anomalous pulmonary venous return, pulmonary atresia, tetralogy of Fallot, and transposition of the great arteries) plus 5 secondary targets for pulse oximetry screening (interrupted aortic arch, coarctation of the aorta, Ebstein's anomaly, single ventricle, and double-outlet right ventricle).
Defined by the percentage of residents below the poverty level in census tract associated with the maternal address at the time of the child's birth.
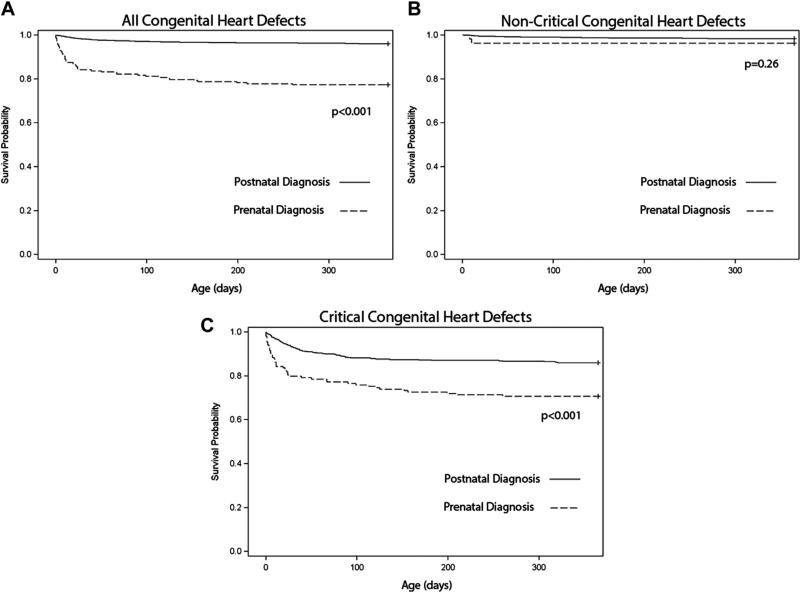
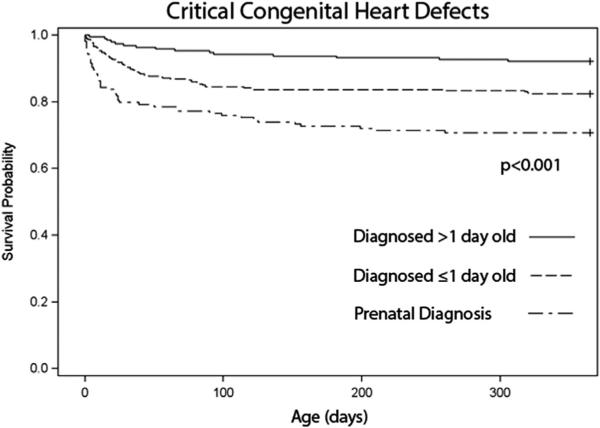
Isolated CHDs accounted for 3,146 cases in the MACDP from 1994 to 2005. Kaplan-Meier survival curves showed significantly decreased 1-year survival rate for prenatally versus postnatally diagnosed CHDs (77% vs 96%, respectively, p <0.001; Figure 2). When stratifying this group into non- (n = 2,455) and CCHDs (n = 691), it was clear that the survival difference was predominantly driven by the CCHD cohort. No difference in survival rate was seen among non-CCHDs (96% vs 98%, p = 0.26; Figure 2), but among CCHDs, prenatally diagnosed infants had lower 1-year survival rate compared with postnatally diagnosed infants (71% vs 86%, respectively, p <0.001; Figure 2). On further analysis of the postnatally diagnosed CCHD cohort, 1-year survival rate was 82% for those diagnosed ≤1 day of life (n = 340) and 92% for those diagnosed beyond ≤1 day of life (n = 189, p <0.001; Figure 3).
Figure 2.
One-year survival rate for infants with isolated CHDs by prenatal versus postnatal diagnosis: Atlanta, Georgia, 1994 to 2005. (A) All CHDs; (B) non-CCHDs; and (C) CCHDs, defined as 7 primary CHD targets for pulse oximetry screening (HLHS, truncus arteriosus, tricuspid atresia, total anomalous pulmonary venous return, pulmonary atresia, tetralogy of Fallot, and transposition of the great arteries) plus 5 secondary targets for pulse oximetry screening (interrupted aortic arch, coarctation of the aorta, Ebstein's anomaly, single ventricle, and double-outlet right ventricle).
Figure 3.
One-year survival rate for infants with isolated CCHDs by age at diagnosis: Atlanta, Georgia, 1994 to 2005.
These findings were corroborated in the adjusted analysis. When examining 1-year mortality rate using proportional hazards regression modeling among isolated CHDs, prenatally diagnosed infants had a hazard of mortality 6.86 times greater than postnatally diagnosed infants (95% confidence interval 4.86 to 9.68), adjusted for gestational age and stratified on maternal race/ethnicity (a variable which violated the proportional hazards assumption but satisfied the no interaction assumption). When the analysis was limited to the CCHD cohort, the adjusted hazard ratio for prenatally versus postnatally diagnosed infants was 2.51 (95% confidence interval 1.72 to 3.66). Among non-CCHDs, the adjusted hazard ratio for prenatally versus postnatally diagnosed infants was 1.79 (95% confidence interval 0.43 to 7.44) but did not meet significance (Table 2).
Table 2.
Stratified Cox proportional hazard ratios for one-year mortality rate for prenatal versus postnatal (referent) diagnosis of congenital heart defects (CHDs): Atlanta, Georgia, 1994 to 2005
| Variable | Hazard Ratio* | 95% Confidence Interval | p Value |
|---|---|---|---|
| All isolated CHDs | 6.86 | 4.86–9.68 | <0.001 |
| Isolated non-CCHDs | 1.79 | 0.43–7.44 | 0.43 |
| Isolated CCHDs | 2.51 | 1.72–3.66 | <0.001 |
All hazard ratios adjusted for gestational age. Models were stratified by maternal race/ethnicity.
Discussion
In this large population-based study, we found significantly decreased 1-year survival rate among infants with prenatally diagnosed CHDs compared with those with postnatally diagnosed CHDs. That is, prenatal diagnosis offered no survival benefit and may even portend a poorer prognosis. This finding is predominantly driven by the decreased 1-year survival rate in children with CCHDs. There was no difference in survival rate among infants with non-CCHDs.
Our study thus adds to a growing body of literature in which the survival benefit of prenatal diagnosis is unclear. Despite Tworetzky et al3 demonstrating a survival benefit among HLHS infants diagnosed prenatally and Franklin et al9 demonstrating benefit for prenatally diagnosed coarctation of the aorta, several other studies have failed to demonstrate such benefit. A recent retrospective analysis of 81 patients with HLHS from 1999 to 2010 found no survival-to-discharge advantage among the 49 prenatally diagnosed patients,10 a finding that has been similarly demonstrated in other single-center studies with regard to HLHS and transposition of the great arteries1,2,11,12 as well as pulmonary atresia with intact ventricular septum.13
What could be a possible explanation for our paradoxical finding of earlier detection being associated with poorer 1-year survival rate? We believe that the answer lies in the severity of disease. That is, there is a spectrum of disease even within specific diagnoses. Those cases that are more severely affected may be more likely to be detected prenatally, and likewise those cases that are more severely affected are more likely to have poorer outcomes. For example, a recent evaluation of children with pulmonary atresia with intact ventricular septum found that those with a prenatal diagnosis represented a more severe spectrum of disease and had poorer neonatal outcomes.14 Similarly, in patients with HLHS, those diagnosed prenatally have been found to be more likely to have various risk factors for mortality compared with those diagnosed postnatally (obstructed pulmonary venous return, right ventricular dysfunction, other associated cardiac defects, low birth weight or prematurity, and noncardiovascular anomalies).14 Therefore, we do not believe that prenatal diagnosis has any causal relation with poorer outcomes. Rather, prenatal diagnosis for a child with a CCHD represents a useful prognostic factor that suggests poorer survival rate compared with a child with a CCHD without prenatal diagnosis. Furthermore, as Figure 2 demonstrates, earlier diagnosis, whether it be prenatal or shortly after birth, is associated with poorer outcomes compared with later diagnosis, likely as a result of severity of disease.
Although our study does not show that prenatal diagnosis is associated with a 1-year survival benefit, there may be other important benefits of prenatal diagnosis. For instance, prenatal diagnosis has been well described to be associated with improvements in preoperative condition, with reductions in morbidity such as hypoxemia, need for invasive respiratory support, and metabolic acidosis.1–5,9–13,15–19 Furthermore, early detection has also been shown to allow for better parental counseling and delivery planning.5,15,20–23 Unfortunately, our population-based study was not able to evaluate long-term outcomes other than survival.
A major strength of this study is its large and diverse population-based cohort. An acknowledged limitation of many studies examining survival outcomes and prenatal diagnosis of CHDs is the difficulty in obtaining adequate patient numbers in single centers.5,21 The MACDP is an active case ascertainment system, which attempts to identify all occurrences of major malformations in the population through a review of a wide variety of clinical records. This review also facilitates accurate reporting of birth defects. These reports are then reviewed by clinical experts in pediatric cardiology and classified in a manner that optimizes accuracy for surveillance and research.24 This large and well-classified system allowed our study to limit analysis to isolated CHDs, thus minimizing the possibility that our findings are due to the poorer outcomes associated with chromosomal abnormalities and extracardiac defects.16,25,26
However, our study is not without limitations. There may have been cases in which prenatal echocardiography was performed at a site that is not covered by the MACDP, and/ or birth records were unable to be matched to echocardio-graphic records. Our need to assume that a lack of echo-cardiographic data meant a postnatal diagnosis is a potential source of misclassification bias, which would likely bias our results toward the null. A second limitation is the lack of data regarding severity. As mentioned previously, we believe that severity of disease, even within a single defect type, may explain our findings. Unfortunately, the MACDP does not collect information regarding disease severity, and we were thus unable to control for this factor. A randomized control study of prenatal echocardiography would overcome this issue, but we do not believe that such a study would be ethical or feasible. Finally, because the MACDP also does not collect information regarding treatment (surgical or otherwise), we do not know the proportion of subjects who underwent surgical intervention. Therefore, our findings should be interpreted on a population basis and should not be construed to estimate surgical survival rate for those prenatally diagnosed versus those postnatally diagnosed.
Acknowledgment
The authors wish to acknowledge the MACDP staff members for their conscientious and skilled data collection efforts and the personnel at the hospitals, clinics, laboratories, and other facilities who helped to contribute data to the MACDP. The authors particularly wish to acknowledge Tiffany Colarusso, MD, MPH, for the efforts in classifying cases for the MACDP.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Kumar RK, Newburger JW, Gauvreau K, Kamenir SA, Hornberger LK. Comparison of outcome when hypoplastic left heart syndrome and transposition of the great arteries are diagnosed prenatally versus when diagnosis of these two conditions is made only postnatally. Am J Cardiol. 1999;83:1649–1653. doi: 10.1016/s0002-9149(99)00172-1. [DOI] [PubMed] [Google Scholar]
- 2.Mahle WT, Clancy RR, McGaurn SP, Goin JE, Clark BJ. Impact of prenatal diagnosis on survival and early neurologic morbidity in neonates with the hypoplastic left heart syndrome. Pediatrics. 2001;107:1277–1282. doi: 10.1542/peds.107.6.1277. [DOI] [PubMed] [Google Scholar]
- 3.Tworetzky W, McElhinney DB, Reddy VM, Brook MM, Hanley FL, Silverman NH. Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation. 2001;103:1269–1273. doi: 10.1161/01.cir.103.9.1269. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet D, Coltri A, Butera G, Fermont L, Le Bidois J, Kachaner J, Sidi D. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation. 1999;99:916–918. doi: 10.1161/01.cir.99.7.916. [DOI] [PubMed] [Google Scholar]
- 5.Kovalchin JP, Silverman NH. The impact of fetal echocardiography. Pediatr Cardiol. 2004;25:299–306. doi: 10.1007/s00246-003-0593-1. [DOI] [PubMed] [Google Scholar]
- 6.Correa A, Cragan JD, Kucik JE, Alverson CJ, Gilboa SM, Balakrishnan R, Strickland MJ, Duke CW, O'Leary LA, Riehle-Colarusso T, Siffel C, Gambrell D, Thompson D, Atkinson M, Chitra J. Reporting birth defects surveillance data 1968-2003. Birth Defects Res A. 2007;79:65–186. doi: 10.1002/bdra.20350. [DOI] [PubMed] [Google Scholar]
- 7.Riehle-Colarusso T, Strickland MJ, Reller MD, Mahle WT, Botto LD, Siffel C, Atkinson M, Correa A. Improving the quality of surveillance data on congenital heart defects in the metropolitan Atlanta congenital defects program. Birth Defects Res A. 2007;79:743–753. doi: 10.1002/bdra.20412. [DOI] [PubMed] [Google Scholar]
- 8.Mahle WT, Newburger JW, Matherne GP, Smith FC, Hoke TR, Koppel R, Gidding SS, Beekman RH, 3rd, Grosse SD. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation. 2009;120:447–458. doi: 10.1161/CIRCULATIONAHA.109.192576. [DOI] [PubMed] [Google Scholar]
- 9.Franklin O, Burch M, Manning N, Sleeman K, Gould S, Archer N. Prenatal diagnosis of coarctation of the aorta improves survival and reduces morbidity. Heart. 2002;87:67–69. doi: 10.1136/heart.87.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kipps AK, Feuille C, Azakie A, Hoffman JI, Tabbutt S, Brook MM, Moon-Grady AJ. Prenatal diagnosis of hypoplastic left heart syndrome in current era. Am J Cardiol. 2011;108:421–427. doi: 10.1016/j.amjcard.2011.03.065. [DOI] [PubMed] [Google Scholar]
- 11.Levey A, Glickstein JS, Kleinman CS, Levasseur SM, Chen J, Gersony WM, Williams IA. The impact of prenatal diagnosis of complex congenital heart disease on neonatal outcomes. Pediatr Cardiol. 2010;31:587–597. doi: 10.1007/s00246-010-9648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munn MB, Brumfield CG, Lau Y, Colvin EV. Prenatally diagnosed hypoplastic left heart syndrome—outcomes after postnatal surgery. J Matern-Fetal Neo M. 1999;8:147–150. doi: 10.1002/(SICI)1520-6661(199907/08)8:4<147::AID-MFM1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Tuo G, Volpe P, Bondanza S, Volpe N, Serafino M, De Robertis V, Zannini L, Pongiglione G, Calevo MG, Marasini M. Impact of prenatal diagnosis on outcome of pulmonary atresia and intact ventricular septum. J Matern-Fetal Neo M. 2012;25:669–674. doi: 10.3109/14767058.2011.587062. [DOI] [PubMed] [Google Scholar]
- 14.Samai C, Gomez C, Russel M, Parra D, Ludomirsky A. The fetus with hypoplastic left heart syndrome: risk factors and outcomes. J Am Coll Cardiol. 2003;41:483. [Google Scholar]
- 15.Berkley EM, Goens MB, Karr S, Rappaport V. Utility of fetal echo-cardiography in postnatal management of infants with prenatally diagnosed congenital heart disease. Prenatal Diag. 2009;29:654–658. doi: 10.1002/pd.2260. [DOI] [PubMed] [Google Scholar]
- 16.Copel JA, Tan AS, Kleinman CS. Does a prenatal diagnosis of congenital heart disease alter short-term outcome? Ultrasound Obst Gyn. 1997;10:237–241. doi: 10.1046/j.1469-0705.1997.10040237.x. [DOI] [PubMed] [Google Scholar]
- 17.Eapen RS, Rowland DG, Franklin WH. Effect of prenatal diagnosis of critical left heart obstruction on perinatal morbidity and mortality. Am J Perinat. 1998;15:237–242. doi: 10.1055/s-2007-993934. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto A, Aoyagi Y, Mitomo M, Endo K, Mochizuki I, Kaneko M, Fukuda Y, Momoi N, Hosoya M. Outcome of fetal echocardiography: a 17 year single-institution experience in Japan. Pediatr Int. 2012 doi: 10.1111/j.1442-200X.2012.03639.x. [DOI] [PubMed] [Google Scholar]
- 19.Landis BJ, Levey A, Levasseur SM, Glickstein JS, Kleinman CS, Simpson LL, Williams IA. Prenatal diagnosis of congenital heart disease and birth outcomes. Pediatr Cardiol. 2013;34:597–605. doi: 10.1007/s00246-012-0504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allan LD, Huggon IC. Counselling following a diagnosis of congenital heart disease. Prenatal Diag. 2004;24:1136–1142. doi: 10.1002/pd.1071. [DOI] [PubMed] [Google Scholar]
- 21.Chung ML, Lee BS, Kim EA, Kim KS, Pi SY, Oh YM, Park IS, Seo DM, Won HS. Impact of fetal echocardiography on trends in disease patterns and outcomes of congenital heart disease in a neonatal intensive care unit. Neonatology. 2010;98:41–46. doi: 10.1159/000264673. [DOI] [PubMed] [Google Scholar]
- 22.Clur SA, Van Brussel PM, Ottenkamp J, Bilardo CM. Prenatal diagnosis of cardiac defects: accuracy and benefit. Prenatal Diag. 2012;32:450–455. doi: 10.1002/pd.3837. [DOI] [PubMed] [Google Scholar]
- 23.Gedikbasi A, Oztarhan K, Yildirim G, Gul A, Ceylan Y. Counseling and outcomes of antenatally diagnosed congenital heart anomalies in Turkey. Anadolu Kardiyol Der. 2011;11:137–145. doi: 10.5152/akd.2011.035. [DOI] [PubMed] [Google Scholar]
- 24.Strickland MJ, Riehle-Colarusso TJ, Jacobs JP, Reller MD, Mahle WT, Botto LD, Tolbert PE, Jacobs ML, Lacour-Gayet FG, Tchervenkov CI, Mavroudis C, Correa A. The importance of nomenclature for congenital cardiac disease: implications for research and evaluation. Cardiol Young. 2008;18(Suppl 2):92–100. doi: 10.1017/S1047951108002515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JE, Jung KL, Kim SE, Nam SH, Choi SJ, Oh SY, Roh CR, Kim JH. Prenatal diagnosis of congenital heart disease: trends in pregnancy termination rate, and perinatal and 1-year infant mortalities in Korea between 1994 and 2005. J Obstet Gynaecol Re. 2010;36:474–478. doi: 10.1111/j.1447-0756.2010.01222.x. [DOI] [PubMed] [Google Scholar]
- 26.Montana E, Khoury MJ, Cragan JD, Sharma S, Dhar P, Fyfe D. Trends and outcomes after prenatal diagnosis of congenital cardiac malformations by fetal echocardiography in a well defined birth population, Atlanta, Georgia, 1990-1994. J Am Coll Cardiol. 1996;28:1805–1809. doi: 10.1016/S0735-1097(96)00381-6. [DOI] [PubMed] [Google Scholar]